CXCR Family and Hematologic Malignancies in the Bone Marrow Microenvironment
Abstract
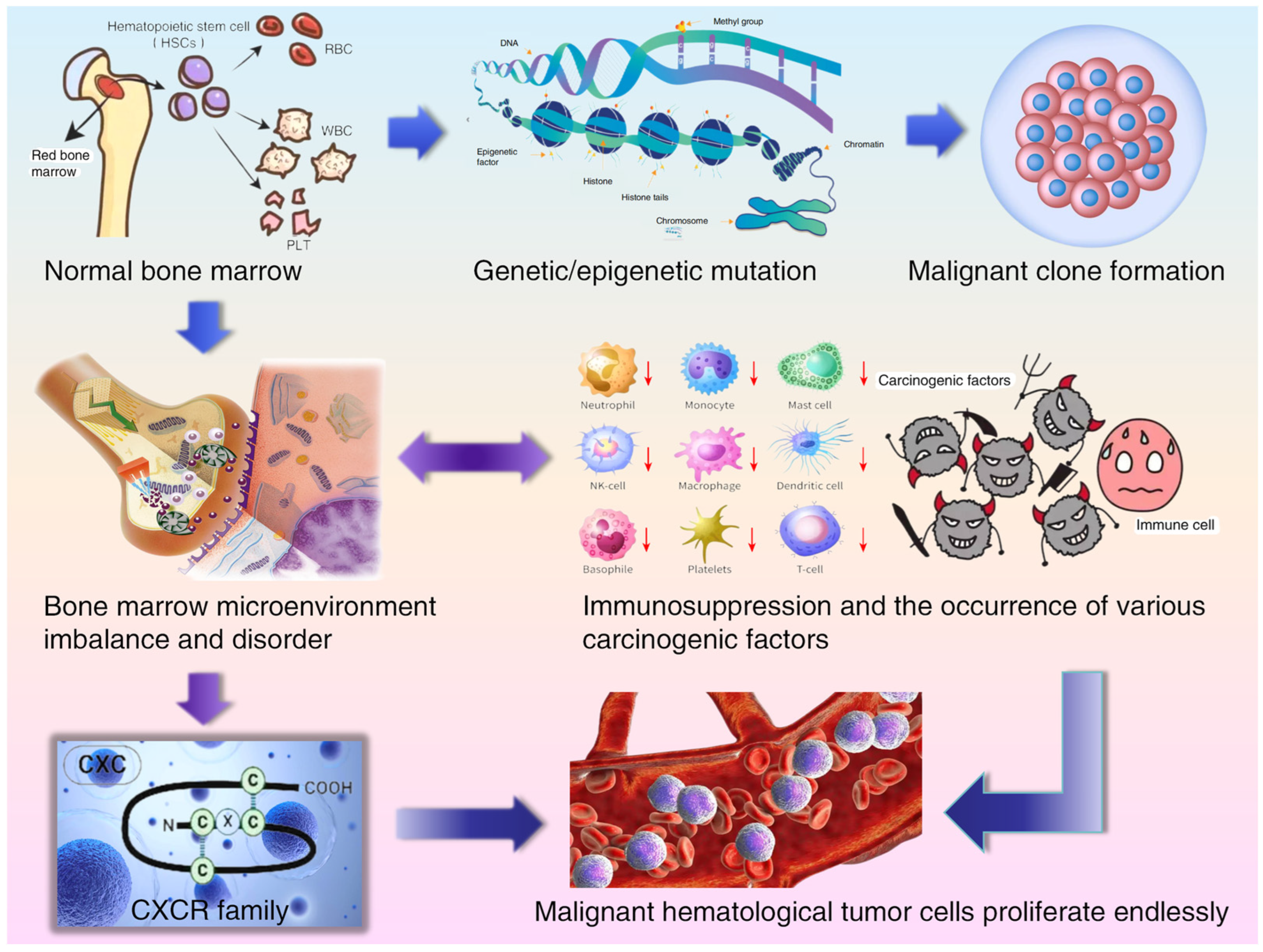
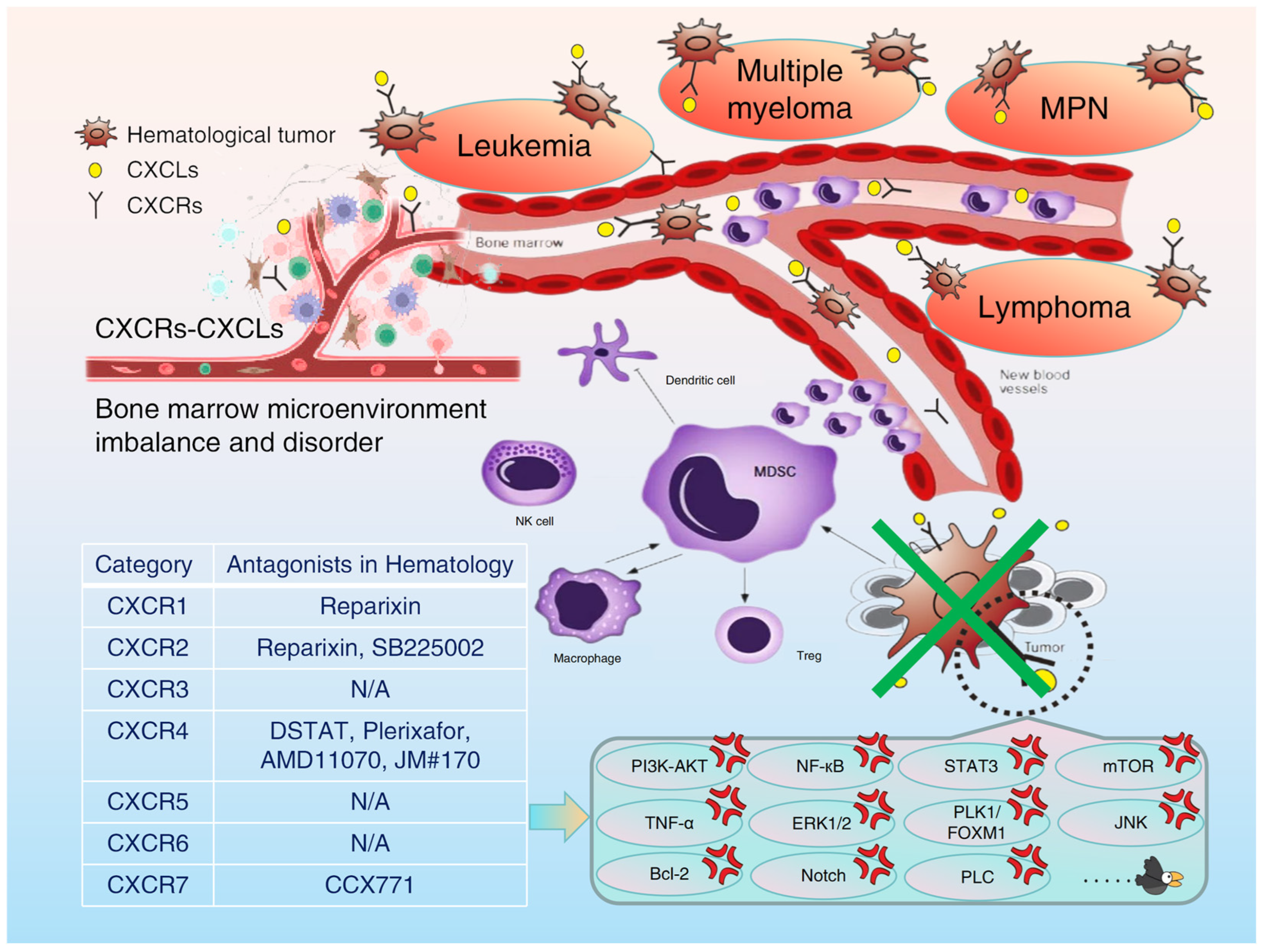
1. Introduction
2. CXCR Family and Myelodysplastic Syndrome (MDS)
3. CXCR Family and Acute Myeloid Leukemia (AML)
4. CXCR Family and Acute Lymphoblastic Leukemia (ALL)
5. CXCR Family and Chronic Myelogenous Leukemia (CML)
6. CXCR Family and Chronic Lymphocytic Leukemia (CLL), Malignant Lymphoma (ML)
7. CXCR Family and Multiple Myeloma (MM)
8. Summary and Prospect
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CXCR | C-X-C motif chemokine receptor |
| CXCL | C-X-C motif chemokine ligand |
| BME | Bone marrow microenvironment |
| TME | Tumor microenvironment |
| MDS | Myelodysplastic syndrome |
| AL | Acute leukemia |
| AML | Acute Myeloid Leukemia |
| ALL | Acute lymphoblastic leukemia |
| CML | Chronic myeloid leukemia |
| CLL | Chronic lymphocytic leukemia |
| ML | Malignant lymphoma |
| CLL/SLL | Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| MM | Multiple myeloma |
| MPN | Myeloproliferative neoplasm |
| HL | Hodgkin lymphoma |
| NHL | Non-Hodgkin lymphoma |
| MDSCs | Myeloid-derived suppressor cells |
| TANs | Tumor-associated neutrophils |
| TAMs | Tumor-associated macrophages |
| Tregs | Regulatory T cells |
| NK cells | Natural killer cells |
| PD1 | Programmed death receptor 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| AKT | Protein kinase B |
| NF-κB | Nuclear factor kappa-B |
| HSCs | Hematopoietic stem cells |
| CR | Complete response |
| DFS | Disease-free survival rate |
| CAR-T | Chimeric antigen receptor T cells |
| PLC | Phospholipase C |
| PBMCs | Peripheral blood mononuclear cells |
| ACT | Adoptive cell transfer therapy |
| STAT3 | Signal transducer and activator of transcription 3 |
| TKIs | Tyrosine kinase inhibitors |
| Ph | Philadelphia chromosome |
| mTOR | Mammalian target of rapamycin |
| MRD | Minimal or molecular residual disease |
| LSCs | Leukemia stem cells |
| TNF-α | Tumor necrosis factor α |
| BMSCs | Bone marrow stromal cells |
| ERK | Extracellular signal-regulated kinase |
| HTLV-1 | Host–human T-cell leukemia virus type 1 |
| ATLL | Adult T-cell leukemia/lymphoma |
| PVL | Proviral load |
| MZL | Marginal zone lymphoma |
| BLR1 | Burkitt lymphoma receptor 1 |
| LPL | Lymphoplasmacytic lymphoma |
| WM | Waldenström macroglobulinemia |
| PLK1 | Polo-like Kinase 1 |
| FOXM1 | Forkhead box protein M1 |
| JNK | Jun N-terminal kinase |
| DLBCL | Diffuse large B-cell lymphoma |
| TEM | Transendothelial migration |
References
- Holstein, S.A.; Lunning, M.A. CAR T-cell therapy in hematologic malignancies: A voyage in progress. Clin. Pharmacol. Ther. 2020, 107, 112–122. [Google Scholar] [CrossRef]
- Xun, Y.; Yang, H.; Li, J.; Wu, F.; Liu, F. CXC chemokine receptors in the tumor microenvironment and an update of antagonist development. Rev. Physiol. Biochem. Pharmacol. 2020, 178, 1–40. [Google Scholar]
- Liu, Y.Q.; Shen, J.Z. Correlation between CXCR1 and malignant tumors. J. Int. Oncology. 2020, 47, 732–736. [Google Scholar]
