Biochar Utilization in Antimicrobial, Anticancer, and Biosensing Applications: A Review
Abstract
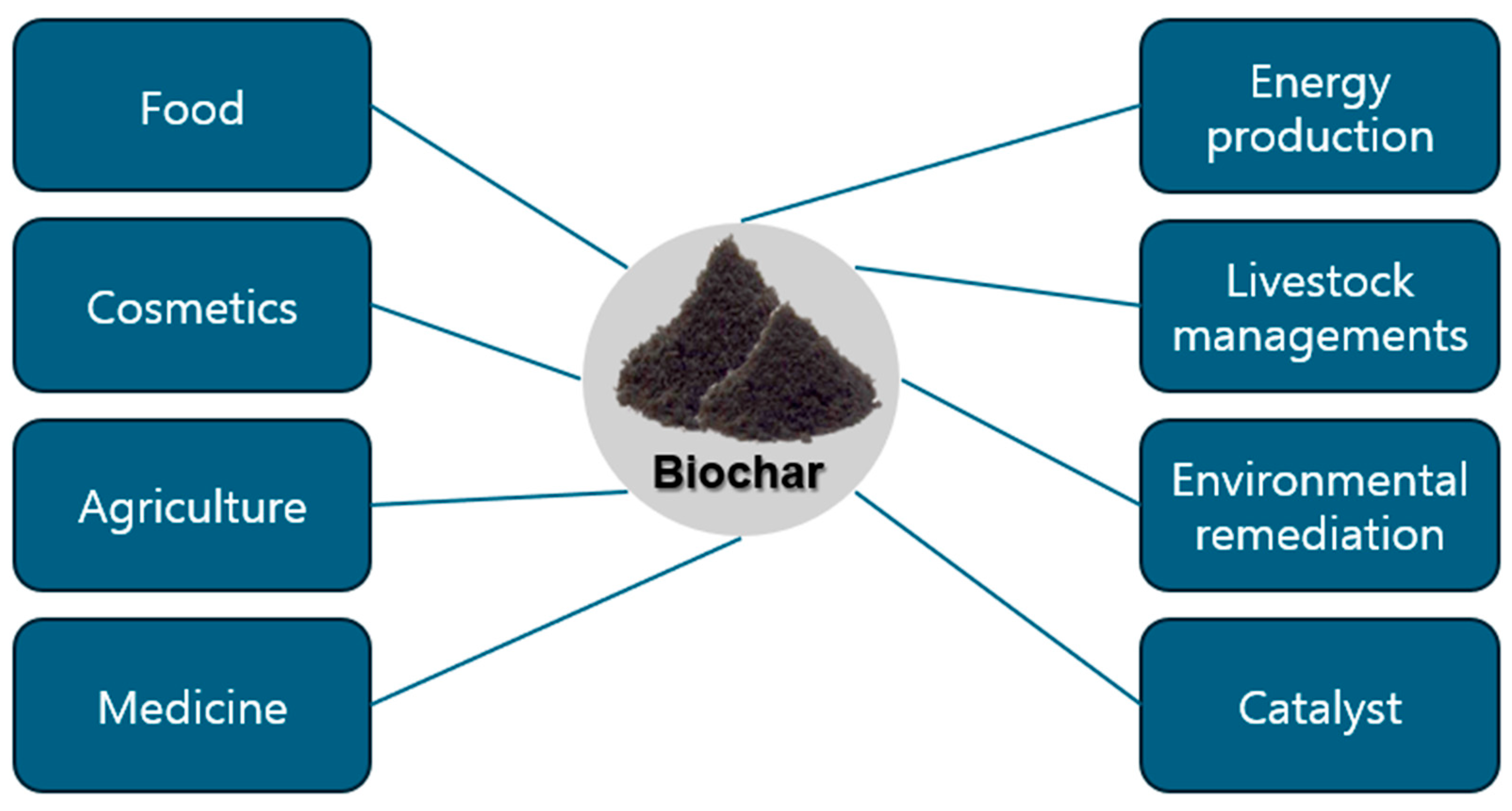
1. Introduction
2. Preparation and Functionalization of Biochar
2.1. Biochar Production
2.2. Biochar Modification
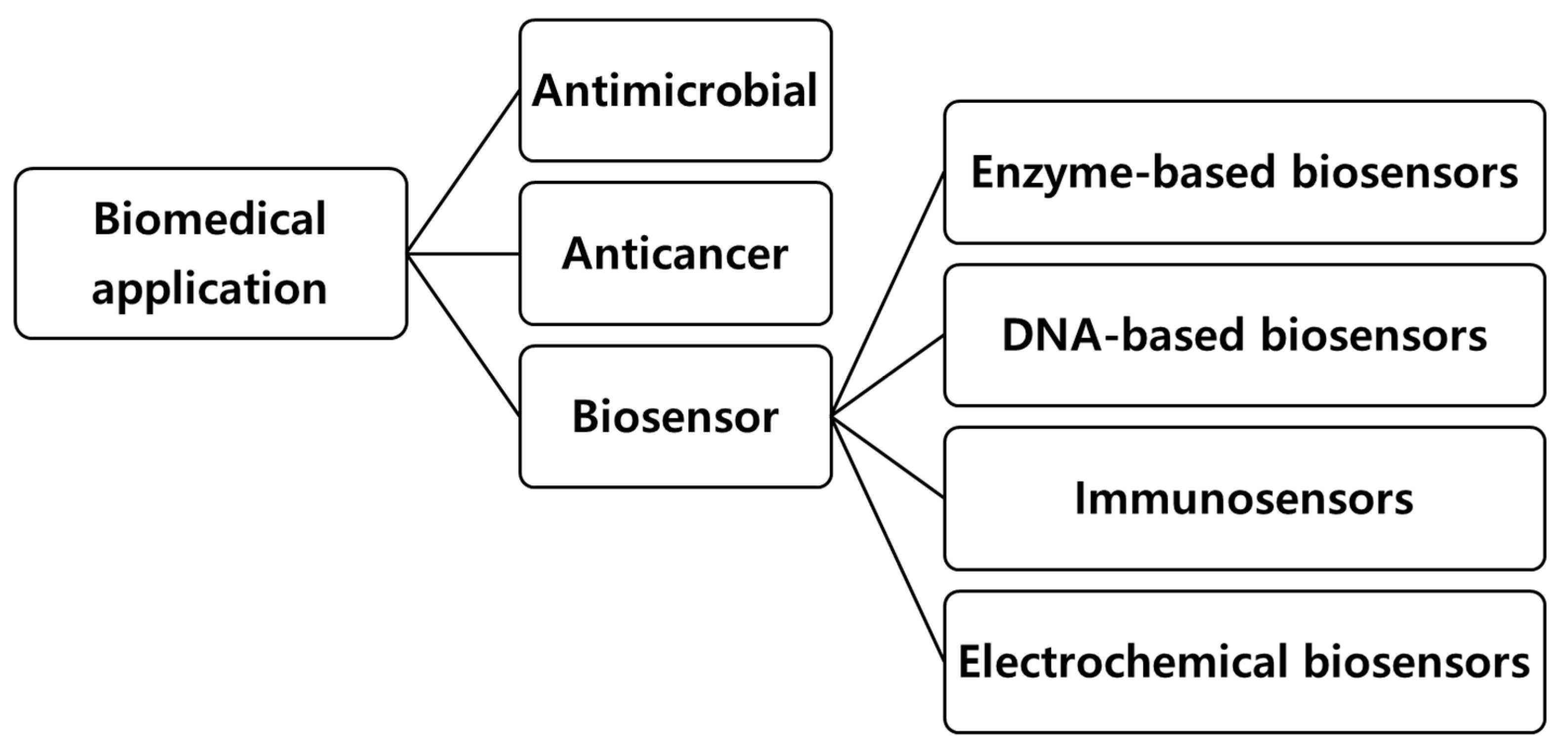
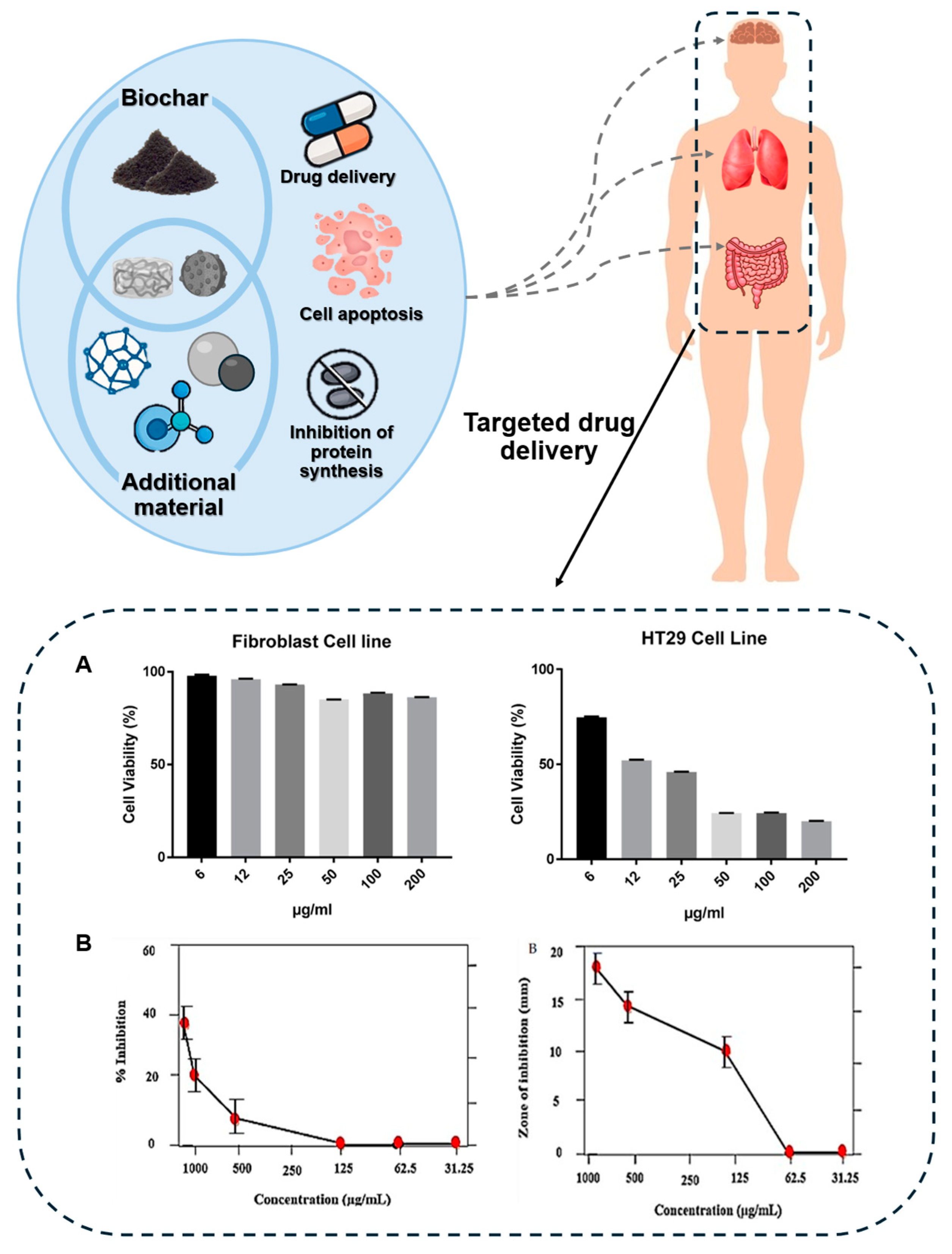
3. Biochar for Biomedical Applications
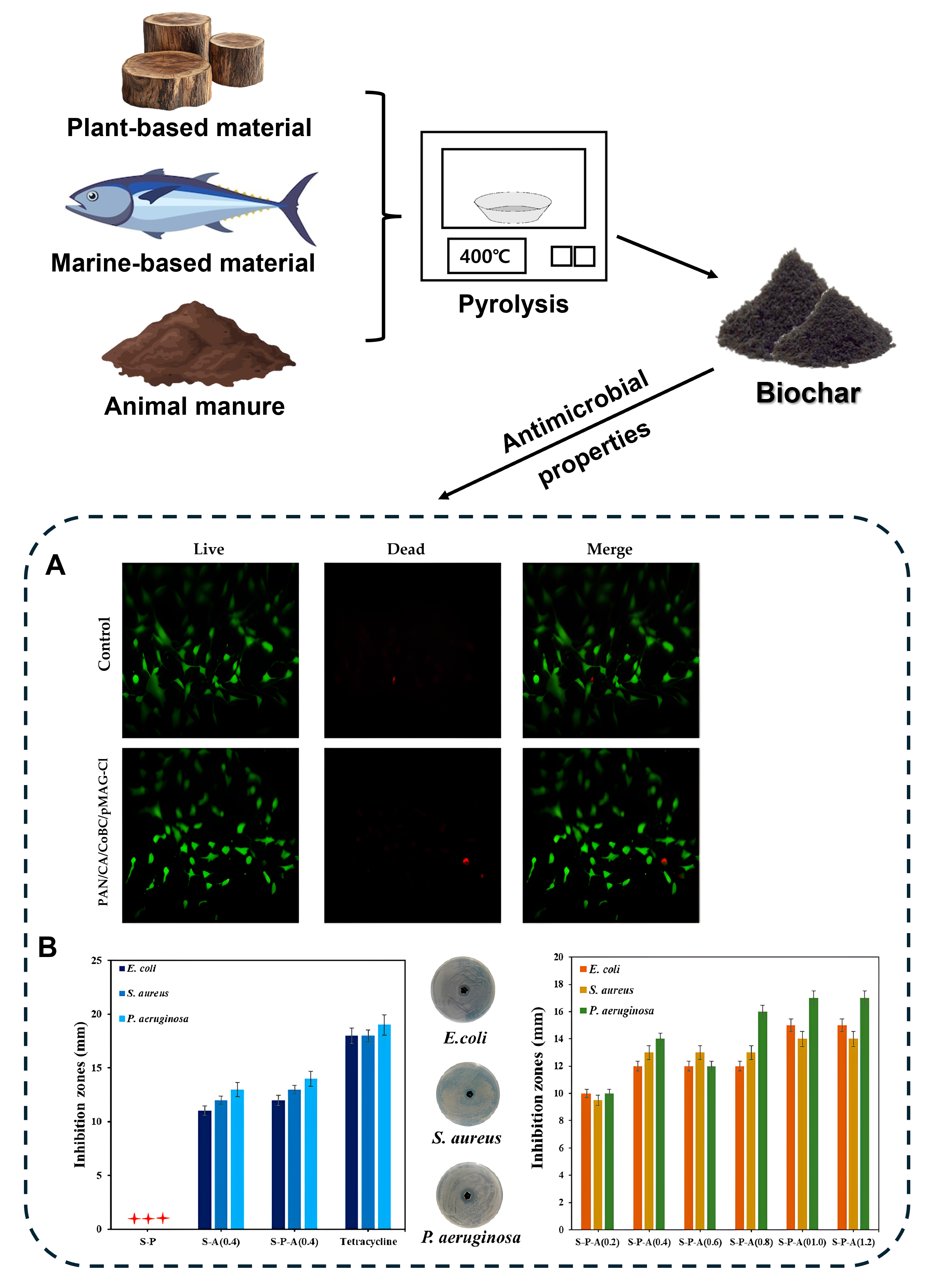
3.1. Biochar in Antimicrobial Applications
| Source | Additional Material | Methods | Target | Effects | Ref. |
|---|---|---|---|---|---|
| Cow dung | Photothermal N-halamine hydrogel | Pyrolysis acidification | E. coli, S. aureus |
| [119] |
| Wood powder and rice husk | Commiphora myrrha (T. Nees) | Oven-dried at 100 °C for 24 h. Pyrolysis (550 °C, 2 h) | S. aureus, P. aeruginosa, S. Enteritidis |
| [121] |
| Hazelnut shells | Boric acid (H3BO3) solutions | Pyrolysis (500 °C, 10 °C/min, 1 h) | C. albicans, S. aureus |
| [127] |
| Maize straw | Iron oxide, quaternary phosphonium salt | Pyrolysis (500 °C, anoxic condition) | E. coli, S. aureus |
| [122] |
| Waste barley distillers’ grains shell | Silver nanoparticles, polyvinyl alcohol-chitosan | Pyrolysis (300 °C, 2 h) | E. coli, S. aureus |
| [128] |
| Potato peels | Glutaraldehyde, sodium alginate | Microwave pyrolysis (20 min, maximum microwave power) | S. aureus, P. aeruginosa E. faecium, E. faecalis L. monocytogenes |
| [123] |
| Coconut husk | Polybutylene adipate terephthalate/Carvacrol | Pyrolysis (70 °C, 4 h and 80 °C, 12 h/vacuum-dried) | E. coli, L. monocytogenes, S. enteritidis, S. aureus |
| [124] |
| Rice husk and cotton | Silver ion | Pyrolysis (480 °C, 5 °C/min, 3 h) | E. coli |
| [117] |
| Atriplex halimus L. | Ag–Cu | Pyrolysis (550 °C, 3 h) | E. coli, K. pneumonia, B. subtilis S. aureus |
| [129] |
| Dried fish scale | Silver, polyvinyl alcohol, alginate gel beads | Pyrolysis (300 °C, 10 °C/min, 2 h) | E. coli, S. aureus, P. aeruginosa |
| [120] |
| Waste fish scale | Nanocellulose, silver, chitosan-polyvinyl alcohol hydrogel | Pyrolysis (300 °C, 2 h) | E. coli, S. aureus, P. aeruginosa |
| [125] |
| Waste fish scale | Carbon substrate, nanosilver | Pyrolysis (500 °C, 10 °C/min, 2 h) | E. coli, S. aureus, P. aeruginosa |
| [126] |
3.2. Biochar in Anticancer Applications
| Source | Additional Material | Methods | Target | Effects | Ref. |
|---|---|---|---|---|---|
| Alder wood chips | Butyrate glycerides | Pyrolysis (450–700 °C, 3 h) | HCT116, HT29 |
| [137] |
| Date seeds | Silver nanoparticles | Pyrolysis (550 °C, 10 °C/min) | HT29 |
| [139] |
| Leaves of Pontederia crassipes L. | Zinc oxide nanoparticles | Pyrolysis (600 °C, 5 °C/min, 4 h) | MCF-7 human breast cancer cell |
| [140] |
| Orange peels | Carbon nanostructures | Hydrothermal carbonization (240 °C, 600 rpm, 1 h) | A549 |
| [138] |
| Date seeds | Emericella dentata | Pyrolysis (550 °C, 2 h) | A549 |
| [141] |
| Mangifera indica bark | Ag/Cu-ZrO2 nanostructure | Pyrolysis (650 °C, 35 °C/50 min, 3 h) | SH-SY5Y cell |
| [135] |
| Maize straw | Zinc oxide nanoparticles | Pyrolysis (600 °C, 6 h/vacuum-dried) | Streptomyces 85E strain |
| [136] |
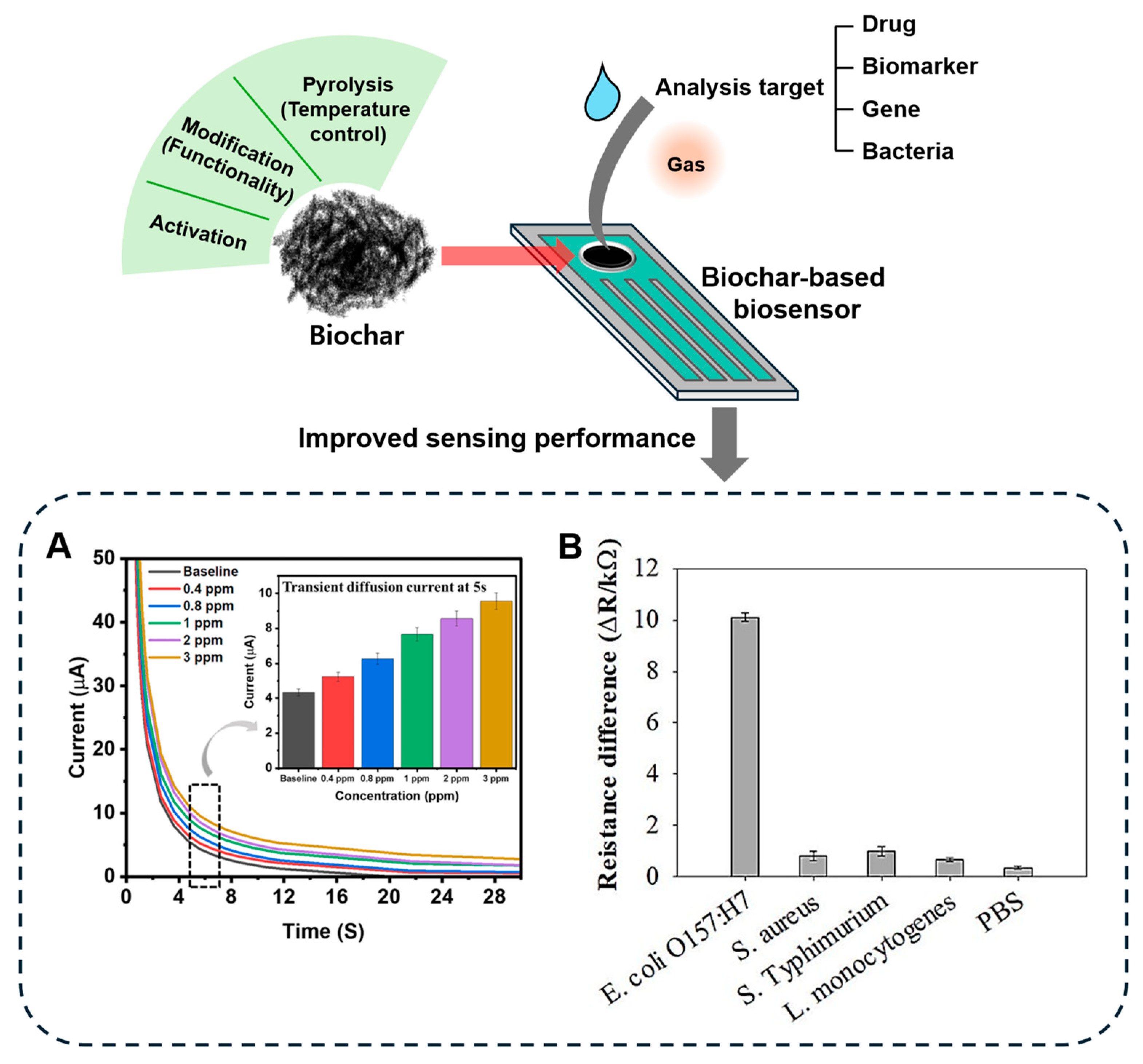
3.3. Biochar in Biosensor Applications
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, L.P.; Xi, F.F.; Tan, W.S.; Meng, X.; Hu, B.W.; Wang, X.K. Review of organic and inorganic pollutants removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Patel, A.K.; Katiyar, R.; Chen, C.W.; Singhania, R.R.; Awasthi, M.K.; Bhatia, S.; Bhaskar, T.; Dong, C.D. Antibiotic bioremediation by new generation biochar: Recent updates. Bioresour. Technol. 2022, 358, 127384. [Google Scholar] [CrossRef]
- Song, G.; Qin, F.Z.; Yu, J.F.; Tang, L.; Pang, Y.; Zhang, C.; Wang, J.J.; Deng, L.F. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2022, 424, 127663. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Paula, A.J.; Ferreira, O.P.; Souza, A.G.; Nepomuceno, F.; Andrade, C.E.; Faria, A.F. Machine Learning and Natural Language Processing Enable a Data-Oriented Experimental Design Approach for Producing Biochar and Hydrochar from Biomass. Chem. Mater. 2022, 34, 979–990. [Google Scholar] [CrossRef]
- Wong, A.; de Lima, D.G.; Ferreira, P.A.; Khan, S.; da Silva, R.A.B.; de Faria, J.L.B.; Sotomayor, M.D.T. Voltammetric sensing of glyphosate in different samples using carbon paste electrode modified with biochar and copper(II) hexadecafluoro-29H,31 phtalocyanine complex. J. Appl. Electrochem. 2021, 51, 761–768. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Li, S.Y.; Li, X.Y.; Deng, Q.Y.; Li, D.G. Three kinds of charcoal powder reinforced ultra-high molecular weight polyethylene composites with excellent mechanical and electrical properties. Mater. Design 2015, 85, 54–59. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Masek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar-Comparison of physical and functional properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef]
- Duku, M.H.; Gu, S.; Ben Hagan, E. Biochar production potential in Ghana—A review. Renew. Sustain. Energy Rev. 2011, 15, 3539–3551. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Zheng, W.; Leng, S.; Ai, Z.; Zhang, W.; Yang, Z.; Yang, J.; Xu, Z.; Cao, J.; et al. A complete review on the oxygen-containing functional groups of biochar: Formation mechanisms, detection methods, engineering, and applications. Sci. Total Environ. 2024, 946, 174081. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedlácek, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Shao, B.; Liu, Z.; Tang, L.; Liu, Y.; Liang, Q.; Wu, T.; Pan, Y.; Zhang, X.; Tan, X.; Yu, J. The effects of biochar on antibiotic resistance genes (ARGs) removal during different environmental governance processes: A review. J. Hazard. Mater. 2022, 435, 129067. [Google Scholar] [CrossRef]
