Uncovering a Novel Role of ROR1 in the Epigenetic Regulation of Tumor Suppressor Gene CREB3L1 in Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment Techniques
2.1.1. Cell Culture
2.1.2. Cell Transfection Techniques
2.1.3. Cell Treatment Techniques
2.2. Protein Extraction and Western Blot Analysis
2.3. DNMT Activity Assay
2.4. Immunofluorescence Analysis
2.5. Chromatin-Immunoprecipitation (ChIP)
2.6. DNA Extraction and Reduced Representation Bisulfite Sequencing
2.7. Statistical Analysis
3. Results
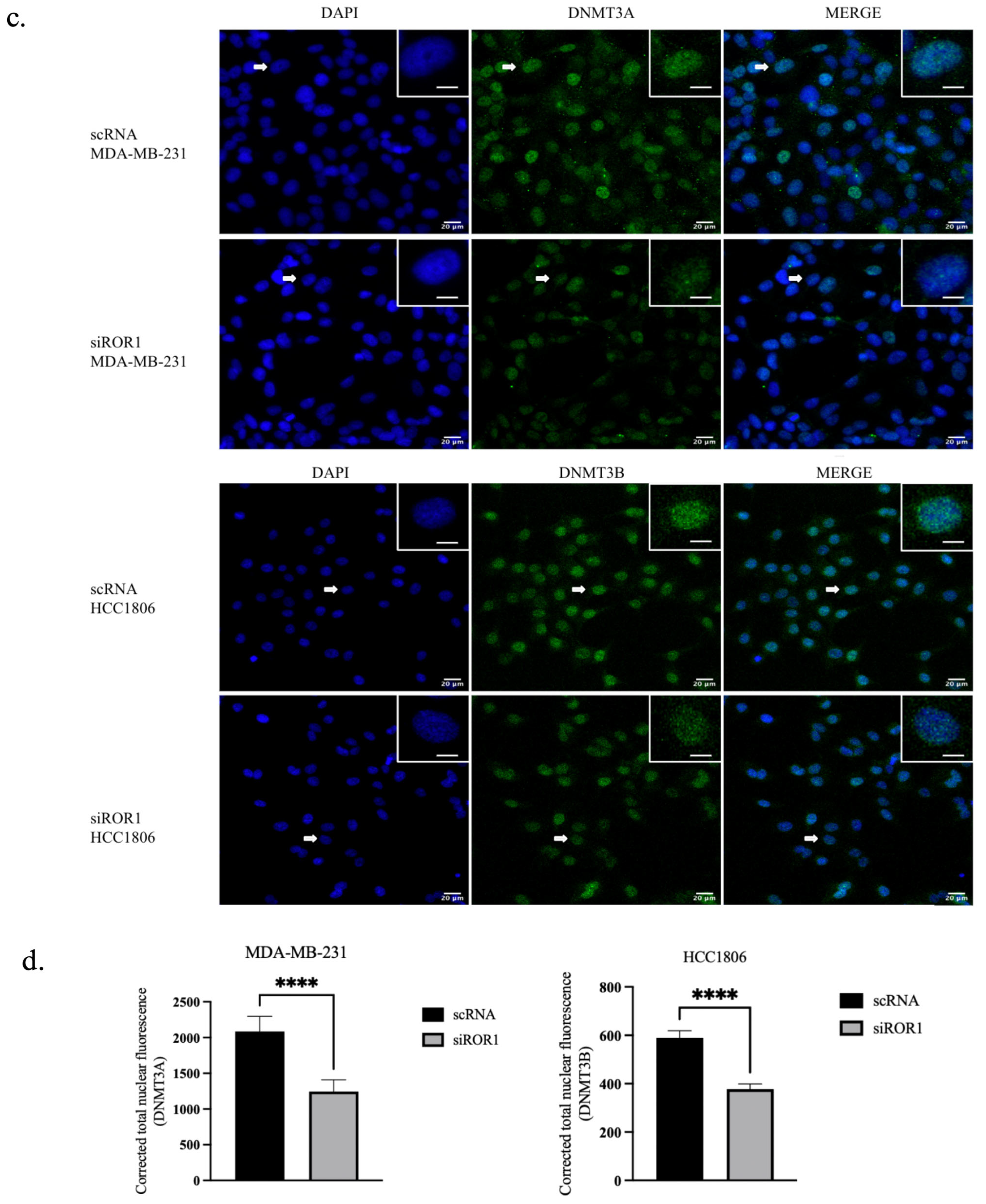
3.1. ROR1 Regulates DNMT3A and DNMT3B Expression and Activity in TNBC Cell Lines
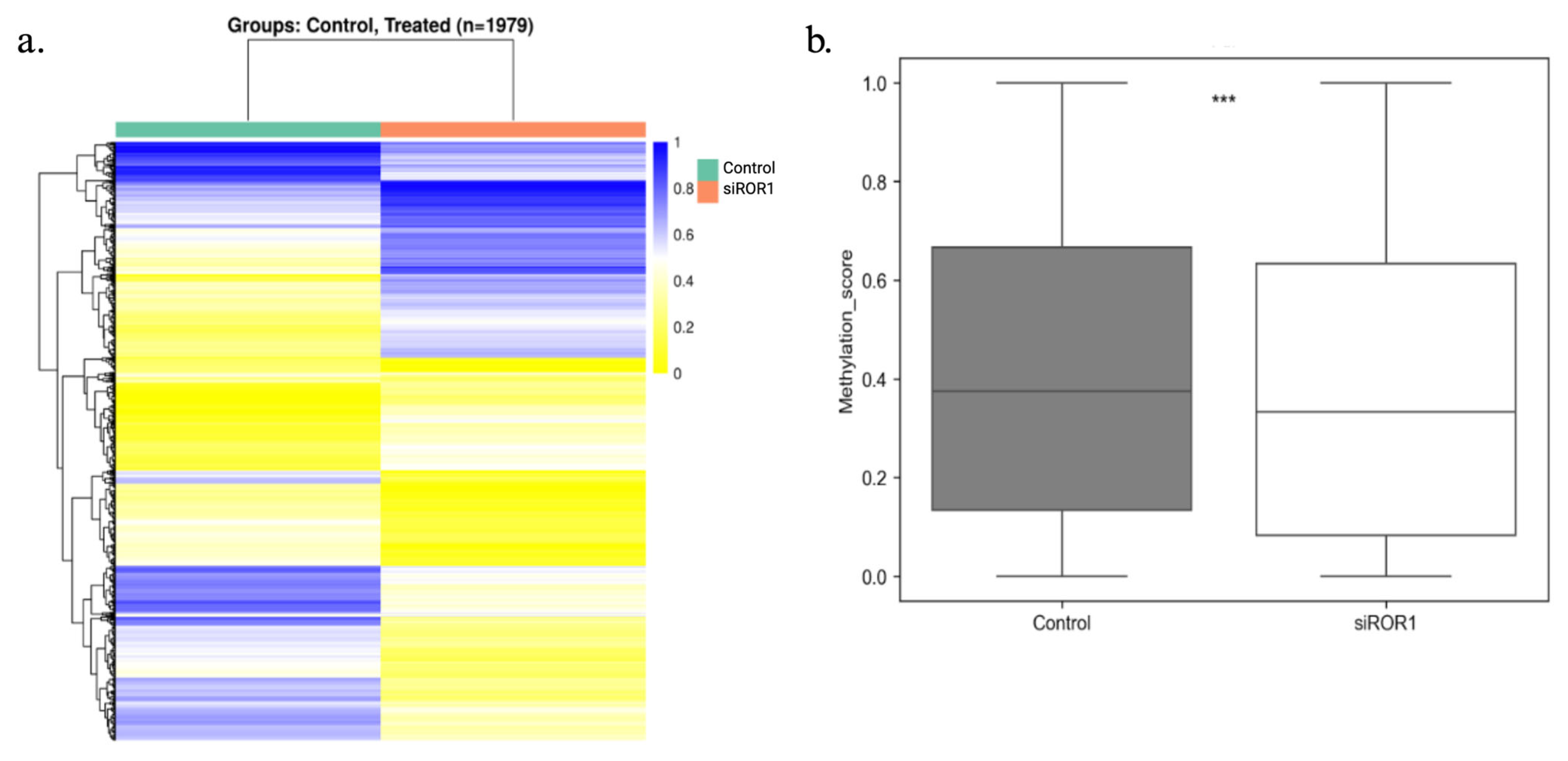
3.2. ROR1 Knockdown Reduces the DNA Methylation Status of the CpG Regions Throughout the MDA-MB-231 Genome
