Cathepsin B Levels Correlate with the Severity of Canine Myositis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Cases
2.2. Histological and Immunohistochemical Examination
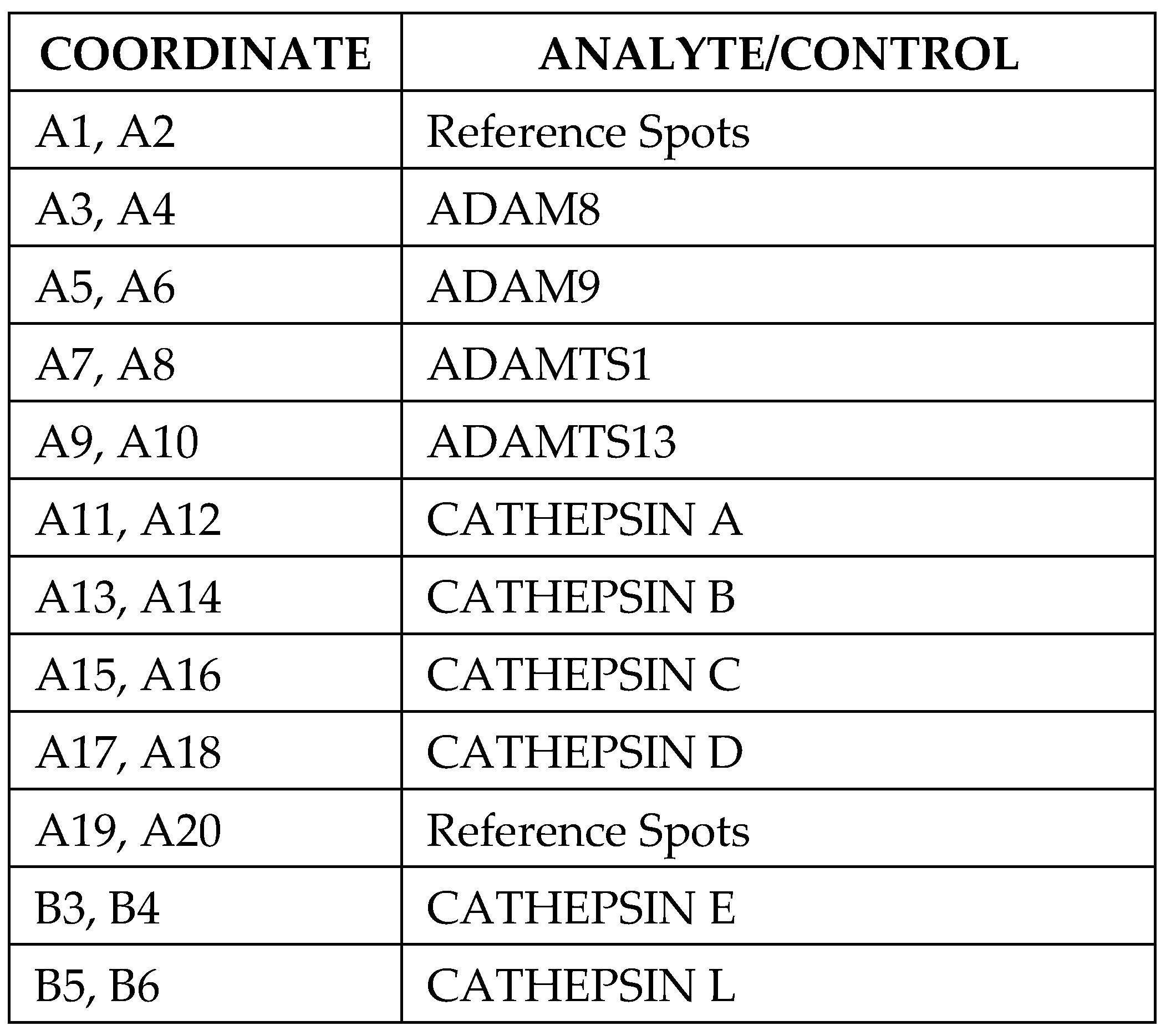
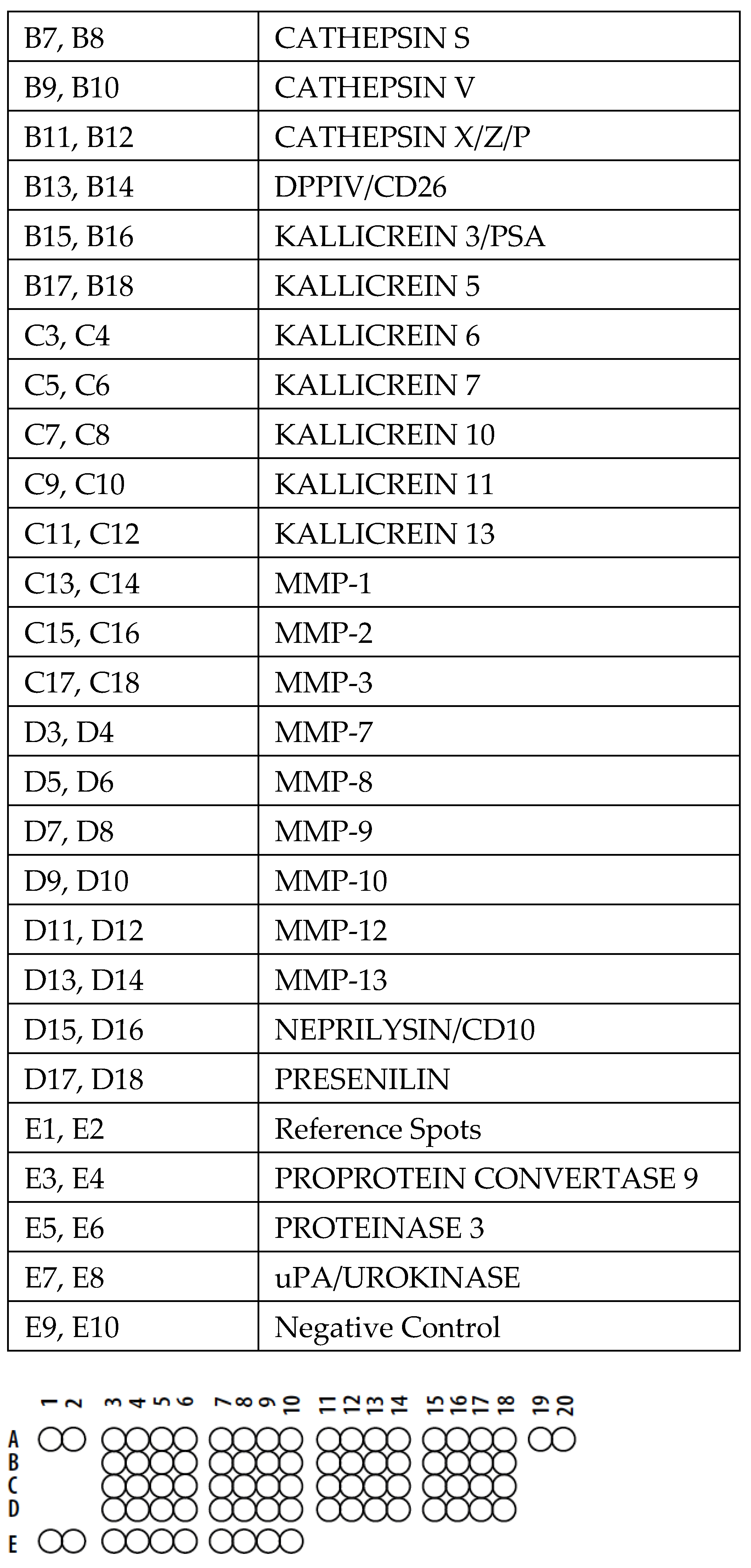
2.3. Proteome Profiler Protease Array
2.4. Western Blotting
2.5. Statistical Analysis
3. Results
3.1. Histopathological Evaluation of IM Severity in Selected Dogs Compared to a Healthy Sample
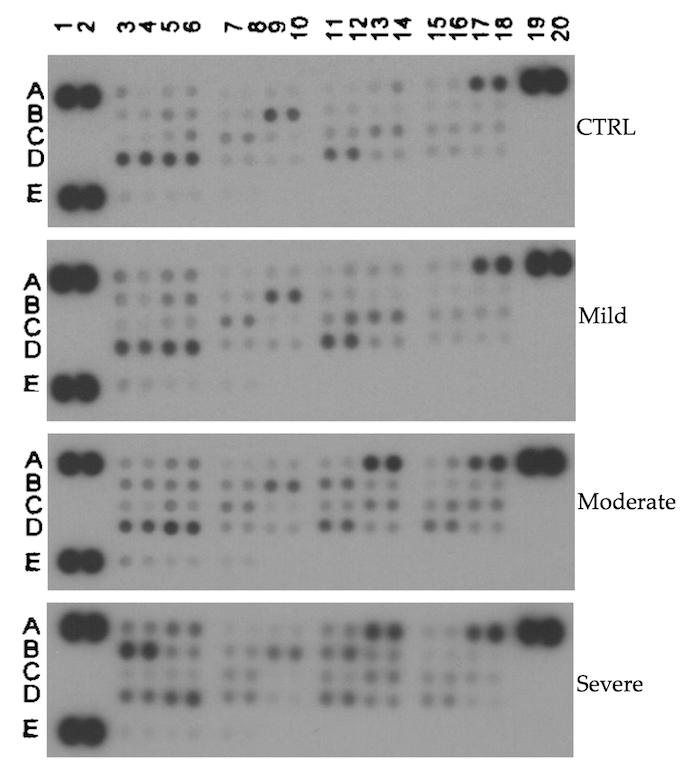
3.2. Proteome Profile Array
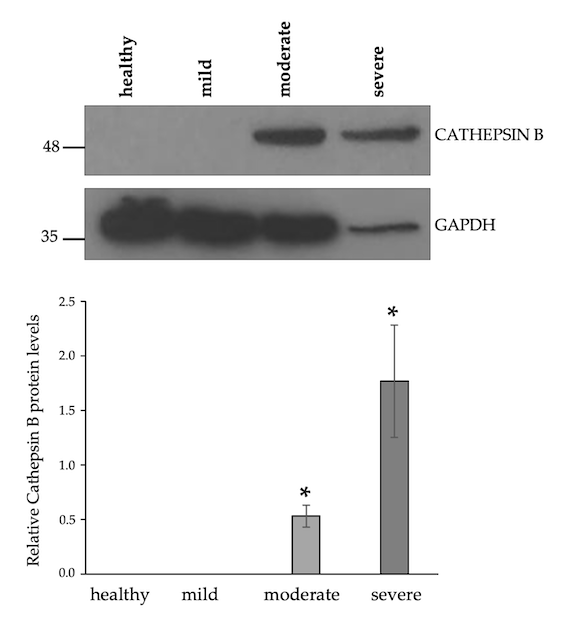
3.3. Upregulation of Cathepsin B Protein Levels in Moderate and Severe IM Muscle Samples
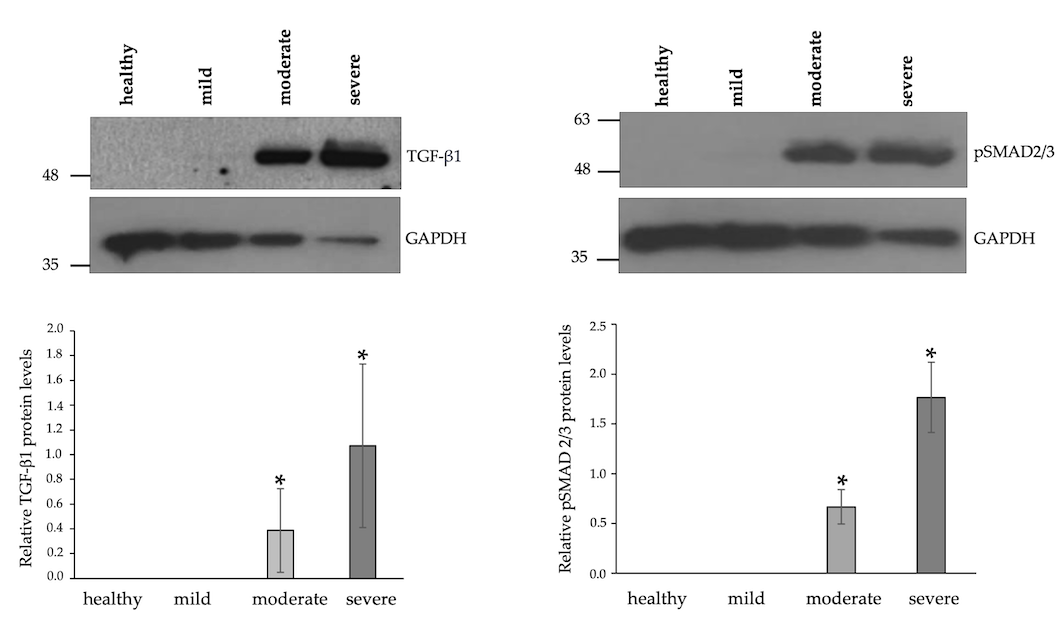
3.4. Increased Expression Levels of TGFβ-1 and Enhanced Phosphorylation of SMAD2/3 in IM-Affected Dogs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| IMs | Inflammatory myopathies |
| GADPH | Glyceraldehyde-3-phosphate dehydrogenase |
| HRP | Horseradish peroxidase |
| TGFβ-1 | Transforming growth factor beta 1 |
References
- Rawlings, D.N.; Salvesen, G. Handbook of Proteolytic Enzymes, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Turk, V.; Stoka, V.; Vasiljeva, O.; Renko, M.; Sun, T.; Turk, B.; Turk, D. Cysteine cathepsins: From structure, function and regulation to new frontiers. Biochim. Biophys. Acta 2012, 1824, 68–88. [Google Scholar] [CrossRef] [PubMed]
- Stoka, V.; Turk, V.; Turk, B. Lysosomal cysteine cathepsins: Signaling pathways in apoptosis. Biol. Chem. 2007, 388, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine cathepsins and their extracellular roles: Shaping the microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef]
- Yadati, T.; Houben, T.; Bitorina, A.; Shiri-Sverdlov, R. The ins and outs of cathepsins: Physiological function and role in disease management. Cells 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized roles for cysteine cathepsins in health and disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef]
- Patel, S.; Homaei, A.; El-Seedi, H.R.; Akhtar, N. Cathepsins: Proteases that are vital for survival but can also be fatal. Biomed. Pharmacother. 2018, 105, 526–532. [Google Scholar] [CrossRef]
