FmocFF Peptide Hydrogel Is a Promising Matrix for Encapsulation and Controlled Release of the Anticancer Peptide Drug Bortezomib
Abstract
1. Introduction
2. Materials and Methods
2.1. Model and Simulation
2.2. Experimental Details/Materials and Methods
Materials
2.3. Methods
2.3.1. BTZ Standard Curve
2.3.2. Gel Formation and Characterization
2.3.3. Rheological Characterization
2.3.4. Casting Fmoc-FF–BTZ Hydrogels and Release Protocol
2.3.5. UV–Visible Spectroscopy
2.3.6. Field Emission-SEM (FESEM)
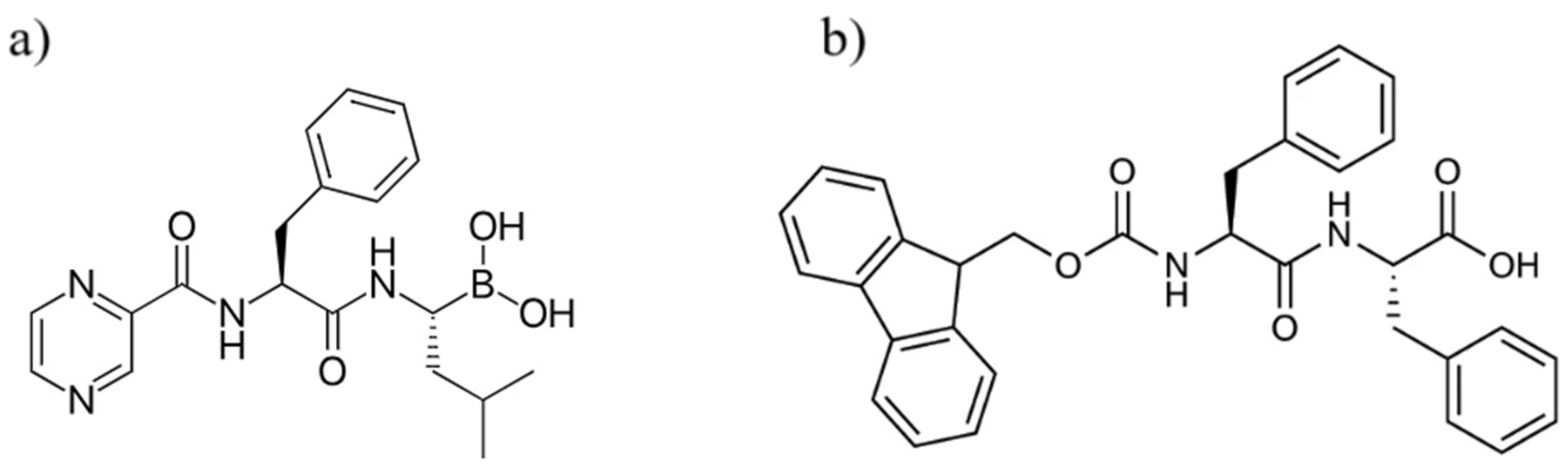
2.3.7. Mass Spectrometry Analysis
2.3.8. Proteasome Inhibitor Assay Protocol
3. Results and Discussion
3.1. Computational Evidence
3.1.1. The Potential of Mean Force (PMF)
3.1.2. Solvent Accessible Surface Area (SASA)
3.1.3. Mean Square Displacement (MSD)
3.2. Experimental Results
3.2.1. Gel Formation Kinetics, Drug Loading, and Encapsulation Efficiency
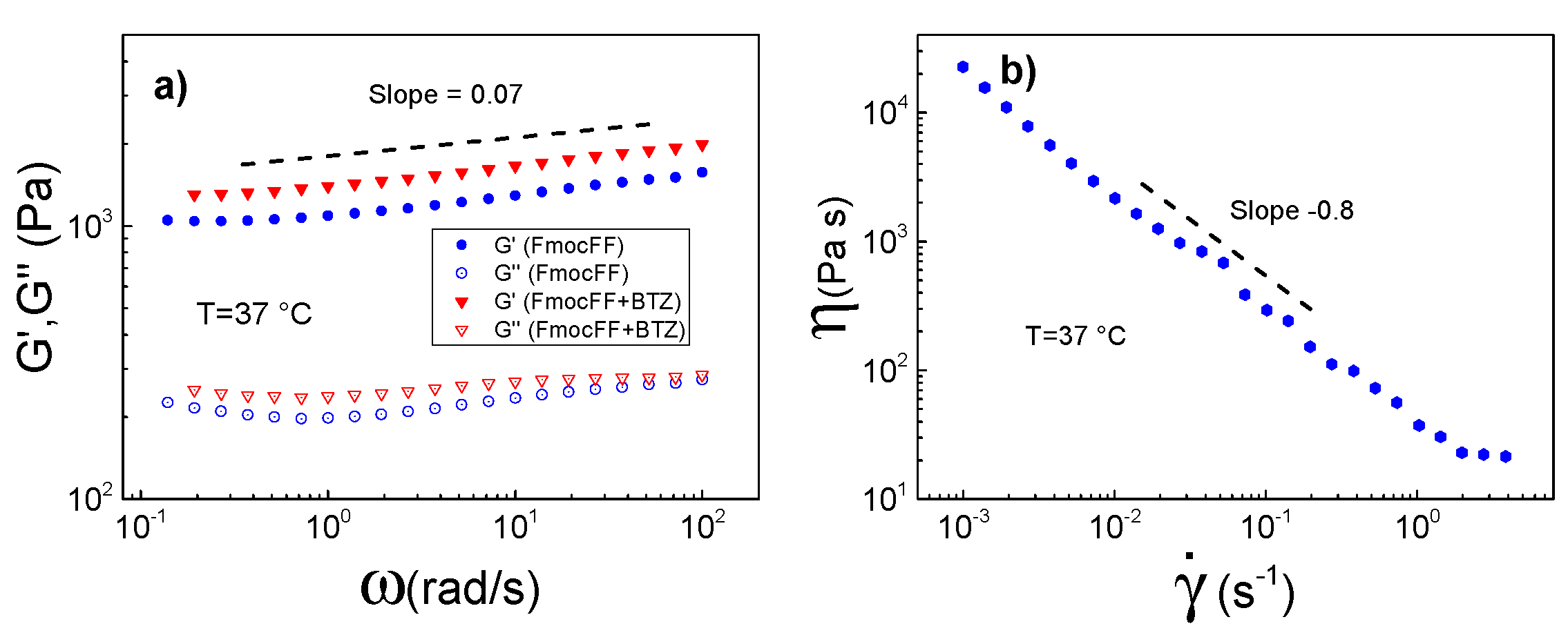
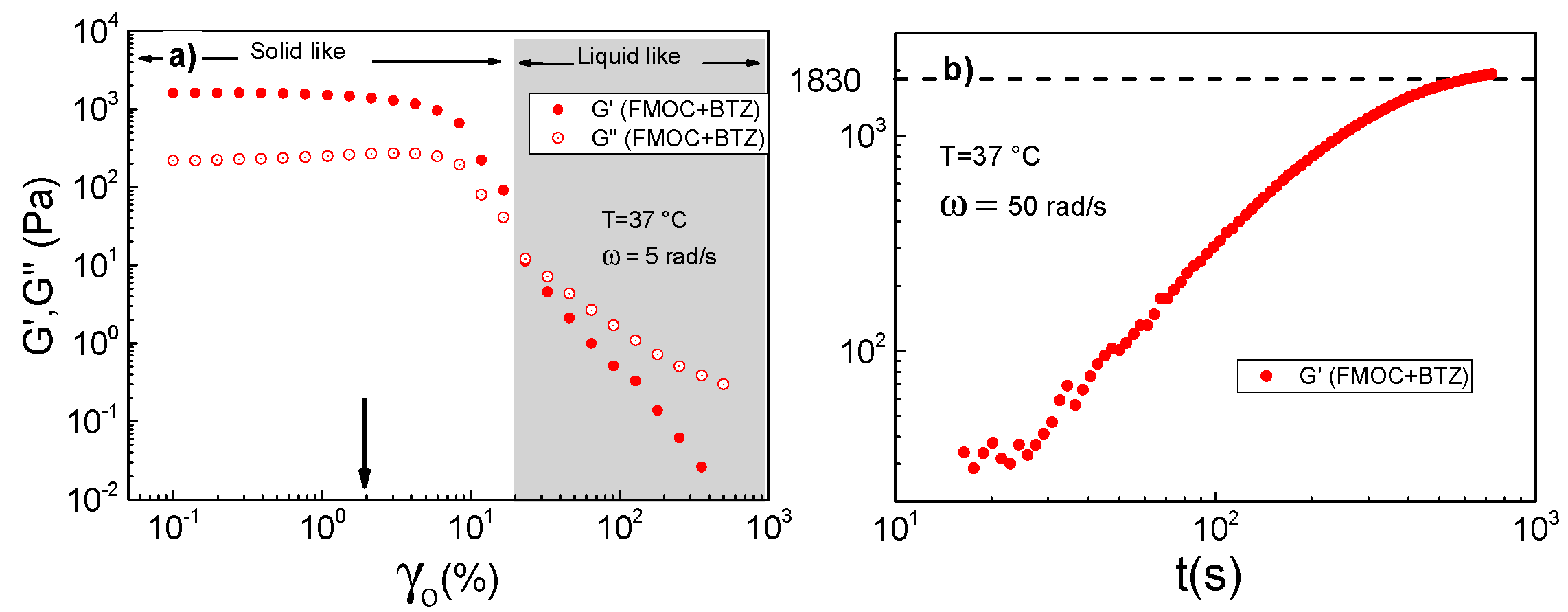
3.2.2. Rheological Properties of FmocFF and FmocFF + BTZ Complexes
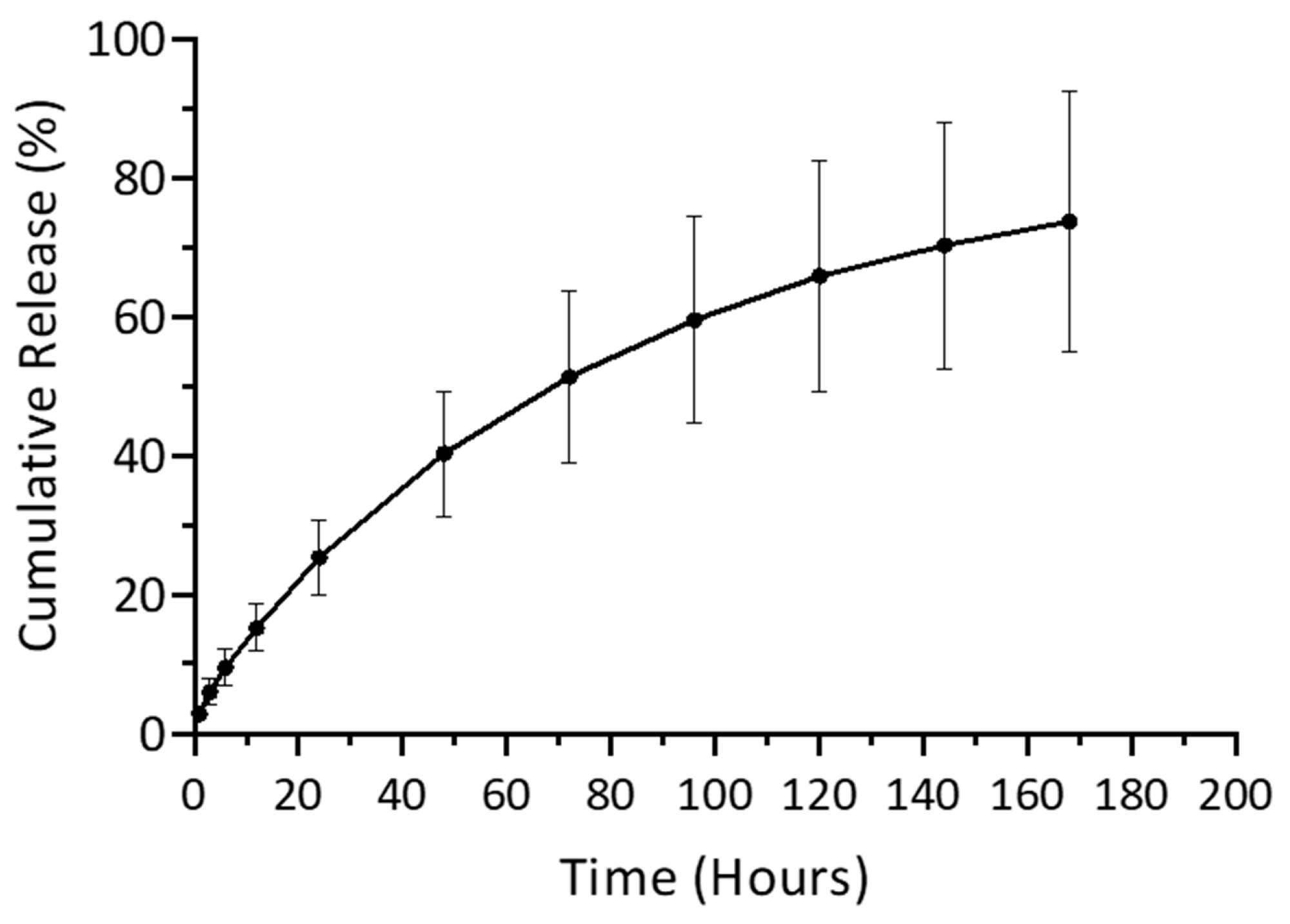
3.2.3. Release Evaluation with UV–Visible Spectroscopy
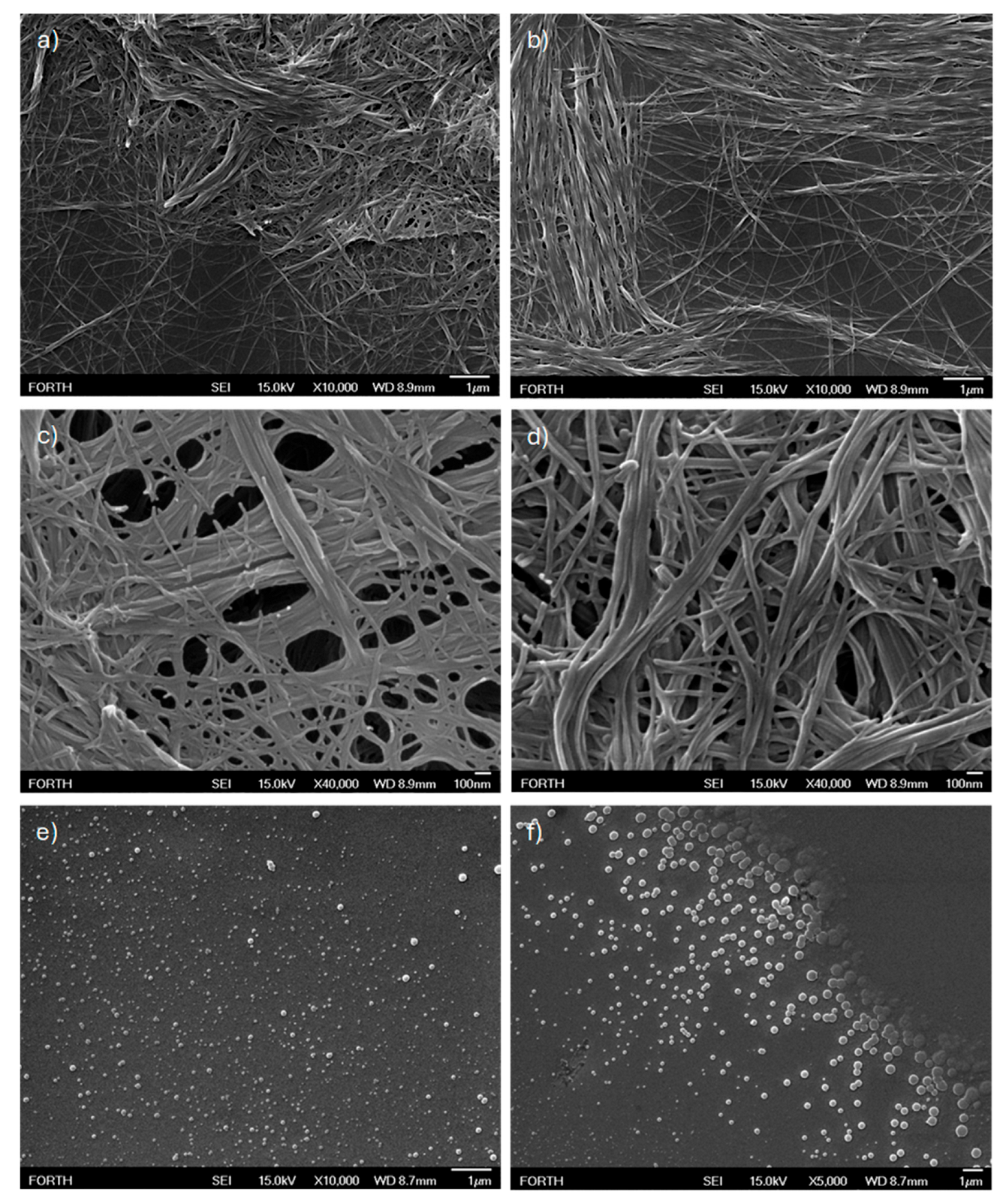
3.2.4. Structural Characterization with FESEM
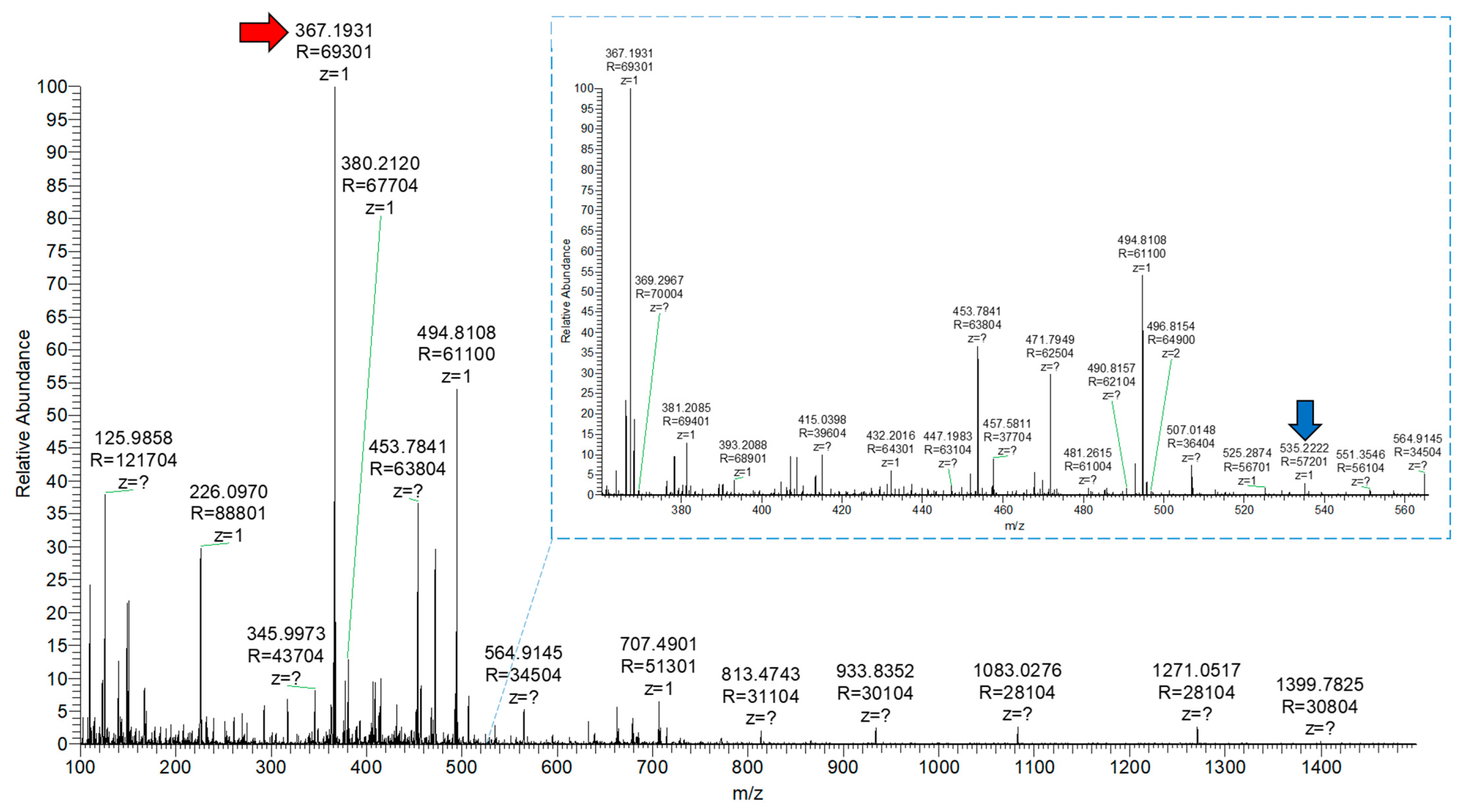
3.2.5. Release Evaluation with Mass Spectrometry
3.2.6. Discussion of the Possible Drug Release Mechanism
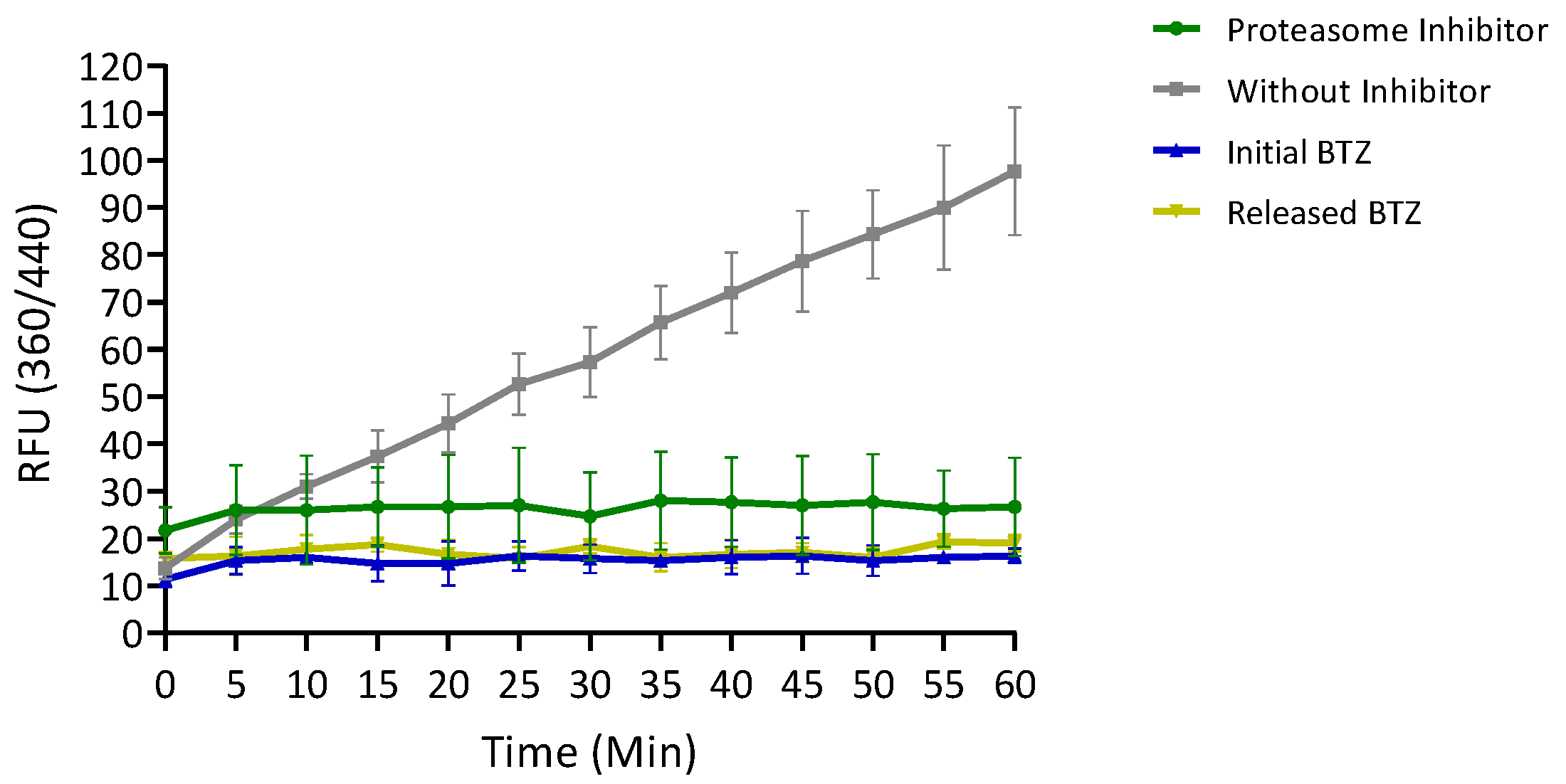
3.2.7. Evaluation of the Inhibitory Activity of Released BTZ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BTZ | Bortezomib |
| MM | Multiple myeloma |
| MCL | Mantle cell lymphoma |
| FmocFF | N-(fluorenylmethyloxycarbonyl)-Phenylalanine-Phenylalanine |
| HFIP | 1,1,1,3,3,3-Hexafluoro-2-propanol |
| DMSO | Dimethyl sulfoxide |
| EtOH | Ethanol |
| AMC | 7-amino-4-methylcoumarin |
| SEM | Scanning Electron Microscopy |
| FESEM | Field Emission Scanning Electron Microscopy |
