Role of 5-HT1A and 5-HT7 Receptors in Memory Regulation and the Importance of Their Coexpression: A Systematic Review
Abstract
1. Introduction
1.1. General Characteristics of the 5-HT1AR
1.1.1. Genetic Aspects
1.1.2. Molecular Structure
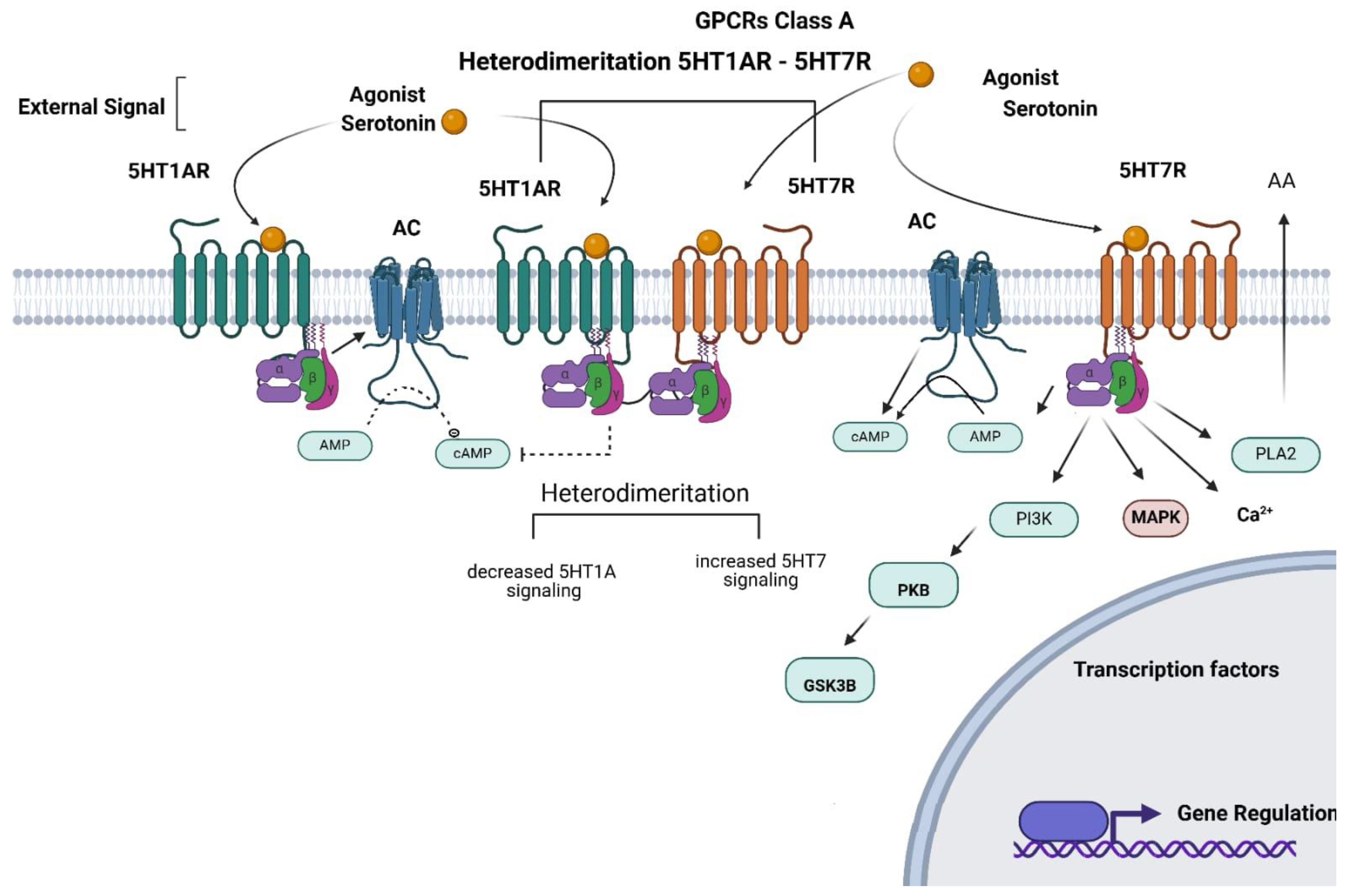
1.1.3. Molecular Signaling
1.1.4. Neuroanatomical Distribution
1.1.5. Functions
1.1.6. Pharmacology
1.2. General Characteristics of the 5-HT7 Receptor
1.2.1. Genetic Aspects
1.2.2. Molecular Structure
1.2.3. Molecular Signaling
1.2.4. Neuroanatomical Distribution
1.2.5. Functions
1.2.6. Pharmacology
| 5-HT1A Serotoninergic Drugs | |||
| Agonists | Ki (nM) | Antagonists | Ki (nM) |
| NLX-101 [63] | 8.7 | WAY-100635 [64] | 0.24 |
| 8-OH-DPAT [65] | 0.58 | NAD-299 [64] | 0.59 |
