Silver Nanoparticles (AgNPs) from Lysinibacillus sp. Culture Broths: Antibacterial Activity, Mechanism Insights, and Synergy with Classical Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms, Culture Media Used, and Cell-Free Broth Preparation
2.2. Green Synthesis of AgNPs
2.3. Determination of the AgNPs’ Physicochemical Properties
2.4. Evaluation of AgNPs’ Antibacterial Activity
2.5. Testing of the Putative Antibacterial Synergy of AgNPs with Classical Antibiotics
2.6. Reactive Oxygen Species (ROS) Production and Bactericidal Activity of the AgNPs
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phylogenetic Identification of the Bacterial Isolate
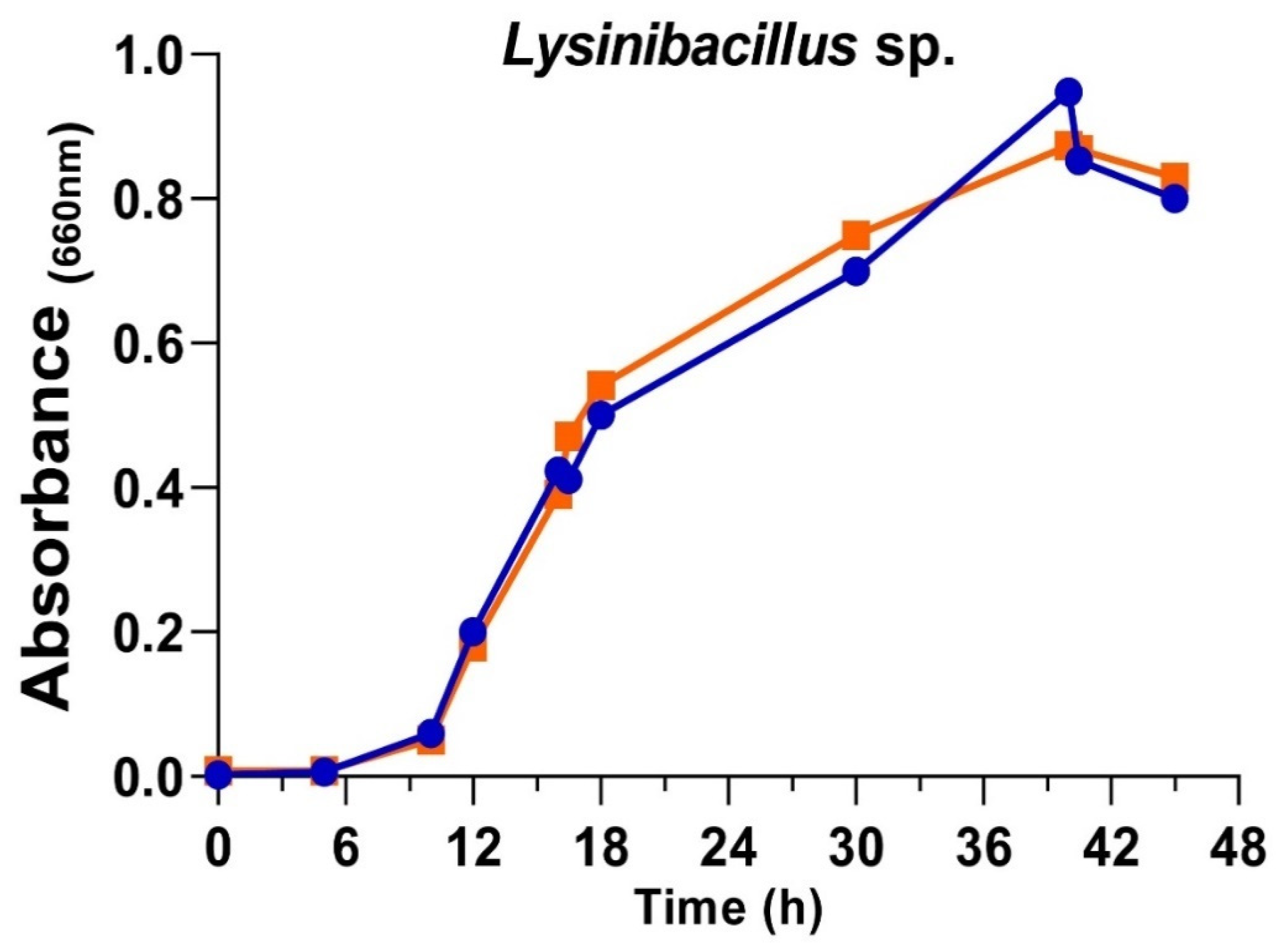
3.2. Growth of Lysinibacillus sp. in Two Culture Conditions
3.3. Synthesis of AgNPs Using Lysinibacillus sp. Culture Broths
3.4. Stability of the AgNPs in the Dark at 4 °C
3.5. Physicochemical Characteristics of the AgNPs
3.5.1. AgNPs’ Elemental Composition Determined by Total Reflection X-Ray Fluorescence (TXRF)
3.5.2. AgNP Crystallinity Determined by X-Ray Diffraction (XRD)
3.5.3. The Shape and Size of the AgNPs Core Determined by Transmission Electron Microscopy (TEM)
3.5.4. Hydrodynamic Size and Z-Potential of the AgNPs Determined by Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS)
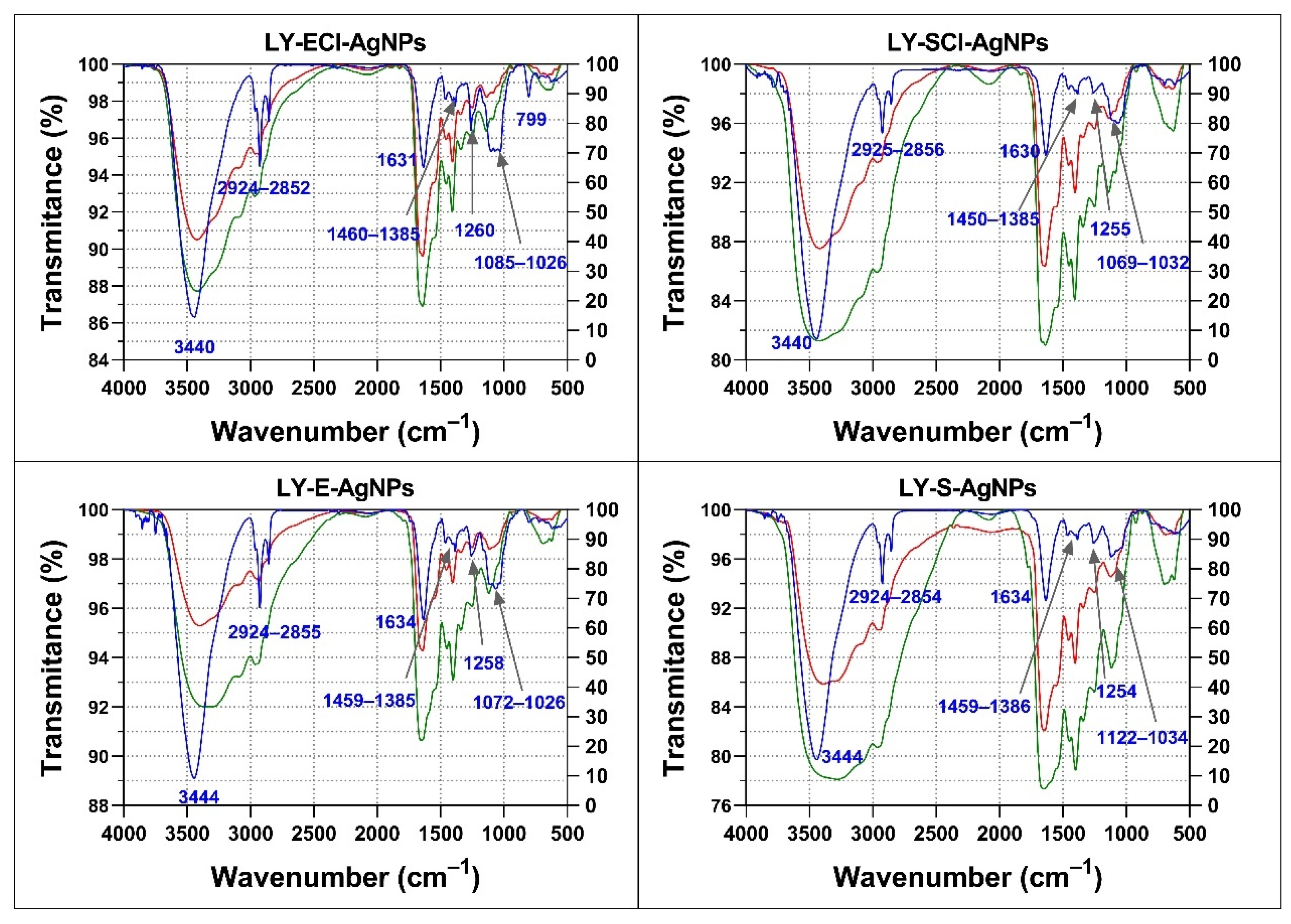
3.5.5. Insights into the AgNPs’ Corona Composition Determined by Fourier Transform Infrared Spectroscopy (FTIR)
3.6. AgNPs’ Antibacterial Activity
3.7. Synergistic Antibacterial Activity of the AgNPs with Classical Antibiotics
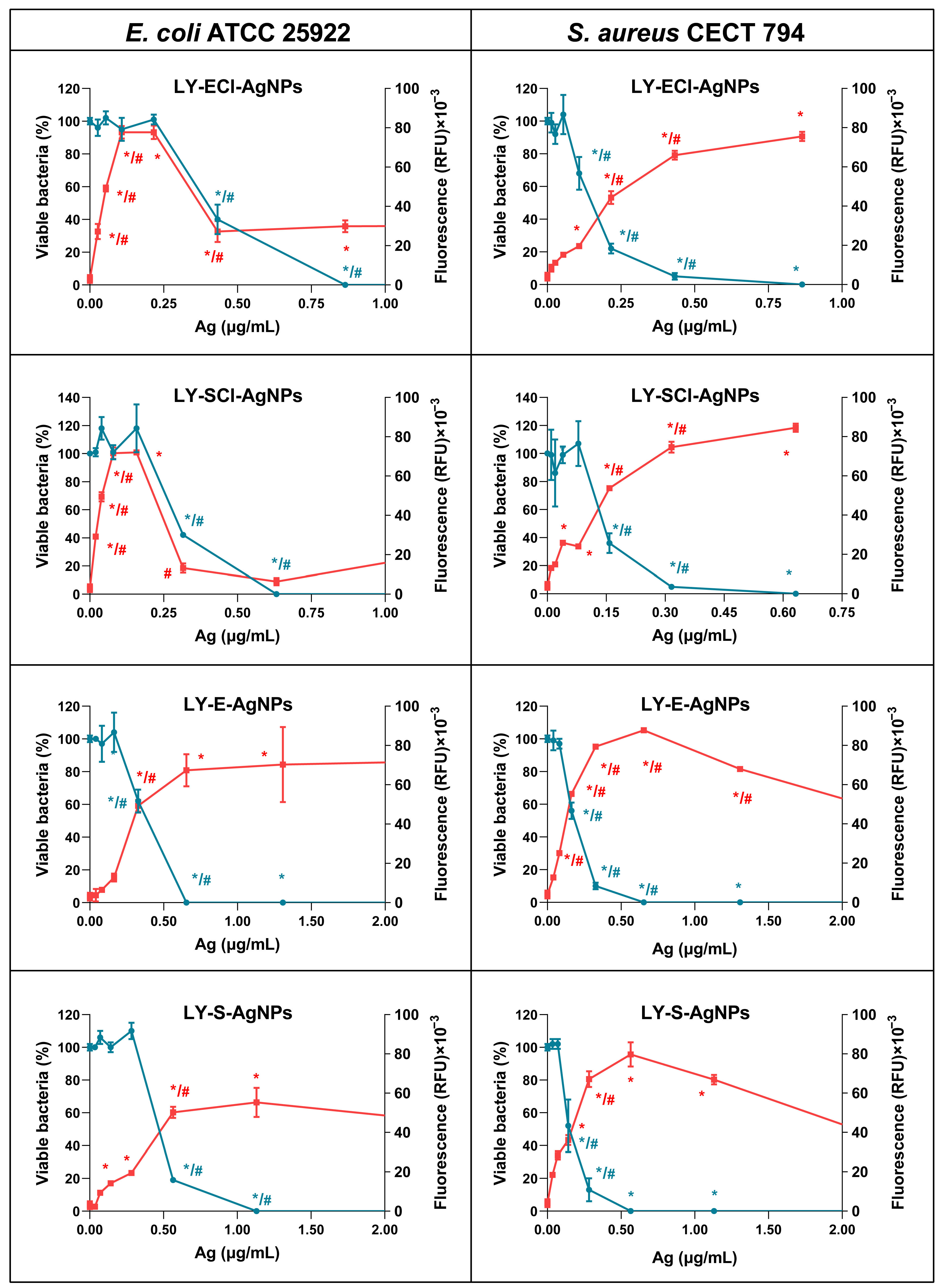
3.8. ROS Production and Bacteria Killing by the AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kariuki, S. Global Burden of Antimicrobial Resistance and Forecasts to 2050. Lancet 2024, 404, 1172–1173. [Google Scholar] [CrossRef]
- Mba, I.E.; Nweze, E.I. Nanoparticles as Therapeutic Options for Treating Multidrug-Resistant Bacteria: Research Progress, Challenges, and Prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Garay, R.; Lara-Ortiz, L.F.; Campos-López, M.; Gonzalez-Rodriguez, D.E.; Gamboa-Lugo, M.M.; Mendoza-Pérez, J.A.; Anzueto-Ríos, Á.; Nicolás-Álvarez, D.E. A Comprehensive Review of Silver and Gold Nanoparticles as Effective Antibacterial Agents. Pharmaceuticals 2024, 17, 1134. [Google Scholar] [CrossRef]
- Mateo, E.M.; Jiménez, M. Silver Nanoparticle-Based Therapy: Can It Be Useful to Combat Multi-Drug Resistant Bacteria? Antibiotics 2022, 11, 1205. [Google Scholar] [CrossRef] [PubMed]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.-A.; Mocan, L. Review on Silver Nanoparticles as a Novel Class of Antibacterial Solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Abdelmoneim, D.; Coates, D.; Porter, G.; Schmidlin, P.; Li, K.C.; Botter, S.; Lim, K.; Duncan, W. In Vitro and in Vivo Investigation of Antibacterial Silver Nanoparticles Functionalized Bone Grafting Substitutes. J. Biomed. Mater. Res. Part. A 2024, 112, 2042–2054. [Google Scholar] [CrossRef]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In Vivo and In Vitro Antimicrobial Activity of Biogenic Silver Nanoparticles against Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- Wali, N.; Shabbir, A.; Wajid, N.; Abbas, N.; Naqvi, S.Z.H. Synergistic Efficacy of Colistin and Silver Nanoparticles Impregnated Human Amniotic Membrane in a Burn Wound Infected Rat Model. Sci. Rep. 2022, 12, 6414. [Google Scholar] [CrossRef]
- Garcia-Reyero, N.; Kennedy, A.J.; Escalon, B.L.; Habib, T.; Laird, J.G.; Rawat, A.; Wiseman, S.; Hecker, M.; Denslow, N.; Steevens, J.A.; et al. Differential Effects and Potential Adverse Outcomes of Ionic Silver and Silver Nanoparticles in Vivo and in Vitro. Environ. Sci. Technol. 2014, 48, 4546–4555. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Ralte, V.; Zohmingliana, H.; Das, S.; Anal, J.M.H.; Lallianrawna, S.; Rokhum, S.L. A Review of Microbes Mediated Biosynthesis of Silver Nanoparticles and Their Enhanced Antimicrobial Activities. Heliyon 2024, 10, e32333. [Google Scholar] [CrossRef] [PubMed]
- Bharose, A.A.; Hajare, S.T.; Gajera, H.P.; Soni, M.; Prajapati, K.K.; Singh, S.C.; Upadhye, V. Bacteria-Mediated Green Synthesis of Silver Nanoparticles and Their Antifungal Potentials against Aspergillus flavus. PLoS ONE 2024, 19, e0297870. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.; Ismail, E.; Maboza, E.; Hussein, A.A.; Adam, R.Z. Green-Synthesized Silver Nanoparticles: Antifungal and Cytotoxic Potential for Further Dental Applications. J. Funct. Biomater. 2023, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Sabaghian, H. Silver Nanoparticles as Antiviral and Antibacterial Agents: A Comprehensive Review of Synthesis Methods and Therapeutic Application. ChemistrySelect 2024, 9, e202304941. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A. Green Nanoparticles: The Future of Nanobiotechnology; Springer: Singapore, 2022; ISBN 9789811671050. [Google Scholar]
- De Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Costa, J.A.S.; da Silva, C.M.P.; Bilal, M.; Iqbal, H.M.N.; Ferreira, L.F.R.; Figueiredo, R.T. Environmental Remediation Potentialities of Metal and Metal Oxide Nanoparticles: Mechanistic Biosynthesis, Influencing Factors, and Application Standpoint. Environ. Technol. Innov. 2021, 24, 101851. [Google Scholar] [CrossRef]
- Saritha, P.; Arunprakash, S.; Srinivasan, P.; Al-Ansari, M.M.; Singh, S.; Dixit, S. Luminescent Silver Nanoparticles Biosynthesis Using Flower Extract: Antibacterial and Anticancer Potential. Luminescence 2024, 39, e70005. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, R.; Yin, T.; Wang, Q.; Guo, Z.; Qiwen, T.; Bilal, M.; He, S.; Zhu, X.; Shi, H.; et al. Novel Bio-Fabrication of Silver Nanoparticles Using the Cell-Free Extract of Lysinibacillus fusiformis sp. and Their Potent Activity against Pathogenic Fungi. Mater. Res. Express 2020, 6, 1250f2. [Google Scholar] [CrossRef]
- Kaiser, K.G.; Delattre, V.; Frost, V.J.; Buck, G.W.; Phu, J.V.; Fernandez, T.G.; Pavel, I.E. Nanosilver: An Old Antibacterial Agent with Great Promise in the Fight against Antibiotic Resistance. Antibiotics 2023, 12, 1264. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Sun, Y.-Z.; Vasantharaj, S.; Ghafarifarsani, H.; Hoseinifar, S.H.; Raeeszadeh, M. Applications of Green Synthesized Metal Nanoparticles—A Review. Biol. Trace Elem. Res. 2024, 202, 360–386. [Google Scholar] [CrossRef]
