Modulation of Kv Channel Gating by Light-Controlled Membrane Thickness
Abstract
1. Introduction
2. Materials and Methods
2.1. KvAP Expression and Purification
2.2. Horizontal Solvent-Depleted Planar Lipid Bilayers (PLBs)
2.3. Optical Measurement and Photoisomerization
2.4. Electrophysiology Recordings
2.5. Electrophysiology Data Analysis
3. Results
3.1. Fast Photoisomerization of OptoDArG Generates Only a Small Transient Increment in Membrane Tension
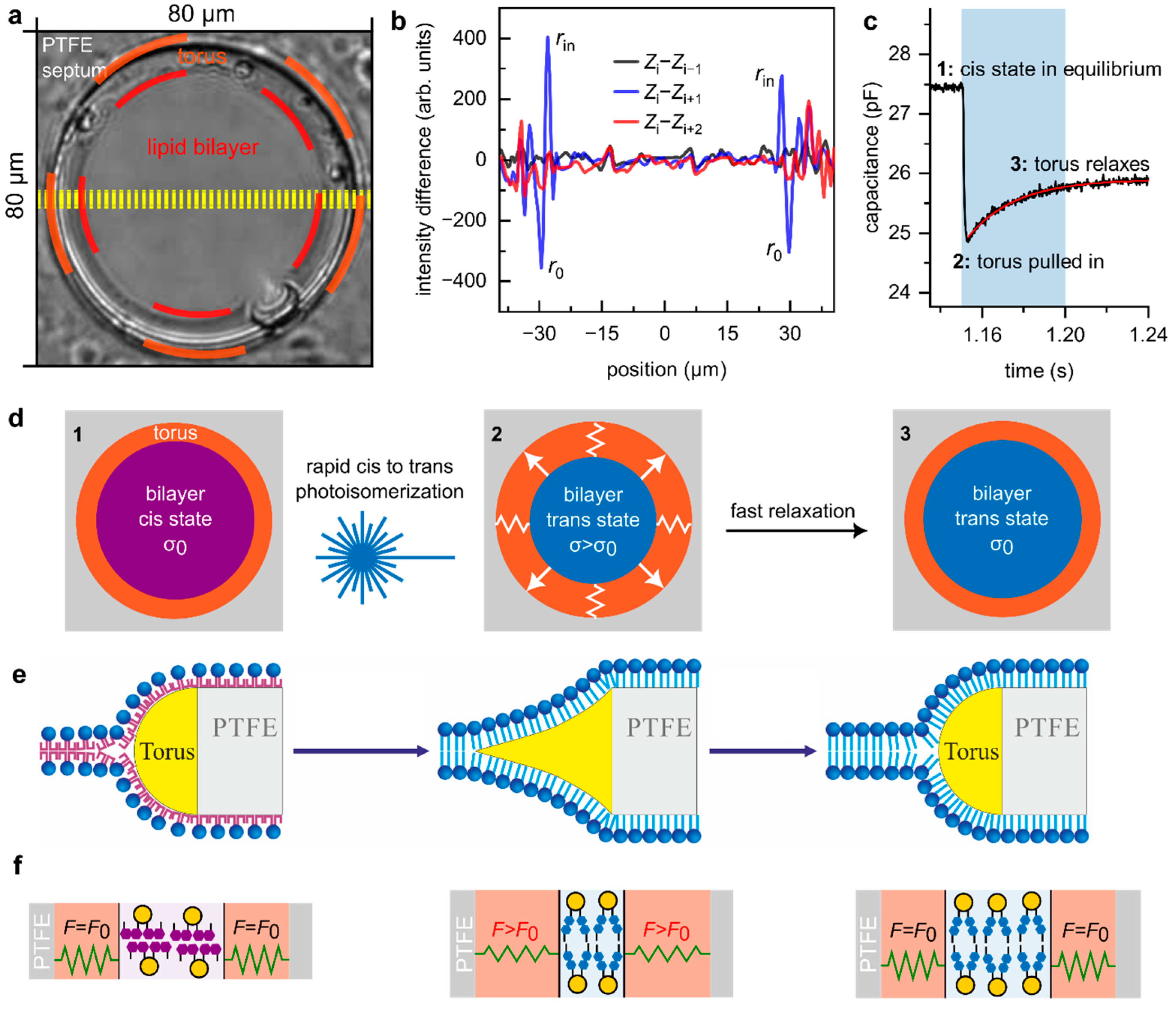
- From the video recordings, we quantified the torus movement by averaging 11-line profiles along the equator of the PLB (yellow lines, Figure 1a), resulting in an average profile for each frame . For seven instances of cis-to-trans photoisomerization (see Supplementary Video), we selected just before light exposure and verified that it was indistinguishable from the previous profile by calculating the difference (black line in Figure 1b). Following blue light exposure, (blue line in Figure 1b) displayed pronounced positive () and negative () deflections, indicating inward torus movement from to . Finally, (red line in 1b) no longer exhibited these peaks, showing that the torus relaxes within less than twice the frame interval of 40 ms, i.e., .