- Liu, Y.Q. The correlation between CXC chemokine receptor family and malignant tumor. J. Hebei Med. University. 2022, 43, 853–859. [Google Scholar]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and chemokine receptors: New targets for cancer immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Bajetto, A.; Thellung, S.; Würth, R.; Florio, T. Drug design strategies focusing on the CXCR4/CXCR7/CXCL12 pathway in leukemia and lymphoma. Expert Opin. Drug Discov. 2016, 11, 1093–1109. [Google Scholar] [CrossRef] [PubMed]
- Chirillo, R.; Aversa, I.; Di Vito, A.; Salatino, A.; Battaglia, A.M.; Sacco, A.; Di Sanzo, M.A.; Faniello, M.C.; Quaresima, B.; Palmieri, C.; et al. FtH-Mediated ROS Dysregulation Promotes CXCL12/CXCR4 Axis Activation and EMT-Like Trans-Differentiation in Erythroleukemia K562 Cells. Front. Oncol. 2020, 10, 698. [Google Scholar] [CrossRef]
- Pillozzi, S.; Bernini, A.; Spiga, O.; Lelli, B.; Petroni, G.; Bracci, L.; Niccolai, N.; Arcangeli, A. Peptides and small molecules blocking the CXCR4/CXCL12 axis overcome bone marrow-induced chemoresistance in acute leukemias. Oncol. Rep. 2019, 41, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Schinke, C.; Giricz, O.; Li, W.; Shastri, A.; Gordon, S.; Barreyro, L.; Bhagat, T.; Bhattacharyya, S.; Ramachandra, N.; Bartenstein, M.; et al. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML Stem cells. Blood 2015, 125, 3144–3152. [Google Scholar] [CrossRef]
- Tang, W.; Li, Z.; Li, X.; Huo, Z. High CXCR2 expression predicts poor prognosis in adult patients with acute myeloid leukemia. Ther. Adv. Hematol. 2020, 11, 2040620720958586. [Google Scholar] [CrossRef]
- Anaya, D.; Santander González, S.P.; Murillo, N.; Ballesteros-Ramírez, R.; Reyes, I.L.; Herrera, M.V.; Solano, J.; Fiorentino, S.; Quijano, S. IL-8 in bone marrow and peripheral blood of patients with B-cell acute lymphoblastic leukemia is associated with high regulatory T cell counts, degree of tumor infiltration and expression of CXCR1 in blasts. Hematol. Transfus. Cell Ther. 2024, 46, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Kupnicka, P.; Barczak, K.; Bosiacki, M.; Ziętek, P.; Chlubek, D.; Baranowska-Bosiacka, I. The Role of CXCR1, CXCR2, CXCR3, CXCR5, and CXCR6 Ligands in Molecular Cancer Processes and Clinical Aspects of Acute Myeloid Leukemia (AML). Cancers 2023, 15, 4555. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhu, J.; Chen, X.; Hu, Y.; Xie, W.; Yao, J.; Huang, S. Risk Stratification in Acute Myeloid Leukemia Using CXCR Gene Signatures: A Bioinformatics Analysis. Front. Oncol. 2020, 10, 584766. [Google Scholar] [CrossRef]
- Kikuchi-Ueda, T.; Tansho, S.; Ono, Y. Enhancement of interleukin-8-induced chemotactic response and reactive oxygen species production in HL-60 cells expressing CXCR1. J. Infect. Chemother. 2012, 18, 283–287. [Google Scholar] [CrossRef]
- Dejean, E.; Foisseau, M.; Lagarrigue, F.; Lamant, L.; Prade, N.; Marfak, A.; Delsol, G.; Giuriato, S.; Gaits-Iacovoni, F.; Meggetto, F. ALK+ALCLs induce cutaneous, HMGB-1-dependent IL-8/CXCL8 production by keratinocytes through NF-κB activation. Blood 2012, 119, 4698–4707. [Google Scholar] [CrossRef] [PubMed]
- Kline, M.; Donovan, K.; Wellik, L.; Lust, C.; Jin, W.; Moon-Tasson, L.; Xiong, Y.; Witzig, T.E.; Kumar, S.; Rajkumar, S.V.; et al. Cytokine and chemokine profiles in multiple myeloma; significance of stromal interaction and correlation of IL-8 production with disease progression. Leuk. Res. 2007, 31, 591–598. [Google Scholar] [CrossRef]
- Vermeersch, G.; Proost, P.; Struyf, S.; Gouwy, M.; Devos, T. CXCL8 and its cognate receptors CXCR1/CXCR2 in primary myelofibrosis. Haematologica 2024, 109, 2060–2072. [Google Scholar] [CrossRef]