- Cocchiaro, P.; De Pasquale, V.; Della Morte, R.; Tafuri, S.; Avallone, L.; Pizard, A.; Moles, A.; Pavone, L.M. The multifaceted role of the lysosomal protease cathepsins in kidney disease. Front. Cell Dev. Biol. 2017, 5, 114. [Google Scholar] [CrossRef]
- De Pasquale, V.; Moles, A.; Pavone, L.M. Cathepsins in the pathophysiology of mucopolysaccharidoses: New perspectives for therapy. Cells 2020, 9, 979. [Google Scholar] [CrossRef]
- Scarcella, M.; d’Angelo, D.; Ciampa, M.; Tafuri, S.; Avallone, L.; Pavone, L.M.; De Pasquale, V. The key role of lysosomal protease cathepsins in viral infections. Int. J. Mol. Sci. 2022, 23, 9089. [Google Scholar] [CrossRef]
- Stoka, V.; Vasiljeva, O.; Nakanishi, H.; Turk, V. The role of cysteine protease cathepsins B, H, C, and X/Z in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2023, 24, 15613. [Google Scholar] [CrossRef]
- Senjor, E.; Kos, J.; Nanut, M.P. Cysteine cathepsins as therapeutic targets in immune regulation and immune disorders. Biomedicines 2023, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Sun, Y.; Zhong, S.; Luo, J.L. The multifaceted roles of cathepsins in immune and inflammatory responses: Implications for cancer therapy, autoimmune diseases, and infectious diseases. Biomark. Res. 2024, 31, 12. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Levesque, D.; Shelton, G.D. Canine inflammatory myopathies: A clinicopathologic review of 200 cases. J. Vet. Intern. Med. 2004, 18, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J. Current classification and management of inflammatory myopathies. J. Neuromuscul. Dis. 2018, 5, 109–129. [Google Scholar] [CrossRef]
- Pavone, L.M.; Rea, S.; Trapani, F.; De Pasquale, V.; Tafuri, S.; Papparella, S.; Paciello, O. Role of serotonergic system in the pathogenesis of fibrosis in canine idiopathic inflammatory myopathies. Neuromuscul. Disord. 2012, 22, 549–557. [Google Scholar] [CrossRef]
- Paciello, O.; Shelton, G.D.; Papparella, S. Expression of major histocompatibility complex class I and class II antigens in canine masticatory muscle myositis. Neuromuscul. Disord. 2007, 17, 313–320. [Google Scholar] [CrossRef]