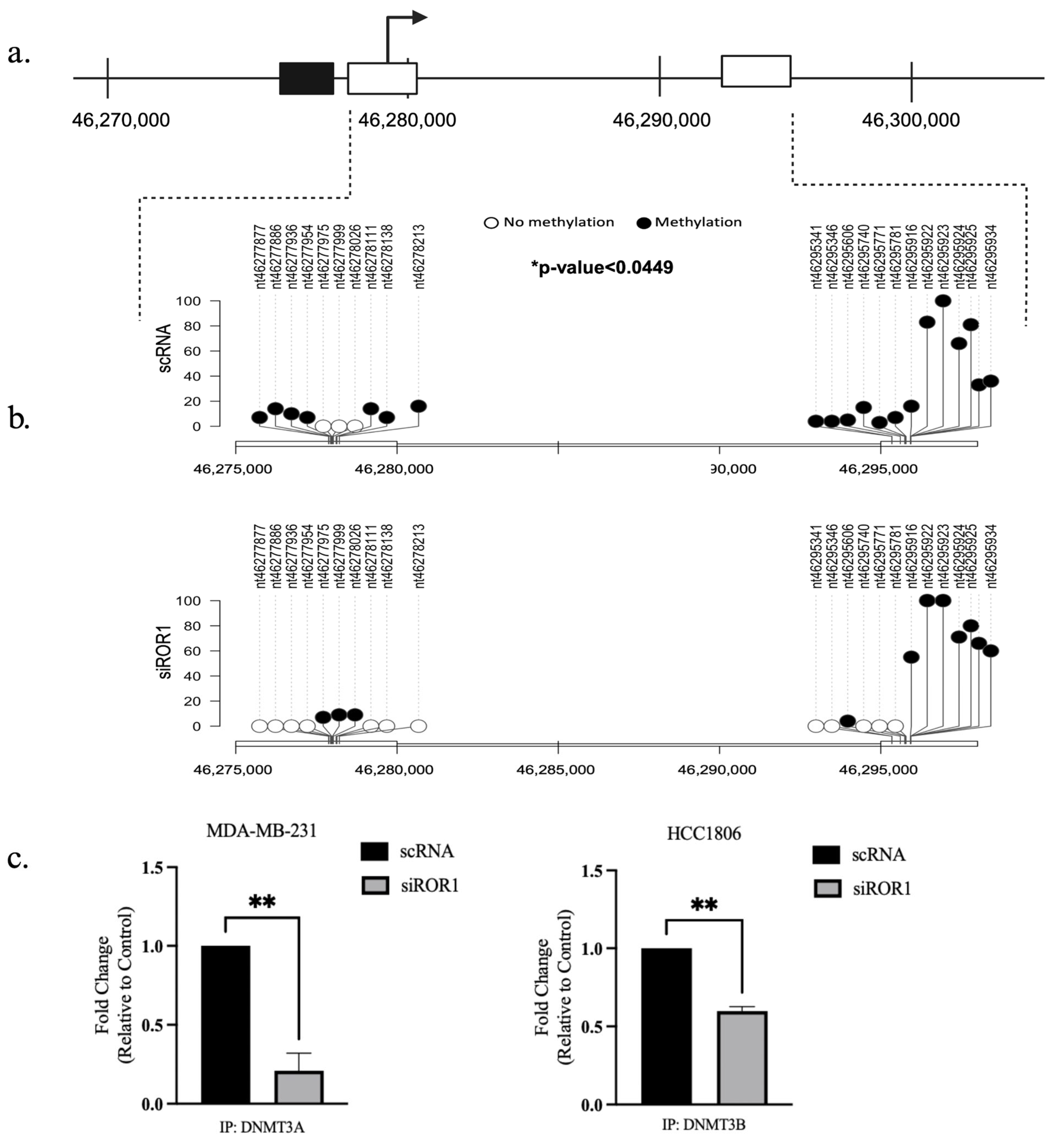
3.3. ROR1 Knockdown Hinders the Physical Binding of DNMT3A and DNMT3B to the Coding Region of the CREB3L1 Gene in TNBC Cells
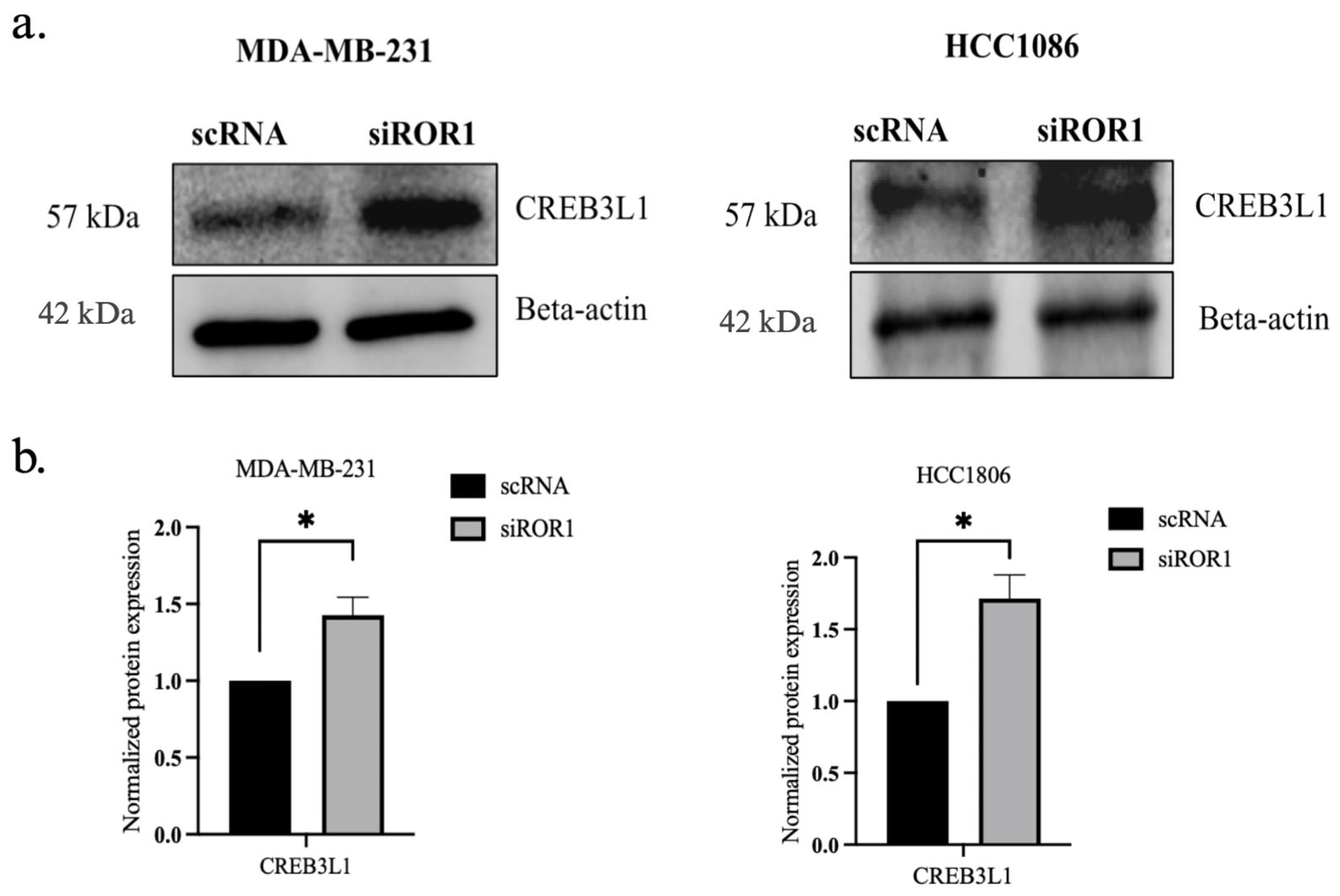
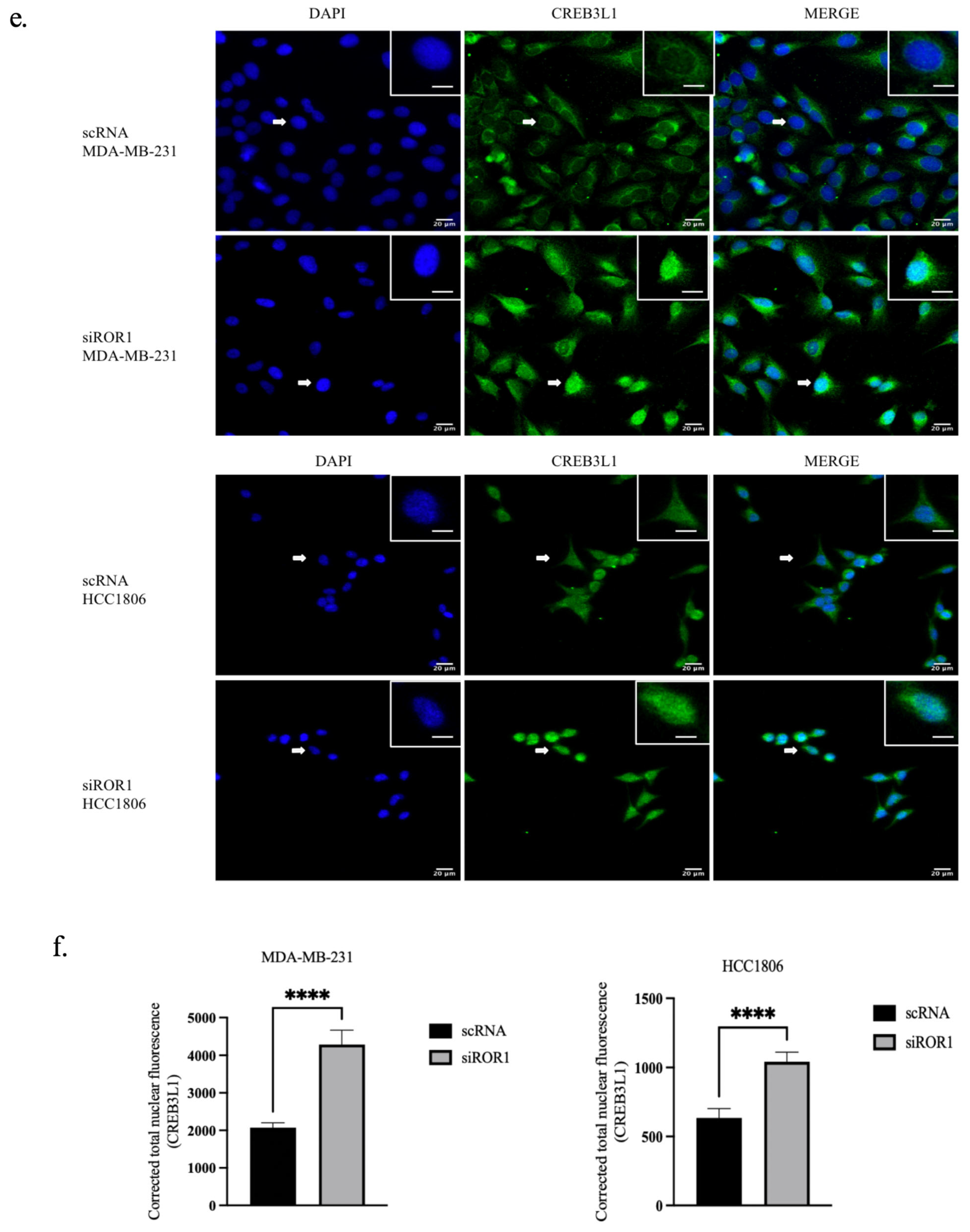
3.4. ROR1 Knockdown Reverses CREB3L1 Silencing in TNBC Cells
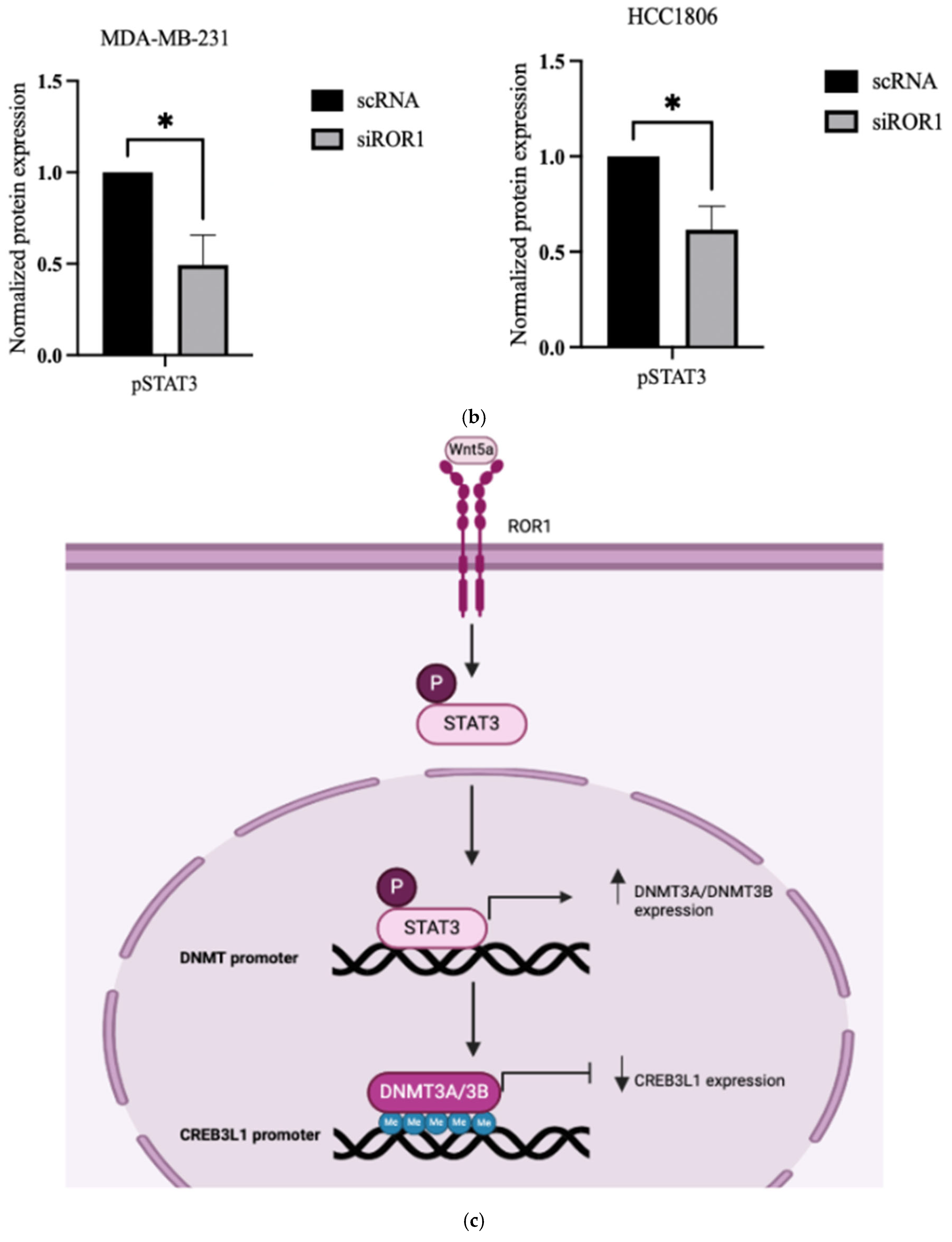
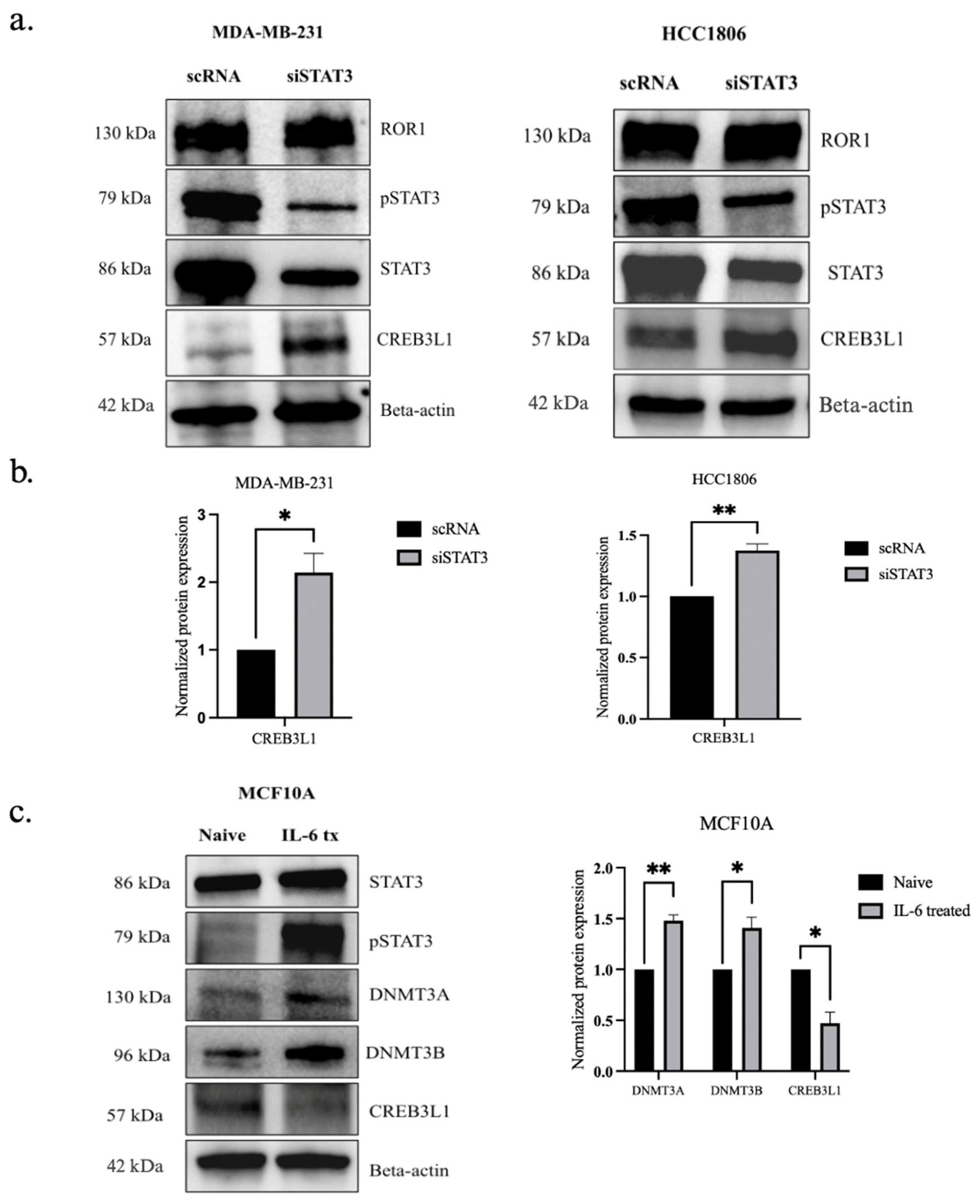
3.5. ROR1 Activates STAT3 Phosphorylation in TNBC Cells
3.6. STAT3 Phosphorylation Has an Inverse Effect on CREB3L1 Expression in TNBC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CREB3L1 | cAMP responsive element binding protein 3-like-1 |
| DNMT | DNA methyltransferase |
| DMR | Differentially methylated region |
| ROR1 | Receptor tyrosine kinase-like orphan receptor 1 |
| RRBS | Reduced representation bisulfite sequencing |
| STAT3 | Signal transducer and activator of transcription 3 protein |
| TNBC | Triple-negative breast cancer |
References
- Cancer Facts for Women | Most Common Cancers in Women. Available online: https://www.cancer.org/cancer/risk-prevention/understanding-cancer-risk/cancer-facts/cancer-facts-for-women.html (accessed on 10 July 2024).
