CIRBP Enhances the Function of Yak Cumulus Cells by Activating AMPK/mTOR-Mediated Mitophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture of Primary Yak Cumulus Cells (YCCs)
2.2. Low-Temperature Treatment and Transfection of YCCs
2.3. Drug Treatment of YCCs
2.4. Immunofluorescence Staining
2.5. RT-PCR
2.6. Detection of E2 and P4 Secretion
2.7. Western Blotting
2.8. Flow Cytometry
2.9. Detection of Ad-mCherry-GFP-LC3B
2.10. Statistical Analysis
3. Results
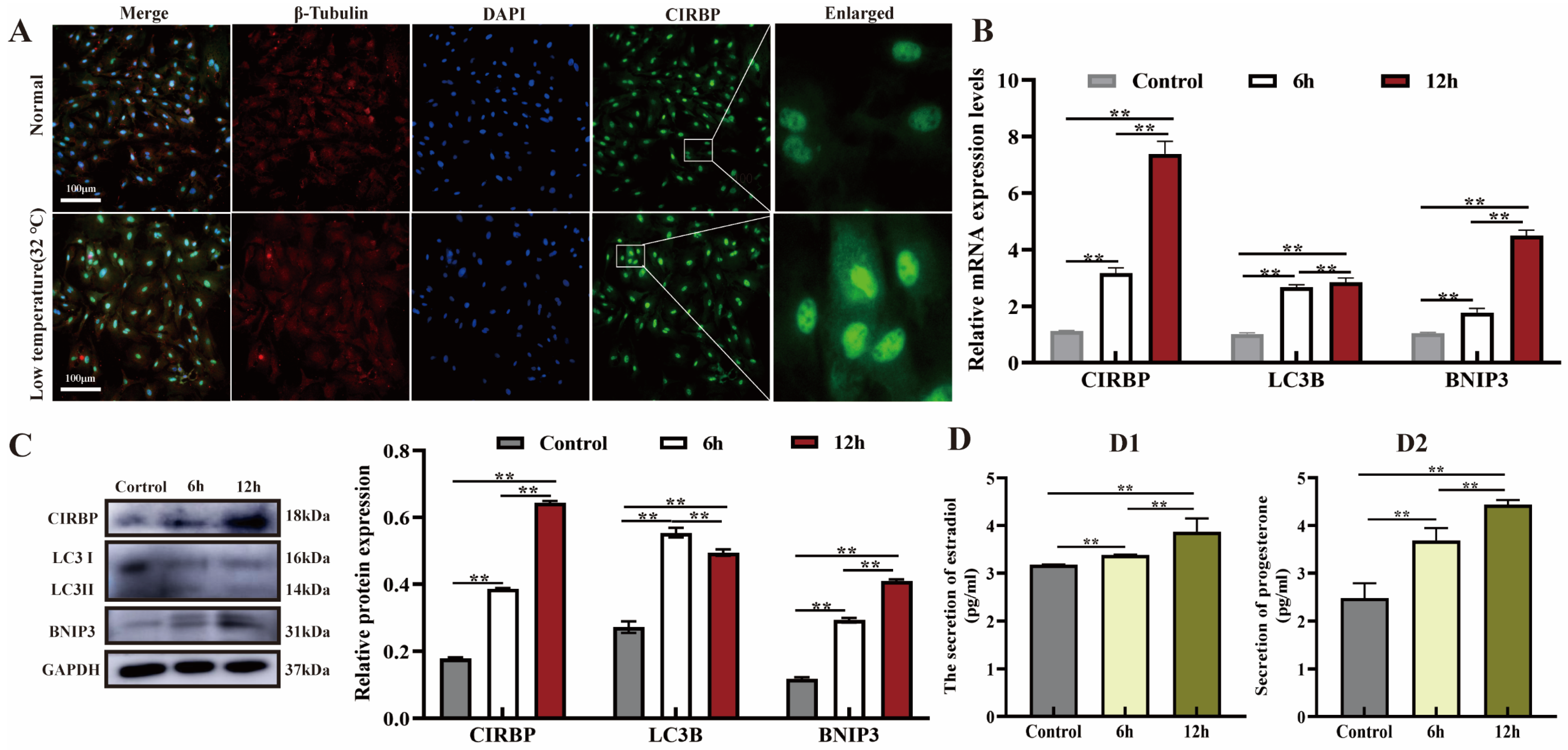
3.1. Effect of Low Temperature on CIRBP Expression, Mitophagy and E2 and P4 Secretion in Cells
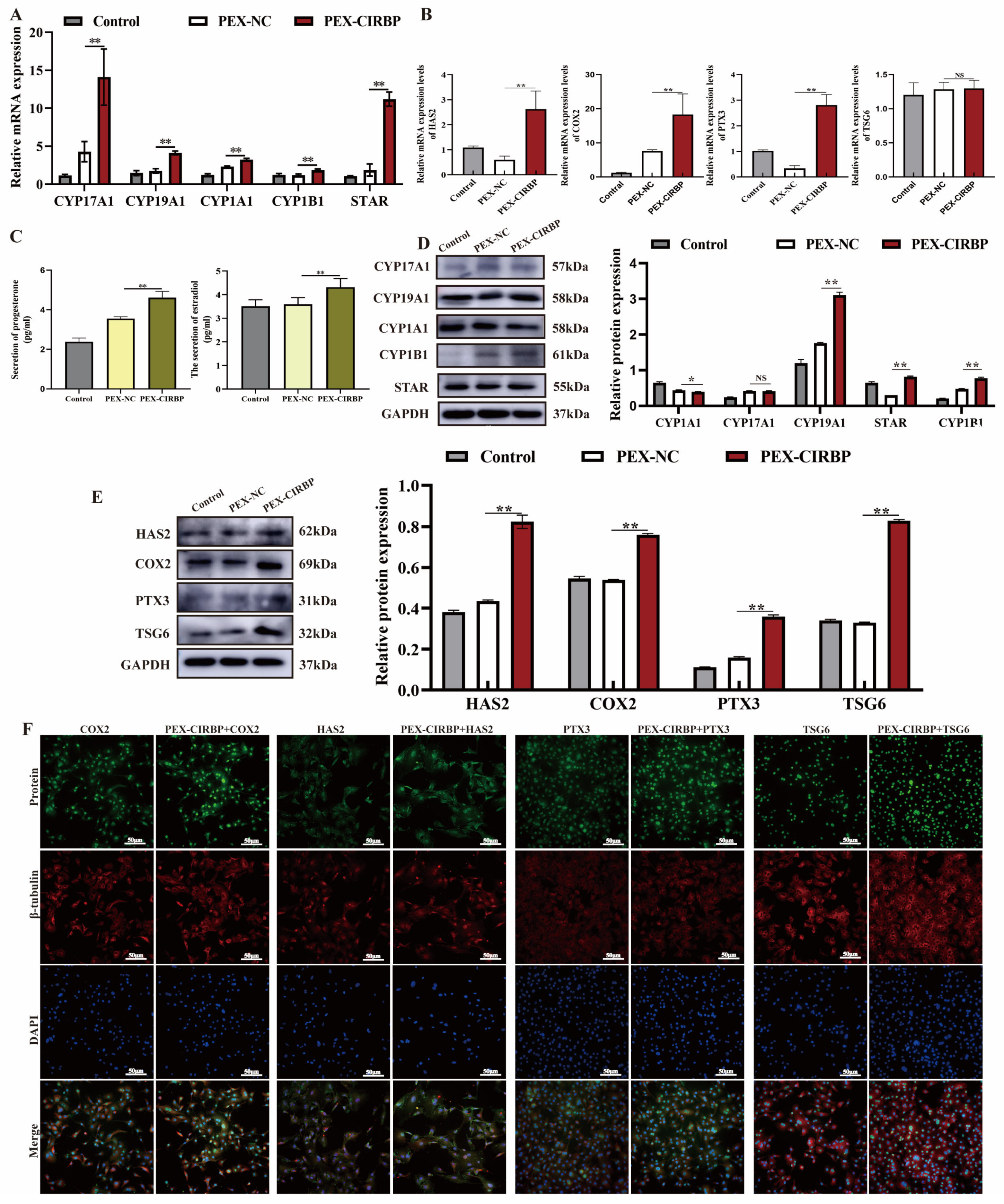
3.2. Overexpression of CIRBP Activates Mitophagy in YCCs
3.3. Overexpression of CIRBP Improves YCC Function and Inhibits Apoptosis
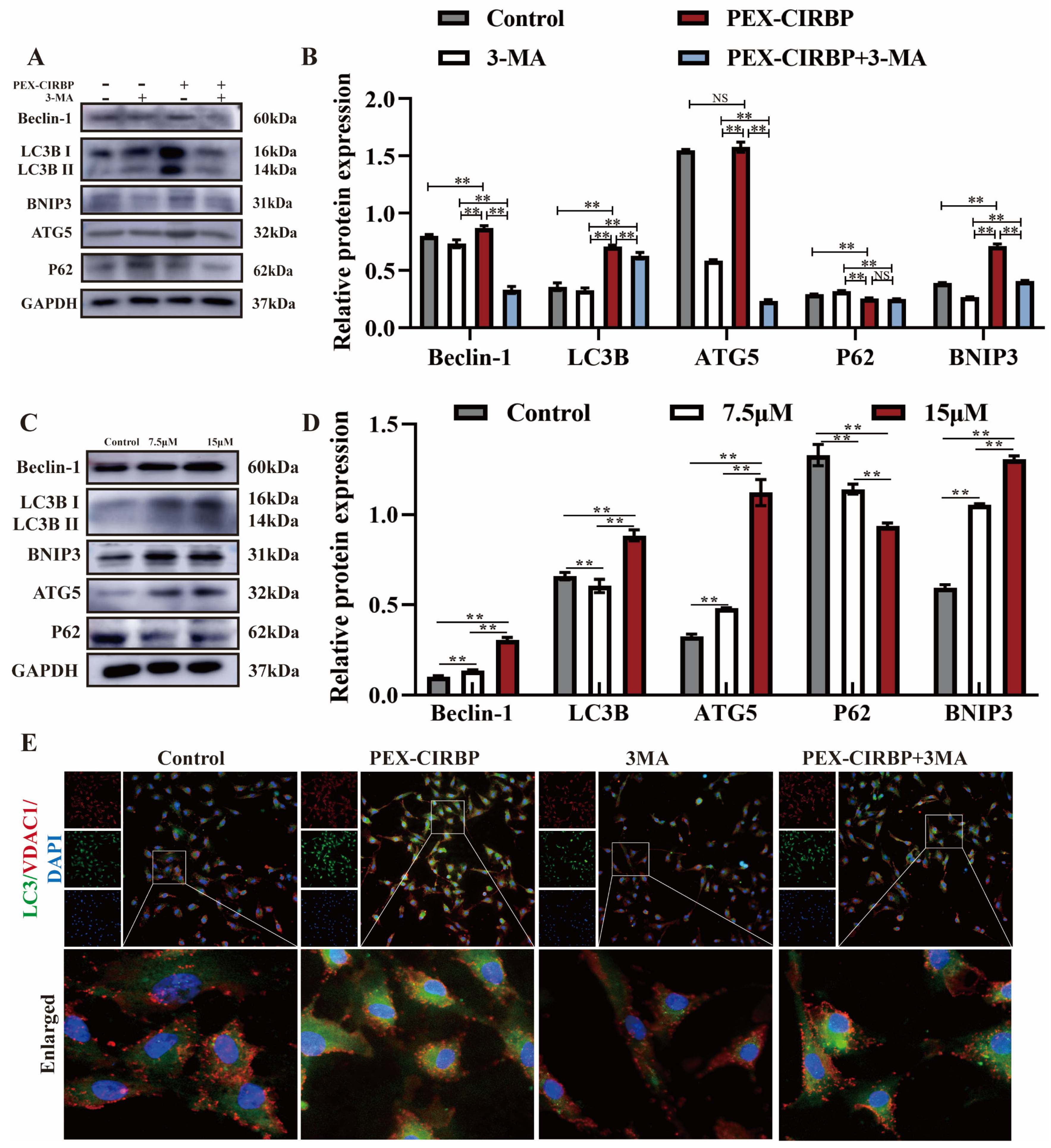
3.4. CIRBP Enhances YCC Function and Inhibits Apoptosis by Activating Mitophagy
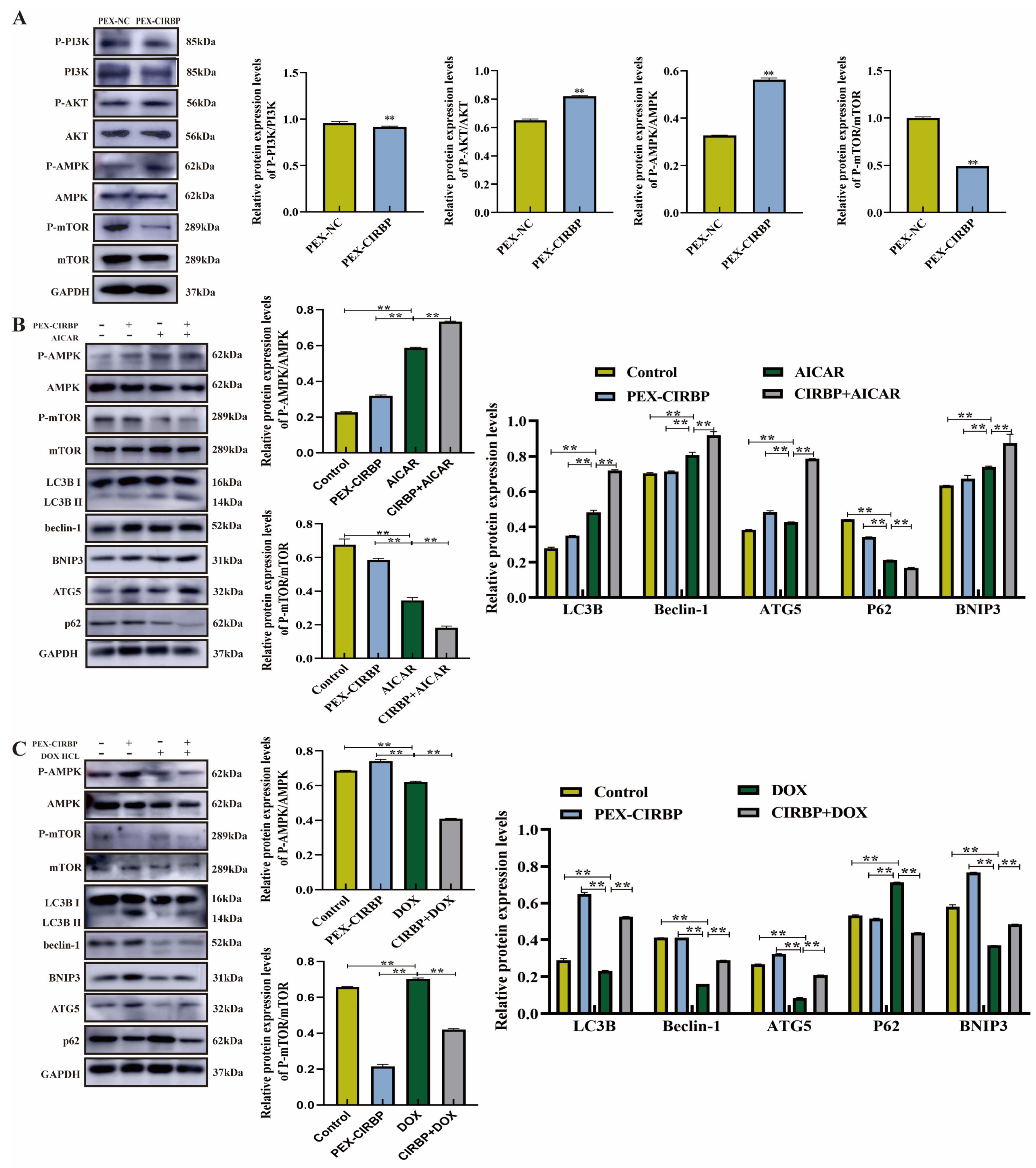
3.5. CIRBP Activated YCC Mitophagy Through the AMPK/mTOR Signaling Pathway
3.6. PI3K/AKT Signaling Pathway Mediates CIRBP’s Regulation of Apoptosis in YCCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| PCD | Programmed cell death |
| GCs | Granulosa cells |
| ANOVA | Analysis of variance |
| YCCs | Yak cumulus cells |
| Sp1 | Specific protein 1 |
| MAPK | Mitogen-activated protein kinase |
| CYP | Cytochrome P450 enzyme |
| CIRBP | Cold-induced RNA-binding protein |
| HIF-1α | Hypoxia-inducible factor 1α |
| AMPK | AMP-activated protein kinase |
| TLR4 | Toll-Like Receptor 4 |
| BNIP3 | BCL2 interacting protein 3 |
| ROS | Reactive oxygen species |
| 3-MA | 3-Methyladenine |
| RAPA | Rapamycin |
| AICAR | Acadesine |
| DOX | Doxorubicin |
References
- Lin, T.Y.; Jia, J.S.; Luo, W.R.; Lin, X.L.; Xiao, S.J.; Yang, J.; Xia, J.W.; Zhou, C.; Zhou, Z.H.; Lin, S.J.; et al. ThermomiR-377-3p-induced suppression of Cirbp expression is required for effective elimination of cancer cells and cancer stem-like cells by hyperthermia. J. Exp. Clin. Cancer Res. 2024, 43, 62. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.; Brenner, M.; Wang, P. Extracellular CIRP (eCIRP) and inflammation. J. Leukoc. Biol. 2019, 106, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Che, H.; Zhang, W.; Wang, J.; Ke, T.; Cao, R.; Meng, S.; Li, D.; Weiming, O.; Chen, J.; et al. Effects of Mild Chronic Intermittent Cold Exposure on Rat Organs. Int. J. Biol. Sci. 2015, 11, 1171–1180. [Google Scholar] [CrossRef]
