Biological Nanocarriers in Cancer Therapy: Cutting Edge Innovations in Precision Drug Delivery
Abstract
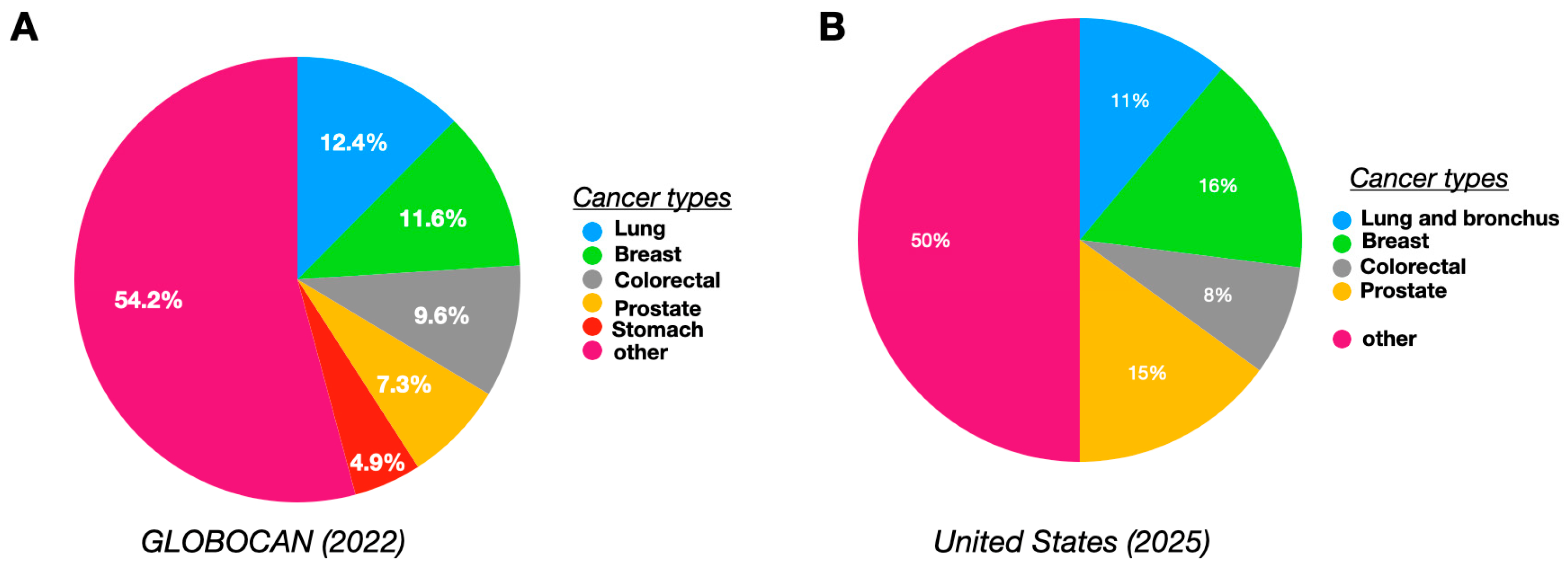
1. Introduction
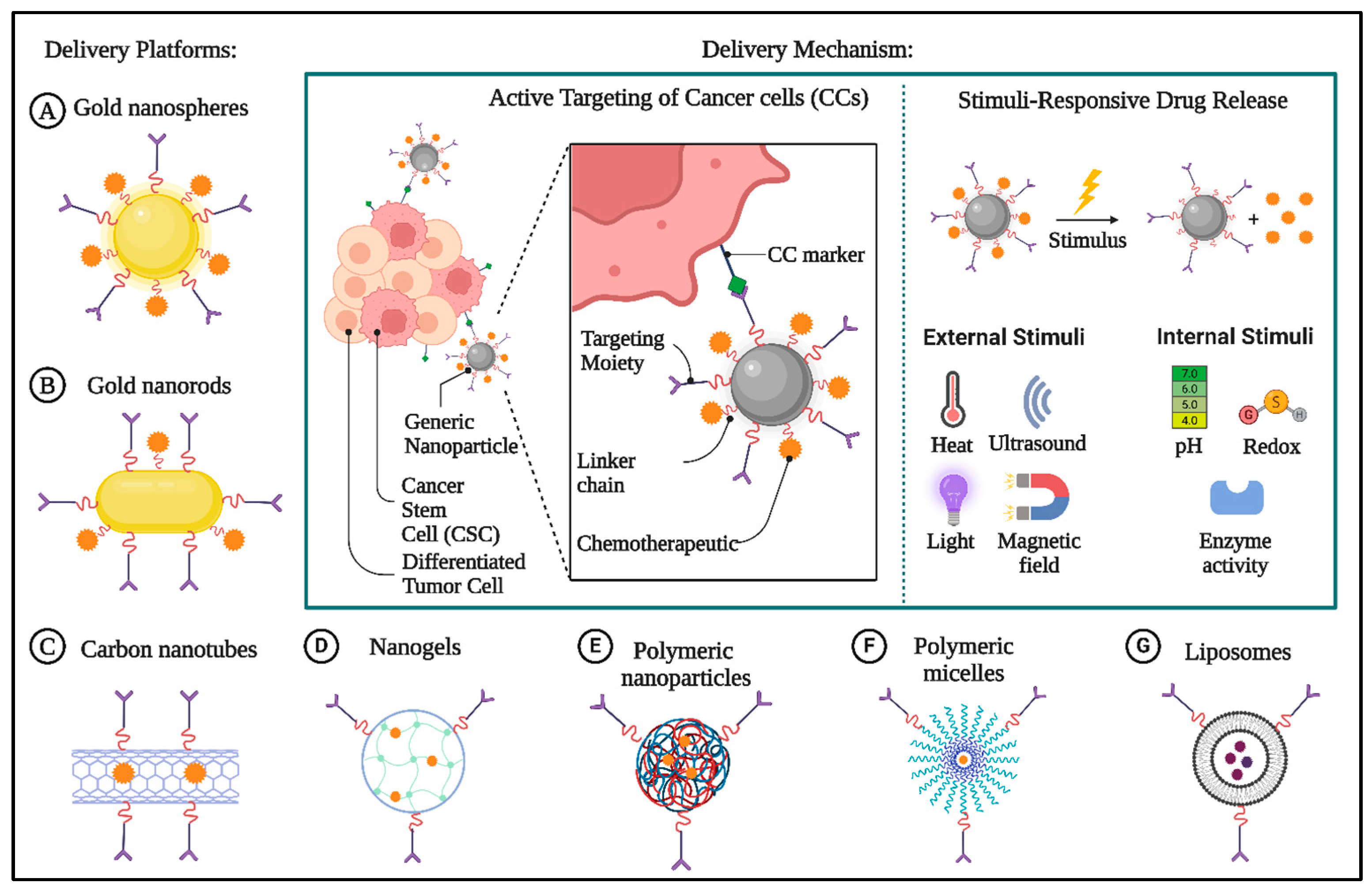
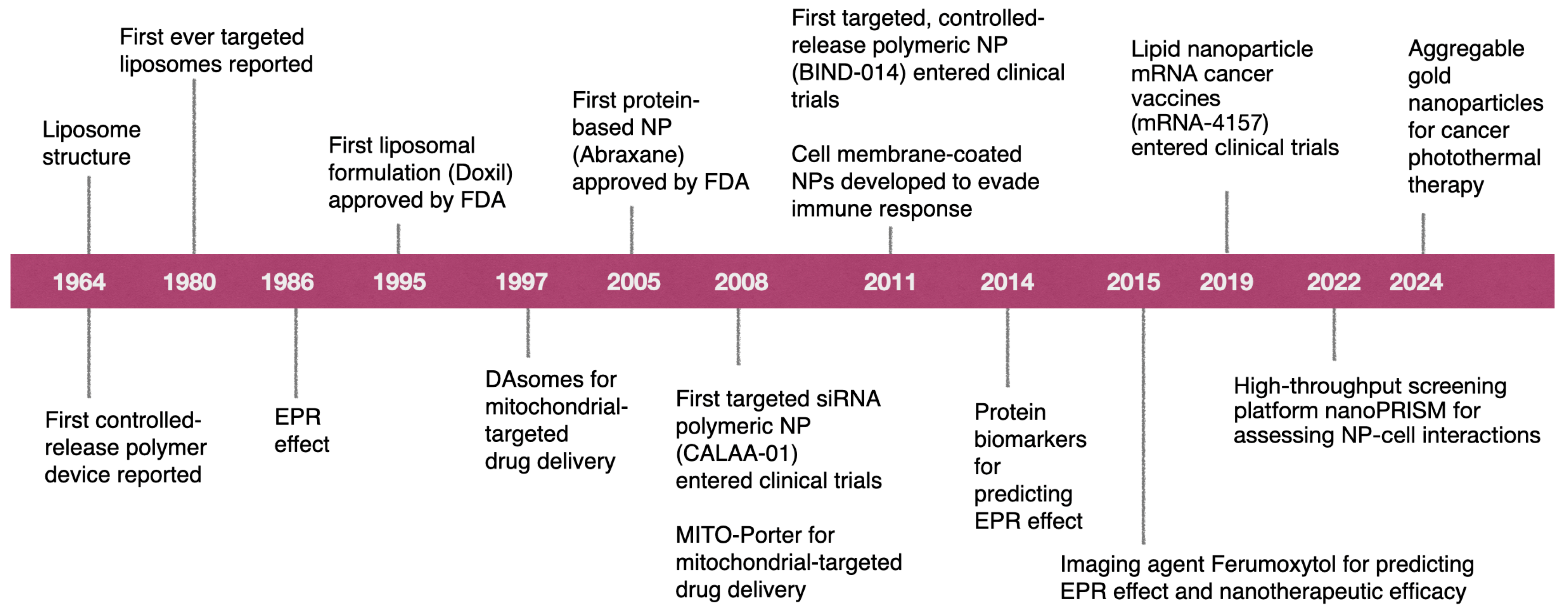
2. Current Status of Cancer Nanomedicine
| Product | Nanoparticle Material | Company | Drug/ Mechanism | Approval (Year) | Indication | References |
|---|---|---|---|---|---|---|
| Hensify (NBTXR3) | Hafnium oxide nanoparticle | Nanobiotix (Paris, France) | Radiotherapy | EMA (2019) | Locally advanced soft tissue sarcoma (STS) | [40] |
| Pazenir | Nanoparticle-bound albumin | Ratiopharm GmbH (Ulm, Germany) | Paclitaxel | EMA (2019) | Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, non-small cell lung cancer | [41,42,43,44] |
| Vyxeos | Liposome | Celator/Jazz Pharma (NJ, USA) | Cytarabine/Daunorubicin | FDA (2017) EMA (2018) | Acute myeloid leukemia | |
| Onivyde | Liposome | Merrimack Pharma (MA, USA) | Irinotecan | FDA (2015) | Pancreatic cancer, colorectal cancer | [42,43,44] |
| NanoTherm | Iron oxide nanoparticles | MagForce Nanotechnologies AG (Berlin, Germany) | Thermal ablation with magnetic field | EMA (2010, 2013) | Glioblastoma, prostate, and pancreatic cancer | |
| Marqibo | Liposome | Talon Therapeutics/Spectrum Pharmaceuticals (MA, USA) | Vincristine | FDA (2012) | Acute lymphoblastic leukemia | |
| Mepact | Liposome | Takeda Pharmaceuticals (Tokyo, Japan) | Mifamurtide MTP-PE | EMA (2009) | Osteosarcoma | [43,44] |
| Genexol-PM | PEG-PLA polymeric micelle | Samyang Biopharmaceuticals (Gyeonggi-do, South Korea) | Paclitaxel | South Korea (2007) | Breast, lung, ovarian cancer | |
| Oncaspar | Polymer protein conjugate | Les Laboratoires Servier (Suresnes, France) | Pegaspargase/L-asparaginase | FDA (1994, 2006) | Acute lymphoblastic leukemia | |
| Abraxane | Nanoparticle-bound albumin | Abraxis/Celgene (NJ, USA) | Paclitaxel | FDA (2005) | Breast and pancreatic cancer, non-small-cell lung cancer | |
| DepoCyt | Liposome | Pacira Pharmaceuticals (NJ, USA) | Cytarabine | FDA (1999) | Neoplastic meningitis | |
| DaunoXome | Liposome | Gilead Sciences (CA, USA) | Daunorubicin | FDA (1996) | Kaposi’s sarcoma | |
| Doxil | Liposome | Johnson and Johnson (NJ, USA) | Doxorubicin | FDA (1995, 1999, 2007), EMA (1996, 2000), Taiwan (1998) | Metastatic breast cancer, ovarian cancer, Kaposi’s sarcoma, multiple myeloma | |
| Lipusu | Liposome | Luye Pharma (China) | Paclitaxel | State Food and Drug Administration of China (2006) | NSCLC, ovarian cancer, and breast cancer | |
| DHP107 | Lipid nanoparticle | Daehwa Pharmaceutical (Gangwon-do, South Korea) | Paclitaxel | South Korea (2016 | Gastric cancer | |
| Apealea | Micelle | Oasmia Pharmaceutical (Uppsala, Sweden) | Paclitaxel | EMA (2018) | Ovarian, peritoneal, and fallopian tube cancer |
| Product | Nanoparticle Material | Company | Active Ingredient | Clinical Trial Number | Indication | References |
|---|---|---|---|---|---|---|