| ESI | Electrospray Ionization |
| MD | Molecular Dynamics |
References
- Hu, Q.Y.; Sun, W.J.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Peng, S.; Yuan, X.M.; Li, H.J.; Wei, Y.N.; Zhou, B.L.; Ding, G.; Bai, J.K. Recent progress in nanocarrier-based drug delivery systems for antitumour metastasis. Eur. J. Med. Chem. 2023, 252, 115259. [Google Scholar] [CrossRef]
- Kumar, V.B.; Ozguney, B.; Vlachou, A.; Chen, Y.; Gazit, E.; Tamamis, P. Peptide Self-Assembled Nanocarriers for Cancer Drug Delivery. J. Phys. Chem. B 2023, 127, 1857–1871. [Google Scholar] [CrossRef]
- Vlachou, A.; Kumar, V.B.; Tiwari, O.S.; Rencus-Lazar, S.; Chen, Y.; Ozguney, B.; Gazit, E.; Tamamis, P. Co-Assembly of Cancer Drugs with Cyclo-HH Peptides: Insights from Simulations and Experiments. ACS Appl. Bio Mater. 2024, 7, 2309–2324. [Google Scholar] [CrossRef]
- Bross, P.F.; Kane, R.; Farrell, A.T.; Abraham, S.; Benson, K.; Brower, M.E.; Bradley, S.; Gobburu, J.V.; Goheer, A.; Lee, S.-L.; et al. Approval Summary for Bortezomib for Injection in the Treatment of Multiple Myeloma. Clin. Cancer Res. 2004, 10, 3954–3964. [Google Scholar] [CrossRef]
- Alwahsh, M.; Farhat, J.; Talhouni, S.; Hamadneh, L.; Hergenröder, R. Bortezomib advanced mechanisms of action in multiple myeloma, solid and liquid tumors along with its novel therapeutic applications. EXCLI J. 2023, 22, 146–168. [Google Scholar] [CrossRef]
- Dou, P.Q.; Zonder, A.J. Overview of Proteasome Inhibitor-Based Anti-cancer Therapies: Perspective on Bortezomib and Second Generation Proteasome Inhibitors versus Future Generation Inhibitors of Ubiquitin-Proteasome System. Curr. Cancer Drug Targets 2014, 14, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, R.; Jiang, X.; Li, Z.; Zhang, B. Progress on the Application of Bortezomib and Bortezomib-Based Nanoformulations. Biomolecules 2022, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Ramakrishnan, V.; Rajkumar, S.V. Bortezomib combination therapy in multiple myeloma. Semin. Hematol. 