An Exogenous NO Donor Provokes Mechanical Alternans in Normal Rat Atria and Impairs Sarcomere Contractility in Right Atrial Cardiomyocytes in Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experimental Model of Paroxysmal AF
2.3. Atrial Cardiomyocyte Isolation
2.4. Measurements of NO Contents in Atrial Cardiomyocytes
2.5. Incubation of Atrial Cardiomyocytes with a NO Donor
2.6. Measurements of SL Length and Sarcomere-Shortening Alternans in Single Atrial Cardiomyocytes
2.7. Statistical Analysis
3. Results
3.1. In AF, NOC-22 Increases NO Levels More in RA Cardiomyocytes than in LA Cardiomyocytes

3.2. In AF, NOC-22 Reduces the Amplitude and Velocities of Sarcomere Shortening and Relengthening Only in RA Cardiomyocytes
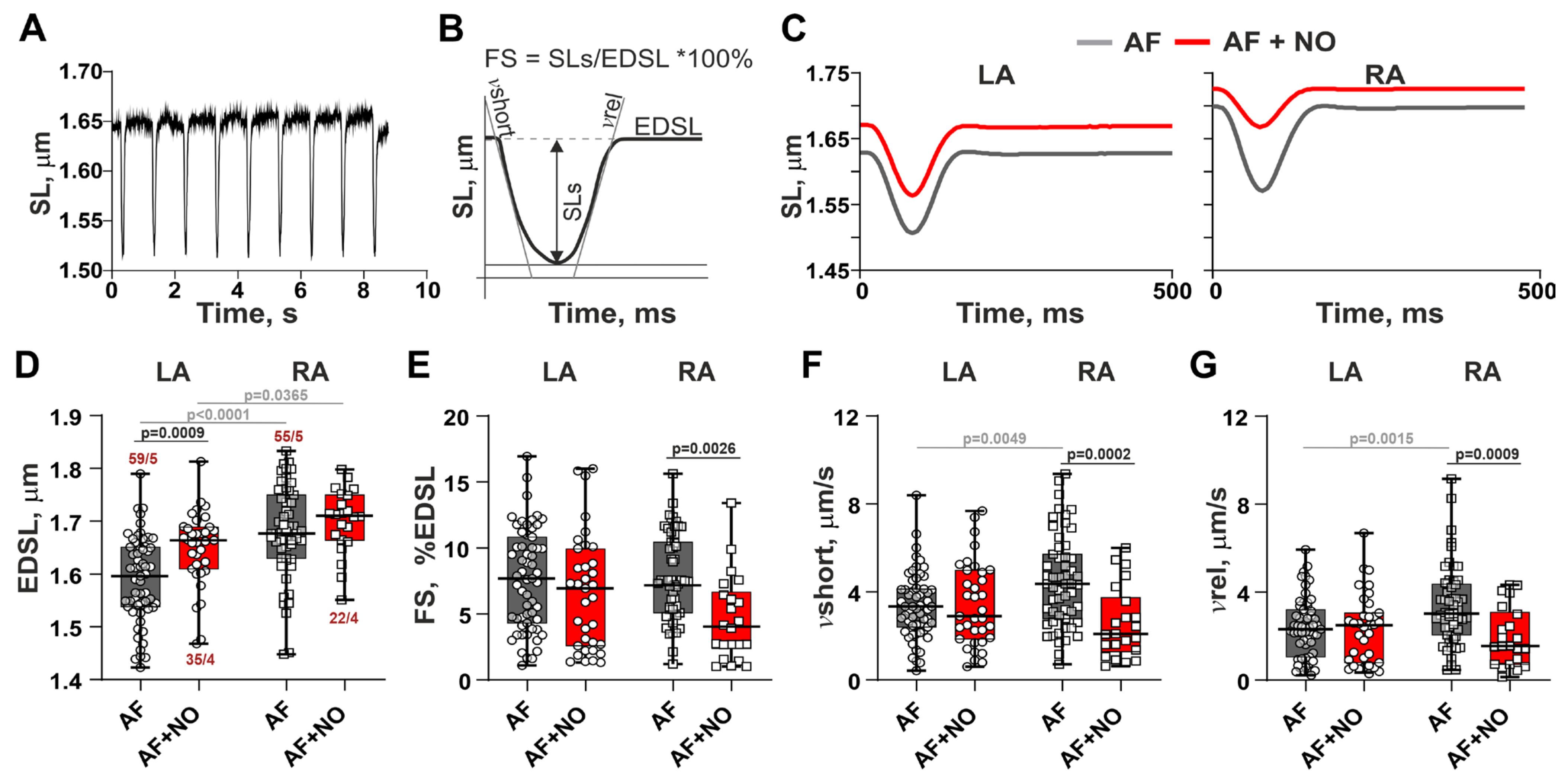
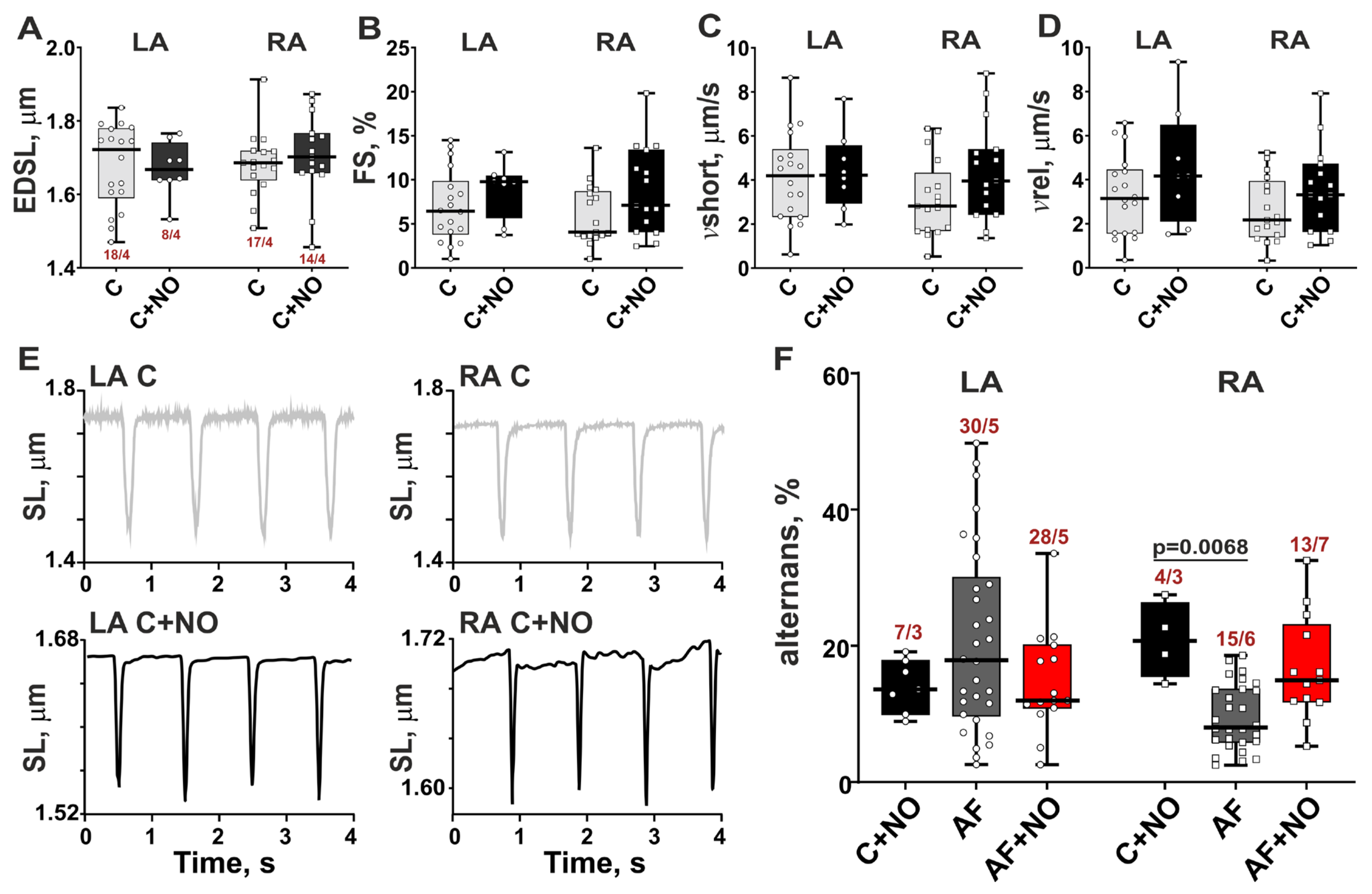
3.3. In AF, NOC-22 Enhances Sarcomere-Shortening Alternans in RA Cardiomyocytes

3.4. In Norm, NOC-22 Provokes Sarcomere-Shortening Alternans in LA and RA Cardiomyocytes
4. Discussion
5. Conclusions
6. Limitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| AF | Atrial fibrillation |
| BH4 | tetrahydrobiopterin |
| CaMKIIδ | Calcium calmodulin dependent protein kinase II |
| cGMP | Cyclic guanosine monophosphate |
| DAF-FM | Diaminofluorescein-FM diacetate |
| EDSL | End-diastolic sarcomere length |
| FS | Fractional sarcomere-shortening amplitude normalized by EDSL |
| LA | Left atrium |
| NO | Nitric oxide |
| NOC-22 | Spermine-NONOate |
| NOS | Nitric oxide synthase |
| PKG | cGMP-dependent protein kinase |
| RA | Right atrium |
| ROS | Reactive oxygen species |
| SL | Sarcomere length |
| vrel | Maximal velocity of sarcomere relengthening |
| vshort | Maximal velocity of sarcomere shortening |
References
- Boycott, H.E.; Nguyen, M.N.; Vrellaku, B.; Gehmlich, K.; Robinson, P. Nitric oxide and mechano-electrical transduction in cardiomyocytes. Front. Physiol. 2020, 11, 606740. [Google Scholar] [CrossRef] [PubMed]