- Kim, S.Y.; Ban, H.J.; Lee, S.; Jin, H.J. Regulation of CIRP by genetic factors of SP1 related to cold sensitivity. Front. Immunol. 2022, 13, 994699. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Ke, D.; Cheng, G.; Hu, S.; Wu, Y.; Wang, Y.; Zhou, F.; Liu, H.; Zhu, C.; Xia, W. The regulation of CIRBP by transforming growth factor beta during heat shock-induced testicular injury. Andrology 2019, 7, 244–250. [Google Scholar] [CrossRef]
- Corre, M.; Lebreton, A. Regulation of cold-inducible RNA-binding protein (CIRBP) in response to cellular stresses. Biochimie 2024, 217, 3–9. [Google Scholar] [CrossRef]
- Sui, M.; Xu, D.; Zhao, W.; Lu, H.; Chen, R.; Duan, Y.; Li, Y.; Zhu, Y.; Zhang, L.; Zeng, L. CIRBP promotes ferroptosis by interacting with ELAVL1 and activating ferritinophagy during renal ischaemia-reperfusion injury. J. Cell. Mol. Med. 2021, 25, 6203–6216. [Google Scholar] [CrossRef]
- Morf, J.; Rey, G.; Schneider, K.; Stratmann, M.; Fujita, J.; Naef, F.; Schibler, U. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science 2012, 338, 379–383. [Google Scholar] [CrossRef]
- Xia, Z.; Zheng, X.; Zheng, H.; Liu, X.; Yang, Z.; Wang, X. Cold-inducible RNA-binding protein (CIRP) regulates target mRNA stabilization in the mouse testis. FEBS Lett. 2012, 586, 3299–3308. [Google Scholar] [CrossRef]
- Masuda, T.; Itoh, K.; Higashitsuji, H.; Higashitsuji, H.; Nakazawa, N.; Sakurai, T.; Liu, Y.; Tokuchi, H.; Fujita, T.; Zhao, Y.; et al. Cold-inducible RNA-binding protein (Cirp) interacts with Dyrk1b/Mirk and promotes proliferation of immature male germ cells in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 10885–10890. [Google Scholar] [CrossRef]
- Gardela, J.; Ruiz-Conca, M.; Olvera-Maneu, S.; Lopez-Bejar, M.; Alvarez-Rodriguez, M. The mRNA expression of the three major described cold-inducible proteins, including CIRBP, differs in the bovine endometrium and ampulla during the estrous cycle. Res. Vet. Sci. 2022, 152, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, P.H.; Tanner, J.A.; Huang, J.D.; Li, M.; Lee, H.F.; Xu, R.H.; Kung, H.F.; Lin, M.C. Cold-inducible RNA binding protein is required for the expression of adhesion molecules and embryonic cell movement in Xenopus laevis. Biochem. Biophys. Res. Commun. 2006, 344, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, Y.; Wang, Y.; Wu, R. Hypoxia activates the unfolded protein response signaling network: An adaptive mechanism for endometriosis. Front. Endocrinol. 