| Docetaxel-PNP | Polymeric nanoparticles | Samyang Biopharmaceuticals Corporation (Gyeonggi-do, South Korea) | Docetaxel | NCT01103791 | Advanced solid malignancies | [41,45,46] |
| ABT-888 | PEGylated liposomes | AbbVie (IL, USA) | Temozolomide and lipo somal and doxorubicin | NCT01113957 | Ovarian cancer | [45,46] |
| LipoVNB | Liposomes | Taiwan Liposome Company (Taipei, Taiwan) | Vinorelbine tartrate | NCT02925000 | Advanced malignancy | |
| FF-10850 | Liposomes | Fujifilm Pharmaceuticals U.S.A., Inc. (MA, USA) | Topotecan | NCT04047251 | Advanced solid tumors | |
| LY01610 | Liposomes | Luye Pharma Group Ltd. China) | Irinotecan hydrochloride | NCT04381910 | Small cell lung cancer | |
| Mitoxantrone hydrochloride liposome injection | Liposomes | CSPC ZhongQi Pharmaceutical Technology Co., Ltd. (Hebei, China) | Mitoxantrone hydrochloride | NCT04927481 | Breast cancer | |
| WGI-0301 | Lipid nanoparticles | Zhejiang Haichang Biotech Co., Ltd. (Zhejiang, China) | AKT-1 antisense oligonucleotide | NCT05267899 | Advanced solid tumors | |
| CDK-004 | Exosomes | Codiak BioSciences (MA, USA) | Antisense oligonucleotide targeting STAT6 | NCT05375604 | Advanced hepatocellular carcinoma, gastric cancer metastatic to liver | |
| OTX-2002 | Lipid nanoparticles | Omega Therapeutics (MA, USA) | Biscistronic mRNA downregulate c-Myc expression | NCT05497453 | Hepatocellular carcinoma |
3. RNA Therapeutics and Gene-Based Delivery
4. Applications of Nanomedicine in Cancer Therapy
4.1. Biological Nanocarriers in Chemotherapy
4.2. Biological Nanocarriers in Gene Therapy
4.3. Biological Nanocarriers in Immunotherapy
5. Challenges and Regulatory Considerations of Cancer Nanomedicines
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Common Cancer Sites—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/common.html (accessed on 6 January 2025).
- Rezaianzadeh, A.; Jalali, M.; Maghsoudi, A.; Mokhtari, A.M.; Azgomi, S.; Dehghani, S.L. The Overall 5-Year Survival Rate of Breast Cancer among Iranian Women: A Systematic Review and Meta-Analysis of Published Studies. Breast Dis. 2017, 37, 63–68. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Altun, İ.; Sonkaya, A. The Most Common Side Effects Experienced by Patients Were Receiving First Cycle of Chemotherapy. Iran. J. Public Health 2018, 47, 1218–1219. [Google Scholar] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To Exploit the Tumor Microenvironment: Passive and Active Tumor Targeting of Nanocarriers for Anti-Cancer Drug Delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in Cancer Diagnosis: Progress, Challenges and Opportunities. J. Hematol. Oncol. 2019, 12, 137. [Google Scholar] [CrossRef]
- Zhao, M.; Mi, D.; Ferdows, B.E.; Li, Y.; Wang, R.; Li, J.; Patel, D.; Kong, N.; Shi, S.; Tao, W. State-of-The-Art Nanotechnologies for the Detection, Recovery, Analysis and Elimination of Liquid Biopsy Components in Cancer. Nano Today 2022, 42, 101361. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Xiong, Q.; Hornburg, D.; Tao, W.; Farokhzad, O.C. Nano-Bio Interactions in Cancer: From Therapeutics Delivery to Early Detection. Acc. Chem. Res. 2021, 54, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and Challenges towards Targeted Delivery of Cancer Therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Islam, M.A.; Xu, Y.; Tao, W.; Ubellacker, J.M.; Lim, M.; Aum, D.; Lee, G.Y.; Zhou, K.; Zope, H.; Yu, M.; et al. Restoration of Tumour-Growth Suppression in Vivo via Systemic Nanoparticle-Mediated Delivery of PTEN MRNA. Nat. Biomed. Eng. 2018, 2, 850–864. [Google Scholar] [CrossRef]