- Power, A.S.; Asamudo, E.U.; Worthington, L.P.; Alim, C.C.; Parackal, R.E.; Wallace, R.S.; Ebenebe, O.V.; Brown, J.H.; Kohr, M.J.; Bers, D.M.; et al. Nitric Oxide Modulates Ca2+ Leak and Arrhythmias via S-Nitrosylation of CaMKII. Circ. Res. 2023, 133, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Sadek, M.S.; Cachorro, E.; El-Armouche, A.; Kämmerer, S. Therapeutic implications for PDE2 and cGMP/cAMP mediated crosstalk in cardiovascular diseases. Int. J. Mol. Sci. 2020, 21, 7462. [Google Scholar] [CrossRef]
- Beneke, K.; Molina, C.E. Molecular basis of atrial fibrillation initiation and maintenance. Hearts 2021, 2, 170–187. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.S.; Cordon, B.; Ma, B.; Xie, J. Nitric oxide: Physiological functions, delivery, and biomedical applications. Adv. Sci. 2023, 10, 2303259. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Goette, A.; Mera, F.; Honeycutt, C.; Feterik, K.; Wilcox, J.N.; Dudley, S.C.; Harrison, D.G.; Langberg, J.J. Downregulation of endocardial nitric oxide synthase expression and nitric oxide production in atrial fibrillation: Potential mechanisms for atrial thrombosis and stroke. Circulation 2002, 106, 2854–2858. [Google Scholar] [CrossRef]
- Reilly, S.N.; Liu, X.; Carnicer, R.; Recalde, A.; Muszkiewicz, A.; Jayaram, R.; Carena, M.C.; Wijesurendra, R.; Stefanini, M.; Surdo, N.C.; et al. Up-regulation of miR-31 in human atrial fibrillation begets the arrhythmia by depleting dystrophin and neuronal nitric oxide synthase. Sci. Transl. Med. 2016, 8, 340ra374. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Ding, W.; Cai, S.; Zhao, Q. Ablation alleviates atrial fibrillation by regulating the signaling pathways of endothelial nitric oxide synthase/nitric oxide via miR-155-5p and miR-24-3p. J. Cell. Biochem. 2019, 120, 4451–4462. [Google Scholar] [CrossRef]
- Ramos-Mondragón, R.; Lozhkin, A.; Vendrov, A.E.; Runge, M.S.; Isom, L.L.; Madamanchi, N.R. NADPH oxidases and oxidative stress in the pathogenesis of atrial fibrillation. Antioxidants 2023, 12, 1833. [Google Scholar] [CrossRef]