| Lurasidone [66] | 6.4 | NAN-190 [65] | 0.97 |
| Flesinoxan [65] | 0.54 | WAY-100135 [65] | 17 |
| 5-HT7 Serotoninergic Drugs | |||
| Agonists | Ki (nM) | Antagonists | Ki (nM) |
| LP-12 [67] | 0.13 | Lurasidone [66] | 0.5 |
| LP-44 [67] | 0.22 | SB-258719 [68] | 31.6 |
| AS-19 [69] | 2.5 | SB-269970 [70] | 1.3 |
| LP-211 [71] | 15 | Vortioxetine [72] | 19 |
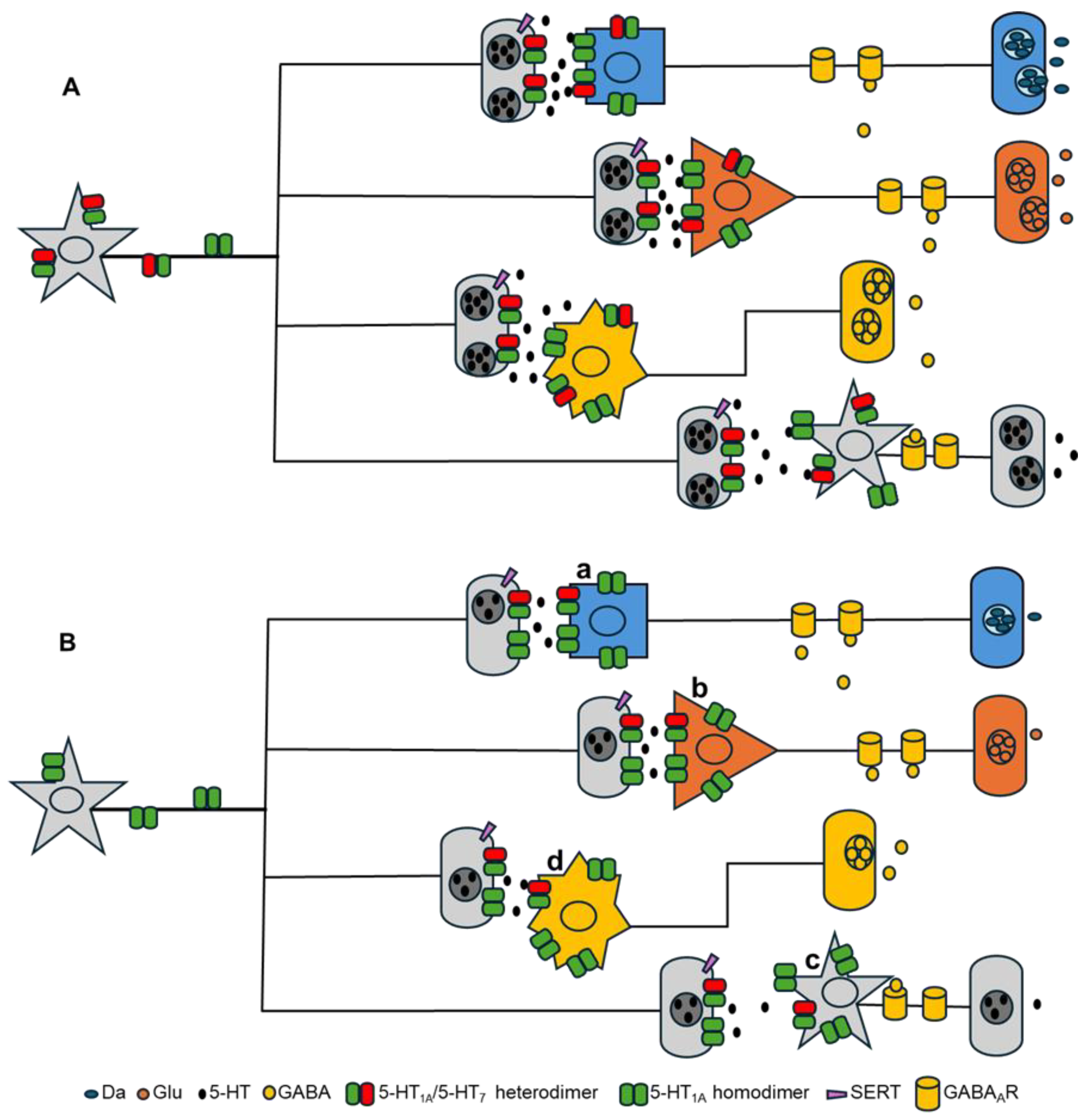
1.3. Oligomerization of 5-HT1AR and 5-HT7R
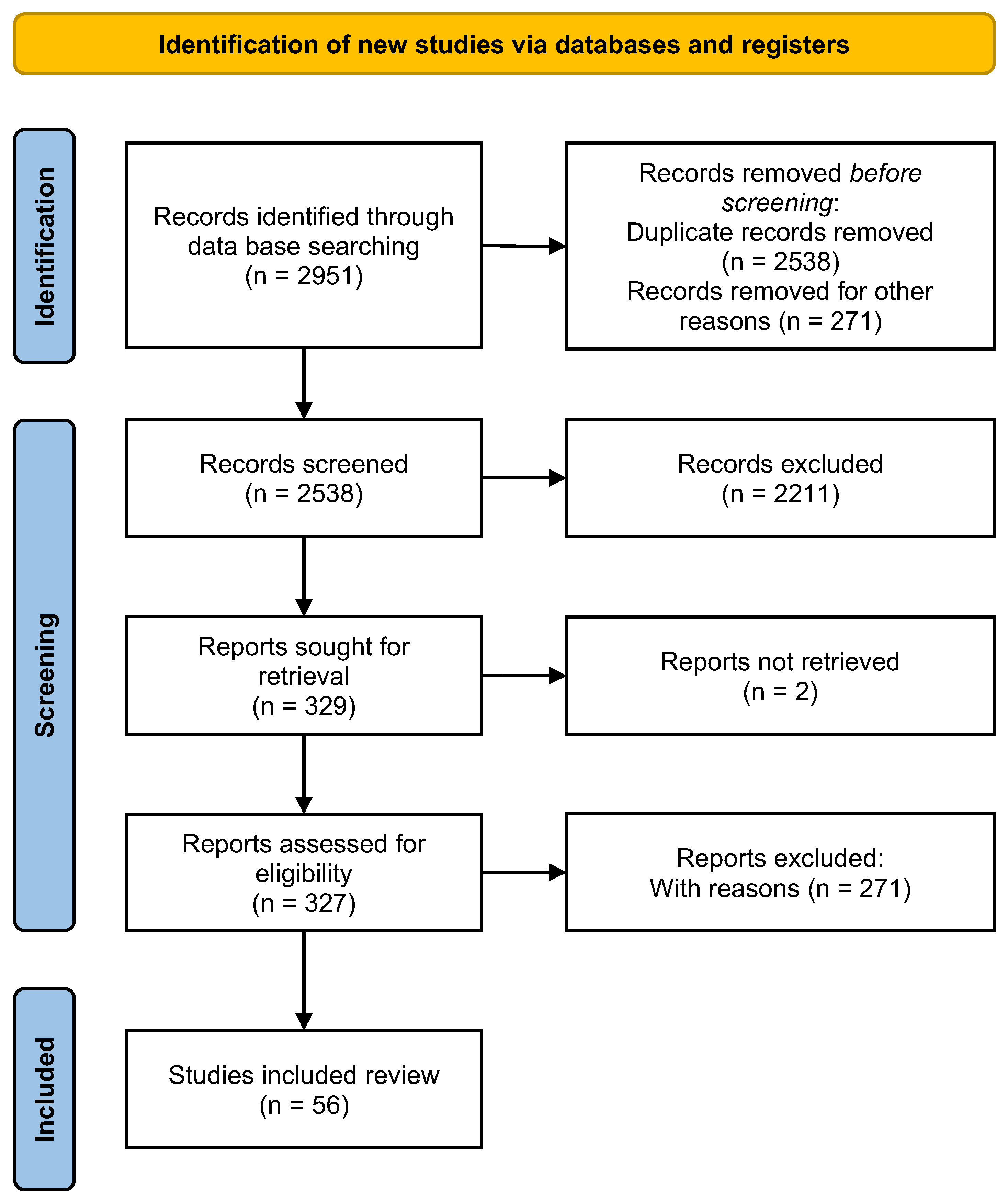
2. Methods
2.1. Literature Review and Information Selection Process
- Reviewed previous neuropharmacological research where serotonergic agonists and antagonists (5-HT1AR and 5-HT7R) were administered to assess memory using different cognitive models.