- Kong, N.; Tao, W.; Ling, X.; Wang, J.; Xiao, Y.; Shi, S.; Ji, X.; Shajii, A.; Gan, S.T.; Kim, N.Y.; et al. Synthetic MRNA Nanoparticle-Mediated Restoration of P53 Tumor Suppressor SensitizesP53-Deficient Cancers to MTOR Inhibition. Sci. Transl. Med. 2019, 11, eaaw1565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhong, D.; Ouyang, J.; He, J.; Qi, Y.; Chen, W.; Zhang, X.; Tao, W.; Zhou, M. Microalgae-Based Oral Microcarriers for Gut Microbiota Homeostasis and Intestinal Protection in Cancer Radiotherapy. Nat. Commun. 2022, 13, 1413. [Google Scholar] [CrossRef]
- Xie, A.; Hanif, S.; Ouyang, J.; Tang, Z.; Kong, N.; Kim, N.Y.; Qi, B.; Patel, D.; Shi, B.; Tao, W. Stimuli-Responsive Prodrug-Based Cancer Nanomedicine. EBioMedicine 2020, 56, 102821. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Liu, C.; Wang, Z.; Deng, L.; Feng, C.; Tao, W.; Xu, X.; Cui, W. Biologically Modified Nanoparticles as Theranostic Bionanomaterials. Prog. Mater. Sci. 2020, 118, 100768. [Google Scholar] [CrossRef]
- Kong, N.; Zhang, H.; Feng, C.; Liu, C.; Xiao, Y.; Zhang, X.; Mei, L.; Kim, J.M.; Tao, W.; Ji, X. Arsenene-Mediated Multiple Independently Targeted Reactive Oxygen Species Burst for Cancer Therapy. Nat. Commun. 2021, 12, 4777. [Google Scholar] [CrossRef]
- Feng, C.; Li, Y.; Ferdows, B.E.; Patel, D.N.; Ouyang, J.; Tang, Z.; Kong, N.; Chen, E.; Tao, W. Emerging Vaccine Nanotechnology: From Defense against Infection to Sniping Cancer. Acta Pharm. Sin. B 2022, 12, 2206–2223. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine Applied to Translational Oncology: A Future Perspective on Cancer Treatment. Nanomedicine: Nanotechnology. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef]
- Huayamares, S.G.; Loughrey, D.; Kim, H.; Dahlman, J.E.; Sorscher, E.J. Nucleic Acid-Based Drugs for Patients with Solid Tumours. Nat. Rev. Clin. Oncol. 2024, 21, 407–427. [Google Scholar] [CrossRef]
- Kommineni, N.; Butreddy, A.; Jyothi, V.G.S.; Angsantikul, P. Freeze-Drying for the Preservation of Immunoengineering Products. iScience 2022, 25, 105127. [Google Scholar] [CrossRef]
- Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol-Derived Nano-Architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19, 1900073. [Google Scholar] [CrossRef] [PubMed]
- Ajith, S.; Almomani, F.; Elhissi, A.; Husseini, G.A. Nanoparticle-Based Materials in Anticancer Drug Delivery: Current and Future Prospects. Heliyon 2023, 9, e21227. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Caelyx Pegylated Liposomal|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/caelyx-pegylated-liposomal (accessed on 22 May 2025).