- Pozios, I.; Vouliotis, A.I.; Dilaveris, P.; Tsioufis, C. Electro-mechanical alterations in atrial fibrillation: Structural, electrical, and functional correlates. J. Cardiovasc. Dev. Dis. 2023, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Gasparova, I.; Kubatka, P.; Opatrilova, R.; Caprnda, M.; Filipova, S.; Rodrigo, L.; Malan, L.; Mozos, I.; Rabajdova, M.; Nosal, V.; et al. Perspectives and challenges of antioxidant therapy for atrial fibrillation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1–14. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, D.; Xu, Y.; Jiang, F.; Zheng, J.; Feng, Y.; Cao, J.; Zhou, X. Nitric oxide-releasing platforms for treating cardiovascular disease. Pharmaceutics 2022, 14, 1345. [Google Scholar] [CrossRef] [PubMed]
- West, M.B.; Rokosh, G.; Obal, D.; Velayutham, M.; Xuan, Y.T.; Hill, B.G.; Rachel, J.; Keith, M.S.; Schrader, J.; Guo, Y.; et al. Cardiac myocyte–specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 2008, 118, 1970–1978. [Google Scholar] [CrossRef]
- Bonilla, I.M.; Sridhar, A.; Györke, S.; Cardounel, A.J.; Carnes, C.A. Nitric oxide synthases and atrial fibrillation. Front. Physiol. 2012, 3, 105. [Google Scholar] [CrossRef]
- Schaefer, A.K.; Kiss, A.; Oszwald, A.; Nagel, F.; Acar, E.; Aliabadi-Zuckermann, A.; Kain, R.; Jakubowski, A.; Ferdinandy, P.; Hallström, S.; et al. Single donor infusion of S-nitroso-human-serum-albumin attenuates cardiac isograft fibrosis and preserves myocardial micro-RNA-126-3p in a murine heterotopic heart transplant model. Transpl. Int. 2022, 35, 10057. [Google Scholar] [CrossRef]
- Lenaerts, I.; Holemans, P.; Pokreisz, P.; Sipido, K.R.; Janssens, S.; Heidbüchel, H.; Willems, R. Nitric oxide delays atrial tachycardia-induced electrical remodelling in a sheep model. Europace 2011, 13, 747–754. [Google Scholar] [CrossRef]
- Gómez, R.; Núñez, L.; Vaquero, M.; Amorós, I.; Barana, A.; de Prada, T.; Macaya, C.; Maroto, L.; Rodríguez, E.; Caballero, R.; et al. Nitric oxide inhibits Kv4.3 and human cardiac transient outward potassium current (Ito1). Cardiovasc. Res. 2008, 80, 375–384. [Google Scholar] [CrossRef]
- Wegener, J.W.; Gödecke, A.; Schrader, J.; Nawrath, H. Effects of nitric oxide donors on cardiac contractility in wild-type and myoglobin-deficient mice. Br. J. Pharmacol. 2002, 136, 415–420. [Google Scholar] [CrossRef][Green Version]