- Thomas, S.; Gonsalves, R.A.; Jose, J.; Zyoud, S.H.; Prasad, A.R.; Garvasis, J. Plant-Based Synthesis, Characterization Approaches, Applications and Toxicity of Silver Nanoparticles: A Comprehensive Review. J. Biotechnol. 2024, 394, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Salnus, S.; Wahab, W.; Arfah, R.; Zenta, F.; Natsir, H.; Muri, M.; Fatimah, F.; Rajab, A.; Armah, Z.; Irfandi, R. A Review on Green Synthesis, Antimicrobial Applications and Toxicity of Silver Nanoparticles Mediated by Plant Extract. Indones. J. Chem. 2022, 22, 1129–1143. [Google Scholar] [CrossRef]
- Jacob, J.M.; Ravindran, R.; Narayanan, M.; Samuel, S.M.; Pugazhendhi, A.; Kumar, G. Microalgae: A Prospective Low Cost Green Alternative for Nanoparticle Synthesis. Curr. Opin. Environ. Sci. Health 2021, 20, 100163. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Kim, H.J.; Jo, J.W.; Rangarajulu, S.K. Macrofungal Mediated Biosynthesis of Silver Nanoparticles and Evaluation of Its Antibacterial and Wound-Healing Efficacy. Int. J. Mol. Sci. 2024, 25, 861. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Sajjad, W.; Wu, F.; Bibi, N.; Shah, K.; Yali, Z.; Wang, W. Green Synthesis of Silver Nanoparticles and Their Shortcomings, Animal Blood a Potential Source for Silver Nanoparticles: A Review. J. Hazard. Mater. Adv. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Han, L.; Kim, Y.S.; Cho, S.; Park, Y. Invertebrate Water Extracts as Biocompatible Reducing Agents for the Green Synthesis of Gold and Silver Nanoparticles. Nat. Prod. Commun. 2013, 8, 1149–1152. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Bhaskar, R.; Seok, Y.J.; Han, S.S. Photocatalytic Degradation, Anticancer, and Antibacterial Studies of Lysinibacillus sphaericus Biosynthesized Hybrid Metal/Semiconductor Nanocomposites. Microorganisms 2023, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Al-khattaf, F.S. Gold and Silver Nanoparticles: Green Synthesis, Microbes, Mechanism, Factors, Plant Disease Management and Environmental Risks. Saudi J. Biol. Sci. 2021, 28, 3624–3631. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Strong Antimicrobial Activity of Silver Nanoparticles Obtained by the Green Synthesis in Viridibacillus sp. Extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef]
- Baltazar-Encarnación, E.; Escárcega-González, C.E.; Vasto-Anzaldo, X.G.; Cantú-Cárdenas, M.E.; Morones-Ramírez, J.R. Silver Nanoparticles Synthesized through Green Methods Using Escherichia coli Top 10 (Ec-Ts) Growth Culture Medium Exhibit Antimicrobial Properties against Nongrowing Bacterial Strains. J. Nanomater. 2019, 2019, 4637325. [Google Scholar] [CrossRef]
- Luo, K.; Jung, S.; Park, K.-H.; Kim, Y.-R. Microbial Biosynthesis of Silver Nanoparticles in Different Culture Media. J. Agric. Food Chem. 2018, 66, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Javani, S.; Marín, I.; Amils, R.; Abad, J.P. Four Psychrophilic Bacteria from Antarctica Extracellularly Biosynthesize at Low Temperature Highly Stable Silver Nanoparticles with Outstanding Antimicrobial Activity. Colloids Surf. A 2015, 483, 60–69. [Google Scholar] [CrossRef]
- Alves, M.F.; Murray, P.G. Biological Synthesis of Monodisperse Uniform-Size Silver Nanoparticles (AgNPs) by Fungal Cell-Free Extracts at Elevated Temperature and pH. J. Fungi 2022, 8, 439. [Google Scholar] [CrossRef]
- Wu, L.; Wei, S.; Cheng, X.; He, N.; Kang, X.; Zhou, H.; Cai, Y.; Ye, Y.; Li, P.; Liang, C. Release of Ions Enhanced the Antibacterial Performance of Laser-Generated, Uncoated Ag Nanoparticles. Colloids Surf. B 2024, 243, 114131. [Google Scholar] [CrossRef]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green Synthesis and Potential Antibacterial Applications of Bioactive Silver Nanoparticles: A Review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Vikulina, A.; Loughlin, M.; Volodkin, D. How Similar Is the Antibacterial Activity of Silver Nanoparticles Coated with Different Capping Agents? RSC Adv. 2023, 13, 10542–10555. [Google Scholar] [CrossRef]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Arsene, M.M.J. Synergy Test for Antibacterial Activity: Towards the Research for a Consensus between the Fractional Inhibitory Concentration (Checkboard Method) and the Increase in Fold Area (Disc Diffusion Method). Clin. Res. Anim. Sci. 2021, 1, CRAS.000519. [Google Scholar]
- Pillai, S.; Moellering, R.; Eliopoulos, G. Antimicrobial Combinations. In Antibiotics in Laboratory Medicine, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; pp. 365–440. [Google Scholar]
- Odds, F.C. Synergy, Antagonism, and What the Chequerboard Puts between Them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, J.; Singh, K. Transformation of Malathion by Lysinibacillus sp. Isolated from Soil. Biotechnol. Lett. 2012, 34, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Park, S.-H.; Oh, S.-H.; Lee, J.-J.; Kwon, K.K.; Kim, S.-J.; Choi, M.; Rha, E.; Lee, H.; Lee, D.-H.; et al. Discovery and Biochemical Characterization of a Methanol Dehydrogenase from Lysinibacillus xylanilyticus. Front. Bioeng. Biotechnol. 2020, 8, 67. [Google Scholar] [CrossRef]
- El-Bendary, M.A. Bacillus thuringiensis and Bacillus sphaericus Biopesticides Production. J. Basic. Microbiol. 2006, 46, 158–170. [Google Scholar] [CrossRef]
- Omole, R.K.; George, R.C.; Adeyemi, O.I.; Torimiro, N.; Saravanan, M.; Agboluaje, E.O.; Xiong, M.P. Spectral Characterization of Silver Nanoparticles Biosynthesized from Lysinibacillus fusiformis and Its Antibacterial Efficacy Against Multidrug-Resistant Bacteria Isolated from Chronic Wounds. BioNanoSci 2024, 14, 688–698. [Google Scholar] [CrossRef]
- Huq, M.A. Green Synthesis of Silver Nanoparticles Using Pseudoduganella eburnea MAHUQ-39 and Their Antimicrobial Mechanisms Investigation against Drug Resistant Human Pathogens. Int. J. Mol. Sci. 2020, 21, 1510. [Google Scholar] [CrossRef]
- Bhatia, D.; Mittal, A.; Malik, D.K. Antimicrobial Potential and in Vitro Cytotoxicity Study of Polyvinyl Pyrollidone-Stabilised Silver Nanoparticles Synthesised from Lysinibacillus boronitolerans. IET Nanobiotechnol. 2021, 15, 427–440. [Google Scholar] [CrossRef]
- Bhatia, D.; Mittal, A.; Malik, D.K. Antimicrobial Activity of PVP Coated Silver Nanoparticles Synthesized by Lysinibacillus varians. 3 Biotech 2016, 6, 196. [Google Scholar] [CrossRef]
