The Novel Imiqualine EAPB02303 Is a Potent Drug for Treating Acute Myeloid Leukemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Primary Cells
2.2. Drugs
2.3. Cell Cycle Analysis
2.4. Annexin V/PI Assay
2.5. Immunoblotting
2.6. Xenograft Animal Studies
2.7. Statistical Analysis
3. Results
3.1. EAPB02303 Abrogates Cell Proliferation of AML Cells with No Cytotoxicity on Human Peripheral Blood Mononuclear Cells
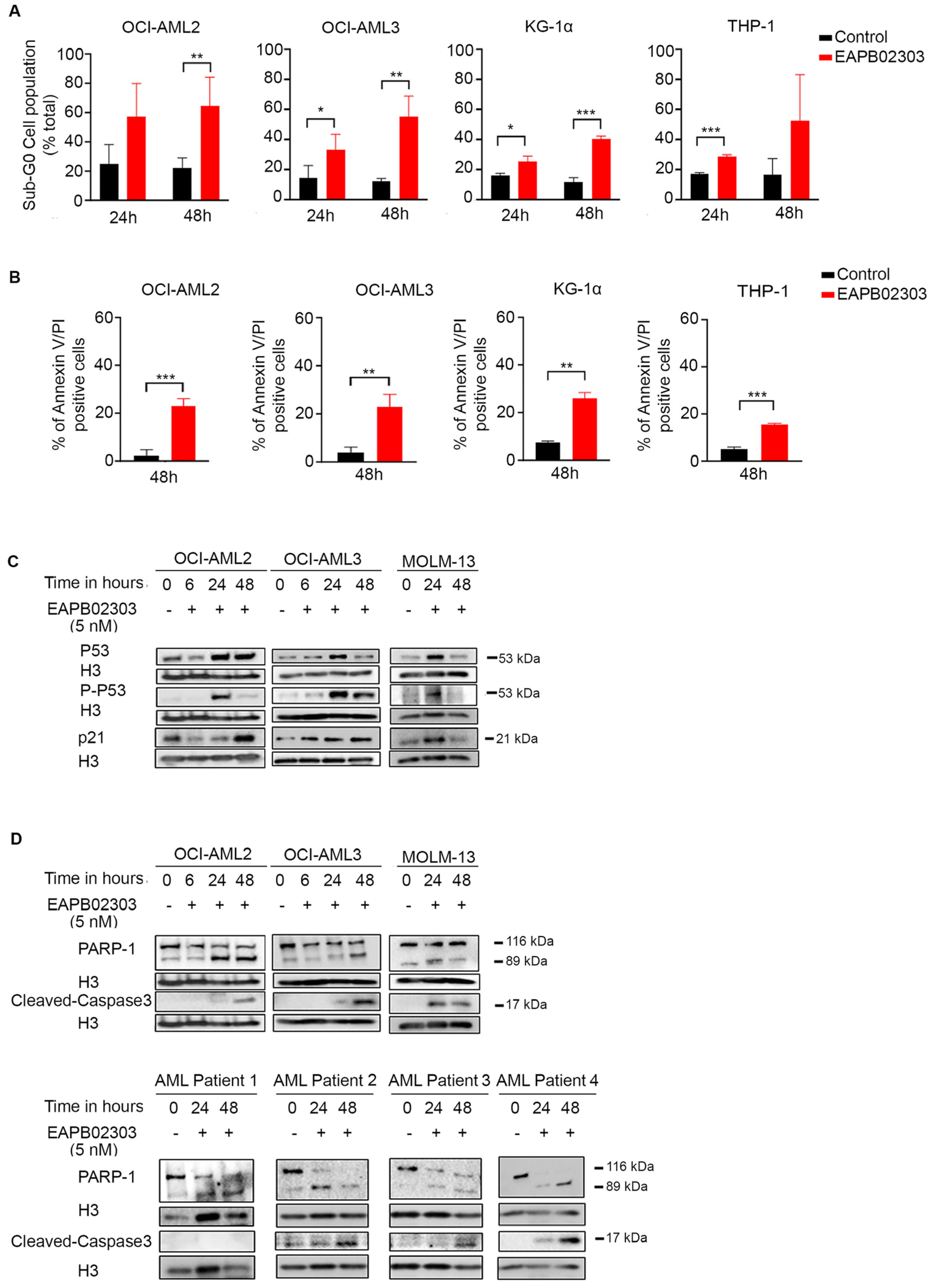
3.2. EAPB02303 Induces Cell Cycle Arrest and Cell Death in AML Cells
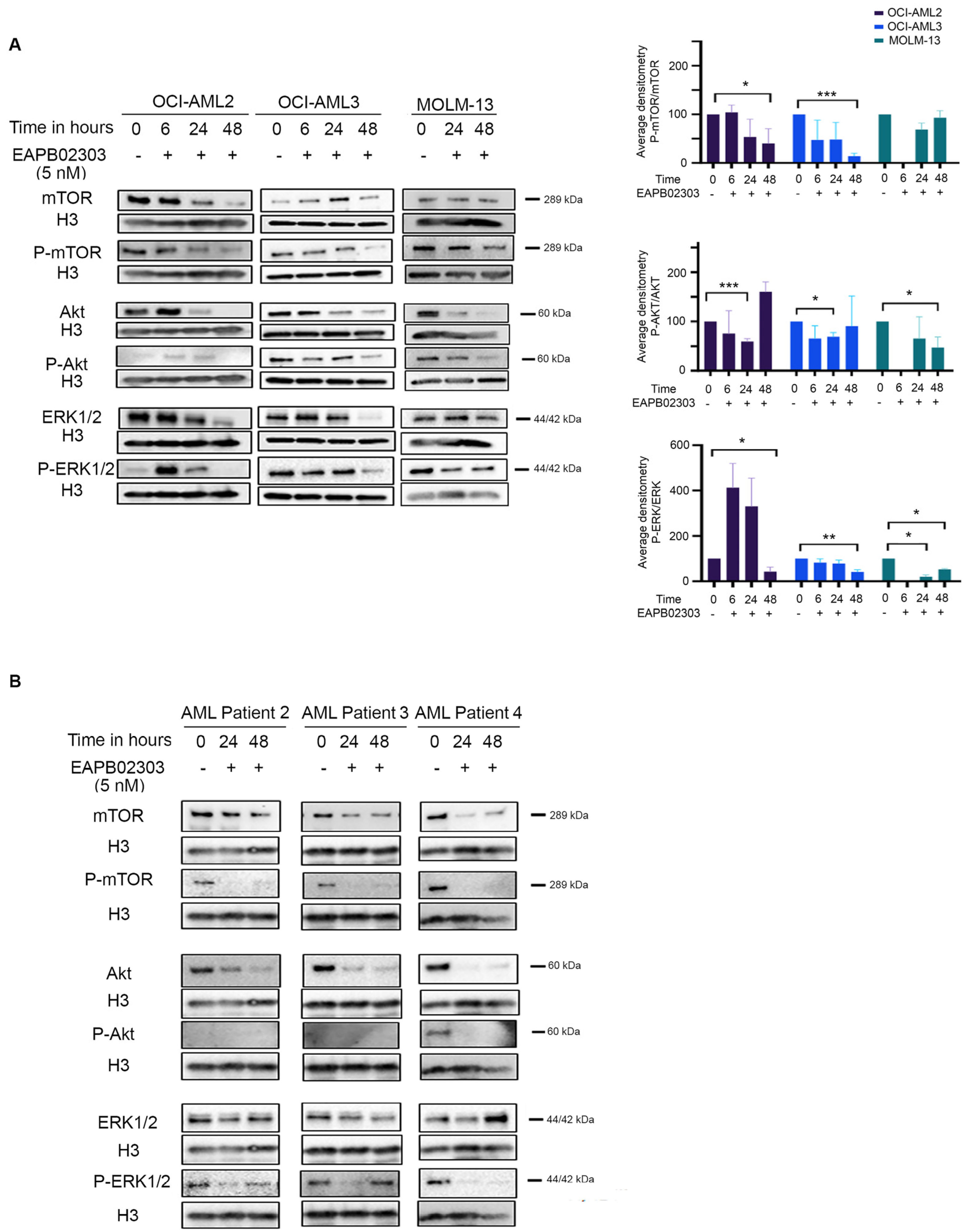
3.3. The Mechanism of Action of EAPB02303 Involves the PI3K/AKT/mTOR Signaling
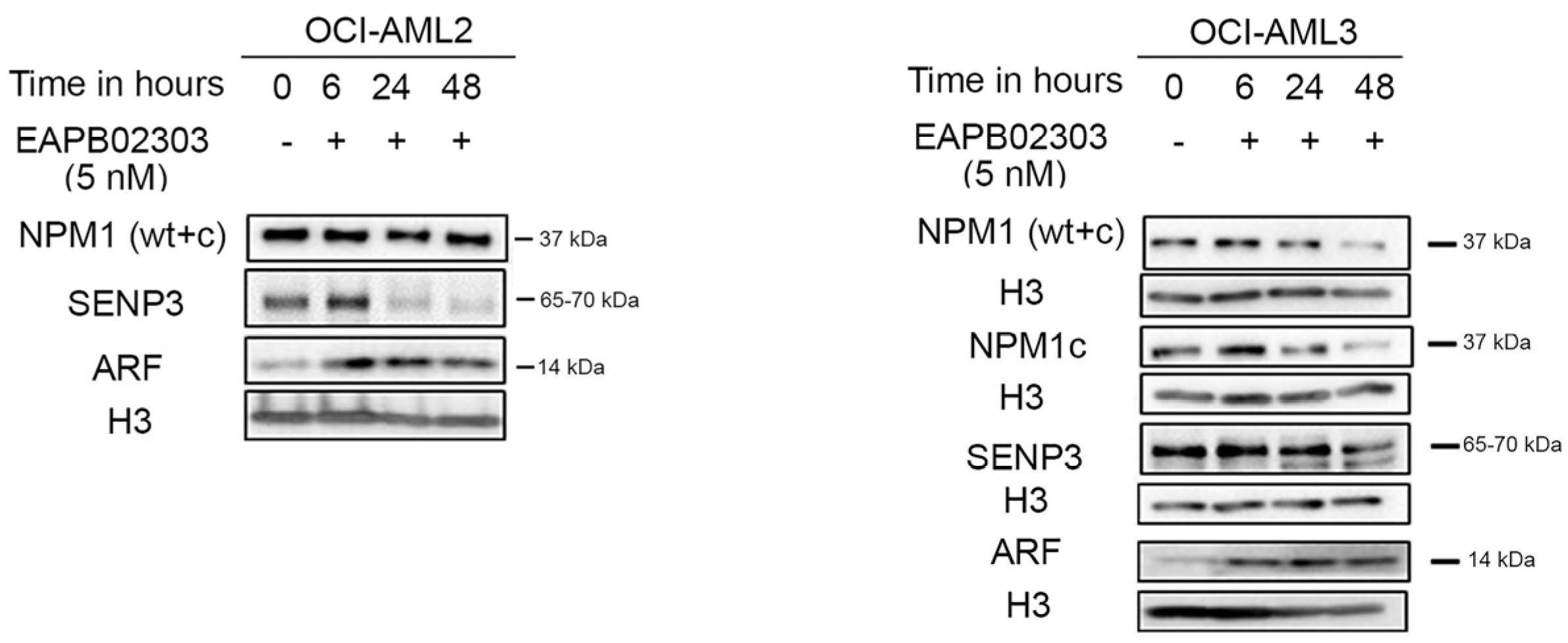
3.4. EAPB02303 Induces NPM1c Degradation, Attenuates SENP3 Expression, and Enhances ARF Levels in NPM1c OCI-AML3
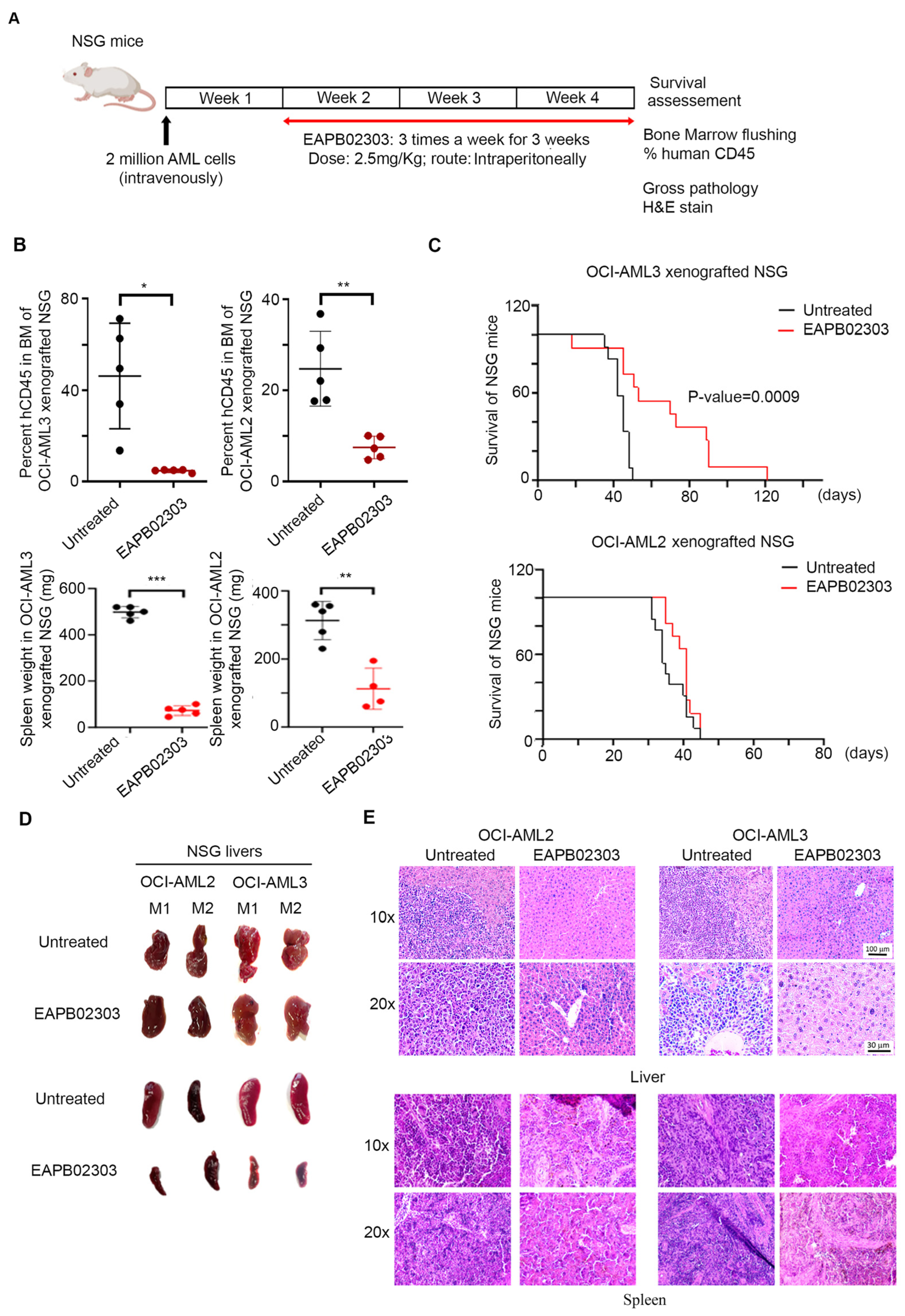
3.5. EAPB02303 Exhibits Potent In Vivo Activity Against AML
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- Vakiti, A.; Reynolds, S.B.; Mewawalla, P. Acute Myeloid Leukemia. In StatPearls; StatPearls Publishing Copyright © 2024; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- SEER Data Base: Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 11 July 2024).
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Rai, K.R.; Holland, J.F.; Glidewell, O.J.; Weinberg, V.; Brunner, K.; Obrecht, J.P.; Preisler, H.D.; Nawabi, I.W.; Prager, D.; Carey, R.W.; et al. Treatment of acute myelocytic leukemia: A study by cancer and leukemia group B. Blood 1981, 58, 1203–1212. [Google Scholar] [CrossRef]
- Kantarjian, H.; Kadia, T.; DiNardo, C.; Daver, N.; Borthakur, G.; Jabbour, E.; Garcia-Manero, G.; Konopleva, M.; Ravandi, F. Acute myeloid leukemia: Current progress and future directions. Blood Cancer J. 2021, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Orduña, G.R.; Ledesma-Martínez, E.; Aguiñiga-Sánchez, I.; Mora-García, M.L.; Weiss-Steider, B.; Santiago-Osorio, E. Inhibitors of Chemoresistance Pathways in Combination with Ara-C to Overcome Multidrug Resistance in AML. A Mini Review. Int. J. Mol. Sci. 2021, 22, 4955. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Pratz, K.W.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Döhner, H.; Récher, C.; Fiedler, W.; Yamamoto, K.; Wang, J.; et al. Long-term follow-up of VIALE-A: Venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 2024, 99, 615–624. [Google Scholar] [CrossRef]
- Wei, A.H.; Loo, S.; Daver, N.G. How I Treat patients with AML using azacitidine and venetoclax. Blood 2024, 145, 1237–1250. [Google Scholar] [CrossRef]
- Othman, J.; Lam, H.P.J.; Leong, S.; Basheer, F.; Abdallah, I.; Fleming, K.; Mehta, P.; Yassin, H.; Laurie, J.; Austin, M.; et al. Real-world outcomes of newly diagnosed AML treated with venetoclax and azacitidine or low-dose cytarabine in the UK NHS. Blood Neoplasia 2024, 1, 100017. [Google Scholar] [CrossRef]
- Kayser, S.; Levis, M.J. The clinical impact of the molecular landscape of acute myeloid leukemia. Haematologica 2023, 108, 308–320. [Google Scholar] [CrossRef]
- Xu, Q.; Simpson, S.-E.; Scialla, T.J.; Bagg, A.; Carroll, M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood 2003, 102, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, I.; Hatfield, K.J.; Grønningsæter, I.S.; Aasebø, E.; Hernandez-Valladares, M.; Hagen, K.M.; Rye, K.P.; Berven, F.S.; Selheim, F.; Reikvam, H.; et al. Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct. Target. Ther. 2019, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, I.; Hatfield, K.J.; Tvedt, T.H.A.; Reikvam, H.; Bruserud, Ø. Clonal Heterogeneity Reflected by PI3K-AKT-mTOR Signaling in Human Acute Myeloid Leukemia Cells and Its Association with Adverse Prognosis. Cancers 2018, 10, 332. [Google Scholar] [CrossRef]
- Kornblau, S.M.; Tibes, R.; Qiu, Y.H.; Chen, W.; Kantarjian, H.M.; Andreeff, M.; Coombes, K.R.; Mills, G.B. Functional proteomic profiling of AML predicts response and survival. Blood 2009, 113, 154–164. [Google Scholar] [CrossRef]