- Fda. Cder Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050718s029lbl.pdf (accessed on 3 March 2025).
- Working, P.K.; Newman, M.S.; Huang, S.K.; Mayhew, E.; Vaage, J.; Lasic, D.D. Pharmacokinetics, Biodistribution and Therapeutic Efficacy of Doxorubicin Encapsulated in Stealth® Liposomes (Doxil®). J. Liposome Res. 1994, 4, 667–687. [Google Scholar] [CrossRef]
- Waheed, I.; Ali, A.; Tabassum, H.; Khatoon, N.; Lai, W.F.; Zhou, X. Lipid-Based Nanoparticles as Drug Delivery Carriers for Cancer Therapy. Front. Oncol. 2024, 14, 1296091. [Google Scholar] [CrossRef]
- Haag, R.; Kratz, F. Polymer Therapeutics: Concepts and Applications. Angew. Chem.—Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef]
- Chen, W.; Liu, X.; Liu, Y.; Bang, Y.; Kim, H.-I. Synthesis of PMMA and PMMA/PS Nanoparticles by Microemulsion Polymerization with a New Vapor Monomer Feeding System. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 364, 145–150. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhao, Z.; Liang, K.; Nan, F.; Li, Y.; Wang, J.; Ge, J.; Wang, P. Recent Advances and Prospects of Carbon Dots in Cancer Nanotheranostics. Mater. Chem. Front. 2020, 4, 449–471. [Google Scholar] [CrossRef]
- Barua, S.; Mitragotri, S. Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects. Nano Today 2014, 9, 223. [Google Scholar] [CrossRef]
- Kumbham, S.; Ajjarapu, S.; Ghosh, B.; Biswas, S. Current Trends in the Development of Liposomes for Chemotherapeutic Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 87, 104854. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via Epr Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer Active Targeting by Nanoparticles: A Comprehensive Review of Literature. J. Cancer Res. Clin. Oncol. 2014, 141, 769. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, Z.; Pan, D.; Gong, Q.; Luo, K.; Liu, Y. Challenges and Opportunities of Nanomedicine: Novel Comprehensive Approaches for Brain Metastasis. Small Struct. 2023, 5, 2300148. [Google Scholar] [CrossRef]
- Tong, F.; Wang, Y.; Gao, H. Progress and Challenges in the Translation of Cancer Nanomedicines. Curr. Opin. Biotechnol. 2024, 85, 103045. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in Cancer Therapy: Challenges, Opportunities, and Clinical Applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Ali, E.S.; Sharker, S.M.; Islam, M.T.; Khan, I.N.; Shaw, S.; Rahman, M.A.; Uddin, S.J.; Shill, M.C.; Rehman, S.; Das, N.; et al. Targeting Cancer Cells with Nanotherapeutics and Nanodiagnostics: Current Status and Future Perspectives. Semin. Cancer Biol. 2021, 69, 52–68. [Google Scholar] [CrossRef]
- Kopeckova, K.; Eckschlager, T.; Sirc, J.; Hobzova, R.; Plch, J.; Hrabeta, J.; Michalek, J. Nanodrugs Used in Cancer Therapy. Biomed. Pap. 2019, 163, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kemp, J.A.; Kwon, Y.J. Cancer Nanotechnology: Current Status and Perspectives. Nano Converg. 2021, 8, 34. [Google Scholar] [CrossRef]
- Gawne, P.J.; Ferreira, M.; Papaluca, M.; Grimm, J. Paolo Decuzzi New Opportunities and Old Challenges in the Clinical Translation of Nanotheranostics. Nat. Rev. Mater. 2023, 8, 783–798. [Google Scholar] [CrossRef] [PubMed]
- Le, B.T.; Raguraman, P.; Kosbar, T.R.; Fletcher, S.; Wilton, S.D.; Veedu, R.N. Antisense Oligonucleotides Targeting Angiogenic Factors as Potential Cancer Therapeutics. Mol. Ther. Nucleic Acids 2019, 14, 142–157. [Google Scholar] [CrossRef]
- Çakan, E.; Lara, O.D.; Szymanowska, A.; Bayraktar, E.; Chavez-Reyes, A.; Lopez-Berestein, G.; Amero, P.; Rodriguez-Aguayo, C. Therapeutic Antisense Oligonucleotides in Oncology: From Bench to Bedside. Cancers 2024, 16, 2940. [Google Scholar] [CrossRef]