- Ramachandra, N.; Gupta, M.; Schwartz, L.; Todorova, T.; Shastri, A.; Will, B.; Steidl, U.; Verma, A. Role of IL8 in myeloid malignancies. Leuk. Lymphoma. 2023, 64, 1742–1751. [Google Scholar] [CrossRef]
- Emadi, S.; Clay, D.; Desterke, C.; Guerton, B.; Maquarre, E.; Charpentier, A.; Jasmin, C.; Le Bousse-Kerdilès, M.-C.; French INSERM Research Network on MMM. IL-8 and its CXCR1 and CXCR2 receptors participate in the control of megakaryocytic proliferation, differentiation, and ploidy in myeloid metaplasia with myelofibrosis. Blood 2005, 105, 464–473. [Google Scholar] [CrossRef]
- Han, H.; Liu, J.; Zhu, S.; Zhao, T. Identification of two key biomarkers CD93 and FGL2 associated with survival of acute myeloid leukaemia by weighted gene co-expression network analysis. J. Cell. Mol. Med. 2024, 28, e18552. [Google Scholar] [CrossRef]
- Cao, H.; Tadros, V.; Hiramoto, B.; Leeper, K.; Hino, C.; Xiao, J.; Pham, B.; Kim, D.H.; Reeves, M.E.; Chen, C.S.; et al. Targeting TKI-Activated NFKB2-MIF/CXCLs-CXCR2 Signaling Pathways in FLT3 Mutated Acute Myeloid Leukemia Reduced Blast Viability. Biomedicines 2022, 10, 1038. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.J.; Kang, K.W.; Lee, B.H.; Park, Y.; Kim, B.S. CXCR2, a novel target to overcome tyrosine kinase inhibitor resistance in chronic myelogenous leukemia cells. Biochem. Pharmacol. 2021, 190, 114658. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lan, T.; Zhang, Q.; Zhou, C.; Liu, P. Myeloma cell-derived CXCL7 facilitates proliferation of tumor cells and occurrence of osteolytic lesions through JAK/STAT3 pathway. Cell. Death Dis. 2025, 16, 74. [Google Scholar] [CrossRef]
- Dunbar, A.J.; Kim, D.; Lu, M.; Farina, M.; Bowman, R.L.; Yang, J.L.; Park, Y.; Karzai, A.; Xiao, W.; Zaroogian, Z.; et al. CXCL8/CXCR2 signaling mediates bone marrow fibrosis and is a therapeutic target in myelofibrosis. Blood 2023, 141, 2508–2519. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, S.; Wu, Y.; Ji, S. The USP35-CXCR3 Axis plays an oncogenic role in JeKo-1 mantle cell lymphoma cells. Integr. Biol. 2024, 16, zyae021. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Hashida, Y.; Matsuo, K.; Kitahata, K.; Ujihara, T.; Murakami, I.; Nakayama, T.; Daibata, M. EBV-positive pyothorax-associated lymphoma expresses CXCL9 and CXCL10 chemokines that attract cytotoxic lymphocytes via CXCR3. Cancer Sci. 2023, 114, 2622–2633. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, V.; Antonangeli, F.; Santoni, A.; Bernardini, G. Targeting of CXCR3 improves anti-myeloma efficacy of adoptively transferred activated natural killer cells. J. Immunother. Cancer 2019, 7, 290. [Google Scholar] [CrossRef]
- Klement, L.; Drube, J. The interplay of FLT3 and CXCR4 in acute myeloid leukemia: An ongoing debate. Front. Oncol. 2023, 13, 1258679. [Google Scholar] [CrossRef]
- Yu, D.H.; Chen, C.; Liu, X.P.; Yao, J.; Li, S.; Ruan, X.L. Dysregulation of miR-138-5p/RPS6KA1-AP2M1 Is Associated With Poor Prognosis in AML. Front. Cell. Dev. Biol. 2021, 9, 641629. [Google Scholar] [CrossRef]
- Roversi, F.M.; Bueno, M.L.P.; Pericole, F.V.; Saad, S.T.O. Hematopoietic Cell Kinase (HCK) Is a Player of the Crosstalk Between Hematopoietic Cells and Bone Marrow Niche Through CXCL12/CXCR4 Axis. Front. Cell. Dev. Biol. 2021, 9, 634044. [Google Scholar] [CrossRef]