- Derici, K.; Samsar, U.; Demirel-Yilmaz, E. Nitric oxide effects depend on different mechanisms in different regions of the rat heart. HeartVessels 2012, 27, 89–97. [Google Scholar] [CrossRef]
- Butova, X.; Myachina, T.; Simonova, R.; Kochurova, A.; Mukhlynina, E.; Kopylova, G.; Shchepkin, D.; Khokhlova, A. The inter-chamber differences in the contractile function between left and right atrial cardiomyocytes in atrial fibrillation in rats. Front. Cardiovasc. Med. 2023, 10, 1203093. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Geng, N.; Chen, Y.; Ren, L.I.; Liu, X.; Wan, J.; Guo, S.; Wang, S. Ranolazine improves oxidative stress and mitochondrial function in the atrium of acetylcholine-CaCl2 induced atrial fibrillation rats. Life Sci. 2016, 156, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Butova, X.; Myachina, T.; Khokhlova, A. A combined Langendorff-injection technique for simultaneous isolation of single cardiomyocytes from atria and ventricles of the rat heart. MethodsX 2021, 8, 101189. [Google Scholar] [CrossRef]
- Majumder, S.; Sinha, S.; Siamwala, J.H.; Muley, A.; Seerapu, H.R.; Kolluru, G.K.; Veeriah, V.; Nagarajan, S.; Sridhara, S.R.C.; Priya, M.K.; et al. A comparative study of NONOate based NO donors: Spermine NONOate is the best suited NO donor for angiogenesis. Nitric Oxide 2014, 36, 76–86. [Google Scholar] [CrossRef]
- Paul, S.; Pan, S.; Mukherjee, A.; De, P. Nitric oxide releasing delivery platforms: Design, detection, biomedical applications, and future possibilities. Mol. Pharm. 2021, 18, 3181–3205. [Google Scholar] [CrossRef]
- Mikhryakova, P.P.; Butova, X.A.; Myachina, T.A.; Simonova, R.A.; Khokhlova, A.D. A study of mechanical alternans in single rat cardiomyocytes in acetylcholine-CaCl2 induced atrial fibrillation. J. Evol. Biochem. Physiol. 2022, 58, S13–S21. [Google Scholar] [CrossRef]
- Iwamiya, S.; Ihara, K.; Nitta, G.; Sasano, T. Atrial Fibrillation and Underlying Structural and Electrophysiological Heterogeneity. Int. J. Mol. Sci. 2024, 25, 10193. [Google Scholar] [CrossRef]