2022, 13, 945578. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, Z.E.; Cirka, H.A.; Billiar, K.L. Mechanical Regulation of Apoptosis in the Cardiovascular System. Ann. Biomed. Eng. 2021, 49, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Bai, L.; Tong, Y.; Guo, S.; Lu, W.; Yuan, Y.; Wang, W.; Jin, Y.; Gao, P.; Liu, J. CIRP attenuates acute kidney injury after hypothermic cardiovascular surgery by inhibiting PHD3/HIF-1alpha-mediated ROS-TGF-beta1/p38 MAPK activation and mitochondrial apoptotic pathways. Mol. Med. 2023, 29, 61. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, T.; Wei, Y.; Peng, J.; Zeng, G.; Zhong, P. Upregulation of CIRP by its agonist prevents the development of heart failure in myocardial infarction rats. BMC Cardiovasc. Disord. 2024, 24, 185. [Google Scholar] [CrossRef]
- Su, F.; Yang, s.; Wang, H.; Qiao, Z.; Zhao, H.; Qu, Z. CIRBP Ameliorates Neuronal Amyloid Toxicity via Antioxidative and Antiapoptotic Pathways in Primary Cortical Neurons. Oxid. Med. Cell Longev. 2020, 2020, 2786139. [Google Scholar] [CrossRef]
- Yang, W.L.; Sharma, A.; Wang, Z.; Li, Z.; Fan, J.; Wang, P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci. Rep. 2016, 6, 26571. [Google Scholar] [CrossRef]
- Liu, W.; Fan, Y.; Ding, H.; Han, D.; Yan, Y.; Wu, R.; Lv, Y.; Zheng, X. Normothermic machine perfusion attenuates hepatic ischaemia-reperfusion injury by inhibiting CIRP-mediated oxidative stress and mitochondrial fission. J. Cell. Mol. Med. 2021, 25, 11310–11321. [Google Scholar] [CrossRef]
- Zhou, J.; Peng, X.; Mei, S. Autophagy in Ovarian Follicular Development and Atresia. Int. J. Biol. Sci. 2019, 15, 726–737. [Google Scholar] [CrossRef]
- Li, Z.; Fan, E.K.; Liu, J.; Scott, M.J.; Li, Y.; Li, S.; Xie, W.; Billiar, T.R.; Wilson, M.A.; Jiang, Y.; et al. Cold-inducible RNA-binding protein through TLR4 signaling induces mitochondrial DNA fragmentation and regulates macrophage cell death after trauma. Cell Death Dis. 2017, 8, e2775. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, S.; Fairman, G.; Vijithakumar, V.; Mak, E.; Cook, D.P.; Pelletier, A.R.; Huard, S.; Vanderhyden, B.C.; Figeys, D.; Lavallee-Adam, M.; et al. Identification of novel lipid droplet factors that regulate lipophagy and cholesterol efflux in macrophage foam cells. Autophagy 2021, 17, 3671–3689. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Lipinska, P. Energy metabolism of follicular environment during oocyte growth and maturation. J. Reprod. Dev. 2020, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhu, S.; Li, Q.; Hu, C.; Pan, C.; Li, H.; Zhu, Y.; Li, X.; Tang, Y.; Ge, R.S. Prenatal diethylhexylphthalate exposure disturbs adult Leydig cell function via epigenetic downregulation of METTL4 expression in male rats. Ecotoxicol. Environ. Saf. 2024, 277, 116391. [Google Scholar] [CrossRef]
- Shao, T.; Ke, H.; Liu, R.; Xu, L.; Han, S.; Zhang, X.; Dang, Y.; Jiao, X.; Li, W.; Chen, Z.J.; et al. Autophagy regulates differentiation of ovarian granulosa cells through degradation of WT1. Autophagy 2022, 18, 1864–1878. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Wang, J.; Han, X.; Yang, X.; Zhang, H.; Zhong, D.; Qiu, S.; Yu, S.; Wang, L.; et al. Hypoxia-Inducible Factor 1alpha Affects Yak Oocyte Maturation and Early Embryonic Development by Regulating Autophagy. Antioxidants 2024, 13, 840. [Google Scholar] [CrossRef]
- Wan, R.; Zhao, Z.; Zhao, M.; Hu, K.; Zhai, J.; Yu, H.; Wei, Q. Characteristics of pulmonary microvascular structure in postnatal yaks. Sci. Rep. 2021, 11, 18265. [Google Scholar] [CrossRef]
- Gotic, I.; Schibler, U. Posttranscriptional mechanisms controlling diurnal gene expression cycles by body temperature rhythms. RNA Biol. 2017, 14, 1294–1298. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction 2010, 139, 685–695. [Google Scholar] [CrossRef]
- Belousov, D.M.; Mikhaylenko, E.V.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. The Dawn of Mitophagy: What Do We Know by Now? Curr. Neuropharmacol. 2021, 19, 170–192. [Google Scholar] [CrossRef]
- Aguilar-Garrido, P.; Otero-Sobrino, A.; Navarro-Aguadero, M.A.; Velasco-Estevez, M.; Gallardo, M. The Role of RNA-Binding Proteins in Hematological Malignancies. Int. J. Mol. Sci. 2022, 23, 9552. [Google Scholar] [CrossRef] [PubMed]
- Thandapani, P.; O’Connor, T.R.; Bailey, T.L.; Richard, S. Defining the RGG/RG motif. Mol. Cell 2013, 50, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Merchant, J.L.; Du, M.; Todisco, A. Sp1 phosphorylation by Erk 2 stimulates DNA binding. Biochem. Biophys. Res. Commun. 1999, 254, 454–461. [Google Scholar] [CrossRef]
- Sun, Y.J.; Ma, S.; Fan, B.; Wang, Y.; Wang, S.R.; Li, G.Y. Therapeutic hypothermia protects photoreceptors through activating Cirbp pathway. Neurochem. Int. 2019, 126, 86–95. [Google Scholar] [CrossRef]