- Afzali, A.M.; Ruck, T.; Wiendl, H.; Meuth, S.G. Animal models in idiopathic inflammatory myopathies: How to overcome a translational roadblock? Autoimmun. Rev. 2017, 16, 478–494. [Google Scholar] [CrossRef]
- Prisco, F.; Papparella, S.; Paciello, O. The correlation between cardiac and skeletal muscle pathology in animal models of idiopathic inflammatory myopathies. Acta Myol. 2020, 3, 313–319. [Google Scholar] [CrossRef]
- Reisenauer, A.; Eickelberg, O.; Wille, A.; Heimburg, A.; Reinhold, A.; Sloane, B.F.; Welte, T.; Bühling, F. Increased carcinogenic potential of myeloid tumor cells induced by aberrant TGF-beta1-signaling and upregulation of cathepsin B. Biol. Chem. 2007, 388, 639–650. [Google Scholar] [CrossRef]
- Paciello, O.; Papparella, S. Histochemical and immunohistological approach to comparative neuromuscular diseases. Folia Histochem. Cytobiol. 2009, 47, 143–152. [Google Scholar] [CrossRef]
- Pasolini, M.P.; Pagano, T.B.; Costagliola, A.; Biase, D.; Lamagna, B.; Auletta, L.; Fatone, G.; Greco, M.; Coluccia, P.; Vincenzo, V.; et al. Inflammatory myopathy in horses with chronic piroplasmosis. Vet. Pathol. 2018, 55, 133–143. [Google Scholar] [CrossRef] [PubMed]
- De Biase, D.; Prisco, F.; Piegari, G.; Ilsami, A.; d’Aquino, I.; Baldassarre, V.; Zito Marino, F.; Franco, R.; Papparella, S.; Paciello, O. RNAScope in situ hybridization as a novel technique for the assessment of c-KIT mRNA expression in canine mast cell tumor. Front. Vet. Sci. 2021, 8, 591961. [Google Scholar] [CrossRef]
- De Pasquale, V.; Pezone, A.; Sarogni, P.; Tramontano, A.; Schiattarella, G.G.; Avvedimento, V.E.; Paladino, S.; Pavone, L.M. EGFR activation triggers cellular hypertrophy and lysosomal disease in NAGLU-depleted cardiomyoblasts, mimicking the hallmarks of mucopolysaccharidosis IIIB. Cell Death Dis. 2018, 9, 40. [Google Scholar] [CrossRef]
- Doppler, K.; Mittelbronn, M.; Lindner, A.; Bornemann, A. Basement membrane remodelling and segmental fibrosis in sporadic inclusion body myositis. Neuromuscul. Disord. 2009, 19, 406–411. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef]