2012, 49, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J. Combination Therapy of Bortezomib with Novel Targeted Agents: An Emerging Treatment Strategy. Clin. Cancer Res. 2010, 16, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Huang, Y.; Torres-Lugo, M.; Ward, J.H.; Zhang, J. Physicochemical, foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng. 2000, 2, 9–29. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as Drug Delivery Systems: A Review of Current Characterization and Evaluation Techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chu, B.; Shi, K.; Wei, X.; Yang, P.; Chen, M.; Tang, M.; Li, S.; Wang, F.; Yang, X.; et al. Subcutaneous injection of a bortezomib-loaded thermosensitive hydrogel for the treatment of multiple myeloma. Chem. Eng. J. 2023, 455, 140600. [Google Scholar] [CrossRef]
- Pu, G.J.; Ren, C.H.; Li, D.X.; Wang, L.; Sun, J.T. A supramolecular hydrogel for the delivery of bortezomib. RSC Adv. 2014, 4, 50145–50147. [Google Scholar] [CrossRef]
- Zhang, S.; Marini, D.M.; Hwang, W.; Santoso, S. Design of nanostructured biological materials through self-assembly of peptides and proteins. Curr. Opin. Chem. Biol. 2002, 6, 865–871. [Google Scholar] [CrossRef]
- Zhang, S.G. Self-assembling peptides: From a discovery in a yeast protein to diverse uses and beyond. Protein Sci. 2020, 29, 2281–2303. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.B.P.; Gomes, V.; Ferreira, P.M.T.; Martins, J.A.; Jervis, P.J. Peptide-Based Supramolecular Hydrogels as Drug Delivery Agents: Recent Advances. Gels 2022, 8, 706. [Google Scholar] [CrossRef]
- Loo, Y.; Wong, Y.C.; Cai, E.Z.; Ang, C.H.; Raju, A.; Lakshmanan, A.; Koh, A.G.; Zhou, H.J.; Lim, T.C.; Moochhala, S.M.; et al. Ultrashort peptide nanofibrous hydrogels for the acceleration of healing of burn wounds. Biomaterials 2014, 35, 4805–4814. [Google Scholar] [CrossRef]