- Xiong, H.; Veedu, R.N.; Diermeier, S.D. Recent Advances in Oligonucleotide Therapeutics in Oncology. Int. J. Mol. Sci. 2021, 22, 3295. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Torchilin, V.P. Efficient Nanocarriers of SiRNA Therapeutics for Cancer Treatment. Transl. Res. 2019, 214, 62–91. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Ang, H.L.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Hushmandi, K.; Delfi, M.; Khan, H.; Ashrafizadeh, M.; et al. Pre-Clinical and Clinical Applications of Small Interfering Rnas (Sirna) and Co-Delivery Systems for Pancreatic Cancer Therapy. Cells 2021, 10, 3348. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Menon, A.; Abd-Aziz, N.; Khalid, K.; Poh, C.L.; Naidu, R. MiRNA: A Promising Therapeutic Target in Cancer. Int. J. Mol. Sci. 2022, 23, 11502. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Xue, Z.; Tsang, C.; Qiao, X.; Dong, L.; Li, H.; Yang, Y.; Yu, B.; Gao, Y. Aptamer Nucleotide Analog Drug Conjugates in the Targeting Therapy of Cancers. Front. Cell Dev. Biol. 2022, 10, 1053984. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Rui, Y.; Green, J.J. Non-Viral Nucleic Acid Containing Nanoparticles as Cancer Therapeutics. Expert Opin. Drug Deliv. 2016, 13, 1475–1487. [Google Scholar] [CrossRef]
- Jiang, X.; Abedi, K.; Shi, J. Polymeric Nanoparticles for RNA Delivery. Ref. Modul. Mater. Sci. Mater. Eng. 2021, V3-555–V3-573. [Google Scholar] [CrossRef]
- Piotrowski-Daspit, A.S.; Kauffman, A.C.; Bracaglia, L.G.; Saltzman, W.M. Polymeric Vehicles for Nucleic Acid Delivery. Adv. Drug Deliv. Rev. 2020, 156, 119–132. [Google Scholar] [CrossRef]
- Karlsson, J.; Rhodes, K.R.; Green, J.J.; Tzeng, S.Y. Poly(Beta-Amino Ester)s as Gene Delivery Vehicles: Challenges and Opportunities. Expert Opin. Drug Deliv. 2020, 17, 1395. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Guo, P.; Wen, W.-C.; Wong, H. Lipid-Based Nanocarriers for RNA Delivery. Curr. Pharm. Des. 2015, 21, 3140–3147. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Lu, X.; Tian, J. Nanoparticle Drug Delivery Systems for Synergistic Delivery of Tumor Therapy. Front. Pharmacol. 2023, 14, 1111991. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, K.; Kraljević Pavelić, S.; Bulog, A.; Agaj, A.; Rojnić, B.; Čolić, M.; Trivanović, D. Nanoparticles in Medicine: Current Status in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 12827. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Kashani, G.K.; Naghib, S.M.; Soleymani, S.; Mozafari, M.R. A Review of DNA Nanoparticles-Encapsulated Drug/Gene/Protein for Advanced Controlled Drug Release: Current Status and Future Perspective over Emerging Therapy Approaches. Int. J. Biol. Macromol. 2024, 268, 131694. [Google Scholar] [CrossRef] [PubMed]
- Shishparenok, A.N.; Furman, V.V.; Zhdanov, D.D. DNA-Based Nanomaterials as Drug Delivery Platforms for Increasing the Effect of Drugs in Tumors. Cancers 2023, 15, 2151. [Google Scholar] [CrossRef]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xiang, J.; Qiu, N.; Wang, Y.; Piao, Y.; Shao, S.; Tang, J.; Zhou, Z.; Shen, Y. Tumor Abnormality-Oriented Nanomedicine Design. Chem. Rev. 2023, 123, 10920–10989. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Tao, X.; Zhai, X.; Zhu, Y.; Li, Y.; Chen, Y.; Dong, D.; Yang, S.; Lv, L. Application of Aptamer-Drug Delivery System in the Therapy of Breast Cancer. Biomed. Pharmacother. 2023, 161, 114444. [Google Scholar] [CrossRef]