- Analyzed 5-HT1AR and 5-HT7R oligomerization on memory to integrate a theory that could correlate 5-HT1A/5HT7 heterodimerization with the pathophysiology of cognitive impairment.
2.2. Data Extraction
2.3. Risk of Bias Assessment in Selected Studies
3. Results
3.1. Selection of Articles and Descriptive Data Analysis
3.2. Effects of 5-HT1AR Agonists on Memory
| References | 5-HT1A Agonist | Animals; Other Previous Treatments (Dose of the 5-HT1A Agonist) | Behavioral Model Main Effect |
|---|---|---|---|
| Nikolaus et al., 2024 [85] | 8-OH-DPAT | Rats ♂ (3 mg/kg; i.p.—15 min) | NORT Cognitive impairment |
| Wang et al., 2020 [84]. | Mice ♂; β-Amyloid protein (1 mg/kg; i.p.—1 h) | - | |
| Janikova et al., 2021 [86]. | Rats ♂ (0.25 mg/kg; sc. During habituation) | CM - | |
| Solís-Guillén et al., 2021 [80]. | Rats ♂ (0.3–0.62 mg/kg; i.p.—30 min) | CS-US Procognitive | |
| Pittalà et al., 2015 [81]. | Mice ♂ (1 mg/kg; s.c.—15 min) | PA Procognitive | |
| du Jardin et al., 2014 [82]. | Flesinoxan | Rats ♂; PCPA (1 mg/kg; i.p.—1 h) | NORT Procognitive |
| van Hagen et al., 2022 [83]. | NLX-101 | Rats ♂ (0.08 mg/kg; i.p.—30 min acutely, 0.32 mg/kg/day chronically × 14 days) | OPS Procognitive |
3.3. Effects of 5-HT1AR Antagonists on Memory
| References | 5-HT1A Antagonist | Animals; Other Previous Treatments (Dose of the 5-HT1A Antagonist) | Behavioral Model Main Effect |
|---|---|---|---|
| Wang et al., 2020 [84]. | WAY-100635 | Mice ♂; β-Amyloid protein (0.5 mg/kg; ip—1 h) | NORT Procognitive |
| Huang et al., 2018 [89]. | Mice ♂; PCP (0.6 mg/kg; i.p.—1 h) | - | |
| Afshar et al., 2018 [87]. | NAD-299 | Rats ♂; STZ (5 μg/0.5 μL; icv) | Procognitive |
| Gharib et al., 2019 [88]. | Rats ♂; low frequency stimulation (LFS) (5 μg/μL; intrahipocampal) | Water maze Procognitive | |
| van Goethem et al., 2015 [90]. | WAY-100635 | Rats ♂; F13714 5-HT1A agonist (0.63 mg/kg; s.c.—1 h) | OPS Cognitive deterioration |
| Solís et al., 2021 [80]. | Rats ♂ (0.3 and 0.6 mg/kg; i.p.—30 min) | CS-US Procognitive |
3.4. Effects of 5-HT7R Agonists on Memory
| References | 5-HT7 Agonist | Animals; Other Previous Treatments (Dose of the 5-HT7 Agonist) | Cognitive Model Main Effect |
|---|---|---|---|
| Huang et al., 2018 [89]. | AS-19 | Mice ♂ (10 mg/kg; i.p.—30 min) | NORT - |
| Westrich et al., 2015 [72]. | Rats ♂ (5 mg/kg; —4 h and—1 h) | Procognitive | |
| Solís et al., 2021 [80]. | LP-211 | Rats ♂ (5–10 mg/kg; i.p. after last session) | CS-US |
| Meneses et al., 2015 [91]. | Rats ♂ (0.5–1 mg/kg; i.p. after last session) | Procognitive | |
| Rats ♂ scopolamine (1 mg/kg; i.p. after last session) | - |
3.5. Effects of 5-HT7R Antagonists on Memory
| References | 5-HT7 Antagonist | Animals; Other Previous Treatments (Diagram of the 5-HT7 Agonist) | Cognitive Model Main Effect |
|---|---|---|---|
| Solís et al., 2021 [80]. | SB-269970 | Rats ♂ (10 mg/kg; sc. Immediately after) | CS-US - |
| Liu et al., 2022 [93]. | Mice ♂; isoflurane (1 mg/kg; i.p.—3 days) | NORT | |
| Westrich et al., 2015 [72]. | Rats ♂ (4 mg/kg; i.p.—24 h and—1 h) | Procognitive | |
| Rats ♂; AS-19 (4 mg/kg; i.p.—24 h and—1 h) | - | ||
| Vortioxetine | Mice ♂ (10 mg/kg; i.p.—24 h and—1 h) | ||
| Jensen et al., 2014 [92]. | Rats ♀; PCPA (10 mg/kg; i.p.—1 h) | Procognitive | |
| Huang et al., 2018 [89]. | Lurasidone | Mice ♂; PCP (0.3 mg/kg; i.p.—30 min) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | adenylyl cyclase |
| ACh | Acetylcholine |
| AS-19 | (2S)-N,N-Dimethyl-5-(1,3,5-trimethylpyrazol-4-yl)-1,2,3,4-tetrahydronaphthalen-2-amine |
| 5-HT1AR | 5-HT1A receptor |
| 5-HT7R | 5-HT7 receptor Linear dichroism |
| 8-OH-DPAT | 8-Hydroxy-2-(di-n-propylamino)tetralin |
| CM | Carousel Maze |
| CNC | central nervous system |
| CS-US, | Conditioned Stimulus–Unconditioned Stimulus |
| DA | dopamine |
| DRN | dorsal raphe nuclei |
| GABA | gamma-aminobutyric acid |
| Glu | glutamate |
| GPCR | G protein-coupled receptor |
| HC | hippocampus |
| LP-211 | N-[(4-cyanophenyl)methyl]-6-[4-(2-phenylphenyl)piperazin-1-yl]hexanamide |
| NAD-299 | (3R)-3-[di(cyclobutyl)amino]-8-fluoro-3,4-dihydro-2H-chromene-5-carboxamide |
| NORT | Novel Object Recognition Test |
| OPS | Object Pattern Separation |
| PA | tested in the Passive Avoidance Task |
| PCP | phencyclidine |
| PFC | prefrontal cortex |
| PLC | phospholipase C |
| SB-269970 | (3-[(2R-2-[2-(4-methylpiperidin-1-yl)ethyl]pyrrolidin-1-yl]sulfonylphenol |
| SERT | serotonin transporter |
| STZ | streptozotocin |
| SYRCLE | Systematic Review Centre for Laboratory Animal Experimentation |
| WAY-100635 | N-[2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-pyridin-2-ylcyclohexanecarboxamide |
References
- Kroeze, W.; Kristiansen, K.; Roth, B. Molecular Biology of Serotonin Receptors-Structure and Function at the Molecular Level. Curr. Top. Med. Chem. 2002, 2, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Roth, B. Multiple Serotonin Receptors: Clinical and Experimental Aspects. Ann. Clin. Psychiatry 1994, 6, 67–78. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar] [CrossRef]