- Loo, Y.; Goktas, M.; Tekinay, A.B.; Guler, M.O.; Hauser, C.A.E.; Mitraki, A. Self-Assembled Proteins and Peptides as Scaffolds for Tissue Regeneration. Adv. Healthc. Mater. 2015, 4, 2557–2586. [Google Scholar] [CrossRef]
- Firoozi, S.; Pahlavan, S.; Ghanian, M.-H.; Rabbani, S.; Tavakol, S.; Barekat, M.; Yakhkeshi, S.; Mahmoudi, E.; Soleymani, M.; Baharvand, H. A Cell-Free SDKP-Conjugated Self-Assembling Peptide Hydrogel Sufficient for Improvement of Myocardial Infarction. Biomolecules 2020, 10, 205. [Google Scholar] [CrossRef]
- Li, Q.; Qi, G.; Lutter, D.; Beard, W.; Souza, C.R.S.; Highland, M.A.; Wu, W.; Li, P.; Zhang, Y.; Atala, A.; et al. Injectable Peptide Hydrogel Encapsulation of Mesenchymal Stem Cells Improved Viability, Stemness, Anti-Inflammatory Effects, and Early Stage Wound Healing. Biomolecules 2022, 12, 1317. [Google Scholar] [CrossRef]
- Martin, J.R.; Beegle, N.L.; Zhu, Y.; Hanisch, E.M. Subcutaneous Administration of Bortezomib: A Pilot Survey of Oncology Nurses. J. Adv. Pract. Oncol. 2015, 6, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kurtin, S.; Knop, C.S.; Milliron, T. Subcutaneous administration of bortezomib: Strategies to reduce injection site reactions. J. Adv. Pract. Oncol. 2012, 3, 406–410. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.E.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl-dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Diaferia, C.; Rosa, E.; Morelli, G.; Accardo, A. Fmoc-Diphenylalanine Hydrogels: Optimization of Preparation Methods and Structural Insights. Pharmaceuticals 2022, 15, 1048. [Google Scholar] [CrossRef]
- Orbach, R.; Adler-Abramovich, L.; Zigerson, S.; Mironi-Harpaz, I.; Seliktar, D.; Gazit, E. Self-Assembled Fmoc-Peptides as a Platform for the Formation of Nanostructures and Hydrogels. Biomacromolecules 2009, 10, 2646–2651. [Google Scholar] [CrossRef]