- Jabbari, A.; Sameiyan, E.; Elnaz Yaghoobi Ramezani, M.; Alibolandi, M.; Abnous, K. Seyed Mohammad Taghdisi Aptamer-Based Targeted Delivery Systems for Cancer Treatment Using DNA Origami and DNA Nanostructures. Int. J. Pharm. 2023, 646, 123448. [Google Scholar] [CrossRef]
- Kumar, M.; Jha, A.; Mishra, B. DNA-Based Nanostructured Platforms as Drug Delivery Systems. Chem Bio Eng. 2024, 1, 179–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Wang, Z.G.; Wang, H.; Li, Y.; Long, Q.; Jiang, S. Advanced Applications of DNA Nanostructures Dominated by DNA Origami in Antitumor Drug Delivery. Front. Mol. Biosci. 2023, 10, 1239952. [Google Scholar] [CrossRef]
- Asakiya, C.; Zhang, Y.; Zhu, L.; Ackah, M.; Tavakoli, S.; Zhu, L.; Huang, K.; Xu, W. Self-Assembled PH-Responsive DNA Nanosponges for Targeted Co-Delivery of Doxorubicin and Capsaicin for Colorectal Cancer Therapy. Biochem. Eng. J. 2023, 195, 108926. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Li, P.; Sheng, J.; Fu, Q. Construction of Smart DNA-Based Drug Delivery Systems for Cancer Therapy. Small 2024, 20, e2306257. [Google Scholar] [CrossRef]
- Mohanty, A.; Park, I.-K.P.-C.N. A Promising Nanomedicine against Cancer. Chonnam Med. J. 2023, 59, 1. [Google Scholar] [CrossRef]
- Hekmat, A.; Haertlé, T.; Leblanc, R.M.; Khan, H.Y.; Khan, R.H.; Saboury, A.A. A Review on Interaction of Nanomaterials of Group-XIV (G14) Elements of the Periodic Table with Proteins and DNA: Applications in Biotechnology and Pharmacy. Bionanoscience 2024, 14, 1978–2003. [Google Scholar] [CrossRef]
- Li, H.-B.; Wang, W.-H.; Wang, Z.-Y. A Meta-Analysis of the Incidence and Risk of Skin Toxicity with Nab-Paclitaxel and Paclitaxel in Cancer Treatment. Am. J. Transl. Res. 2023, 15, 4279–4290. [Google Scholar] [PubMed]
- Gopi, S.; Amalraj, A.; Sreeraj, T.R. Advanced Nanomaterials for Biological: Nutraceutical, and Medicinal Applications; Apple Academic Press: New York, NY, USA, 2024; ISBN 9781774913468. [Google Scholar]
- Lee, J.; Hyun, D.-H. The Interplay between Intracellular Iron Homeostasis and Neuroinflammation in Neurodegenerative Diseases. Antioxidants 2023, 12, 918. [Google Scholar] [CrossRef] [PubMed]
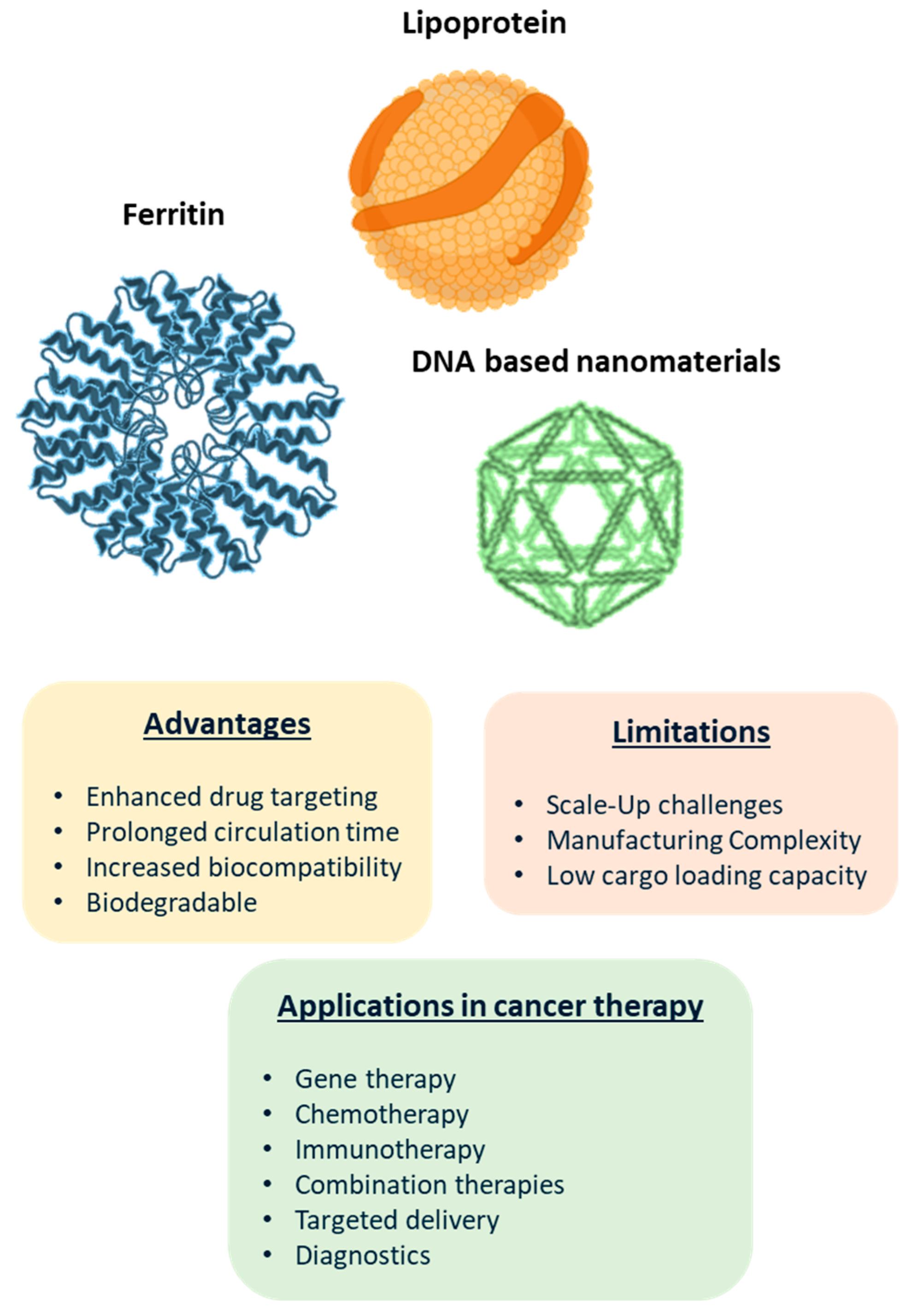
- Zhang, J.; Cheng, D.; He, J.; Hong, J.; Yuan, C.; Liang, M. Cargo Loading within Ferritin Nanocages in Preparation for Tumor-Targeted Delivery. Nat. Protoc. 2021, 16, 4878–4896. [Google Scholar] [CrossRef]
- Patel, K.K.; Kashfi, K.L. Lipoproteins and cancer: The Role of HDL-C, LDL-C, and Cholesterol-Lowering Drugs. Biochem. Pharmacol. 2022, 196, 114654. [Google Scholar] [CrossRef]
- Busatto, S.; Walker, S.A.; Grayson, W.; Pham, A.; Tian, M.; Nesto, N.; Barklund, J.; Wolfram, J. Lipoprotein-Based Drug Delivery. Adv. Drug Deliv. Rev. 2020, 159, 377–390. [Google Scholar] [CrossRef]
- Hou, S.; Hasnat, M.; Chen, Z.; Liu, Y.; Faran Ashraf Baig, M.M.; Liu, F.; Chen, Z. Application Perspectives of Nanomedicine in Cancer Treatment. Front. Pharmacol. 2022, 13, 909526. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Maiseyeu, A.L.-D.L.N. Mechanisms of Targeting, Biology, and Theranostic Potential. Drug Deliv. 2021, 28, 408–421. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; Zheng, L. HDL Structure. Adv. Exp. Med. Biol. 2022, 1377, 1–11. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum Protein-Based Nanoparticles for Cancer Diagnosis and Treatment. J. Control. Release 2021, 329, 997–1022. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Xin, Y.; Li, R.; Ge, Y.; Feng, C.; Xu, X. Targeted Biomimetic Nanoparticles for Synergistic Combination Chemotherapy of Paclitaxel and Doxorubicin. Mol. Pharm. 2016, 14, 107–123. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Alnasser, S.M. Review on Mechanistic Strategy of Gene Therapy in the Treatment of Disease. Gene 2021, 769, 145246. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, Q.; Huang, X.; Koo, S.; Kong, N.; Tao, W. MRNA-Based Cancer Therapeutics. Nat. Rev. Cancer 2023, 23, 526–543. [Google Scholar] [CrossRef]