- Wu, L.; Sun, H.L.; Gao, Y.; Hui, K.L.; Xu, M.M.; Zhong, H.; Duan, M.L. Therapeutic Hypothermia Enhances Cold-Inducible RNA-Binding Protein Expression and Inhibits Mitochondrial Apoptosis in a Rat Model of Cardiac Arrest. Mol. Neurobiol. 2017, 54, 2697–2705. [Google Scholar] [CrossRef]
- Weng, W.; He, Z.; Ma, Z.; Huang, J.; Han, Y.; Feng, Q.; Qi, W.; Peng, Y.; Wang, J.; Gu, J.; et al. Tufm lactylation regulates neuronal apoptosis by modulating mitophagy in traumatic brain injury. Cell Death Differ. 2025, 32, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Tao, J.; Zhao, P.J.; Tang, W.; Xu, J.P.; Zhang, K.Q.; Zou, C.G. Adiponectin receptor PAQR-2 signaling senses low temperature to promote C. elegans longevity by regulating autophagy. Nat. Commun. 2019, 10, 2602. [Google Scholar] [CrossRef]
- Ruperez, C.; Blasco-Roset, A.; Kular, D.; Cairo, M.; Ferrer-Curriu, G.; Villarroya, J.; Zamora, M.; Crispi, F.; Villarroya, F.; Planavila, A. Autophagy is Involved in Cardiac Remodeling in Response to Environmental Temperature Change. Front. Physiol. 2022, 13, 864427. [Google Scholar] [CrossRef]
- Texada, M.J.; Malita, A.; Christensen, C.F.; Dall, K.B.; Faergeman, N.J.; Nagy, S.; Halberg, K.A.; Rewitz, K. Autophagy-Mediated Cholesterol Trafficking Controls Steroid Production. Dev. Cell 2019, 48, 659–671. [Google Scholar] [CrossRef]
- Yang, L.; He, Z.; Hu, L.; Tang, H.; Geng, Y.; Tan, Q.; Zhang, Y.; Wen, Y.; Wu, W.; Gu, H.; et al. Ti3C2 nanosheet-induced autophagy derails ovarian functions. J. Nanobiotechnology 2024, 22, 242. [Google Scholar] [CrossRef]
- Neutelings, T.; Lambert, C.A.; Nusgens, B.V.; Colige, A.C. Effects of mild cold shock (25 degrees C) followed by warming up at 37 degrees C on the cellular stress response. PLoS ONE 2013, 8, e69687. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, C.; Lu, H.; Zhou, Y.; Guan, R.; Wang, J.; Zhang, Q.; Ke, T.; Aschner, M.; Zhang, W.; et al. Hypoxia causes mitochondrial dysfunction and brain memory disorder in a manner mediated by the reduction of Cirbp. Sci. Total. Environ. 2022, 806 Pt 3, 151228. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, Z.; Li, H.; Liao, K. Dihydroartemisinin Reduces Irradiation-Induced Mitophagy and Radioresistance in Lung Cancer A549 Cells via CIRBP Inhibition. Life 2022, 12, 1129. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Xing, J.; Li, J.; Li, Z.; Jian, D.; Wang, Y.; Wang, S.; Li, R.; Zhang, W.; et al. CIRBP-OGFR axis safeguards against cardiomyocyte apoptosis and cardiotoxicity induced by chemotherapy. Int. J. Biol. Sci. 2022, 18, 2882–2897. [Google Scholar] [CrossRef]