- Rosa, M.; Gallo, E.; Pellegrino, P.; Mercurio, F.A.; Leone, M.; Cascione, M.; Carrese, B.; Morelli, G.; Accardo, A.; Diaferia, C. Inclusion of Cationic Amphiphilic Peptides in Fmoc-FF Generates Multicomponent Functional Hydrogels. ACS Appl. Bio Mater. 2024, 8, 488–502. [Google Scholar] [CrossRef]
- Choe, R.; Yun, S.I. Fmoc-diphenylalanine-based hydrogels as a potential carrier for drug delivery. e-Polymers 2020, 20, 458–468. [Google Scholar] [CrossRef]
- Shim, J.; Kang, J.; Yun, S.I. Chitosan-dipeptide hydrogels as potential anticancer drug delivery systems. Int. J. Biol. Macromol. 2021, 187, 399–408. [Google Scholar] [CrossRef]
- Gallo, E.; Diaferia, C.; Smaldone, G.; Rosa, E.; Pecoraro, G.; Morelli, G.; Accardo, A. Fmoc-FF hydrogels and nanogels for improved and selective delivery of dexamethasone in leukemic cells and diagnostic applications. Sci. Rep. 2024, 14, 9940. [Google Scholar] [CrossRef] [PubMed]
- Bellotto, O.; Kralj, S.; De Zorzi, R.; Geremia, S.; Marchesan, S. Supramolecular hydrogels from unprotected dipeptides: A comparative study on stereoisomers and structural isomers. Soft Matter 2020, 16, 10151–10157. [Google Scholar] [CrossRef]
- Scarel, M.; Marchesan, S. Diketopiperazine Gels: New Horizons from the Self-Assembly of Cyclic Dipeptides. Molecules 2021, 26, 3376. [Google Scholar] [CrossRef]
- Tao, K.; Levin, A.; Adler-Abramovich, L.; Gazit, E. Fmoc-modified amino acids and short peptides: Simple bio-inspired building blocks for the fabrication of functional materials. Chem. Soc. Rev. 2016, 45, 3935–3953. [Google Scholar] [CrossRef]
- Divanach, P.; Fanouraki, E.; Mitraki, A.; Harmandaris, V.; Rissanou, A.N. Self-Assembly of Phenylalanine-Leucine, Leucine-Phenylalanine, and Cyclo(-leucine-phenylalanine) Dipeptides through Simulations and Experiments. J. Phys. Chem. B 2023, 127, 4208–4219. [Google Scholar] [CrossRef] [PubMed]