- Göthert, M. 5-Hydroxytryptamine receptors. An example for the complexity of chemical transmission of information in the brain. Arzneimittelforschung 1992, 42, 238–246. [Google Scholar] [PubMed]
- Ray, R.S.; Corcoran, A.E.; Brust, R.D.; Kim, J.C.; Richerson, G.B.; Nattie, E.; Dimecki, S.M. Impaired Respiratory and Body Temperature Control Upon Acute Serotonergic Neuron Inhibition. Science 2011, 333, 637–642. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The Expanded Biology of Serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Airan, R.D.; Meltzer, L.A.; Roy, M.; Gong, Y.; Chen, H.; Deisseroth, K. High-Speed Imaging Reveals Neurophysiological Links to Behavior in an Animal Model of Depression. Science 2007, 317, 819–823. [Google Scholar] [CrossRef]
- Singh, D.; Singh, P.; Srivastava, P.; Kakkar, D.; Pathak, M.; Tiwari, A.K. Development and challenges in the discovery of 5-HT1A and 5-HT7 receptor ligands. Bioorg. Chem. 2023, 131, 106254. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, G.; Santagada, V.; Perissutti, E.; Fiorino, F. Derivatives as 5HT1A receptor ligands-past and present. Curr. Med. Chem. 2005, 12, 1721–1753. [Google Scholar] [CrossRef]
- Banasr, M.; Hery, M.; Printemps, R.; Daszuta, A. Serotonin-Induced Increases in Adult Cell Proliferation and Neurogenesis are Mediated Through Different and Common 5-HT Receptor Subtypes in the Dentate Gyrus and the Subventricular Zone. Neuropsychopharmacology 2004, 29, 450–460. [Google Scholar] [CrossRef]
- Noto, B.; Klempin, F.; Alenina, N.; Bader, M.; Fink, H.; Sander, S.E. Increased adult neurogenesis in mice with a permanent overexpression of the postsynaptic 5-HT1A receptor. Neurosci. Lett. 2016, 633, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Miheau, J.; Barbara, V.M. Stimulation of 5-HT1A receptors by systemic or medial septum injection induces anxiogenic-like effects and facilitates acquisition of a spatial discrimination task in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 1999, 23, 1113–1133. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.J.; Hedlund, P.B. The 5-HT7 receptor in learning and memory. Hippocampus 2012, 22, 762–771. [Google Scholar] [CrossRef]
- Meneses, A. Effects of the 5-HT7 receptor antagonists SB-269970 and DR 4004 in autoshaping Pavlovian/instrumental learning task. Behav. Brain Res. 2004, 155, 275–282. [Google Scholar] [CrossRef]
- Sarkisyan, G.; Hedlund, P.B. The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav. Brain Res. 2009, 202, 26–31. [Google Scholar] [CrossRef]
- Riad, M.; Garcia, S.; Watkins, K.C.; Jodoin, N.; Doucet, E.; Langlois, X.; Mestikawy, S.; Hamon, M.; Descarries, L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J. Comp. Neurol. 2000, 417, 181–194. [Google Scholar] [CrossRef]
- Pazos, A.; Palacios, J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985, 346, 205–230. [Google Scholar] [CrossRef] [PubMed]
- Teitler, M.; Toohey, N.; Knight, J.A.; Klein, M.T.; Smith, C. Clozapine and other competitive antagonists reactivate risperidone-inactivated h5-HT7 receptors: Radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology 2010, 212, 687–697. [Google Scholar] [CrossRef][Green Version]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M.; et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef]
- Millan, M.J.; Agid, Y.; Brüne, M.; Bullmore, E.T.; Carter, C.S.; Clayton, N.S.; Connor, R.; Davis, S.; Deakin, B.; DeRubeis, R.J.; et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012, 11, 141–168. [Google Scholar] [CrossRef]
- Kobilka, B.K.; Frielle, T.; Collins, S.; Yang-Feng, T.; Kobilka, T.S.; Francke, U.; Lefkowitz, R.J.; Caron, M.G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature 1987, 329, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Fargin, A.; Raymond, J.R.; Lohse, M.J.; Kobilka, B.K.; Caron, M.G.; Lefkowitz, R.J. The genomic clone G-21 which resembles a β-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature 1988, 335, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Oakey, R.J.; Caron, M.G.; Lefkowitz, R.J.; Seldin, M.F. Genomic organization of adrenergic and serotonin receptors in the mouse: Linkage mapping of sequence-related genes provides a method for examining mammalian chromosome evolution. Genomics 1991, 10, 338–344. [Google Scholar] [CrossRef]
- Albert, P.R.; Zhou, Q.Y.; Van Tol, H.H.; Bunzow, J.R.; Civelli, O. Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J. Biol. Chem. 1990, 265, 5825–5832. [Google Scholar] [CrossRef]
- Ou, X.M.; Jafar-Nejad, H.; Storring, J.M.; Meng, J.H.; Lemonde, S.; Albert, P.R. Novel Dual Repressor Elements for Neuronal Cell-specific Transcription of the Rat 5-HT1A Receptor Gene. J. Biol. Chem. 2000, 275, 8161–8168. [Google Scholar] [CrossRef]