- Xu, R.; Wang, J.; Wang, M.; Gao, L.; Zhang, R.; Zhao, L.; Liu, B.; Han, X.; Baloch, A.R.; Cui, Y.; et al. Exosomes Derived from Yak Follicular Fluid Increase 2-Hydroxyestradiol Secretion by Activating Autophagy in Cumulus Cells. Animals 2022, 12, 3174. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, T.; Liu, Y.; Liu, W.; Zhao, X.; Zhang, G.; Wang, J.; Zhang, Y. Screening and Mechanism Study of Three Antagonistic Drugs, Oxysophoridine, Rutin, and Phellodendrine, against Zearalenone-Induced Reproductive Toxicity in Ovine Oocytes. Antioxidants 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ling, Z.; Luan, D.; Kang, J.; Dong, X.; Quan, F. HD-sEVs in bovine follicular fluid regulate granulosa cell apoptosis and estradiol secretion through the autophagy pathway. Theriogenology 2023, 212, 91–103. [Google Scholar] [CrossRef]
- Li, N.; Xu, H.; Liu, X.; Gao, R.; He, J.; Ding, Y.; Li, F.; Geng, Y.; Mu, X.; Chen, X. Exposure to benzo(a)pyrene suppresses mitophagy via ANT1-PINK1-Parkin pathway in ovarian corpus luteum during early pregnancy. Sci. Total. Environ. 2022, 814, 152759. [Google Scholar] [CrossRef]
- Ma, Q.; Shen, M.; Wu, J.; Ye, C.; Tan, Y. Mechanism Research of DHEA Treatment Improving Diminished Ovarian Reserve by Attenuating the AMPK-SIRT1 Signaling and Mitophagy. Reprod. Sci. 2024, 31, 2059–2072. [Google Scholar] [CrossRef]
- Zhu, H.L.; Shi, X.T.; Xu, X.F.; Xiong, Y.W.; Yi, S.J.; Zhou, G.X.; Liu, W.B.; Huang, M.M.; Gao, L.; Zhang, C.; et al. Environmental cadmium exposure induces fetal growth restriction via triggering PERK-regulated mitophagy in placental trophoblasts. Environ. Int. 2021, 147, 106319. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Y.; Guo, L. 17beta-estradiol protects INS-1 insulinoma cells from mitophagy via G protein-coupled estrogen receptors and the PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018, 41, 2839–2846. [Google Scholar] [CrossRef]
- Lu, M.; Ge, Q.; Wang, G.; Luo, Y.; Wang, X.; Jiang, W.; Liu, X.; Wu, C.L.; Xiao, Y.; Wang, X. CIRBP is a novel oncogene in human bladder cancer inducing expression of HIF-1alpha. Cell Death Dis. 2018, 9, 1046. [Google Scholar] [CrossRef]
- Yin, H.; Wang, C.; Guo, H.; Li, X.; Liu, J. The mechanism of nickel-induced autophagy and its role in nephrotoxicity. Ecotoxicol. Environ. Saf. 2024, 273, 116150. [Google Scholar] [CrossRef]