- Piya, S.; Mu, H.; Bhattacharya, S.; Lorenzi, P.L.; Davis, R.E.; McQueen, T.; Ruvolo, V.; Baran, N.; Wang, Z.; Qian, Y.; et al. BETP degradation simultaneously targets acute myelogenous leukemia stem cells and the microenvironment. J. Clin. Investig. 2019, 129, 1878–1894. [Google Scholar] [CrossRef] [PubMed]
- Macanas-Pirard, P.; Quezada, T.; Navarrete, L.; Broekhuizen, R.; Leisewitz, A.; Nervi, B.; Ramírez, P.A. The CCL2/CCR2 Axis Affects Transmigration and Proliferation but Not Resistance to Chemotherapy of Acute Myeloid Leukemia Cells. PLoS ONE 2017, 12, e0168888. [Google Scholar] [CrossRef] [PubMed]
- Ondrisova, L.; Mraz, M. Genetic and Non-Genetic Mechanisms of Resistance to BCR Signaling Inhibitors in B Cell Malignancies. Front. Oncol. 2020, 10, 591577. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.L.; Rinella, S.P.; Hess, N.J.; Turicek, D.P.; Kabakov, S.A.; Zhu, F.; Bouchlaka, M.N.; Olson, S.L.; Cho, M.M.; Quamine, A.E.; et al. CXCR4 allows T cell acute lymphoblastic leukemia to escape from JAK1/2 and BCL2 inhibition through CNS infiltration. Leuk. Lymphoma. 2021, 62, 1167–1177. [Google Scholar] [CrossRef]
- Arnaud, M.P.; Vallée, A.; Robert, G.; Bonneau, J.; Leroy, C.; Varin-Blank, N.; Rio, A.G.; Troadec, M.B.; Galibert, M.D.; Gandemer, V. CD9, a key actor in the dissemination of lymphoblastic leukemia, modulating CXCR4-mediated migration via RAC1 signaling. Blood 2015, 126, 1802–1812. [Google Scholar] [CrossRef]
- Montresor, A.; Toffali, L.; Rigo, A.; Ferrarini, I.; Vinante, F.; Laudanna, C. CXCR4- and BCR-triggered integrin activation in B-cell chronic lymphocytic leukemia cells depends on JAK2-activated Bruton’s tyrosine kinase. Oncotarget 2018, 9, 35123–35140. [Google Scholar] [CrossRef]
- Montresor, A.; Toffali, L.; Mirenda, M.; Rigo, A.; Vinante, F.; Laudanna, C. JAK2 tyrosine kinase mediates integrin activation induced by CXCL12 in B-cell chronic lymphocytic leukemia. Oncotarget 2015, 6, 34245–34257. [Google Scholar] [CrossRef]
- Ganghammer, S.; Hutterer, E.; Hinterseer, E.; Brachtl, G.; Asslaber, D.; Krenn, P.W.; Girbl, T.; Berghammer, P.; Geisberger, R.; Egle, A.; et al. CXCL12-induced VLA-4 activation is impaired in trisomy 12 chronic lymphocytic leukemia cells: A role for CCL21. Oncotarget 2015, 6, 12048–12060. [Google Scholar] [CrossRef]
- Scupoli, M.T.; Donadelli, M.; Cioffi, F.; Rossi, M.; Perbellini, O.; Malpeli, G.; Corbioli, S.; Vinante, F.; Krampera, M.; Palmieri, M.; et al. Bone marrow stromal cells and the upregulation of interleukin-8 production in human T-cell acute lymphoblastic leukemia through the CXCL12/CXCR4 axis and the NF-kappaB and JNK/AP-1 pathways. Haematologica 2008, 93, 524–532. [Google Scholar] [CrossRef]
- Redondo-Muñoz, J.; Escobar-Díaz, E.; Samaniego, R.; Terol, M.J.; García-Marco, J.A.; García-Pardo, A. MMP-9 in B-cell chronic lymphocytic leukemia is up-regulated by alpha4beta1 integrin or CXCR4 engagement via distinct signaling pathways, localizes to podosomes, and is involved in cell invasion and migration. Blood 2006, 108, 3143–3151. [Google Scholar] [CrossRef]
- Białopiotrowicz, E.; Górniak, P.; Noyszewska-Kania, M.; Puła, B.; Makuch-Łasica, H.; Nowak, G.; Bluszcz, A.; Szydłowski, M.; Jabłonska, E.; Piechna, K.; et al. Microenvironment-induced PIM kinases promote CXCR4-triggered mTOR pathway required for chronic lymphocytic leukaemia cell migration. J. Cell. Mol. Med. 2018, 22, 3548–3559. [Google Scholar] [CrossRef]