- Ceribelli, A.; Tonutti, A.; Isailovic, N.; De Santis, M.; Selmi, C. Interstitial lung disease associated with inflammatory myositis: Autoantibodies, clinical phenotypes, and progressive fibrosis. Front. Med. 2023, 10, 1068402. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, M.; Yang, X.; Zhou, X.; Zhang, S. The role of cathepsin B in pathophysiologies of non-tumor and tumor tissues: A systematic review. J. Cancer 2023, 14, 2344–2358. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Yu, X.; Huang, Q.; Fang, W.; Li, J.; Bonventre, J.V.; Sukhova, G.K.; Libby, P.; Shi, G.P. Differential roles of cysteinyl cathepsins in TGF-β signaling and tissue fibrosis. iScience 2019, 19, 607–622. [Google Scholar] [CrossRef]
- Chen, W.J.; Lin, I.H.; Lee, C.W.; Chen, Y.F. Aged skeletal muscle retains the ability to remodel extracellular matrix for degradation of collagen deposition after muscle injury. Int. J. Mol. Sci. 2021, 22, 2123. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Kumamoto, T.; Ueyama, H.; Sugihara, R.; Kominami, E.; Goll, D.E.; Tsuda, T. Calpain and cathepsins in the skeletal muscle of inflammatory myopathies. Eur. Neurol. 1997, 37, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; de Andrés, I.; Illa, I. Cathepsins are upregulated by IFN-gamma/STAT1 in human muscle culture: A possible active factor in dermatomyositis. J. Neuropathol. Exp. Neurol. 2001, 60, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Nogalska, A.; D’Agostino, C.; Terracciano, C.; Engel, W.K.; Askanas, V. Impaired autophagy in sporadic inclusion-body myositis and in endoplasmic reticulum stress-provoked cultured human muscle fibers. Am. J. Pathol. 2010, 177, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ni, L.; Wang, Q. Administration of cathepsin B inhibitor CA-074Me reduces inflammation and apoptosis in polymyositis. J. Dermatol. Sci. 2013, 72, 158–167. [Google Scholar] [CrossRef]
- Shi, J.; Tang, M.; Zhou, S.; Xu, D.; Zhao, J.; Wu, C.; Wang, Q.; Tian, X.; Li, M.; Zeng, X. Programmed cell death pathways in the pathogenesis of idiopathic inflammatory myopathies. Front. Immunol. 2021, 12, 783616. [Google Scholar] [CrossRef]
- Ha, S.D.; Martins, A.; Khazaie, K.; Han, J.; Chan, B.M.; Kim, S.O. Cathepsin B is involved in the trafficking of TNF-alpha-containing vesicles to the plasma membrane in macrophages. J. Immunol. 2008, 181, 690–697. [Google Scholar] [CrossRef]