- Min, Y.H.; Eom, J.I.; Cheong, J.W.; Maeng, H.O.; Kim, J.Y.; Jeung, H.K.; Lee, S.T.; Lee, M.H.; Hahn, J.S.; Ko, Y.W. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: Its significance as a prognostic variable. Leukemia 2003, 17, 995–997. [Google Scholar] [CrossRef]
- Chen, W.; Drakos, E.; Grammatikakis, I.; Schlette, E.J.; Li, J.; Leventaki, V.; Staikou-Drakopoulou, E.; Patsouris, E.; Panayiotidis, P.; Medeiros, L.J.; et al. mTOR signaling is activated by FLT3 kinase and promotes survival of FLT3- mutated acute myeloid leukemia cells. Mol. Cancer 2010, 9, 292. [Google Scholar] [CrossRef]
- Wei, A.H.; Sadawarte, S.; Catalano, J.; Hills, R.; Avery, S.; Patil, S.S.; Burnett, A.; Spencer, A. A Phase Ib Study Combining the mTOR Inhibitor Everolimus (RAD001) with Low-Dose Cytarabine in Untreated Elderly AML. Blood 2010, 116, 3299. [Google Scholar] [CrossRef]
- Tan, P.; Tiong, I.S.; Fleming, S.; Pomilio, G.; Cummings, N.; Droogleever, M.; McManus, J.; Schwarer, A.; Catalano, J.; Patil, S.; et al. The mTOR inhibitor everolimus in combination with azacitidine in patients with relapsed/refractory acute myeloid leukemia: A phase Ib/II study. Oncotarget 2017, 8, 52269–52280. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, E.; Camilla, V.; Teresa, M.M.; Kristian, R.; Kim, T.-M. Targeting of PI3K/AKT signaling and DNA damage response in acute myeloid leukemia: A novel therapeutic strategy to boost chemotherapy response and overcome resistance. Cancer Drug Resist. 2021, 4, 984–995. [Google Scholar]
- Tseng, C.-Y.; Fu, Y.-H.; Ou, D.-L.; Lu, J.-W.; Hou, H.-A.; Lin, L.-I. Anti-leukemia effects of omipalisib in acute myeloid leukemia: Inhibition of PI3K/AKT/mTOR signaling and suppression of mitochondrial biogenesis. Cancer Gene Ther. 2023, 30, 1691–1701. [Google Scholar] [CrossRef]