- Rissanou, A.N.; Georgilis, E.; Kasotakis, E.; Mitraki, A.; Harmandaris, V. Effect of Solvent on the Self-Assembly of Dialanine and Diphenylalanine Peptides. J. Phys. Chem. B 2013, 117, 3962–3975. [Google Scholar] [CrossRef]
- Divanach, P.; Fanouraki, E.; Mitraki, A.; Harmandaris, V.; Rissanou, A.N. Investigating the complexation propensity of self-assembling dipeptides with the anticancer peptide-drug Bortezomib: A computational study. Soft Matter 2023, 19, 8684–8697. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Chatterjee, S.; Debenedetti, P.G.; Stillinger, F.H.; Lynden-Bell, R.M. A computational investigation of thermodynamics, structure. dynamics and solvation behavior in modified water models. J. Chem. Phys. 2008, 128, 124511. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Zeng, S.; Ji, Y.; Shen, Y.; Zhu, R.; Wang, X.; Chen, L.; Chen, J. Molecular dynamics simulations of loading and unloading of drug molecule bortezomib on graphene nanosheets. RSC Adv. 2020, 10, 8744–8750. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef]
- Leach, A.R. Molecular Modelling: Principles and Applications, 2nd ed.; Prentice Hall: Harlow, UK, 2001. [Google Scholar]
- Neumann, R.M. Entropic approach to Brownian movement. Am. J. Phys. 1980, 48, 354–357. [Google Scholar] [CrossRef]
- Shrake, A.; Rupley, J.A. Environment and exposure to solvent of protein atoms. Lysozyme and insulin. J. Mol. Biol. 1973, 79, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Eisenhaber, F.; Lijnzaad, P.; Argos, P.; Sander, C.; Scharf, M. The double cubic lattice method: Efficient approaches to numerical integration of surface area and volume and to dot surface contouring of molecular assemblies. J. Comput. Chem. 1995, 16, 273–284. [Google Scholar] [CrossRef]