- El-Bendary, M.A.; Abdelraof, M.; Moharam, M.E.; Elmahdy, E.M.; Allam, M.A. Potential of Silver Nanoparticles Synthesized Using Low Active Mosquitocidal Lysinibacillus sphaericus as Novel Antimicrobial Agents. Prep. Biochem. Biotechnol. 2021, 51, 926–935. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, W.-H.; Song, Y.-P.; Wang, Q.; Kan, Y.-F.; Wang, S.-Y.; Xia, J.-L.; Bilal, M.; Zhu, X.-Y.; Wang, Z.-X.; et al. Characterization, Antimicrobial, and Antioxidant Potentialities of First-Time Isolated Silver Nanoparticles Synthesizing Protein Secreted by Lysinibacillus sphaericus. Process Biochem. 2022, 122, 230–237. [Google Scholar] [CrossRef]
- Pernas-Pleite, C.; Conejo-Martínez, A.M.; Fernández Freire, P.; Hazen, M.J.; Marín, I.; Abad, J.P. Microalga Broths Synthesize Antibacterial and Non-Cytotoxic Silver Nanoparticles Showing Synergy with Antibiotics and Bacterial ROS Induction and Can Be Reused for Successive AgNP Batches. Int. J. Mol. Sci. 2023, 24, 16183. [Google Scholar] [CrossRef] [PubMed]
- Pernas-Pleite, C.; Conejo-Martínez, A.M.; Marín, I.; Abad, J.P. Green Extracellular Synthesis of Silver Nanoparticles by Pseudomonas alloputida, Their Growth and Biofilm-Formation Inhibitory Activities and Synergic Behavior with Three Classical Antibiotics. Molecules 2022, 27, 7589. [Google Scholar] [CrossRef]
- Eduardo-Correia, B.; Morales-Filloy, H.; Abad, J.P. Bacteria From the Multi-Contaminated Tinto River Estuary (SW, Spain) Show High Multi-Resistance to Antibiotics and Point to Paenibacillus spp. as Antibiotic-Resistance-Dissemination Players. Front. Microbiol. 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Eduardo Correia, B. Abundancia, Diversidad y Perfiles de Multirresistencia de Bacterias Cultivables Resistentes a Los Antibióticos en la ría de Huelva y La Chorrera de Despeñalaguna (Guadalajara). Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2016. [Google Scholar]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical Review on Biofilm Methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef]
- Allend, S.O.; Garcia, M.O.; da Cunha, K.F.; de Albernaz, D.T.F.; da Silva, M.E.; Ishikame, R.Y.; Panagio, L.A.; Nakazaro, G.; Reis, G.F.; Pereira, D.B.; et al. Biogenic Silver Nanoparticle (Bio-AgNP) Has an Antibacterial Effect against Carbapenem-resistant Acinetobacter baumannii with Synergism and Additivity When Combined with Polymyxin B. J. Appl. Microbiol. 2022, 132, 1036–1047. [Google Scholar] [CrossRef]
- Gebauer, J.S.; Treuel, L. Influence of Individual Ionic Components on the Agglomeration Kinetics of Silver Nanoparticles. J. Colloid. Interface Sci. 2011, 354, 546–554. [Google Scholar] [CrossRef]
- Xu, J.-M.; Lu, C.; Wang, W.-J.; Du, Z.-Y.; Pan, J.-J.; Cheng, F.; Wang, Y.-S.; Liu, Z.-Q.; Zheng, Y.-G. Strain Screening and Particle Formation: A Lysinibacillus boronitolerans for Self-Healing Concrete. Appl. Environ. Microbiol. 2022, 88, e00804-22. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Matter, I.A.; Eida, M.F.; Moawad, H.; Oh, Y.-K. Influence of Nitrogen Source and Growth Phase on Extracellular Biosynthesis of Silver Nanoparticles Using Cultural Filtrates of Scenedesmus obliquus. Appl. Sci. 2019, 9, 1465. [Google Scholar] [CrossRef]
- Tikariha, S.; Banerjee, S.; Dev, A.; Singh, S. Growth Phase-Dependent Synthesis of Gold Nanoparticles Using Bacillus licheniformis. In Proceedings of the Applications of Biotechnology for Sustainable Development; Mukhopadhyay, K., Sachan, A., Kumar, M., Eds.; Springer: Singapore, 2017; pp. 121–128. [Google Scholar]
- Fernández-Llamosas, H.; Castro, L.; Blázquez, M.L.; Díaz, E.; Carmona, M. Biosynthesis of Selenium Nanoparticles by Azoarcus sp. CIB. Microb. Cell Factories 2016, 15, 109. [Google Scholar] [CrossRef] [PubMed]
- Kaler, A.; Jain, S.; Banerjee, U.C. Green and Rapid Synthesis of Anticancerous Silver Nanoparticles by Saccharomyces boulardii and Insight into Mechanism of Nanoparticle Synthesis. BioMed Res. Int. 2013, 2013, e872940. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaur, K.; Singh, A.; Kaur, K. Biological and Physical Applications of Silver Nanoparticles with Emerging Trends of Green Synthesis. In Engineered Nanomaterials—Health and Safety; IntechOpen: Rijeka, Croatia, 2019; ISBN 978-1-83880-412-1. [Google Scholar]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, Purification and Characterization of Silver Nanoparticles Using Escherichia coli. Colloids Surf. B 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Chen, A.; Contreras, L.M.; Keitz, B.K. Imposed Environmental Stresses Facilitate Cell-Free Nanoparticle Formation by Deinococcus radiodurans. Appl. Environ. Microbiol. 2017, 83, e00798-17. [Google Scholar] [CrossRef]
- Jalab, J.; Abdelwahed, W.; Kitaz, A.; Al-Kayali, R. Green Synthesis of Silver Nanoparticles Using Aqueous Extract of Acacia cyanophylla and Its Antibacterial Activity. Heliyon 2021, 7, e08033. [Google Scholar] [CrossRef]
- Sun, Y. Controlled Synthesis of Colloidal Silver Nanoparticles in Organic Solutions: Empirical Rules for Nucleation Engineering. Chem. Soc. Rev. 2013, 42, 2497–2511. [Google Scholar] [CrossRef]
- Pinto, V.V.; Ferreira, M.J.; Silva, R.; Santos, H.A.; Silva, F.; Pereira, C.M. Long Time Effect on the Stability of Silver Nanoparticles in Aqueous Medium: Effect of the Synthesis and Storage Conditions. Colloids Surf. A 2010, 364, 19–25. [Google Scholar] [CrossRef]
- Pham, T.-L. Toxicity of Silver Nanoparticles to Tropical Microalgae Scenedesmus acuminatus, Chaetoceros gracilis and Crustacean Daphnia lumholtzi. Turk. J. Fish. Aquat. Sci. 2019, 19, 1009–1016. [Google Scholar] [CrossRef]
- Dede, A.; Aytekin-Aydin, M.T.; Güven, K. Green Biosynthesis of Silver Nanoparticles from Olive and Walnut-Related Bacteria, Synthesis, Characterization, and Antimicrobial Activity. Indian. J. Microbiol. 2023, 63, 658–667. [Google Scholar] [CrossRef]
- Taipe Huisa, A.J.; Estrella Josende, M.; Gelesky, M.A.; Fernandes Ramos, D.; López, G.; Bernardi, F.; Monserrat, J.M. Açaí (Euterpe oleracea Mart.) Green Synthesis of Silver Nanoparticles: Antimicrobial Efficacy and Ecotoxicological Assessment. Environ. Sci. Pollut. Res. 2024, 31, 12005–12018. [Google Scholar] [CrossRef]
- Abd Alamer, I.S.; Tomah, A.A.; Ahmed, T.; Li, B.; Zhang, J. Biosynthesis of Silver Chloride Nanoparticles by Rhizospheric Bacteria and Their Antibacterial Activity against Phytopathogenic Bacterium Ralstonia solanacearum. Molecules 2022, 27, 224. [Google Scholar] [CrossRef] [PubMed]