- Zou, T.; Chen, Q.; Chen, C.; Liu, G.; Ling, Y.; Pang, Y.; Xu, Y.; Cheng, K.; Zhu, W.; Wang, R.-X.; et al. Moricizine prevents atrial fibrillation by late sodium current inhibition in atrial myocytes. J. Thorac. Dis. 2022, 14, 2187. [Google Scholar] [CrossRef]
- Shiroshita-Takeshita, A.; Brundel, B.J.; Lavoie, J.; Nattel, S. Prednisone prevents atrial fibrillation promotion by atrial tachycardia remodeling in dogs. Cardiovasc. Res. 2006, 69, 865–875. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, F.; Yang, X.; Xia, Y. The key role of uric acid in oxidative stress, inflammation, fibrosis, apoptosis, and immunity in the pathogenesis of atrial fibrillation. Front. Cardiovasc. Med. 2021, 8, 641136. [Google Scholar] [CrossRef]
- Rodrigo, R.; González-Montero, J.; Sotomayor, C.G. Novel combined antioxidant strategy against hypertension, acute myocardial infarction and postoperative atrial fibrillation. Biomedicines 2021, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Barouch, L.A.; Harrison, R.W.; Skaf, M.W.; Rosas, G.O.; Cappola, T.P.; Kobeissi, Z.A.; Hobai, I.A.; Lemmon, C.A.; Burnett, A.L.; O’Rourke, B.; et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature 2002, 416, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Zhang, M.; Ly, O.T.; Chen, H.; Sridhar, A.; Lambers, E.; Chalazan, B.; Youn, S.-W.; Maienschein-Cline, M.; Feferman, L.; et al. Human induced pluripotent stem cell-derived atrial cardiomyocytes carrying an SCN5A mutation identify nitric oxide signaling as a mediator of atrial fibrillation. Stem Cell Rep. 2021, 16, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Rozmaritsa, N.; Christ, T.; Van Wagoner, D.R.; Haase, H.; Stasch, J.P.; Matschke, K.; Ravens, U. Attenuated response of L-type calcium current to nitric oxide in atrial fibrillation. Cardiovasc. Res. 2014, 101, 533–542. [Google Scholar] [CrossRef]
- Korantzopoulos, P.; Letsas, K.; Fragakis, N.; Tse, G.; Liu, T. Oxidative stress and atrial fibrillation: An update. Free. Radic. Res. 2018, 52, 1199–1209. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of atrial fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef]