- Darici, S.; Alkhaldi, H.; Horne, G.; Jørgensen, H.G.; Marmiroli, S.; Huang, X. Targeting PI3K/Akt/mTOR in AML: Rationale and Clinical Evidence. J. Clin. Med. 2020, 9, 2934. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Bolli, N.; Liso, A.; Martelli, M.P.; Mannucci, R.; Pileri, S.; Nicoletti, I. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: Molecular basis and clinical implications. Leukemia 2009, 23, 1731–1743. [Google Scholar] [CrossRef]
- Haindl, M.; Harasim, T.; Eick, D.; Muller, S. The nucleolar SUMO-specific protease SENP3 reverses SUMO modification of nucleophosmin and is required for rRNA processing. EMBO Rep. 2008, 9, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Grant, S. ATRA and ATO team up against NPM1. Blood 2015, 125, 3369–3371. [Google Scholar] [CrossRef]
- El Hajj, H.; Dassouki, Z.; Berthier, C.; Raffoux, E.; Ades, L.; Legrand, O.; Hleihel, R.; Sahin, U.; Tawil, N.; Salameh, A.; et al. Retinoic acid and arsenic trioxide trigger degradation of mutated NPM1, resulting in apoptosis of AML cells. Blood 2015, 125, 3447–3454. [Google Scholar] [CrossRef]
- Martelli, M.P.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Pierangeli, S.; Mulas, F.; Pacini, R.; Tabarrini, A.; Pettirossi, V.; Rossi, R.; et al. Arsenic trioxide and all-trans retinoic acid target NPM1 mutant oncoprotein levels and induce apoptosis in NPM1-mutated AML cells. Blood 2015, 125, 3455–3465. [Google Scholar] [CrossRef]
- Skayneh, H.; Jishi, B.; Hleihel, R.; Hamie, M.; El Hajj, R.; Deleuze-Masquefa, C.; Bonnet, P.A.; El Sabban, M.; El Hajj, H. EAPB0503, an Imidazoquinoxaline Derivative Modulates SENP3/ARF Mediated SUMOylation, and Induces NPM1c Degradation in NPM1 Mutant AML. Int. J. Mol. Sci. 2022, 23, 3421. [Google Scholar] [CrossRef] [PubMed]
- Rudy, S.J. Imiquimod (Aldara): Modifying the immune response. Dermatol. Nurs. 2002, 14, 268–270. [Google Scholar]