- Tun, W.S.T.; Hongsing, N.; Sirithongsuk, P.; Nasompak, S.; Daduang, S.; Klaynongsruang, S.; Taweechaisupapong, S.; Chareonsudjai, S.; Prangkio, P.; Kosolwattana, S.; et al. The Synergistic Action of Silver Nanoparticles and Ceftazidime against Antibiotic-Resistant Burkholderia pseudomallei: A Modifying Treatment. Process Biochem. 2024, 136, 351–361. [Google Scholar] [CrossRef]
- Aabed, K.; Mohammed, A.E. Synergistic and Antagonistic Effects of Biogenic Silver Nanoparticles in Combination with Antibiotics Against Some Pathogenic Microbes. Front. Bioeng. Biotechnol. 2021, 9, 652362. [Google Scholar] [CrossRef]
- Ghiuta, I.; Croitoru, C.; Kost, J.; Wenkert, R.; Munteanu, D. Bacteria-Mediated Synthesis of Silver and Silver Chloride Nanoparticles and Their Antimicrobial Activity. Appl. Sci. 2021, 11, 3134. [Google Scholar] [CrossRef]
- da Silva Ferreira, V.; ConzFerreira, M.E.; Lima, L.M.T.R.; Frasés, S.; de Souza, W.; Sant’Anna, C. Green Production of Microalgae-Based Silver Chloride Nanoparticles with Antimicrobial Activity against Pathogenic Bacteria. Enzyme Microb. Technol. 2017, 97, 114–121. [Google Scholar] [CrossRef]
- Restrepo, C.V.; Villa, C.C. Synthesis of Silver Nanoparticles, Influence of Capping Agents, and Dependence on Size and Shape: A Review. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100428. [Google Scholar] [CrossRef]
- Cheon, J.Y.; Kim, S.J.; Rhee, Y.H.; Kwon, O.H.; Park, W.H. Shape-Dependent Antimicrobial Activities of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 2773–2780. [Google Scholar] [CrossRef]
- Soleimani, F.F.; Saleh, T.; Shojaosadati, S.A.; Poursalehi, R. Green Synthesis of Different Shapes of Silver Nanostructures and Evaluation of Their Antibacterial and Cytotoxic Activity. BioNanoSci 2018, 8, 72–80. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef]
- Amaro, F.; Morón, Á.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Metallic Nanoparticles—Friends or Foes in the Battle against Antibiotic-Resistant Bacteria? Microorganisms 2021, 9, 364. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Wang, Z.; Zhao, Y. Controlled Synthesis of Ag Nanoparticles with Different Morphologies and Their Antibacterial Properties. Mater. Sci. Eng. C 2013, 33, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Bajrami, D.; Hossain, S.I.; Barbarossa, A.; Sportelli, M.C.; Picca, R.A.; Gentile, L.; Mastrolonardo, F.; Rosato, A.; Carocci, A.; Colabufo, N.A.; et al. A Scalable Route to Quaternary Ammonium-Functionalized AgCl Colloidal Antimicrobials Inhibiting Food Pathogenic Bacteria and Biofilms. Heliyon 2024, 10, e25260. [Google Scholar] [CrossRef] [PubMed]
- Suganya, M.; Preethi, P.S.; Narenkumar, J.; Prakash, A.A.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Nanthini, A.U.R. Synthesis of Silver Nanoparticles from Indian Red Yeast Rice and Its Inhibition of Biofilm in Copper Metal in Cooling Water Environment. Environ. Sci. Pollut. Res. 2022, 29, 77800–77808. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.K.; Philip, D. Catalytic Degradation of Organic Dyes Using Biosynthesized Silver Nanoparticles. Micron 2014, 56, 54–62. [Google Scholar] [CrossRef]
- Nobbmann, U. Polydispersity–What Does It Mean for DLS and Chromatography. 2014. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/insights/polydispersity-what-does-it-mean-for-dls-and-chromatography (accessed on 4 May 2025).
- Worldwide Malvern Instruments. Dynamic Light Scattering, Common Terms Defined; Inform White Paper: Malvern, UK, 2017; Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/whitepapers/wp111214dlstermsdefined (accessed on 4 May 2025).
- Meléndrez, M.F.; Cárdenas, G.; Arbiol, J. Synthesis and Characterization of Gallium Colloidal Nanoparticles. J. Colloid. Interface Sci. 2010, 346, 279–287. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Patel, V.R.; Agrawal, Y.K. Nanosuspension: An Approach to Enhance Solubility of Drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy. In Analytical Techniques in Forensic Science; Wolstenholme, R., Jickells, S., Forbes, S., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 145–160. ISBN 978-1-119-37342-1. [Google Scholar]
- Ferro, L.; Gojkovic, Z.; Gorzsás, A.; Funk, C. Statistical Methods for Rapid Quantification of Proteins, Lipids, and Carbohydrates in Nordic Microalgal Species Using ATR–FTIR Spectroscopy. Molecules 2019, 24, 3237. [Google Scholar] [CrossRef]
- Pietsch, F.; Heidrich, G.; Nordholt, N.; Schreiber, F. Prevalent Synergy and Antagonism Among Antibiotics and Biocides in Pseudomonas aeruginosa. Front. Microbiol. 2021, 11, 615618. [Google Scholar] [CrossRef]
- Li, W.-R.; Sun, T.-L.; Zhou, S.-L.; Ma, Y.-K.; Shi, Q.-S.; Xie, X.-B.; Huang, X.-M. A Comparative Analysis of Antibacterial Activity, Dynamics, and Effects of Silver Ions and Silver Nanoparticles against Four Bacterial Strains. Int. Biodeterior. Biodegrad. 2017, 123, 304–310. [Google Scholar] [CrossRef]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of Silver Nanoparticles and Silver Ions on Growth and Adaptive Response Mechanisms of Pseudomonas putida Mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef]
- Musini, A.; Pravalika, E.; Preethi, M.G.; Sri, I.J. Microbiologically Synthesized Nanoparticles and Their Role in Biofilm Inhibition. In Microbial Processes for Synthesizing Nanomaterials; Maddela, N.R., Rodríguez Díaz, J.M., Branco da Silva Montenegro, M.C., Prasad, R., Eds.; Springer Nature: Singapore, 2023; pp. 285–315. ISBN 978-981-9928-08-8. [Google Scholar]
- Salvioni, L.; Galbiati, E.; Collico, V.; Alessio, G.; Avvakumova, S.; Corsi, F.; Tortora, P.; Prosperi, D.; Colombo, M. Negatively Charged Silver Nanoparticles with Potent Antibacterial Activity and Reduced Toxicity for Pharmaceutical Preparations. Int. J. Nanomed. 2017, 12, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez Rodriguez, C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver Nanoparticles Enhance the Efficacy of Aminoglycosides against Antibiotic-Resistant Bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic Resistance: What Is so Special about Multidrug-Resistant Gram-Negative Bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.M.; Alsaleh, N.B.; Aljasham, A.T.; Tawfik, E.A.; Almutairi, M.M.; Assiri, M.A.; Alkholief, M.; Almutairi, M.M. Silver Nanoparticle-Based Combinations with Antimicrobial Agents against Antimicrobial-Resistant Clinical Isolates. Antibiotics 2022, 11, 1219. [Google Scholar] [CrossRef]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef]