- Hafeez, A.; Pan, T.W.; Tian, J.H.; Cai, K.Z. Modified Biochars and Their Effects on Soil Quality: A Review. Environments 2022, 9, 60. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Komnitsas, K.A.; Zaharaki, D. Morphology of modified biochar and its potential for phenol removal from aqueous solutions. Front. Environ. Sci. 2016, 4, 26. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Khan, A.; Ghosh, T.; Kim, J.T.; Rhim, J.W. Advances and prospects for biochar utilization in food processing and packaging applications. Sustain. Mater. Technol. 2024, 39, e00831. [Google Scholar] [CrossRef]
- Joshi, M.; Bhatt, D.; Srivastava, A. Enhanced Adsorption Efficiency through Biochar Modification: A Comprehensive Review. Ind. Eng. Chem. Res. 2023, 62, 13748–13761. [Google Scholar] [CrossRef]
- Zhuo, Q.; Liang, Y.; Hu, Y.; Shi, M.; Zhao, C.; Zhang, S. Applications of biochar in medical and related environmental fields: Current status and future perspectives. Carbon Res. 2023, 2, 32. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 377. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Yu, C.G.; Fu, L. Biochar-based materials for electroanalytical applications: An overview. Green Anal. Chem. 2023, 7, 100081. [Google Scholar] [CrossRef]
- Florian, G.F.; Ragoubi, M.; Leblanc, N.; Taouk, B.; Abdelouahed, L. Biochar Production and Its Potential Application for Biocomposite Materials: A Comprehensive Review. J. Compos. Sci. 2024, 8, 220. [Google Scholar] [CrossRef]
- Malode, S.J.; Pandiaraj, S.; Alodhayb, A.; Shetti, N.P. Carbon Nanomaterials for Biomedical Applications: Progress and Outlook. ACS Appl. Bio Mater. 2024, 7, 752–777. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ogunwa, K.I.; Chukwuike, V.I.; Nnadozie, O.O.; Ehikhase, C. Synthesis and Characterization of Carbonaceous Materials for Medical Applications: A comprehensive Review. BioMed 2024, 4, 464–492. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, Y.; Han, N.; Li, X.; Geng, H.; Wang, X.; Cui, Y.; Wang, S. Mesoporous carbon nanomaterials in drug delivery and biomedical application. Drug Deliv. 2017, 24, 94–107. [Google Scholar] [CrossRef]
- Zhang, H.; Hay, A.G. Magnetic biochar derived from biosolids via hydrothermal carbonization: Enzyme immobilization, immobilized-enzyme kinetics, environmental toxicity. J. Hazard. Mater. 2020, 384, 121272. [Google Scholar] [CrossRef]
- Tan, X.F.; Zhu, S.S.; Wang, R.P.; Chen, Y.D.; Show, P.L.; Zhang, F.F.; Ho, S.H. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, W.Y.; Peng, H.B.; Pan, B.; Xing, B.S. Functional Biochar and Its Balanced Design. ACS Environ. Au 2021, 2, 115–127. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Duan, W.Y.; Chang, Z.F.; Du, W.; Chen, F.Y.; Li, F.F.; Oleszczuk, P. Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity. Sustainability 2023, 15, 7745. [Google Scholar] [CrossRef]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of Biochar-Based Functional Materials: Toward a Sustainable Platform Carbon Material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef]
- Saraugi, S.S.; Routray, W. Advances in sustainable production and applications of nano-biochar. Sci. Total Environ. 2024, 955, 176883. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, Z.; Huang, S.; Zhang, H.; Yang, H.; Liu, Y.; Zhang, K.; Zeng, Y. Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials 2024, 14, 1933. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.C.B.D.; Evaristo, R.B.W.; Dutra, R.C.; Suarez, P.A.Z.; Silveira, E.A.; Ghesti, G.F. Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification. Sustainability 2025, 17, 2685. [Google Scholar] [CrossRef]
- Ma, J.; Zheng, L.B.; Yu, F. Current status and future prospects of biochar application in electrochemical energy storage devices: A bibliometric review. Desalination 2024, 581, 117597. [Google Scholar] [CrossRef]
- Varkolu, M.; Gundekari, S.; Palla, V.C.S.; Kumar, P.; Bhattacharjee, S.; Vinodkumar, T. Recent Advances in Biochar Production, Characterization, and Environmental Applications. Catalysts 2025, 15, 243. [Google Scholar] [CrossRef]
- Prabakar, P.; Mustafa Mert, K.; Muruganandam, L.; Sivagami, K. A comprehensive review on biochar for electrochemical energy storage applications: An emerging sustainable technology. Front. Energy Res. 2024, 12, 1448520. [Google Scholar] [CrossRef]
- Amalina, F.; Abd Razak, A.S.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar production techniques utilizing biomass waste-derived materials and environmental applications-A review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; He, M.J.; Wang, L.; Yan, J.H.; Ma, B.; Zhu, X.H.; Ok, Y.S.; Mechtcherine, V.; Tsang, D.C.W. Biochar as construction materials for achieving carbon neutrality. Biochar 2022, 4, 59. [Google Scholar] [CrossRef]
- Hayder, G.; Naim, R.M. Biochar-based nanocomposites from waste biomass: A sustainable approach for wastewater treatment and renewable bioenergy. Front. Agric. Sci. Eng. 2025, 12, 117–147. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Aslani, A.; Holghoomi, R.; Fathi-Karkan, S.; Rahdar, A.; Kharaba, Z.; Pandey, S. Harnessing bio-waste for biomedical applications: A new horizon in sustainable healthcare. Eur. J. Med. Chem. Rep. 2024, 12, 100234. [Google Scholar] [CrossRef]
- Sadegh, F.; Sadegh, N.; Wongniramaikul, W.; Apiratikul, R.; Choodum, A. Adsorption of volatile organic compounds on biochar: A review. Process Saf. Environ. 2024, 182, 559–578. [Google Scholar] [CrossRef]