- Brooks, C.L. Computer simulation of liquids. J. Solut. Chem. 1989, 18, 99. [Google Scholar] [CrossRef]
- Orbach, R.; Mironi-Harpaz, I.; Adler-Abramovich, L.; Mossou, E.; Mitchell, E.P.; Forsyth, V.T.; Gazit, E.; Seliktar, D. The Rheological and Structural Properties of Fmoc-Peptide-Based Hydrogels: The Effect of Aromatic Molecular Architecture on Self-Assembly and Physical Characteristics. Langmuir 2012, 28, 2015–2022. [Google Scholar] [CrossRef]
- Winter, H.H.; Chambon, F. Analysis of linear viscoelasticity of a cross-linking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlassopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine To Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Apostolidou, C.P.; Kokotidou, C.; Platania, V.; Nikolaou, V.; Landrou, G.; Nikoloudakis, E.; Charalambidis, G.; Chatzinikolaidou, M.; Coutsolelos, A.G.; Mitraki, A. Antimicrobial Potency of Fmoc-Phe-Phe Dipeptide Hydrogels with Encapsulated Porphyrin Chromophores Is a Promising Alternative in Antimicrobial Resistance. Biomolecules 2024, 14, 226. [Google Scholar] [CrossRef] [PubMed]
- Yuran, S.; Razvag, Y.; Reches, M. Coassembly of Aromatic Dipeptides into Biomolecular Necklaces. Acs Nano 2012, 6, 9559–9566. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. “PubChem Compound Summary for CID 387447, Bortezomib” PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Bortezomib (accessed on 12 May 2024).
- Truong, W.T.; Su, Y.Y.; Gloria, D.; Braet, F.; Thordarson, P. Dissolution and degradation of Fmoc-diphenylalanine self-assembled gels results in necrosis at high concentrations in vitro. Biomater. Sci. 2015, 3, 298–307. [Google Scholar] [CrossRef]
- Moslabeh, F.G.Z.; Miar, S.; Habibi, N. In Vitro Self-Assembly of a Modified Diphenylalanine Peptide to Nanofibers Induced by the Eye Absent Enzyme and Alkaline Phosphatase and Its Activity against Breast Cancer Cell Proliferation. ACS Appl. Bio Mater. 2023, 6, 164–170. [Google Scholar] [CrossRef]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for pH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef]