- Chapa González, C.; González García, L.I.; Burciaga Jurado, L.G.; Carrillo Castillo, A. Bactericidal Activity of Silver Nanoparticles in Drug-Resistant Bacteria. Braz. J. Microbiol. 2023, 54, 691–701. [Google Scholar] [CrossRef]
- Fathil, M.A.M.; Taufeq, F.Y.F.; Abdalla, S.S.I.; Katas, H. Roles of Chitosan in Synthesis, Antibacterial and Anti-Biofilm Properties of Bionano Silver and Gold. RSC Adv. 2022, 12, 19297–19312. [Google Scholar] [CrossRef]
- Panáček, A.; Smékalová, M.; Kilianová, M.; Prucek, R.; Bogdanová, K.; Večeřová, R.; Kolář, M.; Havrdová, M.; Płaza, G.A.; Chojniak, J.; et al. Strong and Nonspecific Synergistic Antibacterial Efficiency of Antibiotics Combined with Silver Nanoparticles at Very Low Concentrations Showing No Cytotoxic Effect. Molecules 2016, 21, 26. [Google Scholar] [CrossRef]
- Li, J.; Rong, K.; Zhao, H.; Li, F.; Lu, Z.; Chen, R. Highly Selective Antibacterial Activities of Silver Nanoparticles Against Bacillus subtilis. J. Nanosci. Nanotechnol. 2013, 13, 6806–6813. [Google Scholar] [CrossRef] [PubMed]
- Levison, M.E. Pharmacodynamics of Antimicrobial Drugs. Infect. Dis. Clin. N. Am. 2004, 18, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Dudhagara, P.; Alagiya, J.; Bhagat, C.; Dudhagara, D.; Ghelani, A.; Desai, J.; Patel, R.; Vansia, A.; Nhiem, D.N.; Chen, Y.-Y.; et al. Biogenic Synthesis of Antibacterial, Hemocompatible, and Antiplatelets Lysozyme Functionalized Silver Nanoparticles through the One-Step Process for Therapeutic Applications. Processes 2022, 10, 623. [Google Scholar] [CrossRef]
- Otari, S.V.; Pati, R.M.; Ghosh, S.J.; Thorat, N.D.; Pawar, S.H. Intracellular Synthesis of Silver Nanoparticle by Actinobacteria and Its Antimicrobial Activity. Spectrochim. Acta Part A 2015, 136, 1175–1180. [Google Scholar] [CrossRef]
- Babapour, E.; Haddadi, A.; Mirnejad, R.; Angaji, S.-A.; Amirmozafari, N. Biofilm Formation in Clinical Isolates of Nosocomial Acinetobacter baumannii and Its Relationship with Multidrug Resistance. Asian Pac. J. Trop. Biomed. 2016, 6, 528–533. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Xie, S. Current Progress and Prospects of Organic Nanoparticles against Bacterial Biofilm. Adv. Colloid. Interface Sci. 2021, 294, 102475. [Google Scholar] [CrossRef]
- Ali, S.G.; Ansari, M.A.; Sajid Jamal, Q.M.; Khan, H.M.; Jalal, M.; Ahmad, H.; Mahdi, A.A. Antiquorum Sensing Activity of Silver Nanoparticles in P. aeruginosa: An in Silico Study. In Silico Pharmacol. 2017, 5, 12. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Sayed, F.A.-Z.; Eissa, N.G.; Shen, Y.; Hunstad, D.A.; Wooley, K.L.; Elsabahy, M. Morphologic Design of Nanostructures for Enhanced Antimicrobial Activity. J. Nanobiotechnol. 2022, 20, 536. [Google Scholar] [CrossRef]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape Dependent Physical Mutilation and Lethal Effects of Silver Nanoparticles on Bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 1, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, P.; Kvitek, L.; Panacek, A.; Prucek, R.; Hrbac, J.; Vecerova, R.; Zboril, R. Comparative Study of Antimicrobial Activity of AgBr and Ag Nanoparticles (NPs). PLoS ONE 2015, 10, e0119202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ghoshal, G.; Goyal, M. Rapid Green Synthesis of Silver Nanoparticles (AgNPs) Using (Prunus persica) Plants Extract: Exploring Its Antimicrobial and Catalytic Activities. J. Nanomed. Nanotechnol. 2017, 8, 452. [Google Scholar]
- Sun, W.; Sanderson, P.E.; Zheng, W. Drug Combination Therapy Increases Successful Drug Repositioning. Drug Discov. Today 2016, 21, 1189–1195. [Google Scholar] [CrossRef]
- Feizi, S.; Cooksley, C.M.; Nepal, R.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Silver Nanoparticles as a Bioadjuvant of Antibiotics against Biofilm-Mediated Infections with Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa in Chronic Rhinosinusitis Patients. Pathology 2022, 54, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Garibo Ruiz, D.; Nefedova, E.; Shkil, N.N.; Shkil, N.A.; Vazquez-Gomez, R.L.; Pestryakov, A.; Bogdanchikova, N. Silver Nanoparticles Targeting the Drug Resistance Problem of Streptococcus dysgalactiae: Susceptibility to Antibiotics and Efflux Effect. Int. J. Mol. Sci. 2022, 23, 6024. [Google Scholar] [CrossRef]
- Ghaffar, N.; Javad, S.; Farrukh, M.A.; Shah, A.A.; Gatasheh, M.K.; AL-Munqedhi, B.M.A.; Chaudhry, O. Metal Nanoparticles Assisted Revival of Streptomycin against MDRS Staphylococcus aureus. PLoS ONE 2022, 17, e0264588. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Mourad, M.I.; Alsewy, F.Z.; Azzam, N.F.A.E.M. Combination of Silver Nanoparticles with Ineffective Antibiotics against Extended Spectrum Beta-Lactamases Producing Isolates at Alexandria Main University Hospital, Egypt. Beni-Suef Univ. J. Basic. Appl. Sci. 2021, 10, 58. [Google Scholar] [CrossRef]
- Abdullah; Jamil, T.; Atif, M.; Khalid, S.; Metwally, K.; Yahya, G.; Moisa, M.; Cavalu, D.S. Recent Advances in the Development of Metal/Metal Oxide Nanoparticle and Antibiotic Conjugates (MNP–Antibiotics) to Address Antibiotic Resistance: Review and Perspective. Int. J. Mol. Sci. 2024, 25, 8915. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology Relating to Methods for the Determination of Susceptibility of Bacteria to Antimicrobial Agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Ballal, A.; Rath, D.; Rath, A. Novel Silver Nanoparticle-Antibiotic Combinations as Promising Antibacterial and Anti-Biofilm Candidates against Multiple-Antibiotic Resistant ESKAPE Microorganisms. Colloids Surfs. B 2024, 236, 113826. [Google Scholar] [CrossRef]
- Trzcińska-Wencel, J.; Wypij, M.; Rai, M.; Golińska, P. Biogenic Nanosilver Bearing Antimicrobial and Antibiofilm Activities and Its Potential for Application in Agriculture and Industry. Front. Microbiol. 2023, 14, 1125685. [Google Scholar] [CrossRef]
- Abdel-Wahab, F.; El Menofy, N.; El- Batal, A.; Mosallam, F.; Abdulall, A. Enhanced Antimicrobial Activity of the Combination of Silver Nanoparticles and Different β Lactam Antibiotics against Methicillin Resistant Staphylococcus aureus Isolates. Azhar Int. J. Pharm. Med. Sci. 2021, 1, 22–31. [Google Scholar] [CrossRef]