- Babu, K.K.B.S.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of biochar from waste biomass using slow pyrolysis: Studies of the effect of pyrolysis temperature and holding time on biochar yield and properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas Production from Biomass Gasification: Influences of Feedstock Properties, Reactor Type, and Reaction Parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Ivanovski, M.; Petrovic, A.; Goricanec, D.; Urbancl, D.; Simonic, M. Exploring the properties of the torrefaction process and its prospective in treating lignocellulosic material. Energies 2023, 16, 6521. [Google Scholar] [CrossRef]
- Orisaleye, J.I.; Jekayinfa, S.O.; Pecenka, R.; Ogundare, A.A.; Akinseloyin, M.O.; Fadipe, O.L. Investigation of the effects of torrefaction temperature and residence time on the fuel quality of corncobs in a fixed-bed reactor. Energies 2022, 15, 5284. [Google Scholar] [CrossRef]
- Devaraja, U.M.A.; Dissanayake, C.L.W.; Gunarathne, D.S.; Chen, W.H. Oxidative torrefaction and torrefaction-based biorefining of biomass: A critical review. Biofuel Res. J. 2022, 9, 1672–1696. [Google Scholar] [CrossRef]
- Xue, Y.T.; Kamali, M.; Aminabhavi, T.M.; Appels, L.; Dewil, R. Tailoring the surface functional groups of biochar for enhanced adsorption and degradation of pharmaceutically active compounds. Chem. Eng. J. 2024, 491, 152037. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Motta, I.L.; Hill, J.M. Modification of biochar with high-energy ball milling: Development of porosity and surface acid functional groups. Bioresour. Technol. Rep. 2021, 15, 100704. [Google Scholar] [CrossRef]
- Mishra, R.K.; Singh, B.; Acharya, B. A comprehensive review on activated carbon from pyrolysis of lignocellulosic biomass: An application for energy and the environment. Carbon Res. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Khandaker, T.; Islam, T.; Nandi, A.; Anik, M.A.M.; Hossain, M.S.; Hasan, M.K.; Hossain, M.S. Biomass-derived carbon materials for sustainable energy applications: A comprehensive review. Sustain. Energ. Fuels 2024, 9, 693–723. [Google Scholar] [CrossRef]
- Dayoub, E.B.; Tóth, Z.; Soós, G.; Anda, A. Chemical and Physical Properties of Selected Biochar Types and a Few Application Methods in Agriculture. Agronomy 2024, 14, 2540. [Google Scholar] [CrossRef]
- Supraja, K.V.; Kachroo, H.; Viswanathan, G.; Verma, V.K.; Behera, B.; Doddapaneni, T.; Kaushal, P.; Ahammad, S.Z.; Singh, V.; Awasthi, M.K.; et al. Biochar production and its environmental applications: Recent developments and machine learning insights. Bioresour. Technol. 2023, 387, 129634. [Google Scholar] [CrossRef]
- Ravindiran, G.; Rajamanickam, S.; Janardhan, G.; Hayder, G.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Sonne, C. Production and modifications of biochar to engineered materials and its application for environmental sustainability: A review. Biochar 2024, 6, 52. [Google Scholar] [CrossRef]
- Yang, J.T.; Zhang, Z.M.; Wang, J.Y.; Zhao, X.L.; Zhao, Y.; Qian, J.Q.; Wang, T.F. Pyrolysis and hydrothermal carbonization of biowaste: A comparative review on the conversion pathways and potential applications of char product. Sustain. Chem. Pharm. 2023, 33, 101106. [Google Scholar] [CrossRef]
- Plavniece, A.; Dobele, G.; Volperts, A.; Zhurinsh, A. Hydrothermal Carbonization vs. Pyrolysis: Effect on the Porosity of the Activated Carbon Materials. Sustainability 2022, 14, 5982. [Google Scholar] [CrossRef]
- Altikat, A.; Alma, M.H.; Altikat, A.; Bilgili, M.E.; Altikat, S. A comprehensive study of biochar yield and quality concerning pyrolysis conditions: A multifaceted approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- Niedzwiecki, L.; Moscicki, K.; Bijl, A.; Owczarek, P.; Arora, A.; Wnukowski, M.; Aragon-Briceno, C.; Vishwajeet; Pawlak-Kruczek, H.; Bramer, E.; et al. Influence of hydrothermal carbonization on catalytic fast pyrolysis of agricultural biomass. Appl. Sci. 2023, 13, 4190. [Google Scholar] [CrossRef]
- Nawaz, A.; Kumar, P. MgO assisted catalytic hydrothermal carbonization followed by pyrolysis of sunflower stalks for the determination of kinetic and thermodynamic parameters. ACS Sustain. Resour. Manag. 2024, 1, 2461–2471. [Google Scholar] [CrossRef]
- Chen, W.H.; Lin, B.J.; Lin, Y.Y.; Chu, Y.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.S.; Ho, S.H.; Culaba, A.B.; et al. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Lin, S.L.; Zhang, H.J.; Chen, W.H.; Song, M.J.; Kwon, E.E. Low-temperature biochar production from torrefaction for wastewater treatment: A review. Bioresour. Technol. 2023, 387, 129588. [Google Scholar] [CrossRef] [PubMed]
- Khairy, M.; Amer, M.; Ibrahim, M.; Ookawara, S.; Sekiguchi, H.; Elwardany, A. The influence of torrefaction on the biochar characteristics produced from sesame stalks and bean husk. Biomass Convers. Biorefinery 2023, 14, 17127–17148. [Google Scholar] [CrossRef]
- Viegas, C.; Nobre, C.; Correia, R.; Gouveia, L.; Goncalves, M. Optimization of biochar production by co-torrefaction of microalgae and lignocellulosic biomass using response surface methodology. Energies 2021, 14, 7330. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; Elhassan, O.; Mansour, S.; McKay, G. Biochar development from thermal TGA studies of individual food waste vegetables and their blended systems. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Li, S.M.; Tasnady, D. Biochar for Soil Carbon Sequestration: Current Knowledge, Mechanisms, and Future Perspectives. C-J. Carbon Res. 2023, 9, 67. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Chiaramonti, D.; Bensaid, S.; Fino, D. Biochar production from slow pyrolysis of biomass under CO2 atmosphere: A review on the effect of CO2 medium on biochar production, characterisation, and environmental applications. J. Environ. Chem. Eng. 2023, 11, 110009. [Google Scholar] [CrossRef]
- Maniscalco, M.; Infurna, G.; Caputo, G.; Botta, L.; Dintcheva, N.T. Slow pyrolysis as a method for biochar production from carob waste: Process investigation and products’ characterization. Energies 2021, 14, 8457. [Google Scholar] [CrossRef]