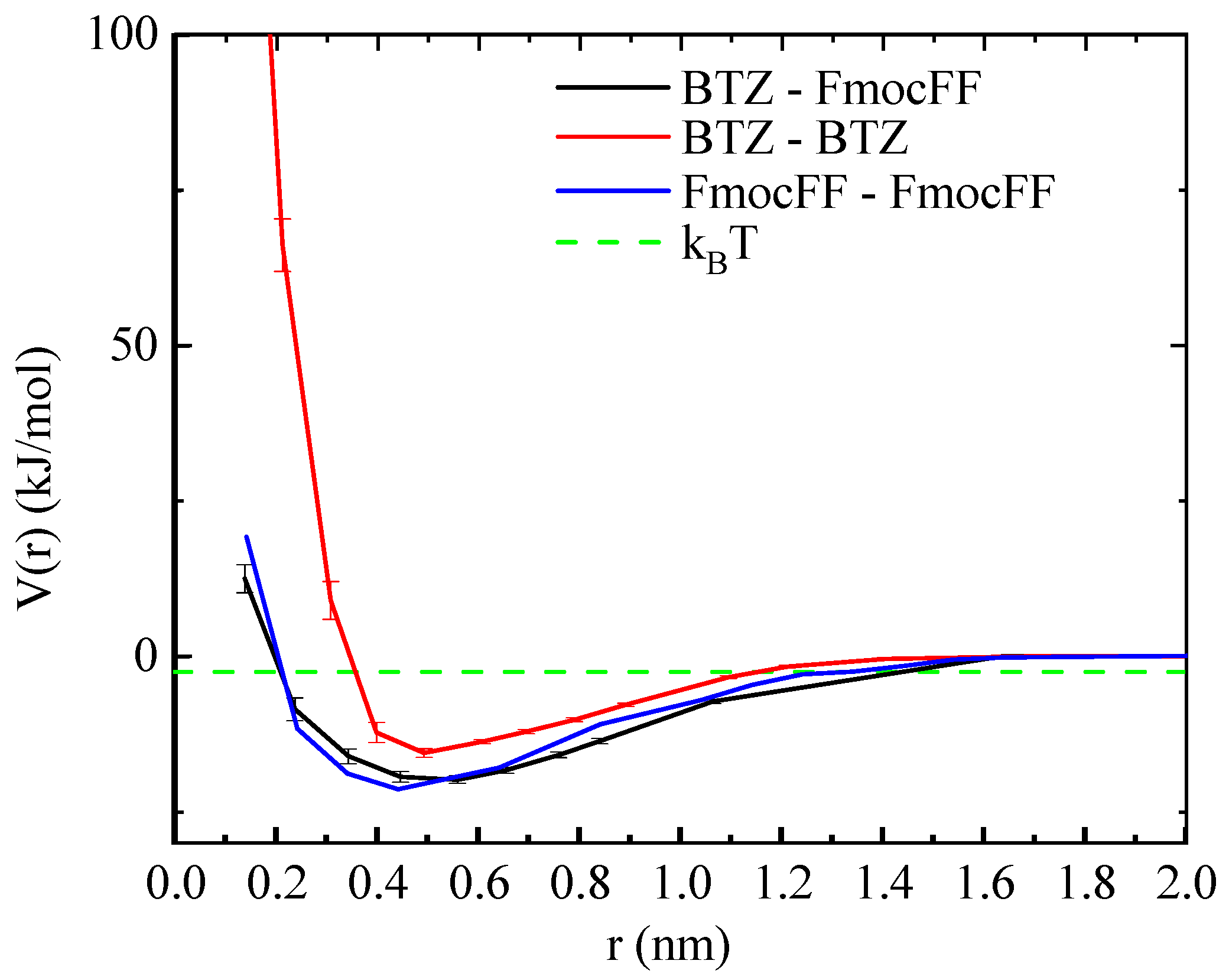
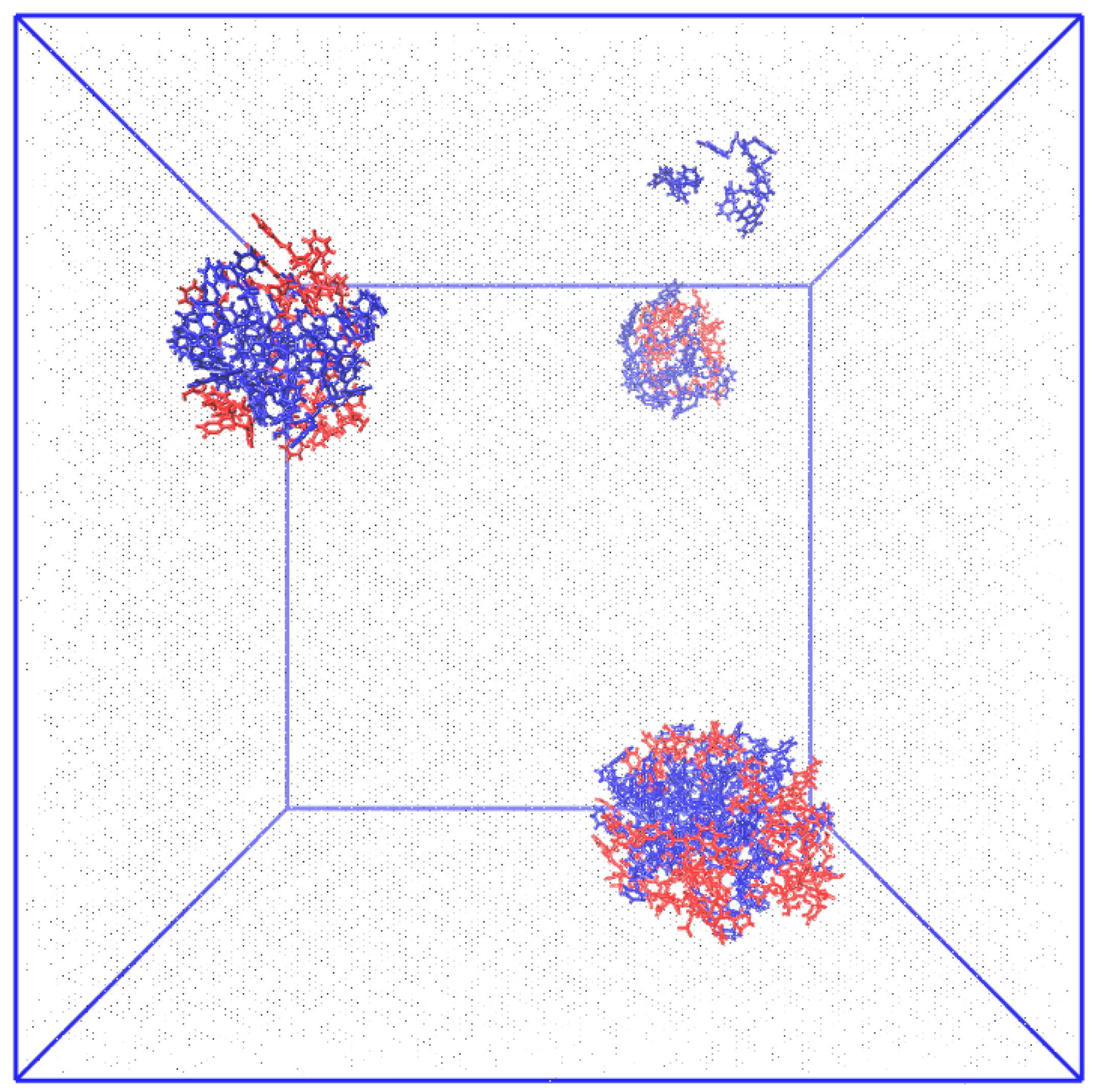
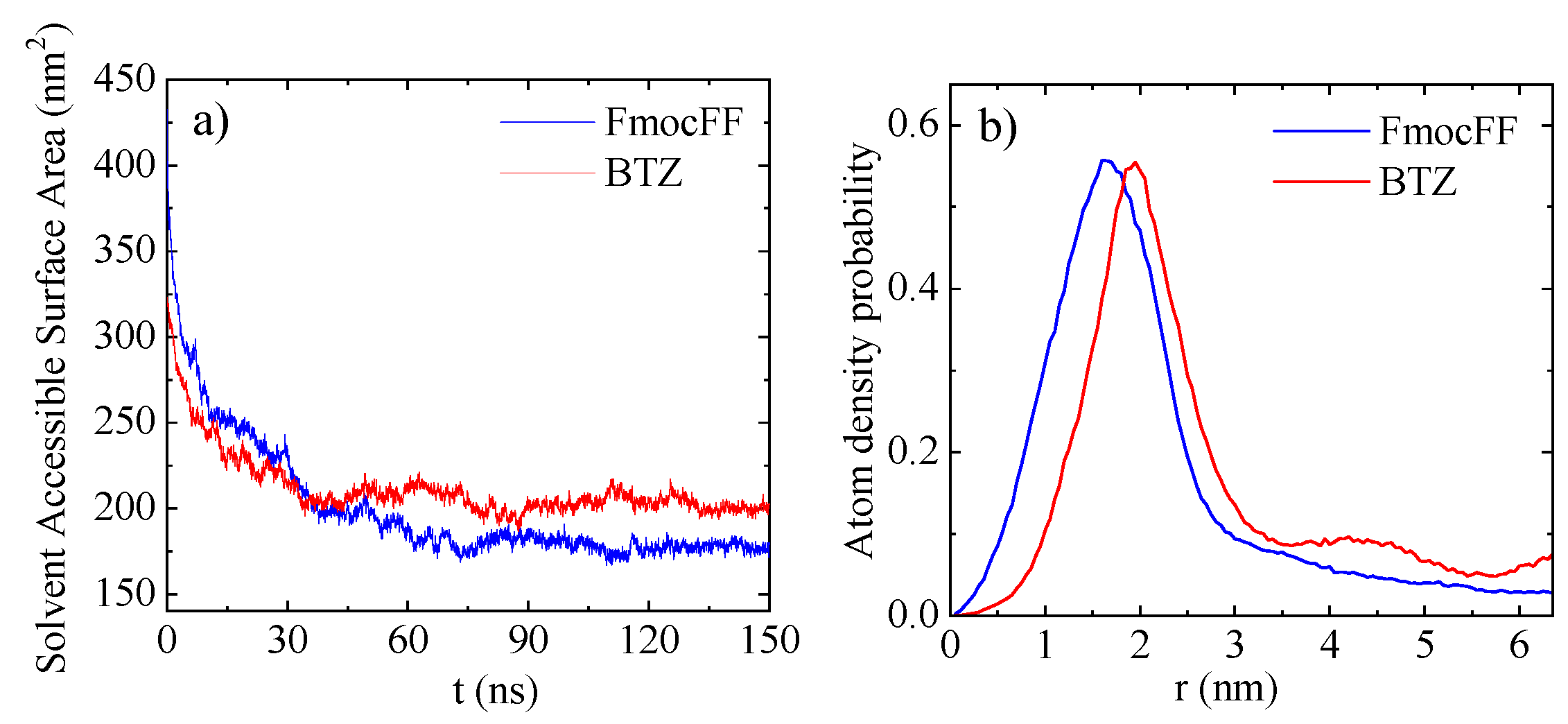
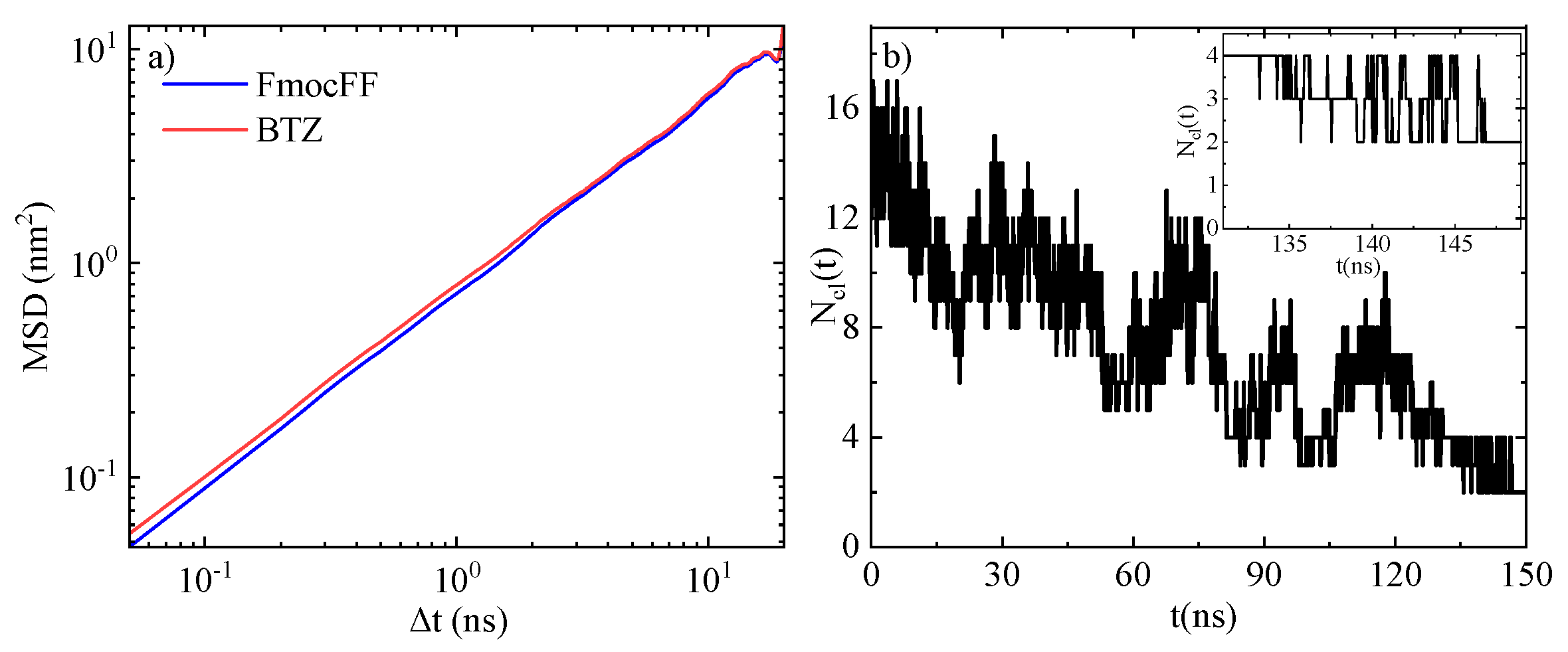
| System | # Molecules | # Water Molecules (SOL) | # Atoms | LBOX (nm) |
|---|---|---|---|---|
| BTZ–FmocFF | 50–50 | 48,379 | 150,387 | 12.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divanach, P.; Noti, A.; Vouvopoulos, P.; Athanasiou, T.; Kountourakis, N.; Harmandaris, V.; Rissanou, A.N.; Mitraki, A. FmocFF Peptide Hydrogel Is a Promising Matrix for Encapsulation and Controlled Release of the Anticancer Peptide Drug Bortezomib. Biomolecules 2025, 15, 839. https://doi.org/10.3390/biom15060839
Divanach P, Noti A, Vouvopoulos P, Athanasiou T, Kountourakis N, Harmandaris V, Rissanou AN, Mitraki A. FmocFF Peptide Hydrogel Is a Promising Matrix for Encapsulation and Controlled Release of the Anticancer Peptide Drug Bortezomib. Biomolecules. 2025; 15(6):839. https://doi.org/10.3390/biom15060839
Chicago/Turabian StyleDivanach, Peter, Antzela Noti, Panagiotis Vouvopoulos, Thanasis Athanasiou, Nikos Kountourakis, Vagelis Harmandaris, Anastassia N. Rissanou, and Anna Mitraki. 2025. "FmocFF Peptide Hydrogel Is a Promising Matrix for Encapsulation and Controlled Release of the Anticancer Peptide Drug Bortezomib" Biomolecules 15, no. 6: 839. https://doi.org/10.3390/biom15060839
APA StyleDivanach, P., Noti, A., Vouvopoulos, P., Athanasiou, T., Kountourakis, N., Harmandaris, V., Rissanou, A. N., & Mitraki, A. (2025). FmocFF Peptide Hydrogel Is a Promising Matrix for Encapsulation and Controlled Release of the Anticancer Peptide Drug Bortezomib. Biomolecules, 15(6), 839. https://doi.org/10.3390/biom15060839