- Al-Momani, H.; Albalawi, H.; Al Balawi, D.; Khleifat, K.M.; Aolymat, I.; Hamed, S.; Albiss, B.A.; Khasawneh, A.I.; Ebbeni, O.; Alsheikh, A.; et al. Enhanced Efficacy of Some Antibiotics in the Presence of Silver Nanoparticles Against Clinical Isolate of Pseudomonas aeruginosa Recovered from Cystic Fibrosis Patients. Int. J. Nanomed. 2024, 19, 12461–12481. [Google Scholar] [CrossRef] [PubMed]
- Kamer, A.M.A.; El Maghraby, G.M.; Shafik, M.M.; Al-Madboly, L.A. Silver Nanoparticle with Potential Antimicrobial and Antibiofilm Efficiency against Multiple Drug Resistant, Extensive Drug Resistant Pseudomonas aeruginosa Clinical Isolates. BMC Microbiol. 2024, 24, 277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Xu, X.; Gao, P.; Xu, Z.; Zhang, Q.; Li, H.; Yan, A.; Kao, R.Y.-T.; Sun, H. Multi-Target Mode of Action of Silver against Staphylococcus aureus Endows It with Capability to Combat Antibiotic Resistance. Nat. Commun. 2021, 12, 3331. [Google Scholar] [CrossRef]
- Wan, G.; Ruan, L.; Yin, Y.; Yang, T.; Ge, M.; Cheng, X. Effects of Silver Nanoparticles in Combination with Antibiotics on the Resistant Bacteria Acinetobacter Baumannii. Int. J. Nanomed. 2016, 11, 3789–3800. [Google Scholar] [CrossRef]
- Murei, A.; Ayinde, W.B.; Gitari, M.W.; Samie, A. Functionalization and Antimicrobial Evaluation of Ampicillin, Penicillin and Vancomycin with Pyrenacantha grandiflora Baill and Silver Nanoparticles. Sci. Rep. 2020, 10, 11596. [Google Scholar] [CrossRef]
- Vazquez-Muñoz, R.; Meza-Villezcas, A.; Fournier, P.G.J.; Soria-Castro, E.; Juarez-Moreno, K.; Gallego-Hernández, A.L.; Bogdanchikova, N.; Vazquez-Duhalt, R.; Huerta-Saquero, A. Enhancement of Antibiotics Antimicrobial Activity Due to the Silver Nanoparticles Impact on the Cell Membrane. PLoS ONE 2019, 14, e0224904. [Google Scholar] [CrossRef]
- Lopez-Carrizales, M.; Velasco, K.; Castillo, C.; Flores, A.; Magaña, M.; Martinez-Castanon, G.; Martinez-Gutierrez, F. In Vitro Synergism of Silver Nanoparticles with Antibiotics as an Alternative Treatment in Multiresistant Uropathogens. Antibiotics 2018, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, Characterization and Evaluation of Antimicrobial and Cytotoxic Activities of Biogenic Silver Nanoparticles Synthesized from Streptomyces xinghaiensis OF1 Strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Hwang, J.H.; Choi, H.; Kim, K.-J.; Lee, D.G. Synergistic Effects between Silver Nanoparticles and Antibiotics and the Mechanisms Involved. J. Med. Microbiol. 2012, 61, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Smekalova, M.; Aragon, V.; Panacek, A.; Prucek, R.; Zboril, R.; Kvitek, L. Enhanced Antibacterial Effect of Antibiotics in Combination with Silver Nanoparticles against Animal Pathogens. Vet. J. 2016, 209, 174–179. [Google Scholar] [CrossRef]
- Maniah, K.; Olyan Al-Otibi, F.; Mohamed, S.; Said, B.A.; Ragab AbdelGawwad, M.; Taha Yassin, M. Synergistic Antibacterial Activity of Biogenic AgNPs with Antibiotics against Multidrug Resistant Bacterial Strains. J. King Saud Univ. Sci. 2024, 36, 103461. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Synergistic Antibacterial Activity of Green Synthesized Silver Nanomaterials with Colistin Antibiotic against Multidrug-Resistant Bacterial Pathogens. Crystals 2022, 12, 1057. [Google Scholar] [CrossRef]
- Panáček, A.; Smékalová, M.; Večeřová, R.; Bogdanová, K.; Röderová, M.; Kolář, M.; Kilianová, M.; Hradilová, Š.; Froning, J.P.; Havrdová, M.; et al. Silver Nanoparticles Strongly Enhance and Restore Bactericidal Activity of Inactive Antibiotics against Multiresistant Enterobacteriaceae. Colloids Surf. B 2016, 142, 392–399. [Google Scholar] [CrossRef]
- Hochvaldová, L.; Panáček, D.; Válková, L.; Prucek, R.; Kohlová, V.; Večeřová, R.; Kolář, M.; Kvítek, L.; Panáček, A. Restoration of Antibacterial Activity of Inactive Antibiotics via Combined Treatment with a Cyanographene/Ag Nanohybrid. Sci. Rep. 2022, 12, 5222. [Google Scholar] [CrossRef]
- Hassan, K.T.; Ibraheem, I.J.; Hassan, O.M.; Obaid, A.S.; Ali, H.H.; Salih, T.A.; Kadhim, M.S. Facile Green Synthesis of Ag/AgCl Nanoparticles Derived from Chara Algae Extract and Evaluating Their Antibacterial Activity and Synergistic Effect with Antibiotics. J. Environ. Chem. Eng. 2021, 9, 105359. [Google Scholar] [CrossRef]
- Rastogi, L.; Kora, A.J.; Sashidhar, R.B. Antibacterial Effects of Gum Kondagogu Reduced/Stabilized Silver Nanoparticles in Combination with Various Antibiotics: A Mechanistic Approach. Appl. Nanosci. 2015, 5, 535–543. [Google Scholar] [CrossRef]
- Ankudze, B.; Neglo, D. Green Synthesis of Silver Nanoparticles from Peel Extract of Chrysophyllum albidum Fruit and Their Antimicrobial Synergistic Potentials and Biofilm Inhibition Properties. Biometals 2023, 36, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic Antibacterial Activity of Silver Nanoparticles Biosynthesized by Carbapenem-Resistant Gram-Negative Bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of Silver Nanoparticles Using Dioscorea bulbifera Tuber Extract and Evaluation of Its Synergistic Potential in Combination with Antimicrobial Agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar] [CrossRef]
- Lee, E.-B.; Lee, K. Silver Nanoparticle-Aminogylcosides Conjugation for Enhanced Control of Pathogenic E. coli O157:H7. J. Inorg. Organomet. Polym. 2024, 34, 2800–2811. [Google Scholar] [CrossRef]
- Barapatre, A.; Aadil, K.R.; Jha, H. Synergistic Antibacterial and Antibiofilm Activity of Silver Nanoparticles Biosynthesized by Lignin-Degrading Fungus. Bioresour. Bioprocess. 2016, 3, 8. [Google Scholar] [CrossRef]
- Ranpariya, B.; Salunke, G.; Karmakar, S.; Babiya, K.; Sutar, S.; Kadoo, N.; Kumbhakar, P.; Ghosh, S. Antimicrobial Synergy of Silver-Platinum Nanohybrids with Antibiotics. Front. Microbiol. 2021, 11, 610968. [Google Scholar] [CrossRef]
- Lin, P.; Wang, F.-Q.; Li, C.-T.; Yan, Z.-F. An Enhancement of Antibacterial Activity and Synergistic Effect of Biosynthesized Silver Nanoparticles by Eurotium cristatum with Various Antibiotics. Biotechnol. Bioprocess E 2020, 25, 450–458. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Nanoparticles against Foodborne Pathogenic Bacteria along with Its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Singh, H.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis of Anisotropic Silver Nanoparticles by Bhargavaea indica and Their Synergistic Effect with Antibiotics against Pathogenic Microorganisms. J. Nanomater. 2015, 2015, 234741. [Google Scholar] [CrossRef]