- Kumar, K.V.; Panwar, N.L. Pyrolysis technologies for biochar production in waste management: A review. Clean. Energy-China 2024, 8, 61–78. [Google Scholar] [CrossRef]
- Vali, N.; Zabihi, S.; Shamim, S.; Mohsenzadeh, A.; Pettersson, A. Slow-pyrolysis of municipal sewage sludge: Biochar characteristics and advanced thermodynamics. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Homagain, K.; Shahi, C.; Luckai, N.; Sharma, M. Biochar-based bioenergy and its environmental impact in Northwestern Ontario Canada: A review. J. Forestry Res. 2014, 25, 737–748. [Google Scholar] [CrossRef]
- Li, S.M. Reviewing Air Pollutants Generated during the Pyrolysis of Solid Waste for Biofuel and Biochar Production: Toward Cleaner Production Practices. Sustainability 2024, 16, 1169. [Google Scholar] [CrossRef]
- Kongto, P.; Palamanit, A.; Pattiya, A.; Promsampao, N.; Sonsupap, S.; Phusunti, N.; Theapparat, Y.; Chanakaewsomboon, I.; Tippayawong, N. Yields and characteristics of bio-oil and biochar from fast pyrolysis and co-pyrolysis of oil palm biomass using innovative twin screw reactor for bio-circular-green economy approach. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Abdullah, N.; Taib, R.M.; Aziz, N.S.M.; Omar, M.R.; Disa, N.M. Banana pseudo-stem biochar derived from slow and fast pyrolysis process. Heliyon 2023, 9, e12940. [Google Scholar] [CrossRef]
- Xu, X.J.; Zhang, M.D.; Qi, C.; Sun, Y.; Yang, L.J.; Gu, X.; Li, Y.P.; Wu, M.B.; Wang, B.; Hu, H. Laser-induced carbonization technology towards biomass-derived carbon materials: Mechanism, preparation and application. Green Chem. 2025, 27, 959–981. [Google Scholar] [CrossRef]
- Lu, S.H.T.; Varanusupakul, P. Laser-induced biochar as a miniaturized sorbent for micro-solid phase extraction of organophosphorus pesticides from environmental waters. Microchem. J. 2025, 212, 113327. [Google Scholar] [CrossRef]
- Selvam, S.M.; Paramasivan, B. Microwave assisted carbonization and activation of biochar for energy-environment nexus: A review. Chemosphere 2022, 286, 131631. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Zhang, X.Y.; Cao, C.C.; Quan, G.X.; Wang, M.; Zimmerman, A.R.; Gao, B. Microwave-assisted pyrolysis derived biochar for volatile organic compounds treatment: Characteristics and adsorption performance. Bioresour. Technol. 2022, 355, 127274. [Google Scholar] [CrossRef]
- Singh, R.; Lindenberger, C.; Chawade, A.; Vivekanand, V. Unveiling the microwave heating performance of biochar as microwave absorber for microwave-assisted pyrolysis technology. Sci. Rep. 2024, 14, 9222. [Google Scholar] [CrossRef]
- Holliday, M.C.; Parsons, D.R.; Zein, S.H. Microwave-assisted hydrothermal carbonisation of waste biomass: The effect of process conditions on hydrochar properties. Processes 2022, 10, 1756. [Google Scholar] [CrossRef]
- Potnuri, R.; Rao, C.S. Synthesis and characterization of biochar obtained from microwave-assisted copyrolysis of torrefied sawdust and polystyrene. ACS Sustain. Resour. Manag. 2024, 1, 2074–2085. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, J.; Yang, J.; Yang, J.; Zeng, X.; Zhou, Y. Ball milling nano-sized biochar: Bibliometrics, preparation, and environmental application. Environ. Sci. Pollut. Res. Int. 2024, 31, 52724–52739. [Google Scholar] [CrossRef] [PubMed]
- Amusat, S.O.; Kebede, T.G.; Dube, S.; Nindi, M.M. Ball-milling synthesis of biochar and biochar-based nanocomposites and prospects for removal of emerging contaminants: A review. J. Water Process Eng. 2021, 41, 101993. [Google Scholar] [CrossRef]
- Li, H.; Ni, Z.; Kang, Z.; Sheng, H.; Wang, Y.; Chen, M.; Qian, L. Research progress on synthesis mechanism and performance evaluation of ball milling biochar-iron based materials. npj Mater. Sustain. 2024, 2, 18. [Google Scholar] [CrossRef]
- Jiang, F.; Wei, C.; Yu, Z.; Ji, L.; Liu, M.; Cao, Q.; Wu, L.; Li, F. Fabrication of iron-containing biochar by one-step ball milling for Cr(VI) and tetracycline removal from wastewater. Langmuir 2023, 39, 18958–18970. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jung, G.B. Effects of pyrolysis and ball-milling on the physicochemical and rhodamine B removal characteristics of rice-bran-derived biochar. Appl. Sci. 2023, 13, 4288. [Google Scholar] [CrossRef]
- Tomczyk, A.; Kondracki, B.; Szewczuk-Karpisz, K. Chemical modification of biochars as a method to improve its surface properties and efficiency in removing xenobiotics from aqueous media. Chemosphere 2023, 312, 137238. [Google Scholar] [CrossRef]
- El-Sharkawy, M.; El-Naggar, A.H.; AL-Huqail, A.A.; Ghoneim, A.M. Acid-modified biochar impacts on soil properties and biochemical characteristics of crops grown in saline-sodic soils. Sustainability 2022, 14, 8190. [Google Scholar] [CrossRef]
- Jiang, H.T.; Li, X.; Dai, Y.J. Phosphoric acid activation of cow dung biochar for adsorbing enrofloxacin in water: Icing on the cake. Environ. Pollut. 2024, 341, 122887. [Google Scholar] [CrossRef]
- Das, T.K.; Basak, S.; Ganguly, S. 2D nanomaterial for microplastic Removal: A critical review. Chem. Eng. J. 2024, 492, 152451. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Duan, X. Mitigating the Health Effects of Aqueous Cr(VI) with Iron-Modified Biochar. Int. J. Environ. Res. Public Health 2022, 19, 1481. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.H.; Amirahmadi, E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Tomczyk, A.; Szewczuk-Karpisz, K. Effect of biochar modification by vitamin C, hydrogen peroxide or silver nanoparticles on its physicochemistry and tetracycline removal. Materials 2022, 15, 5379. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in preparation and application of modified biochar for improving heavy metal ion removal from wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Mihoub, A.; Amin, A.A.; Motaghian, H.R.; Saeed, M.F.; Naeem, A. Citric Acid (CA)-Modified Biochar Improved Available Phosphorus Concentration and Its Half-Life in a P-Fertilized Calcareous Sandy Soil. J. Soil Sci. Plant Nutr. 2022, 22, 465–474. [Google Scholar] [CrossRef]