- The rate of decrease in at the onset of blue light exposure is rapid and largely determined by irradiance and azobenzene photoisomerization kinetics [26]. reaches a minimum as OptoDArG is quantitatively photoisomerized (2 in Figure 1c), resulting in bilayer thickening and a reduction in as the torus is pulled inwards. Then, relaxes with an estimated time constant (monoexponential fit to the relaxation in ; red line in Figure 1c). This increase in is consistent with the observed increase in as the torus retracts. Hence, we conclude that the relaxation in torus position and reflect the same underlying phenomenon.
3.2. Functional Reconstitution of KvAP into Solvent-Depleted PLBs Containing OptoDArG
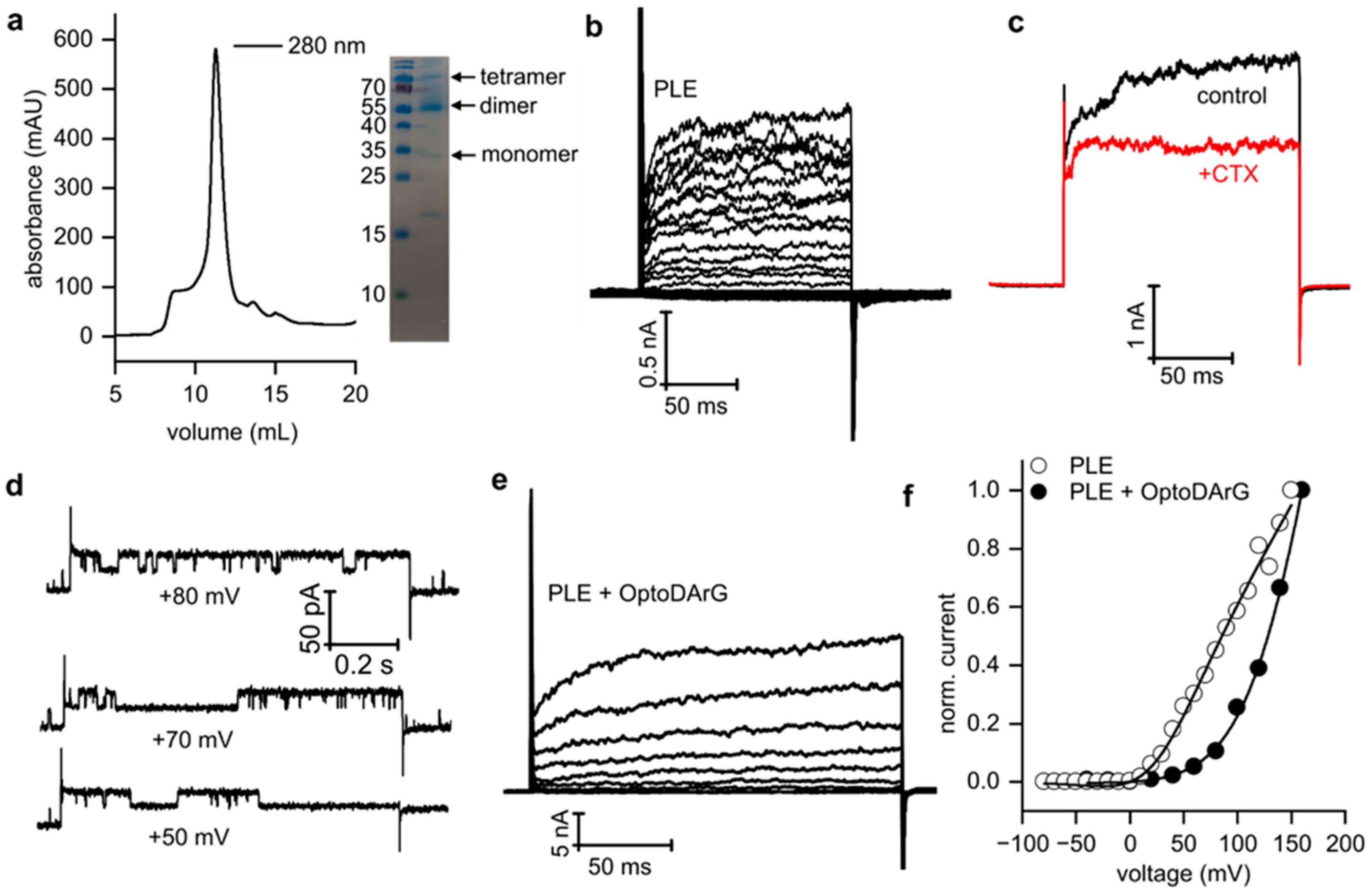
3.3. Voltage-Dependent Gating of KvAP Is Influenced by Solvent Content and the Presence of Non-Phospholipids
3.4. Rapid Photoisomerization of Membrane-Embedded OptoDArG Gates KvAP

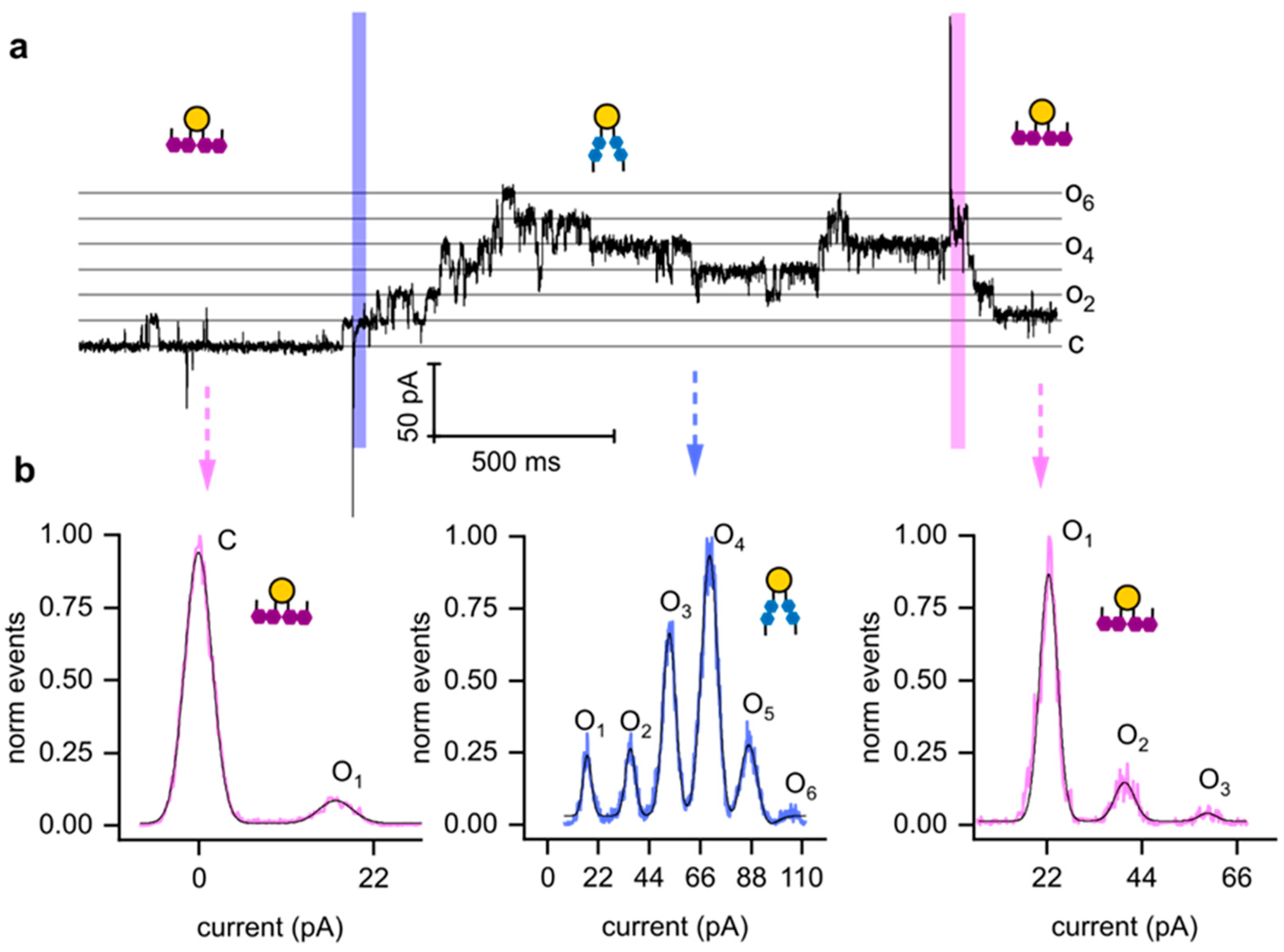
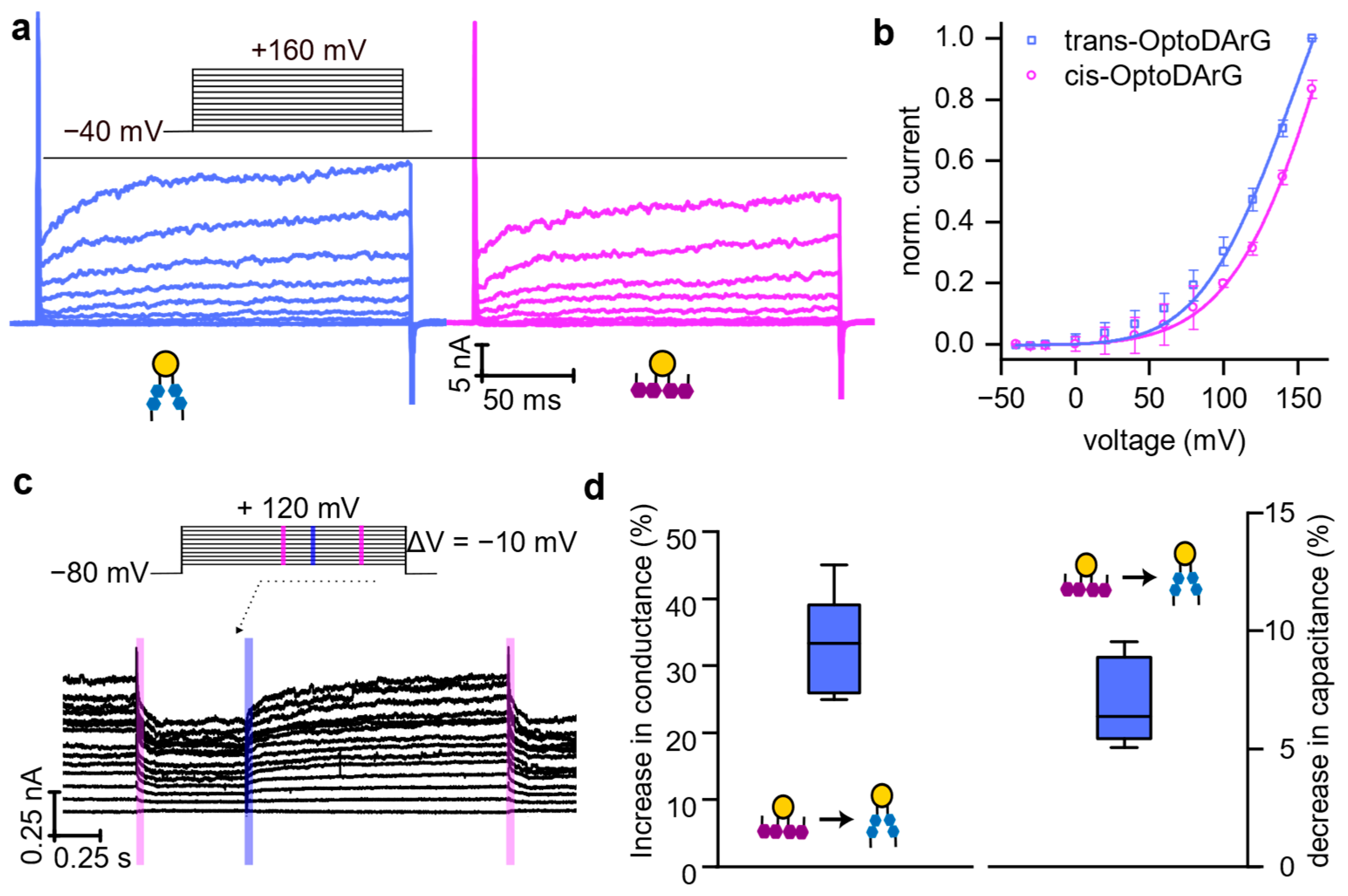
4. Discussion
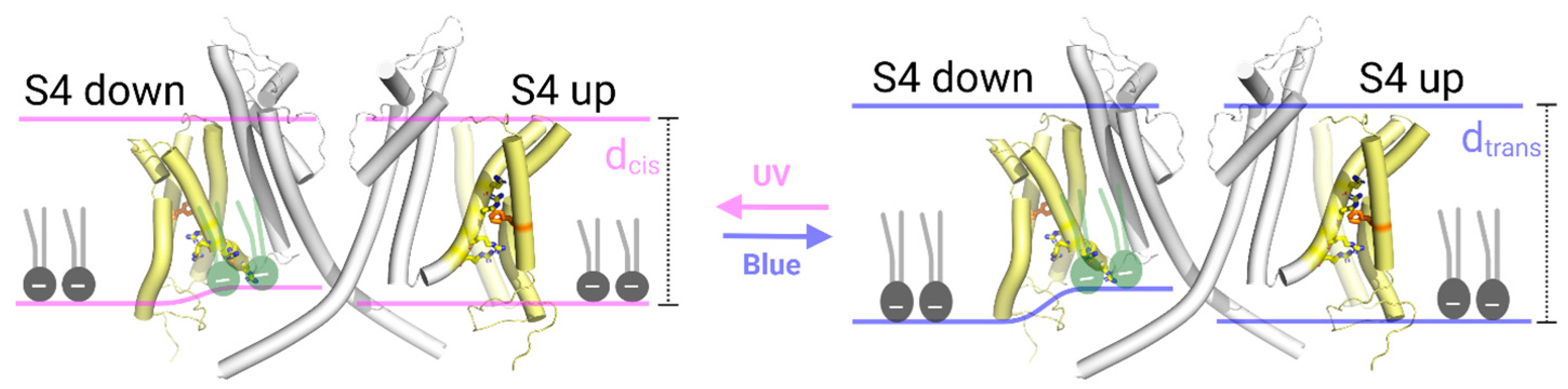
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latorre, R.; Miller, C. Conduction and selectivity in potassium channels. J. Membr. Biol. 1983, 71, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.M.; Nimigean, C.M. Voltage-gated potassium channels: A structural examination of selectivity and gating. Cold Spring Harb. Perspect. Biol. 2016, 8, a029231. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, F. The voltage-sensor structure in a voltage-gated channel. Trends Biochem. Sci. 2005, 30, 166–168. [Google Scholar] [CrossRef]