- Al-Otibi, F.O.; Yassin, M.T.; Al-Askar, A.A.; Maniah, K. Green Biofabrication of Silver Nanoparticles of Potential Synergistic Activity with Antibacterial and Antifungal Agents against Some Nosocomial Pathogens. Microorganisms 2023, 11, 945. [Google Scholar] [CrossRef]
- Suárez, C.; Gudiol, F. Antibióticos betalactámicos. Enferm. Infecc. Microbiol. Clin. 2009, 27, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.A.; Malek, N.A.N.N.; Matmin, J.; Asraf, M.H.; Susanto, H.; Din, S.M.; Shamsuddin, M. Synergistic Antibacterial Effect of Persicaria odorata Synthesised Silver Nanoparticles with Antibiotics on Drug-Resistant Bacteria. Inorg. Chem. Commun. 2024, 159, 111725. [Google Scholar] [CrossRef]
- Loho, T.; Dharmayanti, A. Colistin: An Antibiotic and Its Role in Multiresistant Gram-Negative Infections. Acta Med. Indones. 2015, 47, 157–168. [Google Scholar] [PubMed]
- Klinger-Strobel, M.; Stein, C.; Forstner, C.; Makarewicz, O.; Pletz, M.W. Effects of Colistin on Biofilm Matrices of Escherichia coli and Staphylococcus aureus. Int. J. Antimicrob. Agents 2017, 49, 472–479. [Google Scholar] [CrossRef]
- Naqvi, S.Z.H.; Kiran, U.; Ali, M.I.; Jamal, A.; Hameed, A.; Ahmed, S.; Ali, N. Combined Efficacy of Biologically Synthesized Silver Nanoparticles and Different Antibiotics against Multidrug-Resistant Bacteria. Int. J. Nanomed. 2013, 8, 3187–3195. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Enhancement of Antibacterial Activity of Capped Silver Nanoparticles in Combination with Antibiotics, on Model Gram-Negative and Gram-Positive Bacteria. Bioinorg. Chem. Appl. 2013, 2013, 871097. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef]
- Kaur, A.; Preet, S.; Kumar, V.; Kumar, R.; Kumar, R. Synergetic Effect of Vancomycin Loaded Silver Nanoparticles for Enhanced Antibacterial Activity. Colloids Surf. B 2019, 176, 62–69. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Mamonova, I.A.; Babushkina, I.V.; Norkin, I.A.; Gladkova, E.V.; Matasov, M.D.; Puchin’yan, D.M. Biological Activity of Metal Nanoparticles and Their Oxides and Their Effect on Bacterial Cells. Nanotechnol. Russ. 2015, 10, 128–134. [Google Scholar] [CrossRef]
- Anees Ahmad, S.; Sachi Das, S.; Khatoon, A.; Tahir Ansari, M.; Afzal, M.; Saquib Hasnain, M.; Kumar Nayak, A. Bactericidal Activity of Silver Nanoparticles: A Mechanistic Review. Mat. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Jahan, I.; Matpan Bekler, F.; Tunç, A.; Güven, K. The Effects of Silver Nanoparticles (AgNPs) on Thermophilic Bacteria: Antibacterial, Morphological, Physiological and Biochemical Investigations. Microorganisms 2024, 12, 402. [Google Scholar] [CrossRef]
- Bellanger, X.; Schneider, R.; Dezanet, C.; Arroua, B.; Balan, L.; Billard, P.; Merlin, C. Zn2+ Leakage and Photo-Induced Reactive Oxidative Species Do Not Explain the Full Toxicity of ZnO Core Quantum Dots. J. Hazard. Mater. 2020, 396, 122616. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Pu, X.-M.; Pan, J.-F.; Xu, J.; Liu, C.; Lu, D. From Antioxidant Defense to Genotoxicity: Deciphering the Tissue-Specific Impact of AgNPs on Marine Clam Ruditapes philippinarum. Aquat. Toxicol. 2024, 270, 106883. [Google Scholar] [CrossRef]
- Liao, W.; McNutt, M.A.; Zhu, W.-G. The Comet Assay: A Sensitive Method for Detecting DNA Damage in Individual Cells. Methods 2009, 48, 46–53. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, H.; Shen, Y.; Zhang, W.; Zhang, L. Antibacterial Activity of Silver Nanoparticles of Different Particle Size against Vibrio natriegens. PLoS ONE 2019, 14, e0222322. [Google Scholar] [CrossRef]
- Gunawan, C.; Faiz, M.B.; Mann, R.; Ting, S.R.S.; Sotiriou, G.A.; Marquis, C.P.; Amal, R. Nanosilver Targets the Bacterial Cell Envelope: The Link with Generation of Reactive Oxygen Radicals. ACS Appl. Mater. Interfaces 2020, 12, 5557–5568. [Google Scholar] [CrossRef] [PubMed]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and Effect of Silver Nanoparticles on the Antibacterial Activity of Different Antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS Mediated Destruction of Cell Membrane, Growth and Biofilms of Human Bacterial Pathogens by Stable Metallic AgNPs Functionalized from Bell Pepper Extract and Quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Fan, Z.; Huang, Y.; Duan, Y.; Tang, Z.; Yang, X. Effects of Silver Nanoparticles and Various Forms of Silver on Nitrogen Removal by the Denitrifier Pseudomonas stutzeri and Their Toxicity Mechanisms. Ecotoxicol. Environ. Saf. 2024, 269, 115785. [Google Scholar] [CrossRef]
- Chang, X.; Niu, S.; Shang, M.; Li, J.; Guo, M.; Zhang, W.; Sun, Z.; Li, Y.; Zhang, R.; Shen, X.; et al. ROS-Drp1-Mediated Mitochondria Fission Contributes to Hippocampal HT22 Cell Apoptosis Induced by Silver Nanoparticles. Redox Biol. 2023, 63, 102739. [Google Scholar] [CrossRef]
- Azizi, M.; Ghourchian, H.; Yazdian, F.; Bagherifam, S.; Bekhradnia, S.; Nyström, B. Anti-Cancerous Effect of Albumin Coated Silver Nanoparticles on MDA-MB 231 Human Breast Cancer Cell Line. Sci. Rep. 2017, 7, 5178. [Google Scholar] [CrossRef]
- Krce, L.; Šprung, M.; Rončević, T.; Maravić, A.; Čikeš Čulić, V.; Blažeka, D.; Krstulović, N.; Aviani, I. Probing the Mode of Antibacterial Action of Silver Nanoparticles Synthesized by Laser Ablation in Water: What Fluorescence and AFM Data Tell Us. Nanomaterials 2020, 10, 1040. [Google Scholar] [CrossRef]
- Samberg, M.E.; Orndorff, P.E.; Monteiro-Riviere, N.A. Antibacterial Efficacy of Silver Nanoparticles of Different Sizes, Surface Conditions and Synthesis Methods. Nanotoxicology 2011, 5, 244–253. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique Cellular Interaction of Silver Nanoparticles: Size-Dependent Generation of Reactive Oxygen Species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef]


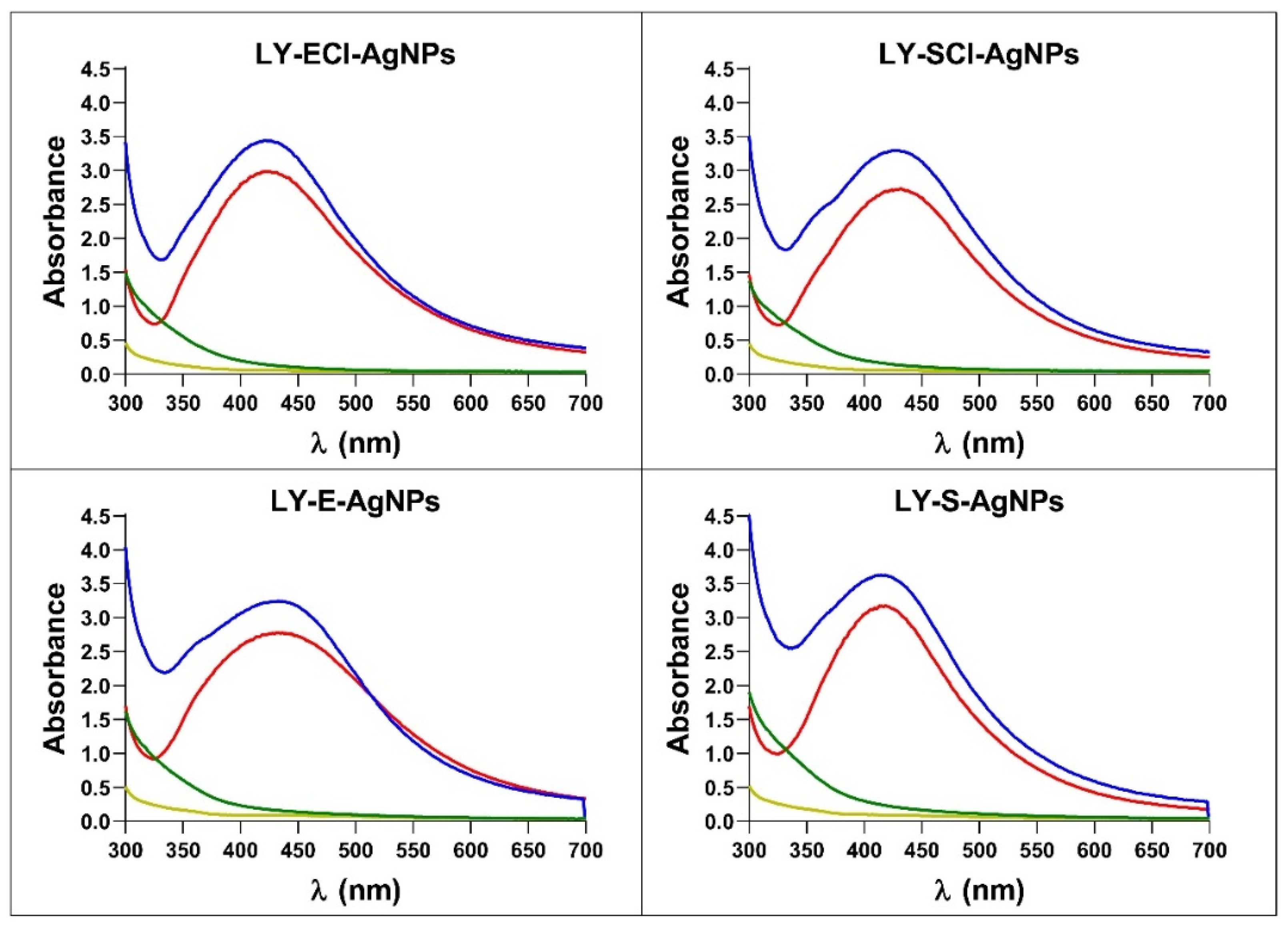


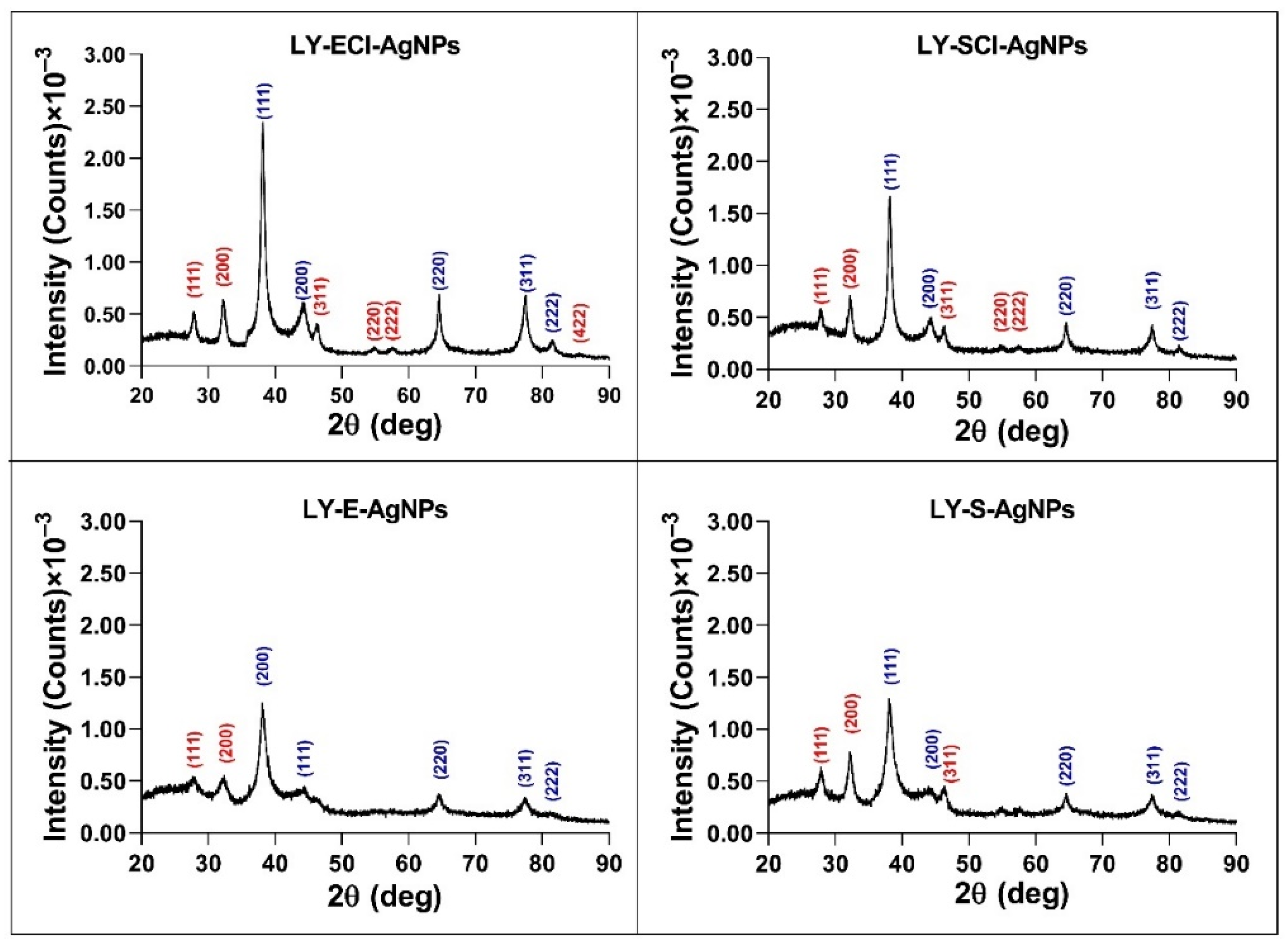
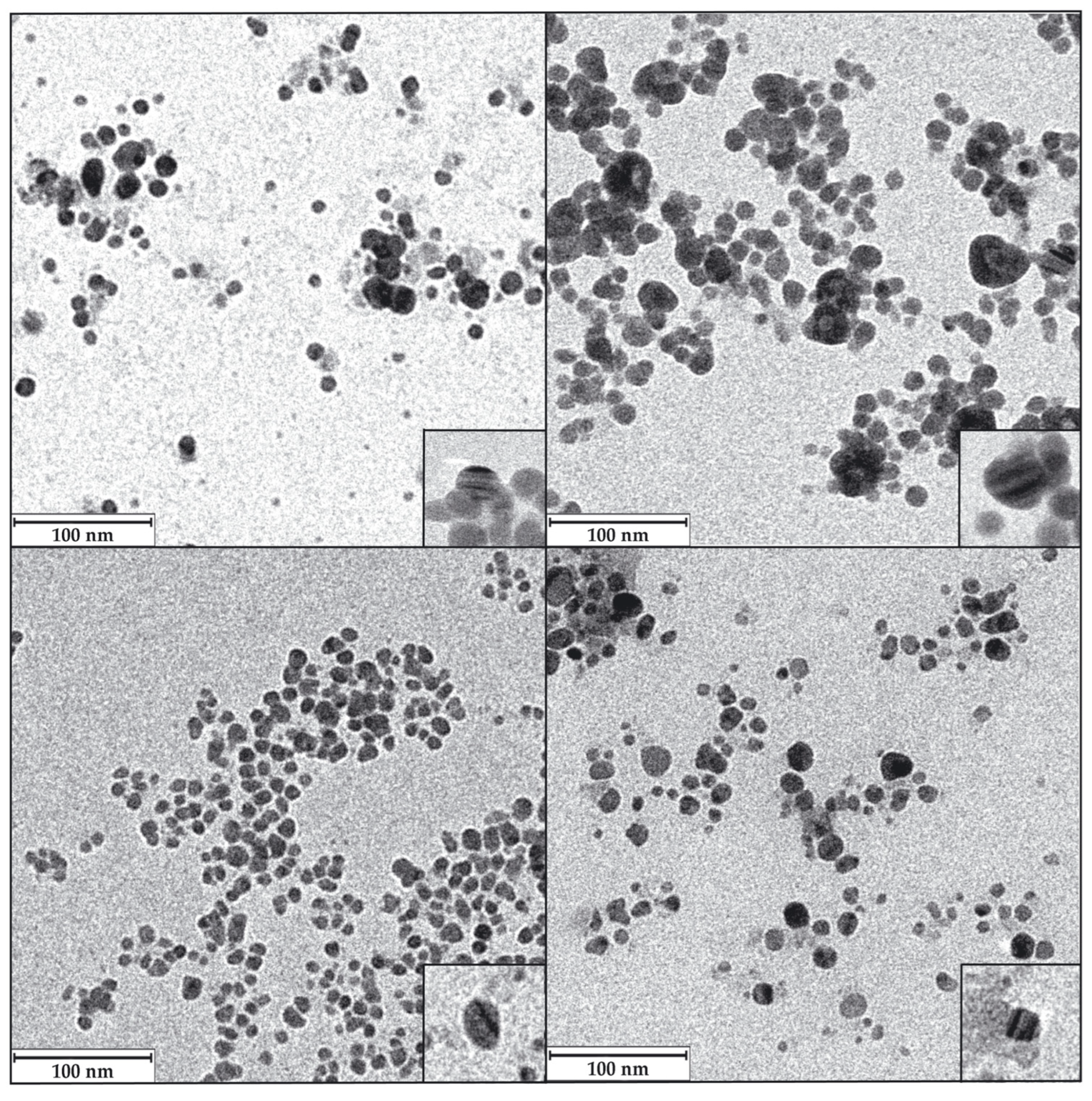

| Microorganism | Culture Medium | Culture Phase | Name of AgNPs |
|---|---|---|---|
| Lysinibacillus sp. | Nutritive medium with NaCl | Exponential | LY-ECl-AgNPs |
| Stationary | LY-SCl-AgNPs | ||
| Nutritive medium without NaCl | Exponential | LY-E-AgNPs | |
| Stationary | LY-S-AgNPs |
| AgNPs | Potential-Z (mV) | Diameter (DLS) (nm) | PDI (DLS) | Diameter (TEM) (nm) | PDI (TEM) |
|---|---|---|---|---|---|