- Dobrev, D.; Heijman, J.; Hiram, R.; Li, N.; Nattel, S. Inflammatory signalling in atrial cardiomyocytes: A novel unifying principle in atrial fibrillation pathophysiology. Nat. Rev. Cardiol. 2023, 20, 145–167. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Tse, G.; Wong, S.T.; Tse, V.; Lee, Y.T.; Lin, H.Y.; Yeo, J.M. Cardiac dynamics: Alternans and arrhythmogenesis. J. Arrhythm. 2016, 32, 411–417. [Google Scholar] [CrossRef]
- Kulkarni, K.; Merchant, F.M.; Kassab, M.B.; Sana, F.; Moazzami, K.; Sayadi, O.; Singh, J.P.; Heist, E.K.; Armoundas, A.A. Cardiac alternans: Mechanisms and clinical utility in arrhythmia prevention. J. Am. Heart Assoc. 2019, 8, e013750. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butova, X.; Myachina, T.; Mikhryakova, P.; Simonova, R.; Shchepkin, D.; Khokhlova, A. An Exogenous NO Donor Provokes Mechanical Alternans in Normal Rat Atria and Impairs Sarcomere Contractility in Right Atrial Cardiomyocytes in Atrial Fibrillation. Biomolecules 2025, 15, 735. https://doi.org/10.3390/biom15050735
Butova X, Myachina T, Mikhryakova P, Simonova R, Shchepkin D, Khokhlova A. An Exogenous NO Donor Provokes Mechanical Alternans in Normal Rat Atria and Impairs Sarcomere Contractility in Right Atrial Cardiomyocytes in Atrial Fibrillation. Biomolecules. 2025; 15(5):735. https://doi.org/10.3390/biom15050735
Chicago/Turabian StyleButova, Xenia, Tatiana Myachina, Polina Mikhryakova, Raisa Simonova, Daniil Shchepkin, and Anastasia Khokhlova. 2025. "An Exogenous NO Donor Provokes Mechanical Alternans in Normal Rat Atria and Impairs Sarcomere Contractility in Right Atrial Cardiomyocytes in Atrial Fibrillation" Biomolecules 15, no. 5: 735. https://doi.org/10.3390/biom15050735
APA StyleButova, X., Myachina, T., Mikhryakova, P., Simonova, R., Shchepkin, D., & Khokhlova, A. (2025). An Exogenous NO Donor Provokes Mechanical Alternans in Normal Rat Atria and Impairs Sarcomere Contractility in Right Atrial Cardiomyocytes in Atrial Fibrillation. Biomolecules, 15(5), 735. https://doi.org/10.3390/biom15050735