- Hannaford, J.; Guo, H.; Chen, X. Involvement of cathepsins B and L in inflammation and cholesterol trafficking protein NPC2 secretion in macrophages. Obesity 2013, 21, 1586–1595. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, M.; Yan, C.; Kong, W.; Lan, F.; Narengaowa; Zhao, S.; Yang, Q.; Bai, Z.; Qing, H.; et al. Cathepsin B in programmed cell death machinery: Mechanisms of execution and regulatory pathways. Cell Death Dis. 2023, 14, 255. [Google Scholar] [CrossRef]
- Nakanishi, H. Microglial cathepsin B as a key driver of inflammatory brain diseases and brain aging. Neural Regen. Res. 2020, 15, 25–29. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, L.; Shen, J.; Wang, Y.; Fu, Z.; Su, S.A.; Cai, Z.; Wang, J.A.; Xiang, M. Cathepsin B aggravates coxsackievirus B3-induced myocarditis through activating the inflammasome and promoting pyroptosis. PLoS Pathog. 2018, 14, e1006872. [Google Scholar] [CrossRef]
- Fang, W.; Deng, Z.; Benadjaoud, F.; Yang, C.; Shi, G.P. Cathepsin B deficiency ameliorates liver lipid deposition, inflammatory cell infiltration, and fibrosis after diet-induced nonalcoholic steatohepatitis. Transl. Res. 2020, 222, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Pišlar, A.; Mitrović, A.; Sabotič, J.; Pečar Fonović, U.; Perišić Nanut, M.; Jakoš, T.; Senjor, E.; Kos, J. The role of cysteine peptidases in coronavirus cell entry and replication: The therapeutic potential of cathepsin inhibitors. PLoS Pathog. 2020, 16, e1009013. [Google Scholar] [CrossRef] [PubMed]
- Canbay, A.; Guicciardi, M.E.; Higuchi, H.; Feldstein, A.; Bronk, S.F.; Rydzewski, R.; Taniai, M.; Gores, G.J. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J. Clin. Investig. 2003, 112, 152–159. [Google Scholar] [CrossRef]
- Halangk, W.; Lerch, M.M.; Brandt-Nedelev, B.; Roth, W.; Ruthenbuerger, M.; Reinheckel, T.; Domschke, W.; Lippert, H.; Peters, C.; Deussing, J. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J. Clin. Investig. 2000, 106, 773–781. [Google Scholar] [CrossRef]
- Hook, G.; Reinheckel, T.; Ni, J.; Wu, Z.; Kindy, M.; Peters, C.; Hook, V. Cathepsin B gene knockout improves behavioral deficits and reduces pathology in models of neurologic disorders. Pharmacol. Rev. 2022, 74, 600–629. [Google Scholar] [CrossRef]