| LY-ECl-AgNPs | −20.6 ± 1.6 | 48.5 ± 0.4 | 0.45 | 9.9 ± 5.5 | 0.31 |
| LY-SCl-AgNPs | −21.6 ± 5.4 | 52.7 ± 0.5 | 0.51 | 14.7 ± 8.3 | 0.32 |
| LY-E-AgNPs | −25.1 ± 3.5 | 80.2 ± 3.6 | 0.43 | 7.5 ± 5.8 | 0.50 |
| LY-S-AgNPs | −8.8 ± 1.4 | 63.3 ± 1.7 | 0.45 | 9.6 ± 5.1 | 0.28 |
| Test Bacteria | AgNPs/AgNO3 | MIC | MBC | IC50 | ICb50 |
|---|---|---|---|---|---|
| E. coli ATCC 25922 | Streptomycin | 16.00 | 16.00 | 5.40 ± 1.06 | 5.83 ± 1.52 |
| AgNO3 | 0.53 | 0.53 | 0.15 ± 0.01 | 0.33 ± 0.03 | |
| LY-ECl-AgNPs | 0.86 | 1.73 | 0.43 ± 0.06 * | 0.62 ± 0.04 */# | |
| LY-SCl-AgNPs | 0.63 | 1.26 | 0.25 ± 0.02 * | 0.34 ± 0.02 * | |
| LY-E-AgNPs | 0.65 | 0.65 | 0.43 ± 0.07 | 0.35 ± 0.02 # | |
| LY-S-AgNPs | 1.13 | 1.13 | 0.55 ± 0.21 | 0.56 ± 0.20 | |
| K. pneumoniae ATCC 29665 | Streptomycin | 4.00 | 4.00 | 2.59 ± 0.18 | 2.29 ± 0.38 |
| AgNO3 | 0.53 | 0.53 | 0.27 ± 0.01 | 0.30 ± 0.07 | |
| LY-ECl-AgNPs | 0.86 | 3.46 | 0.49 ± 0.11 | 0.50 ± 0.02 | |
| LY-SCl-AgNPs | 1.31 | 2.61 | 0.41 ± 0.09 | 0.73 ± 0.15 # | |
| LY-E-AgNPs | 1.31 | 2.61 | 0.45 ± 0.50 | 0.78 ± 0.20 | |
| LY-S-AgNPs | 2.26 | 2.26 | 0.61 ± 0.41 | 1.11 ± 0.04 # | |
| P. aeruginosa CECT 108 | Streptomycin | 16.00 | 16.00 | 4.50 ± 0.27 | 6.43 ± 0.75 |
| AgNO3 | 0.27 | 0.53 | 0.03 ± 0.01 | 0.16 ± 0.02 | |
| LY-ECl-AgNPs | 0.43 | 1.73 | 0.13 ± 0.02 * | 0.25 ± 0.02 * | |
| LY-SCl-AgNPs | 0.32 | 0.63 | 0.08 ± 0.01 */# | 0.18 ± 0.01 */# | |
| LY-E-AgNPs | 1.31 | 1.31 | 0.16 ± 0.12 | 0.45 ± 0.21 | |
| LY-S-AgNPs | 0.57 | 0.57 | 0.36 ± 0.06 # | 0.42 ± 0.01 # | |
| S. aureus CECT 794 | Streptomycin | 32.00 | 32.00 | 4.15 ± 0.11 | 5.45 ± 0.92 |
| AgNO3 | 2.12 | 4.24 | 0.56 ± 0.02 | 1.28 ± 0.15 | |
| LY-ECl-AgNPs | 6.92 | 13.83 | 1.54 ± 0.05 * | 1.76 ± 0.16 # | |
| LY-SCl-AgNPs | 5.06 | 10.12 | 0.87 ± 0.26 */# | 2.04 ± 0.36 # | |
| LY-E-AgNPs | 5.23 | 10.46 | 1.65 ± 0.23 * | 2.88 ± 0.18 */# | |
| LY-S-AgNPs | 9.05 | 18.10 | 3.24 ± 0.26 */# | 5.20 ± 0.27 */# | |
| S. epidermidis ATCC 12228 | Streptomycin | >256.00 | >256.00 | >256.00 | >256.00 |
| AgNO3 | 1.06 | 4.24 | 0.27 ± 0.01 | 0.90 ± 0.35 | |
| LY-ECl-AgNPs | 1.73 | 6.92 | 0.48 ± 0.08 # | 0.92 ± 0.21 # | |
| LY-SCl-AgNPs | 1.26 | 5.06 | 0.42 ± 0.06 # | 0.93 ± 0.20 | |
| LY-E-AgNPs | 2.61 | 10.46 | 1.46 ± 0.14 */# | 1.71 ± 0.24 # | |
| LY-S-AgNPs | 4.52 | 36.20 | 0.67 ± 0.08 */# | 1.46 ± 0.71 | |
| B. subtilis 168 | Streptomycin | 64.00 | >256.00 | 7.58 ± 0.82 | 14.09 ± 5.80 |
| AgNO3 | 1.06 | 1.06 | 0.27 ± 0.08 | 0.40 ± 0.13 | |
| LY-ECl-AgNPs | 1.73 | 1.73 | 0.95 ± 0.16 # | 1.45 ± 0.21 * | |
| LY-SCl-AgNPs | 2.61 | 5.06 | 0.75 ± 0.10 | 0.83 ± 0.14 */# | |
| LY-E-AgNPs | 2.61 | 10.46 | 2.88 ± 0.58 # | 1.19 ± 0.07 * | |
| LY-S-AgNPs | 4.52 | 9.04 | 2.52 ± 0.91 | 2.65 ± 0.48 */# |
| Antibiotic | AgNO3 | LY-ECl-AgNPs | LY-SCl-AgNPs | LY-E-AgNPs | LY-S-AgNPs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| E.C. | S.A. | E.C. | S.A. | E.C. | S.A. | E.C. | S.A. | E.C. | S.A. | ||
| Ap | FICI | 2.000 | 0.250 | 0.625 | 0.500 | 2.000 | 0.375 | 1.000 | 0.375 | 2.000 | 0.375 |
| MF | 1 | 8 | 2 | 2 | 1 | 4 | 2 | 4 | 1 | 4 | |
| Cc | FICI | 0.375 | 0.750 | 0.313 | 2.000 | 0.188 | 2.000 | 0.250 | 2.000 | 0.375 | 1.000 |
| MF | 4 | 4 | 4 | 1 | 8 | 1 | 8 | 1 | 4 | 2 | |
| Co | FICI | 0.188 | 0.094 | 0.157 | 0.063 | 0.125 | 0.063 | 0.094 | 0.063 | 0.094 | 0.063 |
| MF | 16 | 32 | 8 | 32 | 16 | 32 | 32 | 32 | 32 | 32 | |
| Cp | FICI | 2.000 | 1.000 | 2.000 | 0.750 | 2.000 | 0.750 | 2.000 | 0.500 | 2.000 | 0.500 |
| MF | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 4 | 1 | 4 | |
| Cz | FICI | 2.000 | 3.000 | 2.000 | 3.000 | 2.000 | 3.000 | 2.000 | 3.000 | 2.000 | 3.000 |
| MF | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Em | FICI | 1.000 | 2.000 | 0.750 | 0.750 | 0.500 | 0.750 | 0.750 | 2.000 | 0.500 | 2.000 |
| MF | 2 | 1 | 2 | 2 | 4 | 2 | 4 | 1 | 4 | 1 | |
| Ep | FICI | 0.750 | 0.750 | 2.000 | 2.000 | 1.000 | 2.000 | 1.000 | 2.000 | 0.500 | 2.000 |
| MF | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 4 | 1 | |
| Km | FICI | 0.047 | 0.094 | 0.039 | 0.078 | 0.039 | 0.078 | 0.039 | 0.046 | 0.035 | 0.078 |
| MF | 64 | 32 | 128 | 64 | 128 | 64 | 128 | 64 | 128 | 32 | |
| Nx | FICI | 1.000 | 2.000 | 2.000 | 1.000 | 1.000 | 2.000 | 2.000 | 2.000 | 1.000 | 2.000 |
| MF | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | |
| Pn | FICI | 0.750 | 0.078 | 1.000 | 0.188 | 0.750 | 0.188 | 1.000 | 0.188 | 1.000 | 0.313 |
| MF | 2 | 64 | 2 | 8 | 4 | 8 | 2 | 8 | 2 | 4 | |
| Rp | FICI | 0.188 | 1.000 | 0.158 | 1.000 | 0.094 | 1.000 | 0.125 | 1.000 | 0.188 | 1.000 |
| MF | 16 | 2 | 8 | 2 | 16 | 2 | 16 | 2 | 16 | 2 | |
| Sm | FICI | 0.047 | 0.031 | 0.063 | 0.047 | 0.180 | 0.125 | 0.125 | 0.094 | 0.094 | 0.094 |
| MF | 64 | 64 | 64 | 64 | 16 | 16 | 16 | 32 | 32 | 32 | |
| Tc | FICI | 0.750 | 0.750 | 0.375 | 1.000 | 0.500 | 2.000 | 0.310 | 2.000 | 0.375 | 0.750 |
| MF | 2 | 4 | 4 | 2 | 4 | 1 | 16 | 1 | 4 | 4 | |
| Tg | FICI | 2.000 | 2.000 | 2.000 | 0.750 | 2.000 | 0.750 | 2.000 | 0.750 | 1.000 | 1.000 |
| MF | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | |
| Vm | FICI | 0.375 | 0.750 | 0.625 | 1.000 | 0.375 | 1.000 | 0.375 | 0.750 | 0.375 | 1.000 |
| MF | 8 | 2 | 2 | 2 | 4 | 2 | 4 | 4 | 4 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pernas-Pleite, C.; Conejo-Martínez, A.M.; Marín, I.; Abad, J.P. Silver Nanoparticles (AgNPs) from Lysinibacillus sp. Culture Broths: Antibacterial Activity, Mechanism Insights, and Synergy with Classical Antibiotics. Biomolecules 2025, 15, 731. https://doi.org/10.3390/biom15050731
Pernas-Pleite C, Conejo-Martínez AM, Marín I, Abad JP. Silver Nanoparticles (AgNPs) from Lysinibacillus sp. Culture Broths: Antibacterial Activity, Mechanism Insights, and Synergy with Classical Antibiotics. Biomolecules. 2025; 15(5):731. https://doi.org/10.3390/biom15050731
Chicago/Turabian StylePernas-Pleite, Carlos, Amparo M. Conejo-Martínez, Irma Marín, and José P. Abad. 2025. "Silver Nanoparticles (AgNPs) from Lysinibacillus sp. Culture Broths: Antibacterial Activity, Mechanism Insights, and Synergy with Classical Antibiotics" Biomolecules 15, no. 5: 731. https://doi.org/10.3390/biom15050731
APA StylePernas-Pleite, C., Conejo-Martínez, A. M., Marín, I., & Abad, J. P. (2025). Silver Nanoparticles (AgNPs) from Lysinibacillus sp. Culture Broths: Antibacterial Activity, Mechanism Insights, and Synergy with Classical Antibiotics. Biomolecules, 15(5), 731. https://doi.org/10.3390/biom15050731