- Saremi, F.; Miroliaei, M.R.; Nejad, M.S.; Sheibani, H. Adsorption of tetracycline antibiotic from aqueous solutions onto vitamin B6-upgraded biochar derived from date palm leaves. J. Mol. Liq. 2020, 318, 114126. [Google Scholar] [CrossRef]
- Nazari, S.; Rahimi, G.; Nezhad, A.K.J. Effectiveness of native and citric acid-enriched biochar of Chickpea straw in Cd and Pb sorption in an acidic soil. J. Environ. Chem. Eng. 2019, 7, 103064. [Google Scholar] [CrossRef]
- Yu, Y.B.; Liu, W.T.; Zhang, Y.N.; Zhang, B.L.; Jin, Y.P.; Chen, S.J.; Tang, S.S.; Su, Y.J.; Yu, X.X.; Chen, G. Chitosan/magnetic biochar composite with enhanced reusability: Synergistic effect of functional groups and multilayer structure. Arab. J. Chem. 2024, 17, 105746. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Wang, C.J.; Han, Q.; Fang, Z.; Gao, Y.R.; Chen, H.B.; Li, J.H.; Yang, X.; Chen, J.F.; Wang, H.L. Recent advances in biochar-based hydrogel composites: Preparation, aquatic environmental applications, and adsorption mechanisms. Processes 2025, 13, 664. [Google Scholar] [CrossRef]
- Rando, G.; Scalone, E.; Sfameni, S.; Plutino, M.R. Functional bio-based polymeric hydrogels for wastewater treatment: From remediation to sensing applications. Gels 2024, 10, 498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Xu, Y.; Mu, X.Y.; Li, S.J.; Liu, X.M.; Lei, Z.Q. Research progress of polysaccharide-based natural polymer hydrogels in water purification. Gels 2023, 9, 249. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K. Hydrogel-biochar composite for agricultural applications and controlled release fertilizer: A step towards pollution free environment. Energy 2022, 242, 122977. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Binh, Q.A.; Nguyen, X.C.; Huyen Nguyen, T.T.; Vo, Q.N.; Nguyen, T.D.; Phuong Tran, T.C.; Hang Nguyen, T.A.; Kim, S.Y.; Nguyen, T.P.; et al. Metal salt-modified biochars derived from agro-waste for effective congo red dye removal. Environ. Res. 2021, 200, 111492. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.S.; Mercl, F.; Száková, J.; Tejnecky, V.; Tlustos, P. The role of low molecular weight organic acids in the release of phosphorus from sewage sludge-based biochar. All Life 2021, 14, 599–609. [Google Scholar] [CrossRef]
- Sheng, X.Y.; Wang, J.K.; Cui, Q.T.; Zhang, W.; Zhu, X.F. A feasible biochar derived from biogas residue and its application in the efficient adsorption of tetracycline from an aqueous solution. Environ. Res. 2022, 207, 112175. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Eyvaz, M.; Al Maskari, T.; Nassani, D.E.; Abu Amr, S.S.; Abujazar, M.S.S.; Bashir, M.J.K. An Overview of Green Bioprocessing of Algae-Derived Biochar and Biopolymers: Synthesis, Preparation, and Potential Applications. Energies 2023, 16, 791. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Selvamani, V.; Yoo, I.K.; Kim, T.W.; Hong, S.H. A Novel Strategy for the Microbial Removal of Heavy Metals: Cell-surface Display of Peptides. Biotechnol. Bioprocess Eng. 2021, 26, 1–9. [Google Scholar] [CrossRef]
- Tu, C.; Wei, J.; Guan, F.; Liu, Y.; Sun, Y.H.; Luo, Y.M. Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ. Int. 2020, 137, 105576. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Pallavi, P.; Shetty, K.; Bhattacharjee, D.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Nair, V. Effective Usage of Biochar and Microorganisms for the Removal of Heavy Metal Ions and Pesticides. Molecules 2023, 28, 719. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sarkar, B.; Aralappanavar, V.K.; Mukhopadhyay, R.; Basak, B.B.; Srivastava, P.; Marchut-Mikolajczyk, O.; Bhatnagar, A.; Semple, K.T.; Bolan, N. Biochar-microorganism interactions for organic pollutant remediation: Challenges and perspectives. Environ. Pollut. 2022, 308, 119609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bo, W.; Wang, T.; Yılmaz, M.; Sharma, G.; Kumar, A.; Shi, H. Multi-mechanism synergistic adsorption of lead and cadmium in water by structure-functionally adapted modified biochar: A review. Desalination Water Treat. 2025, 322, 101156. [Google Scholar] [CrossRef]
- Mukherjee, D.; Sil, M.; Goswami, A.; Bhattacharya, D.; Nag, M.; Lahiri, D.; Sharma, K.; Verma, R. Synthesis, modification and antimicrobial potential of biochar and its modifications against water-borne pathogens: A review. Results Surf. Interfaces 2025, 18, 100438. [Google Scholar] [CrossRef]
- Xiang, L.L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.H.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N.; et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, P.; Uzunov, I.; Popova, T.; Voykova, D.; Avramova, I.; Mehandjiev, D. Biochars as a solution for silver removal and antimicrobial activity in aqueous systems. Appl. Sci. 2025, 15, 2796. [Google Scholar] [CrossRef]
- Gao, Y.; Pramanik, A.; Patibandla, S.; Gates, K.; Hill, G.; Ignatius, A.; Ray, P.C. Development of human host defense antimicrobial peptide-conjugated biochar nanocomposites for combating broad-spectrum superbugs. ACS Appl. Bio Mater. 2020, 3, 7696–7705. [Google Scholar] [CrossRef]
- Chu, M.; Zhao, S.; Yuan, T.; Yan, J.; Sheng, X.; Lan, S.; Dong, A. Photothermal-Induced Multimodal Antibacterial Dressing Comprising N-Halamine Hydrogel Loaded with Cow Dung Biochar for Infected Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 56653–56665. [Google Scholar] [CrossRef]