- Cha, A.; Bezanilla, F. Characterizing Voltage-Dependent Conformational Changes in the ShakerK + Channel with Fluorescence. Neuron 1997, 19, 1127–1140. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Bezanilla, F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 1974, 63, 533–552. [Google Scholar] [CrossRef]
- Swartz, K.J. Sensing voltage across lipid membranes. Nature 2008, 456, 891–897. [Google Scholar] [CrossRef]
- Li, L.; Vorobyov, I.; Allen, T.W. The Different Interactions of Lysine and Arginine Side Chains with Lipid Membranes. J. Phys. Chem. B 2013, 117, 11906–11920. [Google Scholar]
- Krepkiy, D.; Mihailescu, M.; Freites, J.A.; Schow, E.V.; Worcester, D.L.; Gawrisch, K.; Tobias, D.J.; White, S.H.; Swartz, K.J. Structure and hydration of membranes embedded with voltage-sensing domains. Nature 2009, 462, 473–479. [Google Scholar] [CrossRef]
- Cuello, L.G.; Jogini, V.; Cortes, D.M.; Pan, A.C.; Gagnon, D.G.; Dalmas, O.; Cordero-Morales, J.F.; Chakrapani, S.; Roux, B.; Perozo, E. Structural basis for the coupling between activation and inactivation gates in K(+) channels. Nature 2010, 466, 272–275. [Google Scholar] [CrossRef]
- Freites, J.A.; Tobias, D.J.; Heijne, G.; White, S.H. Interface connections of a transmembrane voltage sensor. Proc. Natl. Acad. Sci. USA 2005, 102, 15059–15064. [Google Scholar] [CrossRef]
- Sands, Z.A.; Sansom, M.S.P. How does a voltage sensor interact with a lipid bilayer? Simulations of a potassium channel domain. Structure 2007, 15, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, S.; Sompornpisut, P.; Intharathep, P.; Roux, B.; Perozo, E. The activated state of a sodium channel voltage sensor in a membrane environment. Proc. Natl. Acad. Sci. USA 2010, 107, 5435–5440. [Google Scholar] [CrossRef]