- Crassini, K.; Pyke, T.; Shen, Y.; Stevenson, W.S.; Christopherson, R.I.; Mulligan, S.P.; Best, O.G. Inhibition of the Raf-1 kinase inhibitory protein (RKIP) by locostatin induces cell death and reduces the CXCR4-mediated migration of chronic lymphocytic leukemia cells. Leuk. Lymphoma 2018, 59, 2917–2928. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Du, J.; Li, Y.; Wang, X.; Zhou, Y.; Yu, X.; Fan, W.; Zhu, Q.; Tong, X.; et al. The Critical Role of PTEN/PI3K/AKT Signaling Pathway in Shikonin-Induced Apoptosis and Proliferation Inhibition of Chronic Myeloid Leukemia. Cell. Physiol. Biochem. 2018, 47, 981–993. [Google Scholar] [CrossRef]
- Cilloni, D.; Saglio, G. Molecular pathways: BCR-ABL. Clin. Cancer Res. 2012, 18, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Iwakami, M.; Muromoto, R.; Inagaki, T.; Kitai, Y.; Kon, S.; Sekine, Y.; Oritani, K.; Matsuda, T. CCR7 is involved in BCR-ABL/STAP-2-mediated cell growth in hematopoietic Ba/F3 cells. Biochem. Biophys. Res. Commun. 2015, 463, 825–831. [Google Scholar] [CrossRef]
- Patterson, S.D.; Copland, M. The Bone Marrow Immune Microenvironment in CML: Treatment Responses, Treatment-Free Remission, and Therapeutic Vulnerabilities. Curr. Hematol. Malig. Rep. 2023, 18, 19–32. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, H.; Li, W.; Yao, J.; Sun, Y.; Li, Z.; Zhao, L.; Guo, Q. CXCL12/CXCR4 axis confers adriamycin resistance to human chronic myelogenous leukemia and oroxylin A improves the sensitivity of K562/ADM cells. Biochem. Pharmacol. 2014, 90, 212–225. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirase, C.; Matsumura, I. [Molecular mechanisms in the resistance of CML stem cells to tyrosine kinase inhibitors and novel targets for achieving a cure]. Rinsho Ketsueki. 2015, 56, 139–149. (In Japanese) [Google Scholar] [PubMed]
- de la Puente, P.; Muz, B.; Azab, F.; Luderer, M.; Azab, A.K. Molecularly targeted therapies in multiple myeloma. Leuk. Res. Treat. 2014, 2014, 976567. [Google Scholar] [CrossRef]
- Alsayed, Y.; Ngo, H.; Runnels, J.; Leleu, X.; Singha, U.K.; Pitsillides, C.M.; Spencer, J.A.; Kimlinger, T.; Ghobrial, J.M.; Jia, X.; et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood 2007, 109, 2708–2717. [Google Scholar] [CrossRef]
- Bila, J.; Katodritou, E.; Guenova, M.; Basic-Kinda, S.; Coriu, D.; Dapcevic, M.; Ibricevic-Balic, L.; Ivanaj, A.; Karanfilski, O.; Zver, S.; et al. Bone Marrow Microenvironment Interplay and Current Clinical Practice in Multiple Myeloma: A Review of the Balkan Myeloma Study Group. J. Clin. Med. 2021, 10, 3940. [Google Scholar] [CrossRef] [PubMed]
- Ullah, T.R. The role of CXCR4 in multiple myeloma: Cells’ journey from bone marrow to beyond. J. Bone Oncol. 2019, 17, 100253. [Google Scholar] [CrossRef]
- Schiano, C.; Soricelli, A.; De Nigris, F.; Napoli, C. New challenges in integrated diagnosis by imaging and osteo-immunology in bone lesions. Expert Rev. Clin. Immunol. 2019, 15, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Gehringer, F.; Weissinger, S.E.; Möller, P.; Wirth, T.; Ushmorov, A. Physiological levels of the PTEN-PI3K-AKT axis activity are required for maintenance of Burkitt lymphoma. Leukemia 2020, 34, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Balsas, P.; Palomero, J.; Eguileor, Á.; Rodríguez, M.L.; Vegliante, M.C.; Planas-Rigol, E.; Sureda-Gómez, M.; Cid, M.C.; Campo, E.; Amador, V. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood 2017, 130, 501–513. [Google Scholar] [CrossRef]