- Lemonde, S.; Turecki, G.; Bakish, D.; Du, L.; Hrdina, P.D.; Bown, C.D.; Sequeira, A.; Kushwaha, N.; Morris, S.J.; Basak, A.; et al. Impaired Repression at a 5-Hydroxytryptamine 1A Receptor Gene Polymorphism Associated with Major Depression and Suicide. J. Neurosci. 2003, 23, 8788–8799. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: Molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 2004, 103, 21–80. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Claustre, Y.; Benavides, J.; Scatton, B. Potential Mechanisms Involved in the Negative Coupling Between Serotonin 5-HT1A Receptors and Carbachol-Stimulated Phosphoinositide Turnover in the Rat Hippocampus. J. Neurochem. 1991, 56, 1276–1285. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2019, 161, 34–45. [Google Scholar] [CrossRef]
- Lanfumey, L.; Hamon, M. Central 5-HT1A receptors: Regional distribution and functional characteristics. Nucl. Med. Biol. 2000, 27, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C.; Cholley, B.; El Mestikawy, S.; Gozlan, H.; Hamon, M. Direct Immunohistochemical Evidence of the Existence of 5-HT1A Autoreceptors on Serotoninergic Neurons in the Midbrain Raphe Nuclei. Eur. J. Neurosci. 1990, 2, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; Piñeyro, G.; el Mansari, M.; Bergeron, R.; de Montigny, C. Role of somatodendritic 5-HT autoreceptors in modulating 5-HT neurotransmission. Ann. N. Y. Acad. Sci. 1998, 861, 204–216. [Google Scholar] [CrossRef]
- Varnäs, K.; Halldin, C.; Hall, H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum. Brain Mapp. 2004, 22, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Riad, M.; Watkins, K.C.; Doucet, E.; Hamon, M.; Descarries, L. Agonist-Induced Internalization of Serotonin-1A Receptors in the Dorsal Raphe Nucleus (Autoreceptors) But Not Hippocampus (Heteroreceptors). J. Neurosci. 2001, 21, 8378–8386. [Google Scholar] [CrossRef]
- Lin, M.T.; Tsay, H.J.; Su, W.H.; Chueh, F.Y. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am. J. Physiol. 1998, 274, 1260–1267. [Google Scholar] [CrossRef]
- Kaufman, J.; DeLorenzo, C.; Choudhury, S.; Parsey, R.V. The 5-HT1A receptor in Major Depressive Disorder. Eur. Neuropsychopharmacol. 2016, 26, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Celada, P.; Bortolozzi, A.; Artigas, F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: Rationale and current status of research. CNS Drugs 2013, 27, 703–716. [Google Scholar] [CrossRef]
- Arvidsson, L.E.; Hacksell, U.; Nilsson, J.L.; Hjorth, S.; Carlsson, A.; Lindberg, P.; Sanchez, D.; Wikstrom, H. 8-Hydroxy-2-(dipropylamino)tetralin, a new centrally acting 5-hydroxytryptamine receptor agonist. J. Med. Chem. 1981, 24, 921–923. [Google Scholar] [CrossRef]
- Bard, J.A.; Zgombick, J.; Adham, N.; Vaysse, P.; Branchek, T.A.; Weinshank, R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993, 268, 23422–23426. [Google Scholar] [CrossRef]
- Lovenberg, T.W.; Baron, B.M.; de Lecea, L.; Miller, J.D.; Prosser, R.A.; Rea, M.A.; Foye, P.E.; Racke, M.; Slone, A.L.; Siegel, B.W. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 1993, 11, 449–458. [Google Scholar] [CrossRef]
- Ruat, M.; Traiffort, E.; Leurs, R.; Tardivel-Lacombe, J.; Diaz, J.; Arrang, J.M.; Schwartz, J.C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. USA 1993, 90, 8547–8551. [Google Scholar] [CrossRef] [PubMed]
- Vanhoenacker, P.; Haegeman, G.; Leysen, J.E. 5-HT7 receptors: Current knowledge and future prospects. Trends Pharmacol. Sci. 2000, 21, 70–77. [Google Scholar] [CrossRef]
- Parajulee, A.; Kim, K. Structural studies of serotonin receptor family. BMB Rep. 2023, 56, 527–536. [Google Scholar] [CrossRef]
- Errico, M.; Crozier, R.A.; Plummer, M.R.; Cowen, D.S. 5-HT7 receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience 2001, 102, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Farley, N.N.; Kertesy, S.B.; Dubyak, G.R.; Cowen, D.S. Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J. Neurochem. 2005, 92, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Kvachnina, E.; Liu, G.; Dityatev, A.; Renner, U.; Dumuis, A.; Richter, D.W.; Dityateva, G.; Schachner, M.; Voyno-Yasenetskaya, T.A.; Ponimaskin, E.G. 5-HT7Receptor Is Coupled to Gα Subunits of Heterotrimeric G12-Protein to Regulate Gene Transcription and Neuronal Morphology. J. Neurosci. 2005, 25, 7821–7830. [Google Scholar] [CrossRef]
- Kobe, F.; Guseva, D.; Jensen, T.P.; Wirth, A.; Renner, U.; Hess, D.; Müller, M.; Medrihan, L.; Zhang, W.; Zhang, M.; et al. 5-HT7 R/G12 Signaling Regulates Neuronal Morphology and Function in an Age-Dependent Manner. J. Neurosci. 2012, 32, 2915–2930. [Google Scholar] [CrossRef]
- Dogrul, A.; Seyrek, M. Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br. J. Pharmacol. 2006, 149, 498–505. [Google Scholar] [CrossRef]
- Neumaier, J.F.; Sexton, T.J.; Yracheta, J.; Diaz, A.M.; Brownfield, M. Localization of 5-HT7 receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J. Chem. Neuroanat. 2001, 21, 63–73. [Google Scholar] [CrossRef]