- Moarbess, G.; El-Hajj, H.; Kfoury, Y.; El-Sabban, M.E.; Lepelletier, Y.; Hermine, O.; Deleuze-Masquéfa, C.; Bonnet, P.-A.; Bazarbachi, A. EAPB0203, a member of the imidazoquinoxaline family, inhibits growth and induces caspase-dependent apoptosis in T-cell lymphomas and HTLV-I–associated adult T-cell leukemia/lymphoma. Blood 2008, 111, 3770–3777. [Google Scholar] [CrossRef]
- Nabbouh, A.I.; Hleihel, R.S.; Saliba, J.L.; Karam, M.M.; Hamie, M.H.; Wu, H.J.M.; Berthier, C.P.; Tawil, N.M.; Bonnet, P.A.; Deleuze-Masquefa, C.; et al. Imidazoquinoxaline derivative EAPB0503: A promising drug targeting mutant nucleophosmin 1 in acute myeloid leukemia. Cancer 2017, 123, 1662–1673. [Google Scholar] [CrossRef]
- Saliba, J.; Deleuze-Masquéfa, C.; Iskandarani, A.; El Eit, R.; Hmadi, R.; Mahon, F.X.; Bazarbachi, A.; Bonnet, P.A.; Nasr, R. EAPB0503, a novel imidazoquinoxaline derivative, inhibits growth and induces apoptosis in chronic myeloid leukemia cells. Anticancer Drugs 2014, 25, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Patinote, C.; Bou Karroum, N.; Moarbess, G.; Deleuze-Masquefa, C.; Hadj-Kaddour, K.; Cuq, P.; Diab-Assaf, M.; Kassab, I.; Bonnet, P.-A. Imidazo[1,2-a]pyrazine, Imidazo[1,5-a]quinoxaline and Pyrazolo[1,5-a]quinoxaline derivatives as IKK1 and IKK2 inhibitors. Eur. J. Med. Chem. 2017, 138, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Patinote, C.; Deleuze-Masquéfa, C.; Kaddour, K.H.; Vincent, L.A.; Larive, R.; Zghaib, Z.; Guichou, J.F.; Assaf, M.D.; Cuq, P.; Bonnet, P.A. Imidazo[1,2-a]quinoxalines for melanoma treatment with original mechanism of action. Eur. J. Med. Chem. 2021, 212, 113031. [Google Scholar] [CrossRef] [PubMed]
- She, M.; Niu, X.; Chen, X.; Li, J.; Zhou, M.; He, Y.; Le, Y.; Guo, K. Resistance of leukemic stem-like cells in AML cell line KG1a to natural killer cell-mediated cytotoxicity. Cancer Lett. 2012, 318, 173–179. [Google Scholar] [CrossRef]