- Schmidt, D.; Jiang, Q.X.; MacKinnon, R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature 2006, 444, 775–779. [Google Scholar] [CrossRef]
- Xu, Y.; Ramu, Y.; Lu, Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature 2008, 451, 826–829. [Google Scholar] [CrossRef]
- Finol-Urdaneta, R.K.; McArthur, J.R.; Juranka, P.F.; French, R.J.; Morris, C.E. Modulation of KvAP unitary conductance and gating by 1-alkanols and other surface active agents. Biophys. J. 2010, 98, 762–772. [Google Scholar] [CrossRef]
- Schmidt, D.; del Marmol, J.; MacKinnon, R. Mechanistic basis for low threshold mechanosensitivity in voltage-dependent K+ channels. Proc. Natl. Acad. Sci. USA 2012, 109, 10352–10357. [Google Scholar] [CrossRef]
- Pinto-Anwandter, B.I.; Bassetto, C.A.B., Jr.; Latorre, R.; Bezanilla, F. Energy landscape of a Kv channel revealed by temperature steps while perturbing its electromechanical coupling. Nat. Commun. 2025, 16, 3379. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]
- Haselwandter, C.A.; Guo, Y.R.; Fu, Z.; MacKinnon, R. Quantitative prediction and measurement of Piezo’s membrane footprint. Proc. Natl. Acad. Sci. USA 2022, 119, e2208027119. [Google Scholar] [CrossRef]
- Haselwandter, C.A.; Guo, Y.R.; Fu, Z.; MacKinnon, R. Elastic properties and shape of the Piezo dome underlying its mechanosensory function. Proc. Natl. Acad. Sci. USA 2022, 119, e2208034119. [Google Scholar] [CrossRef]
- Wiggins, P.; Phillips, R. Analytic models for mechanotransduction: Gating a mechanosensitive channel. Proc. Natl. Acad. Sci. USA 2004, 101, 4071–4076. [Google Scholar] [CrossRef] [PubMed]