- Orlowski, G.M.; Sharma, S.; Colbert, J.D.; Bogyo, M.; Robertson, S.A.; Kataoka, H.; Chan, F.K.; Rock, K.L. Frontline Science: Multiple cathepsins promote inflammasome-independent, particle-induced cell death during NLRP3-dependent IL-1β activation. J. Leukoc. Biol. 2017, 102, 7–17. [Google Scholar] [CrossRef]
- Allan, E.R.; Yates, R.M. Redundancy between cysteine cathepsins in murine experimental autoimmune encephalomyelitis. PLoS ONE 2015, 10, e0128945. [Google Scholar] [CrossRef]
- Pečar Fonović, U.; Kos, J.; Mitrović, A. Compensational role between cathepsins. Biochimie 2024, 226, 62–76. [Google Scholar] [CrossRef]
- de Mingo, Á.; de Gregorio, E.; Moles, A.; Tarrats, N.; Tutusaus, A.; Colell, A.; Fernandez-Checa, J.C.; Morales, A.; Marí, M. Cysteine cathepsins control hepatic NF-κB-dependent inflammation via sirtuin-1 regulation. Cell Death Dis. 2016, 7, e2464. [Google Scholar] [CrossRef]
- Wiendl, H.; Lautwein, A.; Mitsdörffer, M.; Krause, S.; Erfurth, S.; Wienhold, W.; Morgalla, M.; Weber, E.; Overkleeft, H.S.; Lochmüller, H.; et al. Antigen processing and presentation in human muscle: Cathepsin S is critical for MHC class II expression and upregulated in inflammatory myopathies. J. Neuroimmunol. 2003, 138, 132–143. [Google Scholar] [CrossRef]
- Yoon, M.C.; Hook, V.; O’Donoghue, A.J. Cathepsin B dipeptidyl carboxypeptidase and endopeptidase activities demonstrated across a broad pH range. Biochemistry 2022, 61, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Kawakibi, T.; Bala, N.; Liu, L.P.; Searcy, L.A.; Denslow, N.D.; Alli, A.A. Decreased MARCKS protein expression in kidney cortex membrane fractions of cathepsin b knockout mice is associated with reduced lysophosphatidylcholine and protein kinase C activity. Biomedicines 2023, 11, 1489. [Google Scholar] [CrossRef] [PubMed]
- Guicciardi, M.E.; Miyoshi, H.; Bronk, S.F.; Gores, G.J. Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: Implications for therapeutic applications. Am. J. Pathol. 2001, 159, 2045–2054. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Turk, D.; Turk, B. The future of cysteine cathepsins in disease management. Trends Pharmacol. Sci. 2017, 38, 873–898. [Google Scholar] [CrossRef]