- Kobyakova, M.; Lomovskaya, Y.; Senotov, A.; Lomovsky, A.; Minaychev, V.; Fadeeva, I.; Shtatnova, D.; Krasnov, K.; Zvyagina, A.; Odinokova, I.; et al. The Increase in the Drug Resistance of Acute Myeloid Leukemia THP-1 Cells in High-Density Cell Culture Is Associated with Inflammatory-like Activation and Anti-Apoptotic Bcl-2 Proteins. Int. J. Mol. Sci. 2022, 23, 7881. [Google Scholar] [CrossRef]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Facchinetti, V.; Liu, D.; Soto, N.; Wei, S.; Jung, S.Y.; Huang, Q.; Qin, J.; Su, B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 2006, 127, 125–137. [Google Scholar] [CrossRef]
- Tamburini, J.; Elie, C.; Bardet, V.; Chapuis, N.; Park, S.; Broët, P.; Cornillet-Lefebvre, P.; Lioure, B.; Ugo, V.; Blanchet, O.; et al. Constitutive phosphoinositide 3-kinase/Akt activation represents a favorable prognostic factor in de novo acute myelogenous leukemia patients. Blood 2007, 110, 1025–1028. [Google Scholar] [CrossRef]
- Makhoul, P.; Galas, S.; Paniagua-Gayraud, S.; Deleuze-Masquefa, C.; Hajj, H.E.; Bonnet, P.-A.; Richaud, M. Uncovering the Molecular Pathways Implicated in the Anti-Cancer Activity of the Imidazoquinoxaline Derivative EAPB02303 Using a Caenorhabditis elegans Model. Int. J. Mol. Sci. 2024, 25, 7785. [Google Scholar] [CrossRef]
- Nepstad, I.; Hatfield, K.J.; Grønningsæter, I.S.; Reikvam, H. The PI3K-Akt-mTOR Signaling Pathway in Human Acute Myeloid Leukemia (AML) Cells. Int. J. Mol. Sci. 2020, 21, 2907. [Google Scholar] [CrossRef]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The Pathogenic Role of PI3K/AKT Pathway in Cancer Onset and Drug Resistance: An Updated Review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Nayak, A.; Muller, S. mTOR signaling regulates nucleolar targeting of the SUMO-specific isopeptidase SENP3. Mol. Cell Biol. 2014, 34, 4474–4484. [Google Scholar] [CrossRef] [PubMed]