- Bonaventure, P.; Nepomuceno, D.; Hein, L.; Sutcliffe, J.G.; Lovenberg, T.; Hedlund, P.B. Radioligand binding analysis of knockout mice reveals 5-hydroxytryptamine7 receptor distribution and uncovers 8-hydroxy-2-(di-n-propylamino)tetralin interaction with α2 adrenergic receptors. Neuroscience 2004, 124, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Berumen, L.C.; Rodríguez, A.; Miledi, R.; García-Alcocer, G. Serotonin Receptors in Hippocampus. Sci. World J. 2012, 2012, 823493. [Google Scholar] [CrossRef]
- Watts, S.W.; Darios, E.S.; Seitz, B.M.; Thompson, J.M. 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol. Res. Perspect. 2015, 3, e00103. [Google Scholar] [CrossRef]
- Irving, H.R.; Tan, Y.Y.; Tochon-Danguy, N.; Liu, H.; Chetty, N.; Desmond, P.V.; Pouton, C.W.; Coupar, I.M. Comparison of 5-HT4 and 5-HT7 receptor expression and function in the circular muscle of the human colon. Life Sci. 2007, 80, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Tonini, M.; Vicini, R.; Cervio, E.; De Ponti, F.; De Giorgio, R.; Barbara, G.; Stanghellini, V.; Dellabianca, A.; Sternini, C. 5-HT7 Receptors Modulate Peristalsis and Accommodation in the Guinea Pig Ileum. Gastroenterology 2005, 129, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Nawoschik, S.P.; Schurman, B.R.; Schmitt, H.L.; Burno, M.; Smith, D.L.; Schechter, L. Identification of a human 5-HT6 receptor variant produced by alternative splicing. Brain research. Brain Res. Mol. Brains Res. 1999, 64, 255–263. [Google Scholar] [CrossRef]
- Speranza, L.; Chambery, A.; Di Domenico, M.; Crispino, M.; Severino, V.; Volpicelli, F.; Leopoldo, M.; Bellenchi, G.; Di Porzio, U.; Perrone-Capano, C. The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology 2013, 67, 155–167. [Google Scholar] [CrossRef]
- Gasbarri, A.; Cifariello, A.; Pompili, A.; Meneses, A. Effect of 5-HT7 antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav. Brain Res. 2008, 195, 164–170. [Google Scholar] [CrossRef]
- Miura, I.; Horikoshi, S.; Ichinose, M.; Suzuki, Y.; Watanabe, K. Lurasidone for the Treatment of Schizophrenia: Design, Development, and Place in Therapy. Drug Des. Dev. Ther. 2023, 17, 3023–3031. [Google Scholar] [CrossRef]
- Smith, C.; Rahman, T.; Toohey, N.; Mazurkiewicz, J.; Herrick-Davis, K.; Teitler, M. Risperidone Irreversibly Binds to and Inactivates the h5-HT 7 Serotonin Receptor. Mol. Pharmacol. 2006, 70, 1264–1270. [Google Scholar] [CrossRef]
- Roberts, C.; Allen, L.; Langmead, C.J.; Hagan, J.J.; Middlemiss, D.N.; Price, G.W. The effect of SB-269970, a 5-HT(7) receptor antagonist, on 5-HT release from serotonergic terminals and cell bodies. Br. J. Pharmacol. 2001, 132, 1574–1580. [Google Scholar] [CrossRef] [PubMed]
- Di Pilato, P.; Niso, M.; Adriani, W.; Romano, E.; Travaglini, D.; Berardi, F.; Colabufo, N.A.; Perrone, R.; Laviola, G.; Lcivita, E.; et al. Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: An overview. Rev. Neurosci. 2014, 25, 401–415. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Martel, J.C.; Assié, M.B.; Buritova, J.; Lauressergues, E.; Cosi, C.; Heusler, P.; Bruins Slot, L.; Colpaert, B.F.C.; Vacher, E.; et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT1A receptor agonist. Br. J. Pharmacol. 2009, 156, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Stenfors, C.; Werner, T.; Ross, S.B. In vivo labelling of the mouse brain 5-hydroxytryptamine1A receptor with the novel selective antagonist 3H-NAD-299. Naunyn Schmiedebergs Arch. Pharmacol. 1998, 357, 500–507. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Gavaudan, S.; Conte, C.; Chaput, C.; Touzard, M.; Verrièle, L.; Audinot, V.; Millan, M.J. Agonist and antagonist actions of antipsychotic agents at 5-HT1A receptors: A []GTPγS binding study. Eur. J. Pharmacol. 1998, 355, 245–256. [Google Scholar] [CrossRef]
- Ishibashi, T.; Horisawa, T.; Tokuda, K.; Ishiyama, T.; Ogasa, M.; Tagashira, R.; Matsumoto, K.; Nishikawa, H.; Ueda, Y.; Toma, S.; et al. Pharmacological Profile of Lurasidone, a Novel Antipsychotic Agent with Potent 5-Hydroxytryptamine 7 (5-HT7) and 5-HT1A Receptor Activity. J. Pharmacol. Exp. Ther. 2010, 334, 171–181. [Google Scholar] [CrossRef]
- Leopoldo, M.; Lacivita, E.; De Giorgio, P.; Fracasso, C.; Guzzetti, S.; Caccia, S.; Contino, M.; Colabufo, N.; Berardi, F.; Perrone, R. Structural Modifications of N-(1,2,3,4-Tetrahydronaphthalen-1-yl)-4-Aryl-1-piperazinehexanamides: Influence on Lipophilicity and 5-HT7 Receptor Activity. Part III. J. Med. Chem. 2008, 51, 5813–5822. [Google Scholar] [CrossRef]
- Forbes, I.T.; Dabbs, S.; Duckworth, D.M.; Jennings, A.J.; King, F.D.; Lovell, P.J.; Brown, A.M.; Collin, L.; Hagan, J.J.; Middlemiss, D.N.; et al. (R)-3,N-dimethyl-N-[1-methyl-3-(4-methyl-piperidin-1-yl) propyl]benzenesulfonamide: The first selective 5-HT7 receptor antagonist. J. Med. Chem. 1998, 41, 655–657. [Google Scholar] [CrossRef] [PubMed]