- Jin, Z.; Xiang, R.; Dai, J.; Wang, Y.; Xu, Z. HIF-1α mediates CXCR4 transcription to activate the AKT/mTOR signaling pathway and augment the viability and migration of activated B cell-like diffuse large B-cell lymphoma cells. Mol. Carcinog. 2023, 62, 676–684. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Shen, J.K.; Zhao, S.Q.; Li, J.M. Clinical significance of chemokine receptor CXCR4 and mammalian target of rapamycin (mTOR) expression in patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2018, 59, 1451–1460. [Google Scholar] [CrossRef]
- Majka, M.; Ratajczak, J.; Kowalska, M.A.; Ratajczak, M.Z. Binding of stromal derived factor-1alpha (SDF-1alpha) to CXCR4 chemokine receptor in normal human megakaryoblasts but not in platelets induces phosphorylation of mitogen-activated protein kinase p42/44 (MAPK), ELK-1 transcription factor and serine/threonine kinase AKT. Eur. J. Haematol. 2000, 64, 164–172. [Google Scholar]
- Opdam, F.J.; Kamp, M.; de Bruijn, R.; Roos, E. Jak kinase activity is required for lymphoma invasion and metastasis. Oncogene 2004, 23, 6647–6653. [Google Scholar] [CrossRef]
- Hiemcke-Jiwa, L.S.; Leguit, R.J.; Jiwa, N.M.; Huibers, M.M.H.; Minnema, M.C. CXCR4 mutations in lymphoplasmacytic lymphoma lead to altered CXCR4 expression. Br. J. Haematol. 2019, 185, 966–969. [Google Scholar] [CrossRef]
- Shin, H.C.; Seo, J.; Kang, B.W.; Moon, J.H.; Chae, Y.S.; Lee, S.J.; Lee, Y.J.; Han, S.; Seo, S.K.; Kim, J.G.; et al. Clinical significance of nuclear factor κB and chemokine receptor CXCR4 expression in patients with diffuse large B-cell lymphoma who received rituximab-based therapy. Korean J. Intern. Med. 2014, 29, 785–792. [Google Scholar] [CrossRef]
- MacDonald, R.J.; Yen, A. CXCR5 overexpression in HL-60 cells enhances chemotaxis toward CXCL13 without anticipated interaction partners or enhanced MAPK signaling. Vitr. Cell. Dev. Biol. Anim. 2018, 54, 725–735. [Google Scholar] [CrossRef]
- Battle, T.E.; Roberson, M.S.; Zhang, T.; Varvayanis, S.; Yen, A. Retinoic acid-induced blr1 expression requires RARalpha, RXR, and MAPK activation and uses ERK2 but not JNK/SAPK to accelerate cell differentiation. Eur. J. Cell. Biol. 2001, 80, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pascutti, M.F.; Jak, M.; Tromp, J.M.; Derks, I.A.; Remmerswaal, E.B.; Thijssen, R.; van Attekum, M.H.; van Bochove, G.G.; Luijks, D.M.; Pals, S.T.; et al. IL-21 and CD40L signals from autologous T cells can induce antigen-independent proliferation of CLL cells. Blood 2013, 122, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Stache, V.; Verlaat, L.; Gätjen, M.; Heinig, K.; Westermann, J.; Rehm, A.; Höpken, U.E. The splenic marginal zone shapes the phenotype of leukemia B cells and facilitates their niche-specific retention and survival. Oncoimmunology 2017, 6, e1323155. [Google Scholar] [CrossRef] [PubMed]
- Kurtova, A.V.; Tamayo, A.T.; Ford, R.J.; Burger, J.A. Mantle cell lymphoma cells express high levels of CXCR4, CXCR5, and VLA-4 (CD49d): Importance for interactions with the stromal microenvironment and specific targeting. Blood 2009, 113, 4604–4613. [Google Scholar] [CrossRef]