- Yoon, M.C.; Christy, M.P.; Phan, V.V.; Gerwick, W.H.; Hook, G.; O’Donoghue, A.J.; Hook, V. Molecular features of CA-074 pH-dependent inhibition of cathepsin B. Biochemistry 2022, 61, 228–238. [Google Scholar] [CrossRef]
- Hook, V.; Hook, G.; Kindy, M. Pharmacogenetic features of cathepsin B inhibitors that improve memory deficit and reduce beta-amyloid related to Alzheimer’s disease. Biol. Chem. 2010, 391, 861–872. [Google Scholar] [CrossRef]
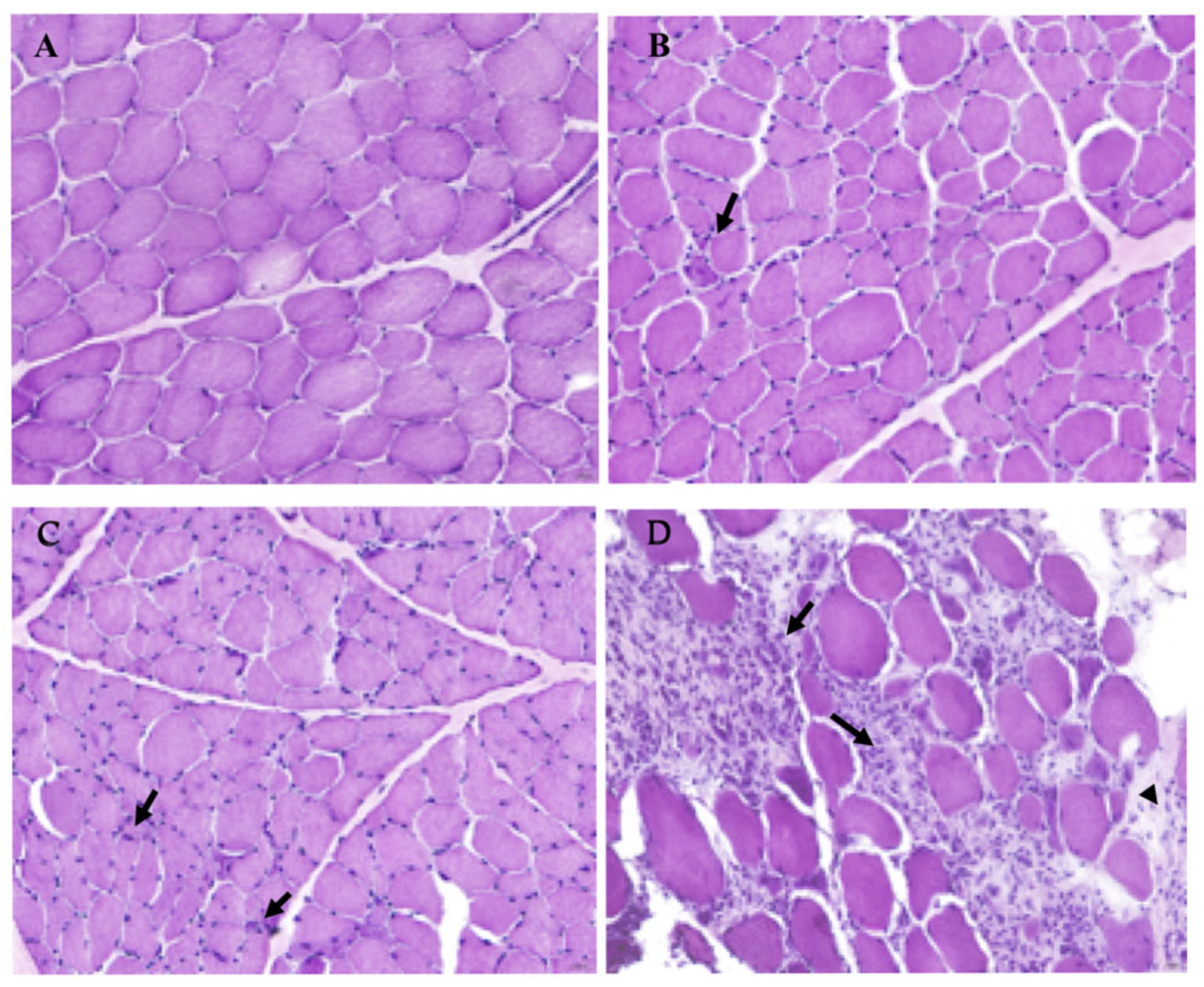
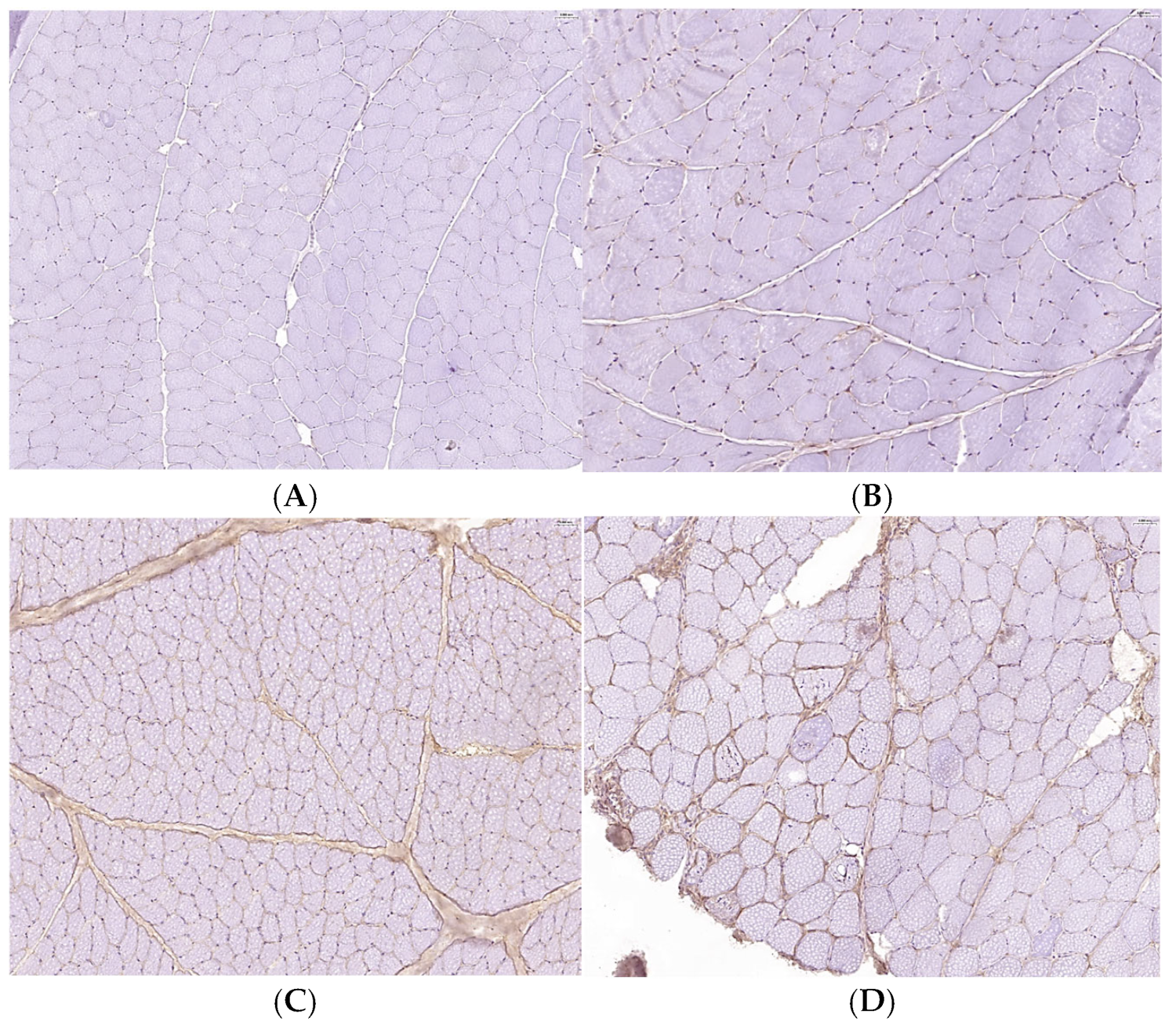

| Case n. | Sex | Age | Diagnosis |
|---|---|---|---|
| 12 | M | 5 years | Mild chronic lymphoplasmacytic myositis and fibrosis |
| 28 | F | 6 months | Mild lymphoplasmacytic myositis |
| 31 | F | 1 year and 2 months | Mild lymphoplasmacytic myositis |
| 21 | F | 5 years | Moderate and multifocal chronic lymphoplasmacytic myositis |
| 24 | M | 3 years | Moderate lymphocytic myositis |
| 32 | F | 5 years | Moderate chronic myositis with fibrosis and severe neurogenic myopathy |
| 7 | M | 8 years | Severe and diffuse chronic lymphoplasmacytic myositis |
| 27 | F | 3 years | Severe lymphoplasmacytic myositis |
| CTRL | M | 6 years | No disorders (healthy) |
| Analyte | Fold Change Mild/Healthy | t-Test |
|---|---|---|
| CATHEPSIN A | 2.23 | 0.014 |
| KALLIKREIN 13 | 1.96 | 0.042 |
| MMP-10 | 1.74 | 0.006 |
| KALLIKREIN 6 | 1.69 | 0.006 |
| PROPROTEIN CONVERTASE 9 | 1.70 | 0.027 |
| ADAMTS13 | 1.65 | 0.036 |
| CATHEPSIN X/Z/P | 1.62 | 0.021 |
| DPPIV/CD26 | 1.61 | 0.041 |
| uPA/UROKINASE | 1.58 | 0.013 |
| PROTEINASE 3 | 1.54 | 0.015 |
| MMP-9 | 1.50 | 0.048 |
| ADAM9 | 1.48 | 0.049 |
| Analyte | Fold Change Moderate/Healthy | t-Test |
|---|---|---|
| CATHEPSIN B | 5.00 | 0.00001 |
| CATHEPSIN X/Z/P | 3.41 | 0.0001 |
| NEPRILYSIN/CD10 | 3.00 | 0.003 |
| DPPIV/CD26 | 2.82 | 0.001 |
| MMP-3 | 2.27 | 0.011 |
| KALLIKREIN 5 | 2.20 | 0.023 |
| CATHEPSIN A | 2.16 | 0.002 |
| PRESENILIN | 2.13 | 0.015 |
| CATHEPSIN S | 2.11 | 0.005 |
| CATHEPSIN E | 1.95 | 0.019 |
| MMP-9 | 1.70 | 0.016 |
| KALLIKREIN 6 | 1.68 | 0.011 |
| ADAMTS1 | 1.61 | 0.049 |