- Brenchat, A.; Romero, L.; García, M.; Pujol, M.; Burgueño, J.; Torrens, A.; Hamon, M.; Baeyens, J.M.; Buschmann, H.; Zamanillo, D.; et al. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain 2009, 141, 239–247. [Google Scholar] [CrossRef]
- Lovell, P.J.; Bromidge, S.M.; Dabbs, S.; Duckworth, D.M.; Forbes, I.T.; Jennings, A.J.; King, F.D.; Middlemiss, D.N.; Rahman, S.K.; Saunders, D.V.; et al. A novel, potent, and selective 5-HT(7) antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolidine-1-sulfonyl)phenol (SB-269970). J. Med. Chem. 2000, 43, 342–345. [Google Scholar] [CrossRef]
- Hedlund, P.B.; Leopoldo, M.; Caccia, S.; Sarkisyan, G.; Fracasso, C.; Martelli, G.; Lacivita, E.; Berardi, F.; Perrone, R. LP-211 is a brain penetrant selective agonist for the serotonin 5-HT(7) receptor. Neurosci. Lett. 2010, 481, 12–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Westrich, L.; Haddjeri, N.; Dkhissi-Benyahya, O.; Sánchez, C. Involvement of 5-HT7 receptors in vortioxetine’s modulation of circadian rhythms and episodic memory in rodents. Neuropharmacology 2015, 89, 382–390. [Google Scholar] [CrossRef][Green Version]
- Rozenfeld, R.; Devi, L.A. Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimers. FASEB J. 2007, 21, 2455–2465. [Google Scholar] [CrossRef]
- Carriba, P.; Navarro, G.; Ciruela, F.; Ferré, S.; Casadó, V.; Agnati, L.; Cortés, A.; Mallol, J.; Fuxe, K.; Canela, E.; et al. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods 2008, 5, 727–733. [Google Scholar] [CrossRef]
- Ferré, S.; Franco, R. Oligomerization of G-protein-coupled receptors: A reality. Curr. Opin. Pharmacol. 2010, 10, 1–5. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Tarakanov, A.O.; Guidolin, D.; Ciruela, F.; Agnati, L.F.; Fuxe, K. Moonlighting characteristics of G protein-coupled receptors: Focus on receptor heteromers and relevance for neurodegeneration. IUBMB Life 2011, 63, 463–472. [Google Scholar] [CrossRef]
- Fernández-Dueñas, V.; Bonaventura, J.; Aso, E.; Luján, R.; Ferré, S.; Ciruela, F. Overcoming the Challenges of Detecting GPCR Oligomerization in the Brain. Curr. Neuropharmacol. 2022, 20, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 89, 105906. [Google Scholar]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Solís-Guillén, R.; Leopoldo, M.; Meneses, A.; Centurión, D. Activation of 5-HT1A and 5-HT7 receptors enhanced a positively reinforced long-term memory. Behav. Brain Res. 2021, 397, 112932. [Google Scholar] [CrossRef]
- Pittalà, V.; Siracusa, M.A.; Salerno, L.; Romeo, G.; Modica, M.N.; Madjid, N.; Ogren, S.O. Analysis of mechanisms for memory enhancement using novel and potent 5-HT1A receptor ligands. Eur. Neuropsychopharmacol. 2015, 25, 1314–1323. [Google Scholar] [CrossRef]
- du Jardin, K.G.; Jensen, J.B.; Sanchez, C.; Pehrson, A.L. Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: A potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. Eur. Neuropsychopharmacol. 2014, 24, 160–171. [Google Scholar] [CrossRef]
- van Hagen, B.T.J.; van Goethem, N.P.; Nelissen, E.; Paes, D.; Koymans, K.; van Hoof, S.; Schreiber, R.; Varney, M.; Adrian Newman-Tancredi, A.; Prickaerts, J. Biased 5-HT1A receptor agonists F13714 and NLX-101 differentially affect pattern separation and neuronal plasticity in rats after acute and chronic treatment. Mol. Cell Neurosci. 2022, 120, 103719. [Google Scholar] [CrossRef]
- Wang, M.; Zong, H.F.; Chang, K.W.; Han, H.; Yasir Rizvi, M.; Iffat Neha, S.; Li, Z.Y.; Yang, W.N.; Qian, Y.H. 5-HT1AR alleviates Aβ-induced cognitive decline and neuroinflammation through crosstalk with NF-κB pathway in mice. Int. Immunopharmacol. 2020, 82, 106354. [Google Scholar] [CrossRef]
- Nikolaus, S.; Chao, O.Y.; Henke, J.; Beu, M.; Fazari, B.; Almeida, F.R.; Abdel-Hafiz, L.; Antke, C.; Hautzel, H.; Mamlins, E.; et al. 5-HT1A and 5-HT2A receptor effects on recognition memory, motor/exploratory behaviors, emotionality and regional dopamine transporter binding in the rat. Behav. Brain Res. 2024, 469, 115051. [Google Scholar] [CrossRef] [PubMed]
- Janikova, M.; Mainerova, K.; Vojtechova, I.; Petrasek, T.; Svoboda, J.; Stuchlik, A. Memantine and Riluzole Exacerbate, Rather Than Ameliorate Behavioral Deficits Induced by 8-OH-DPAT Sensitization in a Spatial Task. Biomolecules 2021, 11, 1007. [Google Scholar] [CrossRef] [PubMed]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Komaki, A.; Asl, S.S. The effect of NAD-299 and TCB-2 on learning and memory, hippocampal BDNF levels and amyloid plaques in Streptozotocin-induced memory deficits in male rats. Psychopharmacology 2018, 235, 2809–2822. [Google Scholar] [CrossRef]
- Gharib, A.; Komaki, A.; Manoochehri Khoshinani, H.; Saidijam, M.; Barkley, V.; Sarihi, A.; Mirnajafi-Zadeh, J. Intrahippocampal 5-HT1A receptor antagonist inhibits the improving effect of low-frequency stimulation on memory impairment in kindled rats. Brain Res. Bull. 2019, 148, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Kwon, S.; Rajagopal, L.; He, W.; Meltzer, H.Y. 5-HT1A parital agonism and 5-HT7 antagonism restore episodic memory in subchronic phencyclidine-treated mice: Role of brain glutamate, dopamine, acetylcholine and GABA. Psychopharmacology 2018, 235, 2795–2808. [Google Scholar] [CrossRef]