- Quemeneur, F.; Sigurdsson, J.K.; Renner, M.; Atzberger, P.J.; Bassereau, P.; Lacoste, D. Shape matters in protein mobility within membranes. Proc. Natl. Acad. Sci. USA 2014, 111, 5083–5087. [Google Scholar] [CrossRef] [PubMed]
- Mandala, V.S.; MacKinnon, R. Voltage-sensor movements in the Eag Kv channel under an applied electric field. Proc. Natl. Acad. Sci. USA 2022, 119, e2214151119. [Google Scholar] [CrossRef]
- Saitov, A.; Akimov, S.A.; Galimzyanov, T.R.; Glasnov, T.N.; Pohl, P. Ordered Lipid Domains Assemble via Concerted Recruitment of Constituents from Both Membrane Leaflets. Phys. Rev. Lett. 2020, 124, 108102. [Google Scholar] [CrossRef]
- Pfeffermann, J.; Eicher, B.; Boytsov, D.; Hannesschlaeger, C.; Galimzyanov, T.R.; Glasnov, T.N.; Pabst, G.; Akimov, S.A.; Pohl, P. Photoswitching of model ion channels in lipid bilayers. J. Photochem. Photobiol. B Biol. 2021, 224, 112320. [Google Scholar] [CrossRef]
- Bassetto, C.A.Z.; Pfeffermann, J.; Yadav, R.; Strassgschwandtner, S.; Glasnov, T.; Bezanilla, F.; Pohl, P. Photolipid excitation triggers depolarizing optocapacitive currents and action potentials. Nat. Commun. 2024, 15, 1139. [Google Scholar] [CrossRef]
- Frank, J.A.; Franquelim, H.G.; Schwille, P.; Trauner, D. Optical Control of Lipid Rafts with Photoswitchable Ceramides. J. Am. Chem. Soc. 2016, 138, 12981–12986. [Google Scholar] [CrossRef]
- Georgiev, V.N.; Grafmüller, A.; Bléger, D.; Hecht, S.; Kunstmann, S.; Barbirz, S.; Lipowsky, R.; Dimova, R. Area Increase and Budding in Giant Vesicles Triggered by Light: Behind the Scene. Adv. Sci. 2018, 5, 1800432. [Google Scholar] [CrossRef]