- Tao, Z.; Shi, A.; Lu, C.; Song, T.; Zhang, Z.; Zhao, J. Breast Cancer: Epidemiology and Etiology. Cell Biochem. Biophys. 2015, 72, 333–338. [Google Scholar] [CrossRef]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, C.-W. Epigenetic Modulations in Triple-Negative Breast Cancer: Therapeutic Implications for Tumor Microenvironment. Pharmacol. Res. 2024, 204, 107205. [Google Scholar] [CrossRef] [PubMed]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Zahraeifard, S.; Xiao, Z.; So, J.Y.; Ahad, A.; Montoya, S.; Park, W.Y.; Sornapudi, T.; Andohkow, T.; Read, A.; Kedei, N.; et al. Loss of Tumor Suppressors Promotes Inflammatory Tumor Microenvironment and Enhances LAG3+T Cell Mediated Immune Suppression. Nat. Commun. 2024, 15, 5873. [Google Scholar] [CrossRef]
- So, J.Y.; Ohm, J.; Lipkowitz, S.; Yang, L. Triple Negative Breast Cancer (TNBC): Non-Genetic Tumor Heterogeneity and Immune Microenvironment: Emerging Treatment Options. Pharmacol. Ther. 2022, 237, 108253. [Google Scholar] [CrossRef]
- Kazanets, A.; Shorstova, T.; Hilmi, K.; Marques, M.; Witcher, M. Epigenetic Silencing of Tumor Suppressor Genes: Paradigms, Puzzles, and Potential. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2016, 1865, 275–288. [Google Scholar] [CrossRef]
- Wong, K.K. DNMT1: A Key Drug Target in Triple-Negative Breast Cancer. Semin. Cancer Biol. 2021, 72, 198–213. [Google Scholar] [CrossRef]
- Karami Fath, M.; Azargoonjahromi, A.; Kiani, A.; Jalalifar, F.; Osati, P.; Akbari Oryani, M.; Shakeri, F.; Nasirzadeh, F.; Khalesi, B.; Nabi-Afjadi, M.; et al. The Role of Epigenetic Modifications in Drug Resistance and Treatment of Breast Cancer. Cell. Mol. Biol. Lett. 2022, 27, 52. [Google Scholar] [CrossRef]
- Zolota, V.; Tzelepi, V.; Piperigkou, Z.; Kourea, H.; Papakonstantinou, E.; Argentou, M.-I.; Karamanos, N.K. Epigenetic Alterations in Triple-Negative Breast Cancer—The Critical Role of Extracellular Matrix. Cancers 2021, 13, 713. [Google Scholar] [CrossRef]
- Man, X.; Li, Q.; Wang, B.; Zhang, H.; Zhang, S.; Li, Z. DNMT3A and DNMT3B in Breast Tumorigenesis and Potential Therapy. Front. Cell Dev. Biol. 2022, 10, 916725. [Google Scholar] [CrossRef]
- Feng, J.; Zhou, Y.; Campbell, S.L.; Le, T.; Li, E.; Sweatt, J.D.; Silva, A.J.; Fan, G. Dnmt1 and Dnmt3a Maintain DNA Methylation and Regulate Synaptic Function in Adult Forebrain Neurons. Nat. Neurosci. 2010, 13, 423–430. [Google Scholar] [CrossRef]
- Dodge, J.E.; Okano, M.; Dick, F.; Tsujimoto, N.; Chen, T.; Wang, S.; Ueda, Y.; Dyson, N.; Li, E. Inactivation of Dnmt3b in Mouse Embryonic Fibroblasts Results in DNA Hypomethylation, Chromosomal Instability, and Spontaneous Immortalization. J. Biol. Chem. 2005, 280, 17986–17991. [Google Scholar] [CrossRef]
- Yu, J.; Zayas, J.; Qin, B.; Wang, L. Targeting DNA Methylation for Treating Triple-Negative Breast Cancer. Pharmacogenomics 2019, 20, 1151–1157. [Google Scholar] [CrossRef]
- Derissen, E.J.; Beijnen, J.H.; Schellens, J.H. Concise Drug Review: Azacitidine and Decitabine. Oncology 2013, 18, 619. [Google Scholar] [CrossRef]
- Dahn, M.L.; Cruickshank, B.M.; Jackson, A.J.; Dean, C.; Holloway, R.W.; Hall, S.R.; Coyle, K.M.; Maillet, H.; Waisman, D.M.; Goralski, K.B.; et al. Decitabine Response in Breast Cancer Requires Efficient Drug Processing and Is Not Limited by Multidrug Resistance. Mol. Cancer Ther. 2020, 19, 1110–1122. [Google Scholar] [CrossRef]
- Borcherding, N.; Kusner, D.; Liu, G.-H.; Zhang, W. ROR1, an Embryonic Protein with an Emerging Role in Cancer Biology. Protein Cell 2014, 5, 496–502. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Cui, B.; Chuang, H.-Y.; Yu, J.; Wang-Rodriguez, J.; Tang, L.; Chen, G.; Basak, G.W.; Kipps, T.J. ROR1 Is Expressed in Human Breast Cancer and Associated with Enhanced Tumor-Cell Growth. PLoS ONE 2012, 7, e31127. [Google Scholar] [CrossRef]
- Yu, J.; Chen, L.; Cui, B.; Widhopf, G.F.; Shen, Z.; Wu, R.; Zhang, L.; Zhang, S.; Briggs, S.P.; Kipps, T.J. Wnt5a Induces ROR1/ROR2 Heterooligomerization to Enhance Leukemia Chemotaxis and Proliferation. J. Clin. Investig. 2016, 126, 585–598. [Google Scholar] [CrossRef]
- Cui, B.; Zhang, S.; Chen, L.; Yu, J.; Widhopf, G.F.; Fecteau, J.-F.; Rassenti, L.Z.; Kipps, T.J. Targeting ROR1 Inhibits Epithelial-Mesenchymal Transition and Metastasis. Cancer Res. 2013, 73, 3649–3660. [Google Scholar] [CrossRef]
- Quezada, M.J.; Lopez-Bergami, P. The Signaling Pathways Activated by ROR1 in Cancer. Cell. Signal. 2023, 104, 110588. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Ghia, E.M.; Huang, J.; Wu, L.; Zhang, J.; Lam, S.; Lei, Y.; He, J.; Cui, B.; et al. Inhibition of Chemotherapy Resistant Breast Cancer Stem Cells by a ROR1 Specific Antibody. Proc. Natl. Acad. Sci. USA 2019, 116, 1370–1377. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Goodpaster, T.; Randolph-Habecker, J.; Hoffstrom, B.G.; Jalikis, F.G.; Koch, L.K.; Berger, C.; Kosasih, P.L.; Rajan, A.; Sommermeyer, D.; et al. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. Clin. Cancer Res. 2017, 23, 3061–3071. [Google Scholar] [CrossRef]
- Fultang, N.; Illendula, A.; Lin, J.; Pandey, M.K.; Klase, Z.; Peethambaran, B. ROR1 Regulates Chemoresistance in Breast Cancer via Modulation of Drug Efflux Pump ABCB1. Sci. Rep. 2020, 10, 1821. [Google Scholar] [CrossRef]