- Ahmed, T.; Liu, F.-C.F.; Wu, X.Y. An Update on Strategies for Optimizing Polymer-Lipid Hybrid Nanoparticle-Mediated Drug Delivery: Exploiting Transformability and Bioactivity of PLN and Harnessing Intracellular Lipid Transport Mechanism. Expert Opin. Drug Deliv. 2024, 21, 245–278. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Gupta, P.; Kumar, S.; Banerjee, M. Lipid Polymer Hybrid Nanoparticles as Potent Vehicles for Drug Delivery in Cancer Therapeutics. Med. Drug Discov. 2023, 20, 100165. [Google Scholar] [CrossRef]
- Karati, D.; Mukherjee, S.; Prajapati, B.; Bose, A.; Paul, S.; Elossaily, G.M.; Roy, S. A Review on Lipid-Polymer Hybrid Nanocarriers in Cancer. J. Drug Deliv. Sci. Technol. 2024, 97, 105827. [Google Scholar] [CrossRef]
- Gencer, A.; Duraloglu, C.; Ozbay, S.; Ciftci, T.T.; Yabanoglu-Ciftci, S.; Arica, B. Recent Advances in Treatment of Lung Cancer: Nanoparticle-Based Drug and SiRNA Delivery Systems. Curr. Drug Deliv. 2020, 17, 103–120. [Google Scholar] [CrossRef]
- Li, D.; Gao, C.; Kuang, M.; Xu, M.; Wang, B.; Luo, Y.; Teng, L.; Xie, J. Nanoparticles as Drug Delivery Systems of RNAi in Cancer Therapy. Molecules 2021, 26, 2380. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Liu, Q.; Tian, R.; Shang, Y.; Liu, F.; Liu, S.; Zhao, S.; Han, Z.; Sun, J.; et al. A Tubular DNA Nanodevice as a SiRNA/Chemo-Drug Co-Delivery Vehicle for Combined Cancer Therapy. Angew. Chemie Int. Ed. 2020, 60, 2594–2598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, C.; An, C.; Zheng, X.; Wen, S.; Chen, W.; Liu, X.; Lv, Z.; Yang, P.; Xu, W.; et al. Application of the CRISPR/Cas9-Based Gene Editing Technique in Basic Research, Diagnosis, and Therapy of Cancer. Mol. Cancer 2021, 20, 126. [Google Scholar] [CrossRef]
- van den Bulk, J.; Verdegaal, E.M.; de Miranda, N.F. Cancer Immunotherapy: Broadening the Scope of Targetable Tumours. Open Biol. 2018, 8, 180037. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhang, H.; Zhu, Y.; Liang, Y.; Yuan, Z.; Li, J.; Li, J.; Li, X.; Jia, Y.; He, T.; et al. Circulating Tumor Cells in Cancer Patients: Developments and Clinical Applications for Immunotherapy. Mol. Cancer 2020, 19, 15. [Google Scholar] [CrossRef]
- Wyatt Shields, C.; Evans, M.A.; Wang, L.L.W.; Baugh, N.; Iyer, S.; Wu, D.; Zhao, Z.; Pusuluri, A.; Ukidve, A.; Pan, D.C.; et al. Cellular Backpacks for Macrophage Immunotherapy. Sci. Adv. 2020, 6, eaaz6579. [Google Scholar] [CrossRef]
- Sorkhabi, A.D.; Khosroshahi, L.M.; Sarkesh, A.; Mardi, A.; Aghebati-Maleki, A.; Aghebati-Maleki, L.; Baradaran, B. The Current Landscape of CAR T-Cell Therapy for Solid Tumors: Mechanisms, Research Progress, Challenges, and Counterstrategies. Front. Immunol. 2023, 14, 1113882. [Google Scholar] [CrossRef]
- Meir, R.; Shamalov, K.; Sadan, T.; Motiei, M.; Yaari, G.; Cohen, C.J.; Popovtzer, R. Fast Image-Guided Stratification Using Anti-Programmed Death Ligand 1 Gold Nanoparticles for Cancer Immunotherapy. ACS Nano 2017, 11, 11127–11134. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, H.; Xiong, P.; He, Y.; Zhou, W.; Wu, C.; Liao, X.; Zhang, W.; Yang, H.; Liu, Y. DNA Origami-Based Composite Nanosandwich for Iteratively Potentiated Chemo-Immunotherapy. J. Control. Release 2025, 379, 452–465. [Google Scholar] [CrossRef]