- Wu, C.; Chen, H.; Zhuang, R.; Zhang, H.; Wang, Y.; Hu, X.; Xu, Y.; Li, J.; Li, Y.; Wang, X.; et al. Betulinic acid inhibits pyroptosis in spinal cord injury by augmenting autophagy via the AMPK-mTOR-TFEB signaling pathway. Int. J. Biol. Sci. 2021, 17, 1138–1152. [Google Scholar] [CrossRef] [PubMed]
- Higashitsuji, H.; Fujita, T.; Higashitsuji, H.; Fujita, J. Mammalian cold-inducible RNA-binding protein facilitates wound healing through activation of AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2020, 533, 1191–1197. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Chen, T.; Wei, C.; Qiao, Y.; Liu, W.; Liang, Y.; Liang, Z.; Chen, C.; Li, D.; et al. Hypoxia-enhanced YAP1-EIF4A3 interaction drives circ_0007386 circularization by competing with CRIM1 pre-mRNA linear splicing and promotes non-small cell lung cancer progression. J. Exp. Clin. Cancer Res. 2024, 43, 200. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, J.; Kang, R.; Tang, D. Mitophagy Receptors in Tumor Biology. Front. Cell Dev. Biol. 2020, 8, 594203. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Li, M.; Gao, D.; Wu, H.; Zhang, S.; Huang, Y.; Guo, K. BNIP3-mediated mitophagy boosts the competitive growth of Lenvatinib-resistant cells via energy metabolism reprogramming in HCC. Cell Death Dis. 2024, 15, 484. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Hu, H.; Zhang, P.; Xie, R.; Cui, W. HIF-1alpha/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2019, 120, 109464. [Google Scholar] [CrossRef]
- Zhu, N.; Li, J.; Li, Y.; Zhang, Y.; Du, Q.; Hao, P.; Li, J.; Cao, X.; Li, L. Berberine Protects Against Simulated Ischemia/Reperfusion Injury-Induced H9C2 Cardiomyocytes Apoptosis In Vitro and Myocardial Ischemia/Reperfusion-Induced Apoptosis In Vivo by Regulating the Mitophagy-Mediated HIF-1alpha/BNIP3 Pathway. Front. Pharmacol. 2020, 11, 367. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, P.; Xia, X.; Cao, C.; Ding, Z.; Li, X. Low ambient temperature exposure increases the risk of ischemic stroke by promoting platelet activation. Sci. Total. Environ. 2024, 912, 169235. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yao, R.; Shi, H.; Liu, Y.; Lian, S.; Yang, Y.; Yang, H.; Li, S. Effects of Cold-inducible RNA-binding Protein (CIRP) on Liver Glycolysis during Acute Cold Exposure in C57BL/6 Mice. Int. J. Mol. Sci. 2019, 20, 1470. [Google Scholar] [CrossRef] [PubMed]