- van Goethem, N.P.; Schreiber, R.; Newman-Tancredi, A.; Varney, M.; Prickaerts, J. Divergent effects of the “biased” 5-HT1 A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br. J. Pharmacol. 2015, 172, 2532–2543. [Google Scholar] [CrossRef]
- Meneses, A.; Perez-Garcia, G.; Liy-Salmeron, G.; Ponce-López, T.; Lacivita, E.; Leopoldo, M. 5-HT7 receptor activation: Procognitive and antiamnesic effects. Psychopharmacology 2015, 232, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.B.; du Jardin, K.G.; Song, D.; Budac, D.; Smagin, G.; Sanchez, C.; Pehrson, A.L. Vortioxetine, but not escitalopram or duloxetine, reverses memory impairment induced by central 5-HT depletion in rats: Evidence for direct 5-HT receptor modulation. Eur. Neuropsychopharmacol. 2014, 24, 148–159. [Google Scholar] [CrossRef]
- Liu, T.; Song, J.; Zhou, Q.; Chu, S.; Liu, Y.; Zhao, X.; Ma, Z.; Xia, T.; Gu, X. The role of 5-HT7R in the memory impairment of mice induced by long-term isoflurane anesthesia. Neurobiol. Learn. Mem. 2022, 188, 107584. [Google Scholar] [CrossRef]
- Stiedl, O.; Pappa, E.; Konradsson-Geuken, Å.; Ögren, S.O. The role of the serotonin receptor subtypes 5-HT1A and 5-HT7 and its interaction in emotional learning and memory. Front. Pharmacol. 2015, 6, 162. [Google Scholar] [CrossRef]
- Iceta, R.; Mesonero, J.E.; Aramayona, J.J.; Alcalde, A.I. Expression of 5-HT1A and 5-HT7 receptors in Caco-2 cells and their role in the regulation of serotonin transporter activity. J. Physiol. Pharmacol. 2009, 60, 157–164. [Google Scholar]
- Rajagopal, L.; Massey, B.W.; Michael, E.; Meltzer, H.Y. Serotonin (5-HT)1A receptor agonism and 5-HT7 receptor antagonism ameliorate the subchronic phencyclidine-induced deficit in executive functioning in mice. Psychopharmacology 2016, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, V.S.; Popova, N.K.; Lacivita, E.; Leopoldo, M.; Ponimaskin, E.G. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci. Ther. 2014, 20, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Capon, A.; De Rood, M.; Verbist, A.; Fruhling, J.; Baleriaux, D. [Contribution of a function test to the measurement of regional blood flow in cerebral ischemia]. Rev. Electroencephalogr. Neurophysiol. Clin. 1974, 4, 217–220. [Google Scholar] [CrossRef]
- Parenti, G.; Crova, M.; Frigo, G. [Intertrochanteric osteotomy of the hip in adults. Indications and technics]. Minerva Ortop. 1969, 20, 642–644. [Google Scholar]
- Danet, M.; Lapiz-Bluhm, S.; Morilak, D.A. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. Int. J. Neuropsychopharmacol. 2010, 13, 997–1009. [Google Scholar] [CrossRef]
- Wallace, A.; Pehrson, A.L.; Sánchez, C.; Morilak, D.A. Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int. J. Neuropsychopharmacol. 2014, 17, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Horiguchi, M.; Felix, A.R.; Meltzer, H.Y. 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. Neuroreport 2012, 23, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Gasbarri, A.; Pompili, A. Serotonergic 5-HT7 receptors and cognition. Rev. Neurosci. 2014, 25, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, G.; Meneses, A. Memory time-course: mRNA 5-HT1A and 5-HT7 receptors. Behav. Brain Res. 2009, 202, 102–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briones-Aranda, A.; Flores-Durán, D.; Romero-Nava, R.; Corzo-Gómez, J.C.; Cruz-Trujillo, R.; Toalá-Sepúlveda, F.; Del-Río-Navarro, B.E.; Huang, F. Role of 5-HT1A and 5-HT7 Receptors in Memory Regulation and the Importance of Their Coexpression: A Systematic Review. Biomolecules 2025, 15, 762. https://doi.org/10.3390/biom15060762
Briones-Aranda A, Flores-Durán D, Romero-Nava R, Corzo-Gómez JC, Cruz-Trujillo R, Toalá-Sepúlveda F, Del-Río-Navarro BE, Huang F. Role of 5-HT1A and 5-HT7 Receptors in Memory Regulation and the Importance of Their Coexpression: A Systematic Review. Biomolecules. 2025; 15(6):762. https://doi.org/10.3390/biom15060762
Chicago/Turabian StyleBriones-Aranda, Alfredo, Daniela Flores-Durán, Rodrigo Romero-Nava, Josselin Carolina Corzo-Gómez, Refugio Cruz-Trujillo, Floribert Toalá-Sepúlveda, Blanca E. Del-Río-Navarro, and Fengyang Huang. 2025. "Role of 5-HT1A and 5-HT7 Receptors in Memory Regulation and the Importance of Their Coexpression: A Systematic Review" Biomolecules 15, no. 6: 762. https://doi.org/10.3390/biom15060762
APA StyleBriones-Aranda, A., Flores-Durán, D., Romero-Nava, R., Corzo-Gómez, J. C., Cruz-Trujillo, R., Toalá-Sepúlveda, F., Del-Río-Navarro, B. E., & Huang, F. (2025). Role of 5-HT1A and 5-HT7 Receptors in Memory Regulation and the Importance of Their Coexpression: A Systematic Review. Biomolecules, 15(6), 762. https://doi.org/10.3390/biom15060762