- Rehm, A.; Anagnostopoulos, I.; Gerlach, K.; Broemer, M.; Scheidereit, C.; Jöhrens, K.; Hübler, M.; Hetzer, R.; Stein, H.; Lipp, M.; et al. Identification of a chemokine receptor profile characteristic for mediastinal large B-cell lymphoma. Int. J. Cancer 2009, 125, 2367–2374. [Google Scholar] [CrossRef]
- Cahir-McFarland, E.D.; Carter, K.; Rosenwald, A.; Giltnane, J.M.; Henrickson, S.E.; Staudt, L.M.; Kieff, E. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J. Virol. 2004, 78, 4108–4119. [Google Scholar] [CrossRef]
- Zhang, G.; Miao, F.; Xu, J.; Wang, R. Mesenchymal stem cells from bone marrow regulate invasion and drug resistance of multiple myeloma cells by secreting chemokine CXCL13. Bosn. J. Basic Med. Sci. 2020, 20, 209–217. [Google Scholar] [CrossRef]
- Yue, Z.; Zhou, Y.; Zhao, P.; Chen, Y.; Yuan, Y.; Jing, Y.; Wang, X. p53 Deletion promotes myeloma cells invasion by upregulating miR19a/CXCR5. Leuk. Res. 2017, 60, 115–122. [Google Scholar] [CrossRef]
- Shi, S.; Xing, H.; Xu, X.; Chai, J.; Lu, Z.; Wang, J.; Wang, B. CXCR6 defines therapeutic subtypes of CD4+ cytotoxic T cell lineage for adoptive cell transfer therapy in pediatric B cell acute lymphoblastic leukemia. Int. Immunopharmacol. 2024, 132, 111972. [Google Scholar] [CrossRef]
- Yang, H.; Qiu, B.; Chen, S.; Xun, Y.; Pan, Y.; Chen, M.; Li, W.X.; Liao, W.; El-Ashram, S.; Yang, A.; et al. Soluble CXCL16 promotes TNF-α-induced apoptosis in DLBCL via the AMAD10-NF-κB regulatory feedback loop. Cell. Biol. Int. 2019, 43, 863–874. [Google Scholar] [CrossRef]
- Mehrpouri, M. The contributory roles of the CXCL12/CXCR4/CXCR7 axis in normal and malignant hematopoiesis: A possible therapeutic target in hematologic malignancies. Eur. J. Pharmacol. 2022, 920, 174831. [Google Scholar] [CrossRef]
- Cui, X.Y.; Tjønnfjord, G.E.; Kanse, S.M.; Dahm, A.E.A.; Iversen, N.; Myklebust, C.F.; Sun, L.; Jiang, Z.X.; Ueland, T.; Campbell, J.J.; et al. Tissue factor pathway inhibitor upregulates CXCR7 expression and enhances CXCL12-mediated migration in chronic lymphocytic leukemia. Sci. Rep. 2021, 11, 5127. [Google Scholar] [CrossRef]
- Yang, X.Y.; Sheng, Y. miR-101 Represses T-Cell Acute Lymphoblastic Leukemia by Targeting CXCR7/STAT3 Axis. Oncol. Res. 2019, 27, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Furuichi, Y.; Kasai, S.; Tamai, M.; Harama, D.; Kagami, K.; Abe, M.; Goi, K.; Inukai, T.; Sugita, K. Chemosensitivity is differentially regulated by the SDF-1/CXCR4 and SDF-1/CXCR7 axes in acute lymphoblastic leukemia with MLL gene rearrangements. Leuk. Res. 2018, 75, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.C.; Ferro, K.P.V.; Duarte, A.D.S.S.; Olalla Saad, S.T. CXCR7 participates in CXCL12-mediated migration and homing of leukemic and normal hematopoietic cells. Stem Cell. Res. Ther. 2018, 9, 34. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, S.Y.; Kim, D.Y.; Moon, J.Y.; Choi, Y.S.; Song, I.C.; Lee, H.J.; Yun, H.J.; Kim, S.; Jo, D.Y. Expression and functional roles of the chemokine receptor CXCR7 in acute myeloid leukemia cells. Blood Res. 2015, 50, 218–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, W.; Ding, Q.; Ding, Y.; Lu, L.; Wang, X.; Zhang, Y.; Zhang, X.; Guo, Q.; Zhao, L. Oroxylin A reverses the drug resistance of chronic myelogenous leukemia cells to imatinib through CXCL12/CXCR7 axis in bone marrow microenvironment. Mol. Carcinog. 2017, 56, 863–876. [Google Scholar] [CrossRef]