- Aleksanyan, M.; Grafmüller, A.; Crea, F.; Georgiev, V.N.; Yandrapalli, N.; Block, S.; Heberle, J.; Dimova, R. Photomanipulation of Minimal Synthetic Cells: Area Increase, Softening, and Interleaflet Coupling of Membrane Models Doped with Azobenzene-Lipid Photoswitches. Adv. Sci. 2023, 10, e2304336. [Google Scholar] [CrossRef]
- Horner, A.; Akimov, S.A.; Pohl, P. Long and short lipid molecules experience the same interleaflet drag in lipid bilayers. Phys. Rev. Lett. 2013, 110, 268101. [Google Scholar] [CrossRef]
- White, S.H. Analysis of the torus surrounding planar lipid bilayer membranes. Biophys. J. 1972, 12, 432–445. [Google Scholar] [CrossRef] [PubMed]
- Sandre, O.; Moreaux, L.; Brochard-Wyart, F. Dynamics of transient pores in stretched vesicles. Proc. Natl. Acad. Sci. USA 1999, 96, 10591–10596. [Google Scholar] [CrossRef]
- Needham, D.; Haydon, D.A. Tensions and free energies of formation of “solventless” lipid bilayers. Measurement of high contact angles. Biophys. J. 1983, 41, 251–257. [Google Scholar] [CrossRef]
- Block, S. Brownian Motion at Lipid Membranes: A Comparison of Hydrodynamic Models Describing and Experiments Quantifying Diffusion within Lipid Bilayers. Biomolecules 2018, 8, 30. [Google Scholar] [CrossRef]
- Cicuta, P.; Keller, S.L.; Veatch, S.L. Diffusion of Liquid Domains in Lipid Bilayer Membranes. J. Phys. Chem. B 2007, 111, 3328–3331. [Google Scholar] [CrossRef]
- Hoomann, T.; Jahnke, N.; Horner, A.; Keller, S.; Pohl, P. Filter gate closure inhibits ion but not water transport through potassium channels. Proc. Natl. Acad. Sci. USA 2013, 110, 10842–10847. [Google Scholar] [CrossRef]
- Ezsias, B.; Wolkenstein, F.; Goessweiner-Mohr, N.; Yadav, R.; Siligan, C.; Posch, S.; Horner, A.; Vargas, C.; Keller, S.; Pohl, P. Enhanced Site-Specific Fluorescent Labeling of Membrane Proteins Using Native Nanodiscs. Biomolecules 2025, 15, 254. [Google Scholar]