- Nie, C.; Ye, J.; Jiang, J.-H.; Chu, X. DNA Nanodevice as a Multi-Module Co-Delivery Platform for Combination Cancer Immunotherapy. J. Colloid Interface Sci. 2024, 667, 1–11. [Google Scholar] [CrossRef]
- Lopes Chaves, L.; Dourado, D.; Prunache, I.-B.; da Silva, P.; dos Santos Lucena, G.; de Souza, Z.; de Moura, P.; Nunes Bordallo, H.; Rocha Formiga, F.; de Souza Rebouças, J. Nanocarriers of Antigen Proteins for Vaccine Delivery. Int. J. Pharm. 2024, 659, 124162. [Google Scholar] [CrossRef]
- Schijns, V.; Fernández-Tejada, A.; Barjaktarović, Ž.; Bouzalas, I.; Brimnes, J.; Chernysh, S.; Gizurarson, S.; Gursel, I.; Jakopin, Ž.; Lawrenz, M.; et al. Modulation of Immune Responses Using Adjuvants to Facilitate Therapeutic Vaccination. Immunol. Rev. 2020, 296, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Safdari, H.A.; Hoque, M. Nanoparticle Mediated Cancer Immunotherapy. Semin. Cancer Biol. 2020, 69, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhao, R.; Qin, Y.; Zhu, J.; Chen, L.-Q.; Di, C.; Han, X.; Cheng, K.; Zhang, Y.; Zhao, Y.; et al. Development of a Cancer Vaccine Using in Vivo Click-Chemistry-Mediated Active Lymph Node Accumulation for Improved Immunotherapy. Adv. Mater. 2021, 33, 2006007. [Google Scholar] [CrossRef] [PubMed]
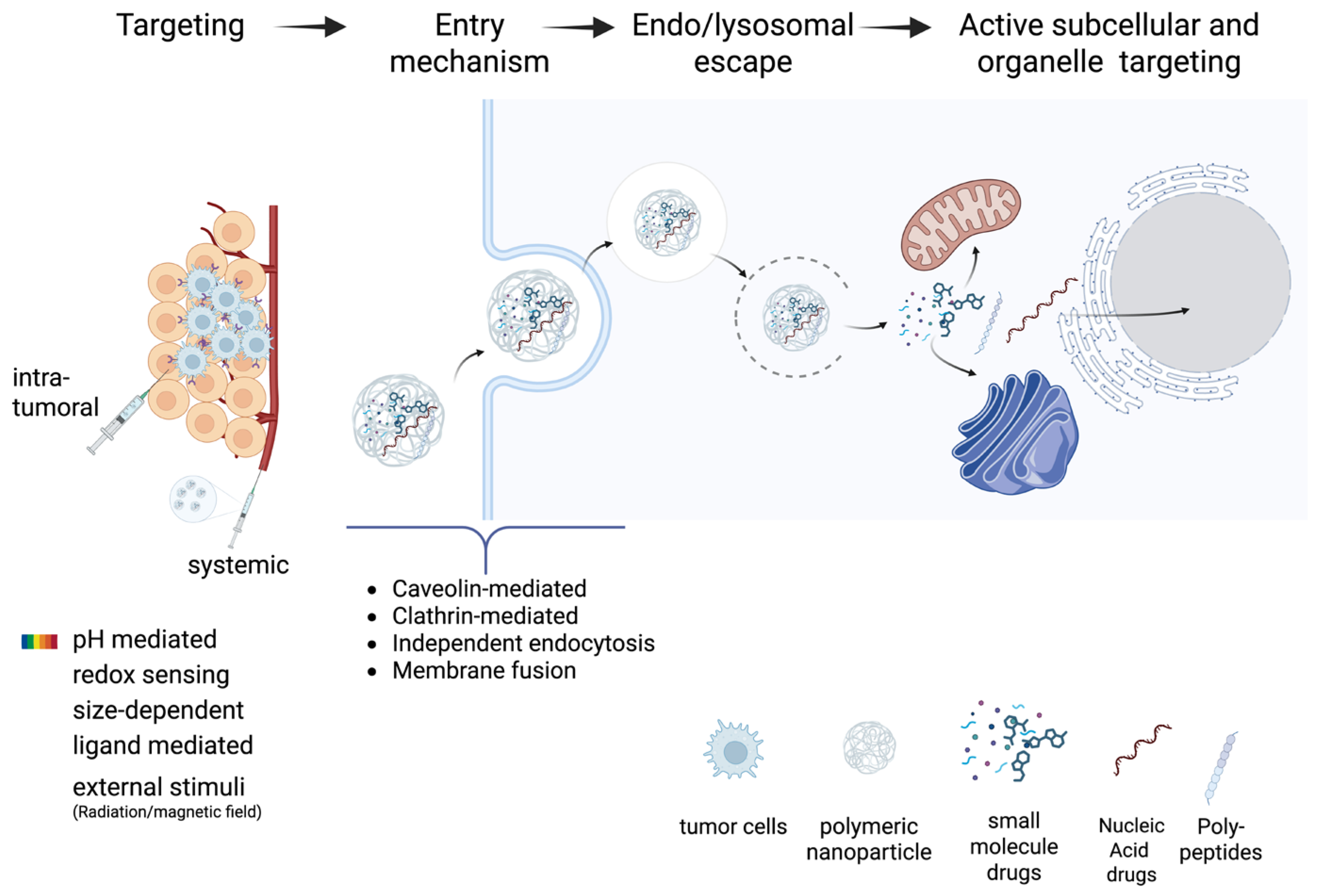
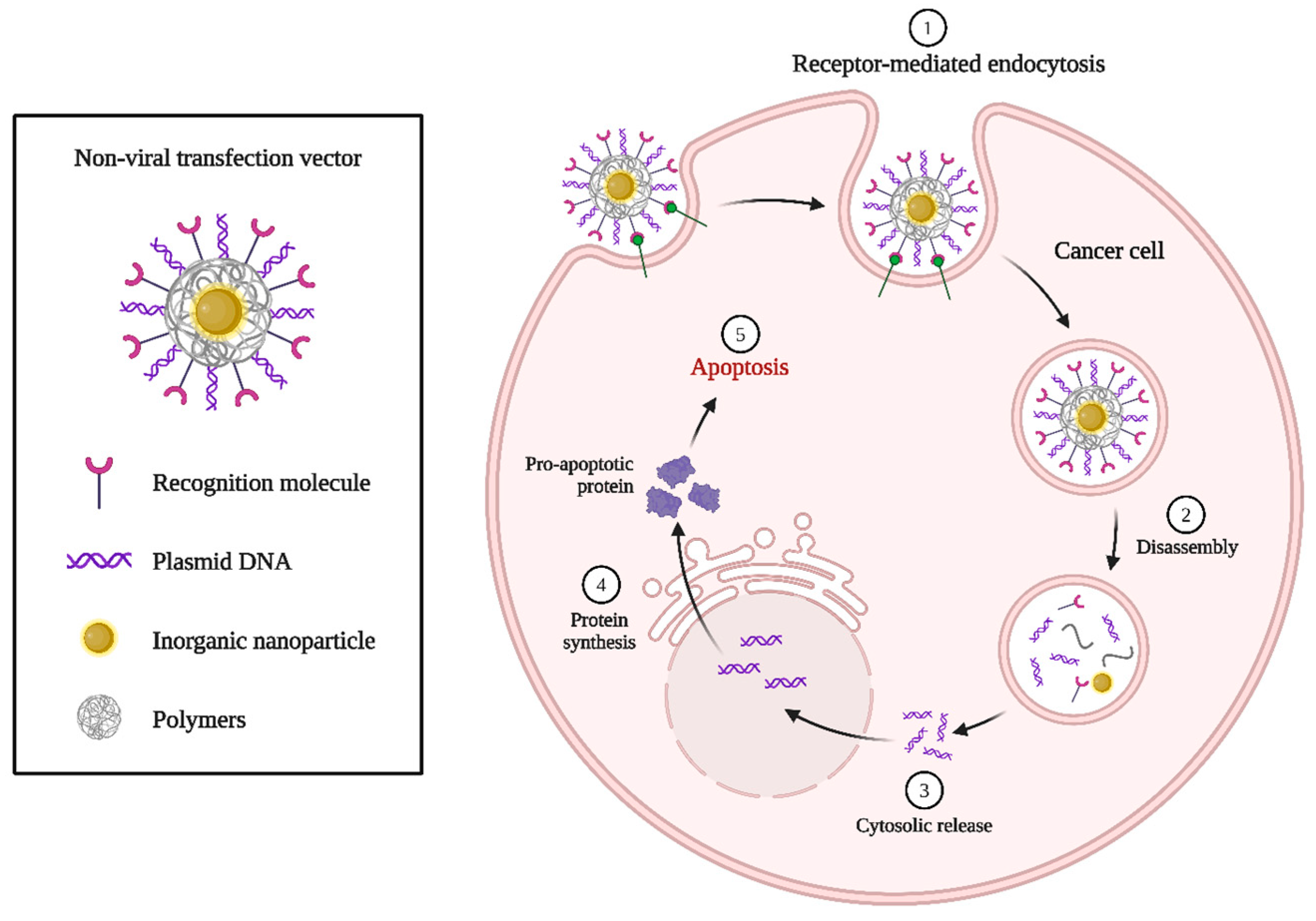
| DNA Nanostructures | Chemotherapeutic Drugs | Effect | Reference |
|---|---|---|---|
| DNA tetrahedron | Doxorubicin | Selectivity and inhibition of breast cancer cells and drug delivery | [68] |
| DNA icosahedron | Selective targeting | [69] | |
| DNA octahedron | Efficient and specific internalization for killing epithelial cancer cells | [70] | |
| DNA triangle and tube | Increased doxorubicin cellular internalization and elevated susceptibility to drug-resistant adenocarcinoma cells | [71] | |
| RCA-based nanostructures | pH-Responsive Drug Delivery | [72] | |
| DNA icosahedron | Platinum | Precise delivery of platinum nanodrugs to cisplatin-resistant cancer | [73] |
| DNA nanorod | Daunorubicin | Circumvent drug-resistance mechanisms in a leukemia mode | [64,71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganpisetti, R.; Giridharan, S.; Vaskuri, G.S.S.J.; Narang, N.; Basim, P.; Dokmeci, M.R.; Ermis, M.; Rojekar, S.; Gholap, A.D.; Kommineni, N. Biological Nanocarriers in Cancer Therapy: Cutting Edge Innovations in Precision Drug Delivery. Biomolecules 2025, 15, 802. https://doi.org/10.3390/biom15060802
Ganpisetti R, Giridharan S, Vaskuri GSSJ, Narang N, Basim P, Dokmeci MR, Ermis M, Rojekar S, Gholap AD, Kommineni N. Biological Nanocarriers in Cancer Therapy: Cutting Edge Innovations in Precision Drug Delivery. Biomolecules. 2025; 15(6):802. https://doi.org/10.3390/biom15060802
Chicago/Turabian StyleGanpisetti, Ramesh, Sanjay Giridharan, G. S. Sainaga Jyothi Vaskuri, Nikesh Narang, Pratap Basim, Mehmet Remzi Dokmeci, Menekse Ermis, Satish Rojekar, Amol D. Gholap, and Nagavendra Kommineni. 2025. "Biological Nanocarriers in Cancer Therapy: Cutting Edge Innovations in Precision Drug Delivery" Biomolecules 15, no. 6: 802. https://doi.org/10.3390/biom15060802
APA StyleGanpisetti, R., Giridharan, S., Vaskuri, G. S. S. J., Narang, N., Basim, P., Dokmeci, M. R., Ermis, M., Rojekar, S., Gholap, A. D., & Kommineni, N. (2025). Biological Nanocarriers in Cancer Therapy: Cutting Edge Innovations in Precision Drug Delivery. Biomolecules, 15(6), 802. https://doi.org/10.3390/biom15060802