- Xie, L.C.; Zhang, Z.C.; He, Y.C. Antibacterial Effect of Polyvinyl Alcohol/Biochar-Nano Silver/Sodium Alginate Gel Beads. Processes 2023, 11, 2330. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.; Sung, Y.W.; Song, H.J.; Yang, S.W.; Han, J.W.; Jo, J.W.; Lee, I.S.; Lee, S.H.; Choi, Y.K.; et al. Evaluation of Antibacterial and Antiviral Compounds from Commiphora myrrha (T.Nees) Engl. Resin and Their Promising Application with Biochar. Appl. Sci. 2023, 13, 10549. [Google Scholar] [CrossRef]
- Fu, Y.H.; Wang, F.; Sheng, H.J.; Xu, M.; Liang, Y.; Bian, Y.R.; Hashsham, S.A.; Jiang, X.; Tiedje, J.M. Enhanced antibacterial activity of magnetic biochar conjugated quaternary phosphonium salt. Carbon 2020, 163, 360–369. [Google Scholar] [CrossRef]
- Abdelwahab, M.S.; El Halfawy, N.M.; El-Naggar, M.Y. Lead adsorption and antibacterial activity using modified magnetic biochar/sodium alginate nanocomposite. Int. J. Biol. Macromol. 2022, 206, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, F.; Botta, L.; Pernice, G.; Garofalo, G.; Gaglio, R. Influence of Biochar on the Properties of Antibacterial PBAT/Carvacrol Films. J. Polym. Environ. 2024, 32, 2780–2796. [Google Scholar] [CrossRef]
- Xie, L.C.; Zhang, Z.C.; He, Y.C.; Jiang, Y. Preparation of Polyvinyl Alcohol-Chitosan Nanocellulose-Biochar Nanosilver Composite Hydrogel and Its Antibacterial Property and Dye Removal Capacity. Processes 2024, 12, 2277. [Google Scholar] [CrossRef]
- Zhang, Z.C.; He, Y.C. Synthesis and Characteristics of a Fish Scale-Based Biochar-Nanosilver Antibacterial Material. Processes 2023, 11, 1992. [Google Scholar] [CrossRef]
- Ates, A.; Aydemir, B.; Öksüz, K.E. Investigation of physicochemical and biological properties of boron-doped biochar. Biomass Convers. Biorefinery 2023, 14, 26355–26369. [Google Scholar] [CrossRef]
- Feng, Q.; Fan, B.; He, Y.C.; Ma, C.L. Antibacterial, antioxidant and fruit packaging ability of biochar-based silver nanoparticles-polyvinyl alcohol-chitosan composite film. Int. J. Biol. Macromol. 2024, 256, 128297. [Google Scholar] [CrossRef] [PubMed]
- Hosny, M.; Fawzy, M.; Eltaweil, A.S. Phytofabrication of bimetallic silver-copper/biochar nanocomposite for environmental and medical applications. J. Environ. Manag. 2022, 316, 115238. [Google Scholar] [CrossRef]
- Rahman, M.A.; Yadab, M.K.; Ali, M.M. Emerging role of extracellular ph in tumor microenvironment as a therapeutic target for cancer immunotherapy. Cells 2024, 13, 1924. [Google Scholar] [CrossRef]
- Wang, Z.L.; Han, J.; Guo, Z.Y.; Wu, H.; Liu, Y.G.; Wang, W.Y.; Zhang, C.P.; Liu, J.N. Ginseng-based carbon dots inhibit the growth of squamous cancer cells by increasing ferroptosis. Front. Oncol. 2023, 13, 1097692. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.L.; Yang, Y.; Qiu, J.H.; Dong, Z.; Ren, Q.Z.; Zuo, Y.Y.; Xia, T.; Chen, W.; Liu, S.J. Binding of benzo[a]pyrene alters the bioreactivity of fine biochar particles toward macrophages leading to deregulated macrophagic defense and autophagy. ACS Nano 2021, 15, 9717–9731. [Google Scholar] [CrossRef]
- Liu, J.; Huo, Z.; Mo, Y.; Huang, X.; Wen, Y.; Yan, X.; Liu, W.; Yan, B.; Zhou, H. Impacts of biochar aging on its interactions with As(III) and the combined cytotoxicity. Environ. Res. 2024, 249, 118430. [Google Scholar] [CrossRef] [PubMed]
- Palem, R.R.; Shimoga, G.; Kang, T.J.; Lee, S.H. Fabrication of multifunctional Guar gum-silver nanocomposite hydrogels for biomedical and environmental applications. Int. J. Biol. Macromol. 2020, 159, 474–486. [Google Scholar] [CrossRef]
- Pathania, D.; Kumar, A.; Saini, A.K.; Saini, R.; Mittal, D.; Sharma, A. Biochar supported Ag/Cu-ZrO2 nano-hetero assembly for enhanced adsorption of heavy metal ions and biomedical applications. Nanotechnol. Environ. Eng. 2024, 9, 189–206. [Google Scholar] [CrossRef]
- Kamal, A.; Haroon, U.; Manghwar, H.; Alamer, K.H.; Alsudays, I.M.; Althobaiti, A.T.; Iqbal, A.; Akbar, M.; Farhana; Anar, M.; et al. Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using L. Molecules 2022, 27, 5333. [Google Scholar] [CrossRef]
- Fei, L.; Propato, A.P.; Lotti, G.; Nardini, P.; Guasti, D.; Polvani, S.; Bani, D.; Galli, A.; Casini, D.; Cantini, G.; et al. Tailor-made Biochar enhances the anti-tumour effects of butyrate-glycerides in colorectal cancer. Biomed. Pharmacother. 2025, 184, 117900. [Google Scholar] [CrossRef] [PubMed]
- Iannazzo, D.; Celesti, C.; Espro, C.; Ferlazzo, A.; Giofrè, S.; Scuderi, M.; Scalese, S.; Gabriele, B.; Mancuso, R.; Ziccarelli, I.; et al. Orange-Peel-Derived Nanobiochar for Targeted Cancer Therapy. Pharmaceutics 2022, 14, 2249. [Google Scholar] [CrossRef]
- Alqaraleh, M.; Khleifat, K.M.; Abu Hajleh, M.N.; Farah, H.S.; Ahmed, K.A. Fungal-Mediated Silver Nanoparticle and Biochar Synergy against Colorectal Cancer Cells and Pathogenic Bacteria. Antibiotics 2023, 12, 597. [Google Scholar] [CrossRef]
- Allah, M.A.A.H.; Ibrahim, H.K.; Alshamsi, H.A. Enhanced adsorption, anticancer and antibacterial potentials of L. extract mediated ecofriendly synthesized ZnO/ biochar nanohybrid. Inorg. Chem. Commun. 2025, 171, 113538. [Google Scholar] [CrossRef]
- Abu Hajleh, M.N.; Al-limoun, M.; Al-Tarawneh, A.; Hijazin, T.J.; Alqaraleh, M.; Khleifat, K.; Al-Madanat, O.Y.; Al Qaisi, Y.; AlSarayreh, A.; Al-Samydai, A.; et al. Synergistic Effects of AgNPs and Biochar: A Potential Combination for Combating Lung Cancer and Pathogenic Bacteria. Molecules 2023, 28, 4757. [Google Scholar] [CrossRef]
- Chalklen, T.; Jing, Q.; Kar-Narayan, S. Biosensors Based on Mechanical and Electrical Detection Techniques. Sensors 2020, 20, 5605. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Bonacin, J.A.; Janegitz, B.C.; Mangrich, A.S.; Marcolino, L.H.; Bergamini, M.F. State-of-the-art and perspectives in the use of biochar for electrochemical and electroanalytical applications. Green Chem. 2021, 23, 5272–5301. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Bushra, B.; Remya, N. Biochar from pyrolysis of rice husk biomass-characteristics, modification and environmental application. Biomass Convers. Biorefinery 2024, 14, 5759–5770. [Google Scholar] [CrossRef]