| MMP-10 | 1.60 | 0.018 |
| ADAMTS13 | 1.58 | 0.054 |
| PROPROTEIN CONVERTASE 9 | 1.56 | 0.072 |
| ADAM9 | 1.53 | 0.035 |
| uPA/UROKINASE | 1.53 | 0.009 |
| PROTEINASE 3 | 1.37 | 0.032 |
| Analyte | Fold Change Severe/Healthy | t-Test |
|---|---|---|
| CATHEPSIN E | 5.71 | 0.00054 |
| CATHEPSIN B | 4.34 | 0.00009 |
| CATHEPSIN X/Z/P | 4.23 | 0.001 |
| DPPIV/CD26 | 3.85 | 0.004 |
| CATHEPSIN A | 3.79 | 0.004 |
| MMP-9 | 2.60 | 0.001 |
| NEPRILYSIN/CD10 | 2.32 | 0.006 |
| ADAM9 | 2.30 | 0.013 |
| CATHEPSIN S | 2.18 | 0.006 |
| MMP-13 | 1.94 | 0.043 |
| KALLIKREIN 6 | 1.87 | 0.010 |
| ADAMTS1 | 1.65 | 0.017 |
| MMP-10 | 1.47 | 0.055 |
| uPA/UROKINASE | 1.43 | 0.047 |
| Analyte | Fold Change Moderate/Severe vs. Healthy/Mild | t-Test |
|---|---|---|
| CATHEPSIN B | 4.38 | 4.5 × 10−10 |
| CATHEPSIN E | 2.93 | 0.007 |
| CATHEPSIN X/Z/P | 2.91 | 0.000003 |
| DPPIV/CD26 | 2.55 | 0.0001 |
| NEPRILYSIN/CD10 | 2.46 | 0.00002 |
| CATHEPSIN A | 1.84 | 0.017 |
| CATHEPSIN S | 1.73 | 0.0002 |
| MMP-9 | 1.72 | 0.002 |
| MMP-3 | 1.57 | 0.012 |
| ADAM9 | 1.54 | 0.024 |
| KALLIKREIN 5 | 1.54 | 0.017 |
| PRESENILIN | 1.53 | 0.0087 |
| MMP-2 | 1.53 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Pasquale, V.; Vaccaro, E.; Rossin, F.; Ciampa, M.; Scarcella, M.; Paciello, O.; Tafuri, S. Cathepsin B Levels Correlate with the Severity of Canine Myositis. Biomolecules 2025, 15, 743. https://doi.org/10.3390/biom15050743
De Pasquale V, Vaccaro E, Rossin F, Ciampa M, Scarcella M, Paciello O, Tafuri S. Cathepsin B Levels Correlate with the Severity of Canine Myositis. Biomolecules. 2025; 15(5):743. https://doi.org/10.3390/biom15050743
Chicago/Turabian StyleDe Pasquale, Valeria, Emanuela Vaccaro, Federica Rossin, Mariangela Ciampa, Melania Scarcella, Orlando Paciello, and Simona Tafuri. 2025. "Cathepsin B Levels Correlate with the Severity of Canine Myositis" Biomolecules 15, no. 5: 743. https://doi.org/10.3390/biom15050743
APA StyleDe Pasquale, V., Vaccaro, E., Rossin, F., Ciampa, M., Scarcella, M., Paciello, O., & Tafuri, S. (2025). Cathepsin B Levels Correlate with the Severity of Canine Myositis. Biomolecules, 15(5), 743. https://doi.org/10.3390/biom15050743