| Primer | Primer Sequence (5′-3′) | Bp | Annealing Temperature |
|---|---|---|---|
| CIRBP | F: TGGATACCGTGGTGGCTCT R: GGCTGCTGTAGTAGTCTCTGG | 161 | 60 °C |
| LC3B | F: TGTTAGGTCAGGCAGTCA R: GTAGTAGGAAGCACTCGTTA | 150 | 56 °C |
| P62 | F: ATCAGCCTCTGGTCCATC R: TTCTCTTGCCTCCGTGTT | 128 | 56 °C |
| ATG5 | F: ATCAATCGGAAACTCATG R: AGATGTTCACTCAGCCAC | 272 | 56 °C |
| Beclin-1 | F: AACCTCAGCCGAAGACTA R: TCAGCCTCTCCTCCTCTA | 253 | 56 °C |
| STAR | F: GGACCTTGATCTCCTTGAC R: CCACACTCTATGAGGAGATG | 178 | 56 °C |
| CYP1A1 | F: GTCCCCTTCACCATCCCA R: CCAAGCCGAAAATAATCACC | 194 | 56 °C |
| CYP1B1 | F: GCTTCCGTCTTGGGCTAC R: GGTCAAAGTCCTCTGGGTTC | 196 | 56 °C |
| CYP11A1 | F: TTTGCCTTTGAGTCCATC R: CCTAAATTCTGTTTTCCGTC | 273 | 56 °C |
| CYP17A1 | F: GCCCAAGACCAAGCACTC R: GGAACCCAAACGAAAGGA | 160 | 56 °C |
| CYP19A1 | F: GAAGCACAGTCACTACATATC R: ATGGAATCAGCACAGATGG | 183 | 56 °C |
| β-actin | F: CGTCCGTGACATCAAGGAGAAGC R: GGAACCGCTCATTGCCGATGG | 141 | 56 °C |
| HAS2 | F: ACAGGCATCTAACGAACCGAG R: AGTAGGACTTGCTCCAGCGG | 135 | 56 °C |
| PTX3 | F: GCTATCGGTCCATAATGCTTG R: CCACCGAGTCACCATTTACC | 150 | 56 °C |
| COX2 | F: CGTTTTCTCGTGAAGCCCTAT R: CAGTACTCGGGAGAGCATATAGG | 230 | 56 °C |
| TSG6 | F: AGCAGTTAGAGGCAGCCAGAAA R: AACACACCACCACACTCCTTTG | 211 | 56 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Cui, Y.; Pan, Y.; Wang, M.; Yu, S.; Xu, R.; Ma, W.; Wang, J.; Zhong, D.; Jiao, Z. CIRBP Enhances the Function of Yak Cumulus Cells by Activating AMPK/mTOR-Mediated Mitophagy. Biomolecules 2025, 15, 759. https://doi.org/10.3390/biom15060759
Zhang R, Cui Y, Pan Y, Wang M, Yu S, Xu R, Ma W, Wang J, Zhong D, Jiao Z. CIRBP Enhances the Function of Yak Cumulus Cells by Activating AMPK/mTOR-Mediated Mitophagy. Biomolecules. 2025; 15(6):759. https://doi.org/10.3390/biom15060759
Chicago/Turabian StyleZhang, Rui, Yan Cui, Yangyang Pan, Meng Wang, Sijiu Yu, Ruihua Xu, Wenbin Ma, Junqian Wang, Donglan Zhong, and Zhengxing Jiao. 2025. "CIRBP Enhances the Function of Yak Cumulus Cells by Activating AMPK/mTOR-Mediated Mitophagy" Biomolecules 15, no. 6: 759. https://doi.org/10.3390/biom15060759
APA StyleZhang, R., Cui, Y., Pan, Y., Wang, M., Yu, S., Xu, R., Ma, W., Wang, J., Zhong, D., & Jiao, Z. (2025). CIRBP Enhances the Function of Yak Cumulus Cells by Activating AMPK/mTOR-Mediated Mitophagy. Biomolecules, 15(6), 759. https://doi.org/10.3390/biom15060759