- Ruta, V.; Jiang, Y.; Lee, A.; Chen, J.; MacKinnon, R. Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature 2003, 422, 180–185. [Google Scholar] [CrossRef]
- Miller, C.; Moczydlowski, E.; Latorre, R.; Phillips, M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature 1985, 313, 316–318. [Google Scholar] [CrossRef]
- MacKinnon, R.; Miller, C. Mechanism of charybdotoxin block of the high-conductance, Ca2+-activated K+ channel. J. Gen. Physiol. 1988, 91, 335–349. [Google Scholar] [CrossRef]
- Gonzalez, C.; Morera, F.J.; Rosenmann, E.; Alvarez, O.; Latorre, R. S3b amino acid residues do not shuttle across the bilayer in voltage-dependent Shaker K+ channels. Proc. Natl. Acad. Sci. USA 2005, 102, 5020–5025. [Google Scholar] [CrossRef]
- Schmidt, D.; MacKinnon, R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 19276–19281. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; MacKinnon, R. Functional analysis of Kv1.2 and paddle chimera Kv channels in planar lipid bilayers. J. Mol. Biol. 2008, 382, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Lee, S.; Llaguno, M.C.; Jiang, Q.-X. bSUM: A bead-supported unilamellar membrane system facilitating unidirectional insertion of membrane proteins into giant vesicles. J. Gen. Physiol. 2016, 147, 77–93. [Google Scholar] [CrossRef]
- Aimon, S.; Manzi, J.; Schmidt, D.; Larrosa, J.A.P.; Bassereau, P.; Toombes, G.E.S. Functional reconstitution of a voltage-gated potassium channel in giant unilamellar vesicles. PLoS ONE 2011, 6, e25529. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; Anderson, L.Y.; Jiang, Q.-X. Lipid-dependent gating of a voltage-gated potassium channel. Nat. Commun. 2011, 2, 250. [Google Scholar] [CrossRef]