- Fultang, N.; Illendula, A.; Chen, B.; Wu, C.; Jonnalagadda, S.; Baird, N.; Klase, Z.; Peethambaran, B. Strictinin, a Novel ROR1-Inhibitor, Represses Triple Negative Breast Cancer Survival and Migration via Modulation of PI3K/AKT/GSK3ß Activity. PLoS ONE 2019, 14, e0217789. [Google Scholar] [CrossRef]
- Spangle, J.M.; Roberts, T.M.; Zhao, J.J. The Emerging Role of PI3K/AKT-Mediated Epigenetic Regulation in Cancer. Biochim. Biophys. Acta 2017, 1868, 123–131. [Google Scholar] [CrossRef]
- Kan, W.; Gao, L.; Chen, J.; Chen, L.; Zhang, G.; Hao, B.; He, M.; Chen, X.; Wang, C. Downregulating DNA Methyltransferase 3B by Suppressing the PI3K/Akt Signaling Pathway Enhances the Chemosensitivity of Glioblastoma to Temozolomide. Mol. Neurobiol. 2024, 61, 7066–7074. [Google Scholar] [CrossRef]
- Mellor, P.; Deibert, L.; Calvert, B.; Bonham, K.; Carlsen, S.A.; Anderson, D.H. CREB3L1 Is a Metastasis Suppressor That Represses Expression of Genes Regulating Metastasis, Invasion, and Angiogenesis. Mol. Cell. Biol. 2013, 33, 4985–4995. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.; Song, Y.; Fan, L.; Lei, T.; He, Y.; Hu, S. The Regulatory Network of CREB3L1 and Its Roles in Physiological and Pathological Conditions. Int. J. Med. Sci. 2024, 21, 123–136. [Google Scholar] [CrossRef]
- Murakami, T.; Saito, A.; Hino, S.; Kondo, S.; Kanemoto, S.; Chihara, K.; Sekiya, H.; Tsumagari, K.; Ochiai, K.; Yoshinaga, K.; et al. Signalling Mediated by the Endoplasmic Reticulum Stress Transducer OASIS Is Involved in Bone Formation. Nat. Cell. Biol. 2009, 11, 1205–1211. [Google Scholar] [CrossRef]
- Ward, A.K.; Mellor, P.; Smith, S.E.; Kendall, S.; Just, N.A.; Vizeacoumar, F.S.; Sarker, S.; Phillips, Z.; Alvi, R.; Saxena, A.; et al. Epigenetic Silencing of CREB3L1 by DNA Methylation Is Associated with High-Grade Metastatic Breast Cancers with Poor Prognosis and Is Prevalent in Triple Negative Breast Cancers. Breast Cancer Res. 2016, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Denard, B.; Jiang, S.; Peng, Y.; Ye, J. CREB3L1 as a Potential Biomarker Predicting Response of Triple Negative Breast Cancer to Doxorubicin-Based Chemotherapy. BMC Cancer 2018, 18, 813. [Google Scholar] [CrossRef] [PubMed]
- Raiter, A.; Lipovetsky, J.; Hyman, L.; Mugami, S.; Ben-Zur, T.; Yerushalmi, R. Chemotherapy Controls Metastasis Through Stimulatory Effects on GRP78 and Its Transcription Factor CREB3L1. Front. Oncol. 2020, 10, 1500. [Google Scholar] [CrossRef]
- Denard, B.; Seemann, J.; Chen, Q.; Gay, A.; Huang, H.; Chen, Y.; Ye, J. The Membrane-Bound Transcription Factor CREB3L1 Is Activated in Response to Virus Infection to Inhibit Proliferation of Virus-Infected Cells. Cell Host Microbe 2011, 10, 65–74. [Google Scholar] [CrossRef]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple Molecular Subtypes of Triple-Negative Breast Cancer Critically Rely on Androgen Receptor and Respond to Enzalutamide In Vivo. Mol. Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.Y.; Woetmann, A.; Raghunath, P.N.; Odum, N.; Wasik, M.A. STAT3 Induces Transcription of the DNA Methyltransferase 1 Gene (DNMT1) in Malignant T Lymphocytes. Blood 2006, 108, 1058–1064. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, P.; Herrmann, A.; Yang, C.; Xin, H.; Wang, Z.; Hoon, D.S.B.; Forman, S.J.; Jove, R.; Riggs, A.D.; et al. Acetylated STAT3 Is Crucial for Methylation of Tumor-Suppressor Gene Promoters and Inhibition by Resveratrol Results in Demethylation. Proc. Natl. Acad. Sci. USA 2012, 109, 7765–7769. [Google Scholar] [CrossRef]
- Wingelhofer, B.; Neubauer, H.A.; Valent, P.; Han, X.; Constantinescu, S.N.; Gunning, P.T.; Müller, M.; Moriggl, R. Implications of STAT3 and STAT5 Signaling on Gene Regulation and Chromatin Remodeling in Hematopoietic Cancer. Leukemia 2018, 32, 1713–1726. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.Y.; Liu, X.; Bhutani, G.; Kantekure, K.; Wasik, M. IL-2R Common γ-Chain Is Epigenetically Silenced by Nucleophosphin–Anaplastic Lymphoma Kinase (NPM-ALK) and Acts as a Tumor Suppressor by Targeting NPM-ALK. Proc. Natl. Acad. Sci. USA 2011, 108, 11977–11982. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-C.; Su, Y.-T.; Chi, C.-C.; Kuo, Y.-C.; Lee, K.-F.; Wu, Y.-C.; Lan, P.-C.; Yang, M.-H.; Chang, T.-S.; Huang, Y.-H. DNMT3b/OCT4 Expression Confers Sorafenib Resistance and Poor Prognosis of Hepatocellular Carcinoma through IL-6/STAT3 Regulation. J. Exp. Clin. Cancer Res. 2019, 38, 474. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.S.B. The STAT3-DNMT1 Connection. JAKSTAT 2012, 1, 257–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piki, E.; Dini, A.; Raivola, J.; Salokas, K.; Zhang, K.; Varjosalo, M.; Pellinen, T.; Välimäki, K.; Veskimäe, K.T.; Staff, S.; et al. ROR1-STAT3 Signaling Contributes to Ovarian Cancer Intra-Tumor Heterogeneity. Cell Death Discov. 2023, 9, 222. [Google Scholar] [CrossRef]
- Li, P.; Harris, D.; Liu, Z.; Liu, J.; Keating, M.; Estrov, Z. Stat3 Activates the Receptor Tyrosine Kinase Like Orphan Receptor-1 Gene in Chronic Lymphocytic Leukemia Cells. PLoS ONE 2010, 5, e11859. [Google Scholar] [CrossRef]
- DNA (Cytosine-5)-Methyltransferase 3A. Available online: https://en.wikipedia.org/wiki/DNA_(cytosine-5)-methyltransferase_3A_Wikipedia (accessed on 10 July 2024).