- Khan, M.A.; Hameed, B.H.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.H. Comparative investigation of the physicochemical properties of chars produced by hydrothermal carbonization, pyrolysis, and microwave-induced pyrolysis of food waste. Polymers 2022, 14, 821. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2021, 297, 126645. [Google Scholar] [CrossRef]
- Chauhan, S.; Dahiya, D.; Sharma, V.; Khan, N.; Chaurasia, D.; Nadda, A.K.; Varjani, S.; Pandey, A.; Bhargava, P.C. Advances from conventional to real time detection of heavy metal(loid)s for water monitoring: An overview of biosensing applications. Chemosphere 2022, 307, 136124. [Google Scholar] [CrossRef]
- Valenga, M.G.P.; Martins, G.; Martins, T.A.C.; Didek, L.K.; Gevaerd, A.; Marcolino-Junior, L.H.; Bergamini, M.F. Biochar: An environmentally friendly platform for construction of a SARS-CoV-2 electrochemical immunosensor. Sci. Total Environ. 2023, 858, 159797. [Google Scholar] [CrossRef]
- Zheng, A.L.T.; Lih, E.T.Y.; Hung, Y.P.; Boonyuen, S.; Al Edrus, S.S.O.; Chung, E.L.T.; Andou, Y. Biochar-based electrochemical sensors: A tailored approach to environmental monitoring. In Analytical Sciences; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar] [CrossRef]
- Kim, S.A.; Kim, E.B.; Imran, M.; Shahzad, K.; Moon, D.H.; Akhtar, M.S.; Ameen, S.; Park, S.H. Naturally manufactured biochar materials based sensor electrode for the electrochemical detection of polystyrene microplastics. Chemosphere 2024, 351, 141151. [Google Scholar] [CrossRef]
- Himani; Amrita; Agarwal, T. Applications of biochar in sensors: Recent advancements and future trends. Mater. Today Chem. 2025, 45, 102646. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S. Biochar derived carbonaceous material for various environmental applications: Systematic review. Environ. Res. 2022, 214, 113857. [Google Scholar] [CrossRef]
- Kalinke, C.; Zanicoski-Moscardi, A.P.; de Oliveira, P.R.; Mangrich, A.S.; Marcolino-Junior, L.H.; Bergamini, M.F. Simple and low-cost sensor based on activated biochar for the stripping voltammetric detection of caffeic acid. Microchem. J. 2020, 159, 105380. [Google Scholar] [CrossRef]
- Banga, I.; Paul, A.; Dhamu, V.N.; Ramasubramanya, A.H.; Muthukumar, S.; Prasad, S. Activated carbon derived from wood biochar for Amperometric sensing of Ammonia for early screening of chronic kidney disease. Int. J. Biol. Macromol. 2023, 253, 126894. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, A.; Jia, F.; Kelso, L.C.; Biswas, S.K.; Muthukumarappan, K.; Cao, C.; Wei, L.; Li, Y. A Novel Activated Biochar-Based Immunosensor for Rapid Detection of E. coli O157:H7. Biosensors 2022, 12, 908. [Google Scholar] [CrossRef]
- Gemeiner, P.; Sarakhman, O.; Hatala, M.; Ház, A.; Roupcová, P.; Mackulak, T.; Barek, J.; Svorc, L. A new generation of fully-printed electrochemical sensors based on biochar/ethylcellulose-modified carbon electrodes: Fabrication, characterization and practical applications. Electrochim. Acta 2024, 487, 144161. [Google Scholar] [CrossRef]
- Kalinke, C.; de Oliveira, P.R.; Marcolino-Júnior, L.H.; Bergamini, M.F. Nanostructures of Prussian blue supported on activated biochar for the development of a glucose biosensor. Talanta 2024, 274, 126042. [Google Scholar] [CrossRef]
- Larasati, L.D.; Ates, A.; Oskay, K.O. Direct co-deposition of binder-free Cu-biochar-based nonenzymatic disposable sensing element for electrochemical glucose detection. Surf. Interfaces 2023, 42, 103355. [Google Scholar] [CrossRef]
- Liu, C.D.; Zhang, N.; Huang, X.; Wang, Q.W.; Wang, X.H.; Wang, S.T. Fabrication of a novel nanocomposite electrode with ZnO-MoO and biochar derived from mushroom biomaterials for the detection of acetaminophen in the presence of DA. Microchem. J. 2021, 161, 105719. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, J.; Wang, Y.; Hasebe, Y.; Zhang, Z. Electrodeposited Ni on a silk-derived carbon modified glassy carbon electrode for non-invasive sensing of glucose in saliva. J. Mater. Chem. C 2025, 13, 6289–6301. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Asadpour-Zeynali, K.; Khataee, A.; Dastborhan, M.; Mokhtarzadeh, A. A PCR-free genosensing platform for detection of Shigella dysenteriae in human plasma samples by porous and honeycomb-like biochar decorated with ultrathin flower-like MoS2 nanosheets incorporated with Au nanoparticles. Chemosphere 2022, 288, 132531. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, G.Y.; Chu, H.Q.; Shen, Z.; Zhang, Y.L.; Abbas, M.H.H.; Albogami, B.Z.; Zhou, L.; Abdelhafez, A.A. Biochar applications for treating potentially toxic elements (PTEs) contaminated soils and water: A review. Front. Bioeng. Biotechnol. 2023, 11, 1258483. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.Y.; Jiang, M.Y.; He, L.Z.; Zhang, Z.R.; Gustave, W.; Vithanage, M.; Niazi, N.K.; Chen, B.; Zhang, X.K.; Wang, H.L.; et al. Challenges in safe environmental applications of biochar: Identifying risks and unintended consequence. Biochar 2025, 7, 12. [Google Scholar] [CrossRef]
- Khan, N.; Chowdhary, P.; Gnansounou, E.; Chaturvedi, P. Biochar and environmental sustainability: Emerging trends and techno-economic perspectives. Bioresour. Technol. 2021, 332, 125102. [Google Scholar] [CrossRef]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Musiol, M.; Rydz, J.; Janeczek, H.; Andrzejewski, J.; Cristea, M.; Musiol, K.; Kampik, M.; Kowalczuk, M. (Bio)degradable biochar composites of PLA/P(3HB-co-4HB) commercial blend for sustainable future-study on degradation and electrostatic properties. Polymers 2024, 16, 2331. [Google Scholar] [CrossRef]