- Zhang, M.; Shan, Y.; Pei, D. Mechanism underlying delayed rectifying in human voltage-mediated activation Eag2 channel. Nat. Commun. 2023, 14, 1470. [Google Scholar] [CrossRef]
- Callahan, K.M.; Mondou, B.; Sasseville, L.; Schwartz, J.-L.; D’Avanzo, N. The influence of membrane bilayer thickness on KcsA channel activity. Channels 2019, 13, 424–439. [Google Scholar] [CrossRef]
- Tao, X.; Lee, A.; Limapichat, W.; Dougherty, D.A.; MacKinnon, R. A gating charge transfer center in voltage sensors. Science 2010, 328, 67–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, R.; Pfeffermann, J.; Goessweiner-Mohr, N.; Glasnov, T.; Akimov, S.A.; Pohl, P. Modulation of Kv Channel Gating by Light-Controlled Membrane Thickness. Biomolecules 2025, 15, 744. https://doi.org/10.3390/biom15050744
Yadav R, Pfeffermann J, Goessweiner-Mohr N, Glasnov T, Akimov SA, Pohl P. Modulation of Kv Channel Gating by Light-Controlled Membrane Thickness. Biomolecules. 2025; 15(5):744. https://doi.org/10.3390/biom15050744
Chicago/Turabian StyleYadav, Rohit, Juergen Pfeffermann, Nikolaus Goessweiner-Mohr, Toma Glasnov, Sergey A. Akimov, and Peter Pohl. 2025. "Modulation of Kv Channel Gating by Light-Controlled Membrane Thickness" Biomolecules 15, no. 5: 744. https://doi.org/10.3390/biom15050744
APA StyleYadav, R., Pfeffermann, J., Goessweiner-Mohr, N., Glasnov, T., Akimov, S. A., & Pohl, P. (2025). Modulation of Kv Channel Gating by Light-Controlled Membrane Thickness. Biomolecules, 15(5), 744. https://doi.org/10.3390/biom15050744