- Ibrahim, M.L.; Klement, J.D.; Lu, C.; Redd, P.S.; Xiao, W.; Yang, D.; Browning, D.D.; Savage, N.M.; Buckhaults, P.J.; Morse, H.C.; et al. Myeloid-Derived Suppressor Cells Produce IL-10 to Elicit DNMT3b-Dependent IRF8 Silencing to Promote Colitis-Associated Colon Tumorigenesis. Cell Rep. 2018, 25, 3036–3046.e6. [Google Scholar] [CrossRef]
- Feng, Y.X.; Jin, D.X.; Sokol, E.S.; Reinhardt, F.; Miller, D.H.; Gupta, P.B. Cancer-Specific PERK Signaling Drives Invasion and Metastasis through CREB3L1. Nat. Commun. 2017, 9, 1079. [Google Scholar] [CrossRef]
- Mellor, P.; Kendall, S.; Smith, S.; Saxena, A.; Anderson, D.H. Reduced CREB3L1 Expression in Triple Negative and Luminal a Breast Cancer Cells Contributes to Enhanced Cell Migration, Anchorage-Independent Growth and Metastasis. PLoS ONE 2022, 17, e0271090. [Google Scholar] [CrossRef]
- Rose, M.; Schubert, C.; Dierichs, L.; Gaisa, N.T.; Heer, M.; Heidenreich, A.; Knüchel, R.; Dahl, E. OASIS/CREB3L1 Is Epigenetically Silenced in Human Bladder Cancer Facilitating Tumor Cell Spreading and Migration in Vitro. Epigenetics 2015, 9, 1626–1640. [Google Scholar] [CrossRef]
- Chien, H.-P.; Ueng, S.-H.; Chen, S.-C.; Chang, Y.-S.; Lin, Y.-C.; Lo, Y.-F.; Chang, H.-K.; Chuang, W.-Y.; Huang, Y.-T.; Cheung, Y.-C.; et al. Expression of ROR1 Has Prognostic Significance in Triple Negative Breast Cancer. Virchows Arch. 2016, 468, 589–595. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, L. Upregulation of Long Non-Coding RNA ROR1-AS1 Promotes Cell Growth and Migration in Bladder Cancer by Regulation of miR-50. PLoS ONE 2020, 15, 1. [Google Scholar] [CrossRef]
- Bayat, A.-A.; Sadeghi, N.; Fatemi, R.; Nowroozi, M.R.; Ohadian Moghadam, S.; Borzuee, M.; Radmanesh, A.; Khodadoost, M.; Sarrafzadeh, A.R.; Zarei, O.; et al. Monoclonal Antibody Against ROR1 Induces Apoptosis in Human Bladder Carcinoma Cells. Avicenna J. Med. Biotechnol. 2020, 12, 165–171. [Google Scholar] [PubMed]
- Yuxiong, W.; Faping, L.; Bin, L.; Yanghe, Z.; Yao, L.; Yunkuo, L.; Yishu, W.; Honglan, Z. Regulatory Mechanisms of the cAMP-Responsive Element Binding Protein 3 (CREB3) Family in Cancers. Biomed. Pharmacother. 2023, 166, 115335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, H.Y.; Marzec, M.; Raghunath, P.N.; Nagasawa, T.; Wasik, M.A. STAT3- and DNA Methyltransferase 1-Mediated Epigenetic Silencing of SHP-1 Tyrosine Phosphatase Tumor Suppressor Gene in Malignant T Lymphocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 6948–6953. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Yi, Y.W.; Hou, S.-J.; Kim, H.J.; Kong, Y.; Bae, I.; Brown, M.L. Disruption of STAT3-DNMT1 Interaction by SH-I-14 Induces Re-Expression of Tumor Suppressor Genes and Inhibits Growth of Triple-Negative Breast Tumor. Oncotarget 2017, 8, 83457–83468. [Google Scholar] [CrossRef]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA Methyltransferase Inhibitors Combination Therapy for the Treatment of Solid Tumor: Mechanism and Clinical Application. Clin. Epigenetics 2021, 13, 166. [Google Scholar] [CrossRef]
- Ye, C.; Jiang, N.; Zheng, J.; Zhang, S.; Zhang, J.; Zhou, J. Epigenetic Therapy: Research Progress of Decitabine in the Treatment of Solid Tumors. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1879, 189066. [Google Scholar] [CrossRef]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and Triple-Negative Breast Cancer: Challenges and Treatment Options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reed, V.L.; Lalu, E.; Yoon, L.; Fultang, N.; Peethambaran, B. Uncovering a Novel Role of ROR1 in the Epigenetic Regulation of Tumor Suppressor Gene CREB3L1 in Triple-Negative Breast Cancer Cells. Biomolecules 2025, 15, 734. https://doi.org/10.3390/biom15050734
Reed VL, Lalu E, Yoon L, Fultang N, Peethambaran B. Uncovering a Novel Role of ROR1 in the Epigenetic Regulation of Tumor Suppressor Gene CREB3L1 in Triple-Negative Breast Cancer Cells. Biomolecules. 2025; 15(5):734. https://doi.org/10.3390/biom15050734
Chicago/Turabian StyleReed, Victoria L., Eric Lalu, Leena Yoon, Norman Fultang, and Bela Peethambaran. 2025. "Uncovering a Novel Role of ROR1 in the Epigenetic Regulation of Tumor Suppressor Gene CREB3L1 in Triple-Negative Breast Cancer Cells" Biomolecules 15, no. 5: 734. https://doi.org/10.3390/biom15050734
APA StyleReed, V. L., Lalu, E., Yoon, L., Fultang, N., & Peethambaran, B. (2025). Uncovering a Novel Role of ROR1 in the Epigenetic Regulation of Tumor Suppressor Gene CREB3L1 in Triple-Negative Breast Cancer Cells. Biomolecules, 15(5), 734. https://doi.org/10.3390/biom15050734