- Sun, Y.; Shaheen, S.M.; Ali, E.F.; Abdelrahman, H.; Sarkar, B.; Song, H.; Rinklebe, J.; Ren, X.A.; Zhang, Z.Q.; Wang, Q. Enhancing microplastics biodegradation during composting using livestock manure biochar. Environ. Pollut. 2022, 306, 119339. [Google Scholar] [CrossRef]
- Ju, S.; Cho, H.Y. Biohybrid nanoparticle-based in situ monitoring of in vivo drug delivery. Biosensors 2023, 13, 1017. [Google Scholar] [CrossRef]
- López, R.P.R.; Vargas, D.C.; Aguilera-Cauich, E.A.; Rivero, J.C.S. Life cycle assessment of biochar from residual lignocellulosic biomass using kon-tiki kilns: Applications in soil amendment and wastewater filtration. Recycling 2024, 9, 125. [Google Scholar] [CrossRef]
- Deng, Q.; Li, A.; Wu, Y.; Sun, L. Life cycle assessment of biochar preparation of chinese traditional medicine residue by low-temperature pyrolysis. In E3S Web of Conferences; EDP Sciences: Les Ulis Cedex, France, 2022; p. 03005. [Google Scholar]
- Shaheen, J.; Fseha, Y.H.; Sizirici, B. Performance, life cycle assessment, and economic comparison between date palm waste biochar and activated carbon derived from woody biomass. Heliyon 2022, 8, e12388. [Google Scholar] [CrossRef]
- Nematian, M.; Keske, C.; Ng’ombe, J.N. A techno-economic analysis of biochar production and the bioeconomy for orchard biomass. Waste Manag. 2021, 135, 467–477. [Google Scholar] [CrossRef]
- Li, X.; Zeng, J.; Zuo, S.; Lin, S.; Chen, G. Preparation, modification, and application of biochar in the printing field: A review. Materials 2023, 16, 5081. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Youn, S.; Pack, S.P. Biomimetic diatom biosilica and its potential for biomedical applications and prospects: A review. Int. J. Mol. Sci. 2024, 25, 2023. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Kim, D.H.; Kim, K.H.; Seo, J.H.; Pack, S.P. Biomimetic scaffolds of calcium-based materials for bone regeneration. Biomimetics 2024, 9, 511. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Ki, M.R.; Pack, S.P. Biominerals and bioinspired materials in biosensing: Recent advancements and applications. Int. J. Mol. Sci. 2024, 25, 4678. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Ki, M.R.; Abdelhamid, M.A.A.; Pack, S.P. Biomimetic materials for skin tissue regeneration and electronic skin. Biomimetics 2024, 9, 278. [Google Scholar] [CrossRef]
- Kim, S.H.; Ki, M.R.; Han, Y.; Pack, S.P. Biomineral-based composite materials in regenerative medicine. Int. J. Mol. Sci. 2024, 25, 6147. [Google Scholar] [CrossRef]
- Min, K.H.; Shin, J.W.; Ki, M.R.; Pack, S.P. Green synthesis of silver nanoparticles on biosilica diatomite: Well-dispersed particle formation and reusability. Process Biochem. 2023, 125, 232–238. [Google Scholar] [CrossRef]
- Kim, D.H.; Min, K.H.; Pack, S.P. Efficient bioactive surface coatings with calcium minerals: Step-wise biomimetic transformation of vaterite to carbonated apatite. Biomimetics 2024, 9, 402. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Pack, S.P. Size control of biomimetic curved-edge vaterite with chiral toroid morphology via sonochemical synthesis. Biomimetics 2024, 9, 174. [Google Scholar] [CrossRef] [PubMed]
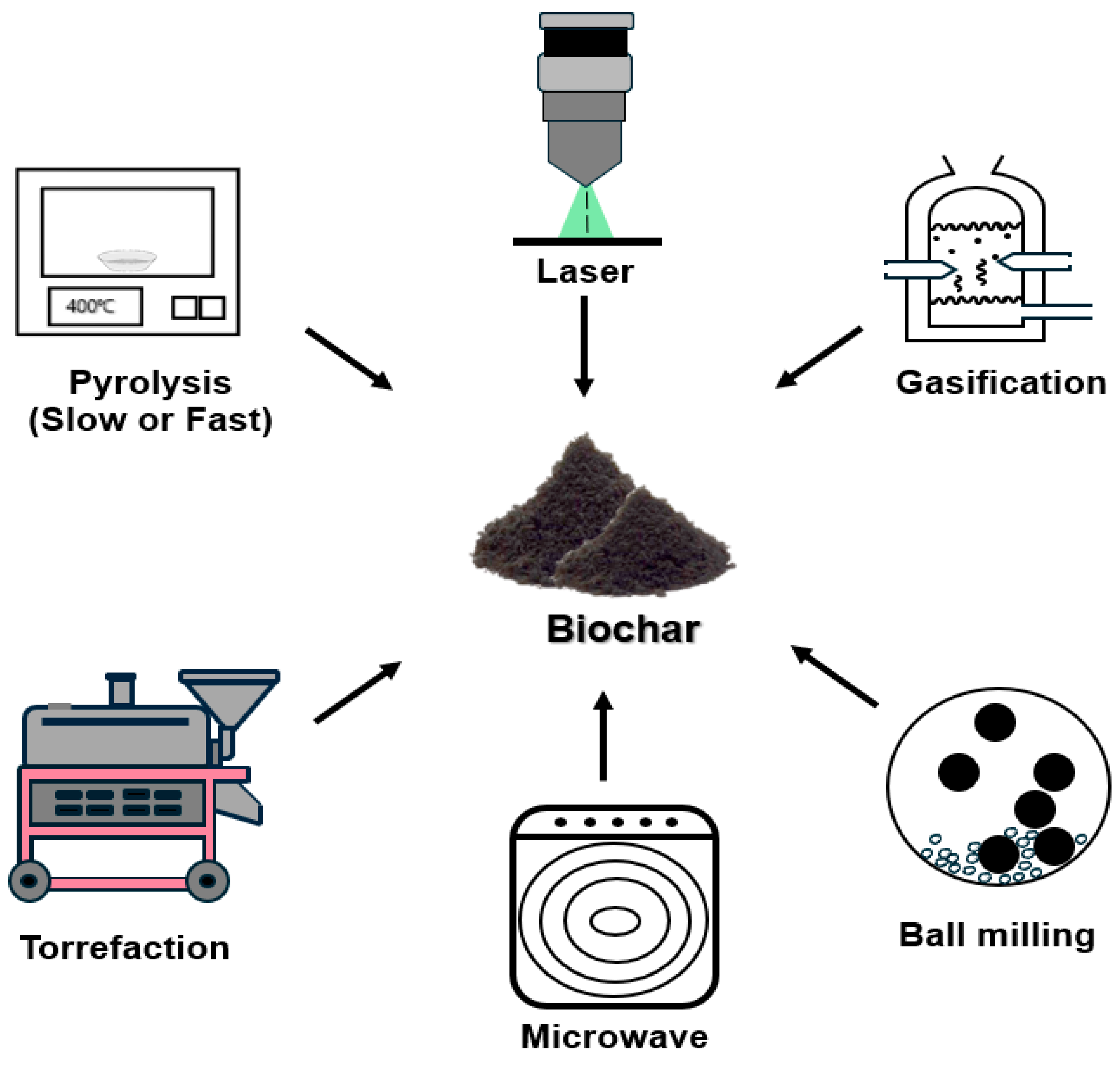

| Methods | Advantages | Limitations | Ref. |
|---|---|---|---|
| Pyrolysis Carbonization |
|
| [46,58,59,60] |
| Hydrothermal Carbonization |
|
| [59,61,62] |
| Torrefaction |
|
| [63,64,65,66] |
| Slow Pyrolysis |
|
| [46,67,68,69,70,71,72] |
| Fast Pyrolysis |
|
| [71,73,74,75,76] |
| Laser-induced Carbonization |
|
| [77,78] |
| Microwave-assisted Carbonization |
|
| [79,80,81,82,83] |
| Ball Milling |
|
| [84,85,86,87,88] |
| Source | Additional Material | Methods | Target | Effects | Ref. |
| Corn and wood | Ethylcellulose | Pyrolysis (470 °C, 25 min) Screen printing | Paracetamol |
| [157] |
| Coster cake | Prussian blue Glucose oxidase | Pyrolysis (400 °C, 5 °C/min for 60 min) Covalent enzyme immobilization | Glucose |
| [158] |
| Raw tea waste | Copper | H3PO4 treatment (60 °C, 30 min) Pyrolysis (500 °C, 1 h) Electrodeposition | Glucose |
| [159] |
| Wood | - | H3PO4 treatment Thermal carbonization (10 °C/min to 400 °C, hold for 3 h) | Ammonia |
| [155] |
| Mushroom | MoO3 ZnO | Thermal calcination (550 °C for 3 h, 2 °C/min) | Acetaminophen |
| [160] |
| Silk | Ni | Pyrolysis (800 °C) Electrochemical deposition | Glucose |
| [161] |
| Pine tree residues | MoS2 Gold nanoparticle | Pyrolysis (600 °C, 1 h) Hydrothermal method | Gene of S. dysenteriae |
| [162] |
| Corn stalk | Anti-E. coli polyclonal antibody | Pyrolysis (250–300 °C) Steam activation (800 °C, 2 mL/min) Antibody immobilization | E. coli O157:H7 |
| [156] |
| Sugarcane bagasse | SARS-CoV-2 S-protein receptor binding domain | Pyrolysis (5 °C/min, 700 °C) Dropping electrode modification | SARS-CoV-2 antibody |
| [149] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, K.H.; Kim, K.H.; Seo, J.-H.; Pack, S.P. Biochar Utilization in Antimicrobial, Anticancer, and Biosensing Applications: A Review. Biomolecules 2025, 15, 760. https://doi.org/10.3390/biom15060760
Min KH, Kim KH, Seo J-H, Pack SP. Biochar Utilization in Antimicrobial, Anticancer, and Biosensing Applications: A Review. Biomolecules. 2025; 15(6):760. https://doi.org/10.3390/biom15060760
Chicago/Turabian StyleMin, Ki Ha, Koung Hee Kim, Joo-Hyung Seo, and Seung Pil Pack. 2025. "Biochar Utilization in Antimicrobial, Anticancer, and Biosensing Applications: A Review" Biomolecules 15, no. 6: 760. https://doi.org/10.3390/biom15060760
APA StyleMin, K. H., Kim, K. H., Seo, J.-H., & Pack, S. P. (2025). Biochar Utilization in Antimicrobial, Anticancer, and Biosensing Applications: A Review. Biomolecules, 15(6), 760. https://doi.org/10.3390/biom15060760