- Boudra, R.; Lagrafeuille, R.; Lours-Calet, C.; de Joussineau, C.; Loubeau-Legros, G.; Chaveroux, C.; Saru, J.P.; Baron, S.; Morel, L.; Beaudoin, C. mTOR transcriptionally and post-transcriptionally regulates Npm1 gene expression to contribute to enhanced proliferation in cells with Pten inactivation. Cell Cycle 2016, 15, 1352–1362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khan, I.; Kaempf, A.; Raghuwanshi, S.; Chesnokov, M.; Zhang, X.; Wang, Z.; Domling, A.; Tyner, J.W.; Camacho, C.; Gartel, A.L. Favorable outcomes of NPM1mut AML patients are due to transcriptional inactivation of FOXM1, presenting a new target to overcome chemoresistance. Blood Cancer J. 2023, 13, 128. [Google Scholar] [CrossRef]

| AGE | GENDER | KARYOTYPE | Disease | NPM1 STATUS | NGS | |
|---|---|---|---|---|---|---|
| P1 | 71 | Female | 46,XX (11) | AML | NPM1c | DNMT3A, IDH2, KDM6A, TET2, FLT3, NOTCH1 |
| P2 | 19 | Male | 46,XY, t(15;17)(q24;q21)(2)/46, XY (7) | APL | WT-NPM1 | N/A |
| P3 | 34 | Male | 49, XY, +8, +9,inv(16)(p13.1q22), +22[cp9]/46, XY (1) | AML | WT-NPM1 | N/A |
| P4 | 72 | Female | 46, XX, del(7)(?p12)del(7)(?q31)(15)/46, XX (5) | AML | WT-NPM1 | DNMT3A, IDH2, TET2, EZH2 |
| P5 | 35 | Male | 46XY (20) | AML | WT-NPM1 | IKZF1, PTPN1, RUNX1 |
| P6 | 54 | Female | 46XX (35) | AML | NPM1c | DNMT3A, GATA1, NPM1, FLT3 |
| Primary Antibodies | Dilution | Species | Company | |
|---|---|---|---|---|
| p53 (DO-1) | sc-126 | 1:200 | Mouse | Santa Cruz Biotechnology, Dallas, TX, USA |
| Phospho-p53 (Ser15) | #9284 | 1:500 | Rabbit | Cell Signaling Technology, Danvers, MA, USA |
| p21 Waf1/Cip1 (12D1) | #9247 | 1:500 | Rabbit | Cell Signaling Technology |
| PARP-1 (F-2) | sc-8007 | 1:500 | Mouse | Santa Cruz Biotechnology |
| Caspase-3 (31A1067) | sc-56053 | 1:500 | Mouse | Santa Cruz Biotechnology |
| Akt (pan) (C67E7) | #4691 | 1:500 | Rabbit | Cell Signaling Technology |
| Phospho-Akt (Ser473) | #9271 | 1:250 | Rabbit | Cell Signaling Technology |
| mTOR (7C10) | #2983 | 1:500 | Rabbit | Cell Signaling Technology |
| Phospho-mTOR (Ser2448) | #2971 | 1:500 | Rabbit | Cell Signaling Technology |
| p44/42 MAPK (Erk1/2) (137F5) | #4695 | 1:500 | Rabbit | Cell Signaling Technology |
| Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) | #4370 | 1:500 | Rabbit | Cell Signaling Technology |
| Anti-nucleophosmin antibody [3A9F1] | ab86712 | 1:1000 | Mouse | Abcam, Cambridge, UK |
| NPM1 (mutant) | PA1-46356 | 1:1000 | Rabbit | Invitrogen, Thermo Fischer Scientific, Waltham, MA, USA |
| SENP3 | ab124790 | 1:500 | Rabbit | Abcam |
| ARF | ab185620 | 1:500 | Rabbit | Abcam |
| Histone (H3) | ab1791 | 1:10,000 | Rabbit | Abcam |
| Horseradish peroxidase (HRP)-conjugated secondary antibodies | ||||
| mouse anti-rabbit IgG-HRP | sc-2357 | 1:5000 | Santa Cruz Biotechnology | |
| m-IgGK BP-HRP | sc-516102 | 1:5000 | Santa Cruz Biotechnology | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhoul, P.; Hleihel, R.; Itani, S.; Hamie, M.; Pagniagua-Gayraud, S.; Patinote, C.; Richaud, M.; Merhi, R.A.; El-Sabban, M.; Galas, S.; et al. The Novel Imiqualine EAPB02303 Is a Potent Drug for Treating Acute Myeloid Leukemia. Biomolecules 2025, 15, 741. https://doi.org/10.3390/biom15050741
Makhoul P, Hleihel R, Itani S, Hamie M, Pagniagua-Gayraud S, Patinote C, Richaud M, Merhi RA, El-Sabban M, Galas S, et al. The Novel Imiqualine EAPB02303 Is a Potent Drug for Treating Acute Myeloid Leukemia. Biomolecules. 2025; 15(5):741. https://doi.org/10.3390/biom15050741
Chicago/Turabian StyleMakhoul, Perla, Rita Hleihel, Shaymaa Itani, Maguy Hamie, Stephanie Pagniagua-Gayraud, Cindy Patinote, Myriam Richaud, Raghida Abou Merhi, Marwan El-Sabban, Simon Galas, and et al. 2025. "The Novel Imiqualine EAPB02303 Is a Potent Drug for Treating Acute Myeloid Leukemia" Biomolecules 15, no. 5: 741. https://doi.org/10.3390/biom15050741
APA StyleMakhoul, P., Hleihel, R., Itani, S., Hamie, M., Pagniagua-Gayraud, S., Patinote, C., Richaud, M., Merhi, R. A., El-Sabban, M., Galas, S., Deleuze-Masquefa, C., Bonnet, P.-A., & El Hajj, H. (2025). The Novel Imiqualine EAPB02303 Is a Potent Drug for Treating Acute Myeloid Leukemia. Biomolecules, 15(5), 741. https://doi.org/10.3390/biom15050741