- Wada, N.; Ikeda, J.; Nojima, S.; Tahara, S.; Ohshima, K.; Okuzaki, D.; Morii, E. Requirement of CXCL12-CXCR7 signaling for CD20(-) CD138(-) double-negative population in lymphoplasmacytic lymphoma. Lab. Investig. 2016, 96, 517–525. [Google Scholar] [CrossRef]
- Zabel, B.A.; Lewén, S.; Berahovich, R.D.; Jaén, J.C.; Schall, T.J. The novel chemokine receptor CXCR7 regulates trans-endothelial migration of cancer cells. Mol. Cancer 2011, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Waldschmidt, J.M.; Simon, A.; Wider, D.; Müller, S.J.; Follo, M.; Ihorst, G.; Decker, S.; Lorenz, J.; Chatterjee, M.; Azab, A.K.; et al. CXCL12 and CXCR7 are relevant targets to reverse cell adhesion-mediated drug resistance in multiple myeloma. Br. J. Haematol. 2017, 179, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.K.; Sahin, I.; Azab, F.; Moschetta, M.; Mishima, Y.; Burwick, N.; Zimmermann, J.; Romagnoli, B.; Patel, K.; Chevalier, E.; et al. CXCR7-dependent angiogenic mononuclear cell trafficking regulates tumor progression in multiple myeloma. Blood 2014, 124, 1905–1914. [Google Scholar] [CrossRef]
- Hellmich, C.; Moore, J.A.; Bowles, K.M.; Rushworth, S.A. Bone Marrow Senescence and the Microenvironment of Hematological Malignancies. Front. Oncol. 2020, 10, 230. [Google Scholar] [CrossRef]
- Hong, Y.; Jeong, S.; Park, M.-J.; Song, W.; Lee, N. Application of Pathomic Features for Differentiating Dysplastic Cells in Patients with Myelodysplastic Syndrome. Bioengineering 2024, 11, 1230. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, K.; Huang, Q.; Wei, C.; Wei, M.; Geng, Z.; Wu, H.; Zhou, T.; Yin, X.; Zhou, Y. Selinexor in combination with venetoclax and decitabine in patients with refractory myelodysplastic syndrome previously exposed to hypomethylating agents: Three case reports. Front. Oncol. 2024, 14, 1477697. [Google Scholar] [CrossRef]
- García, R.; Alkayyali, T.; Gomez, L.M.; Wright, C.; Chen, W.; Oliver, D.; Koduru, P. Recurrent cytogenetic abnormalities reveal alterations that promote progression and transformation in myelodysplastic syndrome. Cancer Genet. 2024, 288–289, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Bakkaloglu, I.G.; Zemheri, I.E.; Kaya, A.H.; Kilicaslan, E. The Impact of Microenvironment and Dysplasia Types on the Prognosis of Myelodysplastic Syndrome. Diagnostics 2024, 14, 2720. [Google Scholar] [CrossRef]
- Xiao, N.; He, X.; Niu, H.; Yu, H.; Cui, N.; Li, H.; Yan, L.; Shao, Z.; Xing, L.; Wang, H. Increased Circulating CD4+CXCR5+ Cells and IgG4 Levels in Patients with Myelodysplastic Syndrome with Autoimmune Diseases. J. Immunol. Res. 2021, 2021, 4302515. [Google Scholar] [CrossRef]
- Huselton, E.; Rettig, M.P.; Campbell, K.; Cashen, A.F.; DiPersio, J.F.; Gao, F.; Jacoby, M.A.; Pusic, I.; Romee, R.; Schroeder, M.A.; et al. Combination of dociparstat sodium (DSTAT), a CXCL12/CXCR4 inhibitor, with azacitidine for the treatment of hypomethylating agent refractory AML and MDS. Leuk. Res. 2021, 110, 106713. [Google Scholar] [CrossRef]
- Yan, Y.; Upadhyaya, R.; Zhang, V.W.; Berg, T. Epigenetic maintenance strategies after allogeneic stem cell transplantation in acute myeloid leukemia. Exp. Hematol. 2022, 109, 1–10.e1. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Yin, Y.; Zeng, M.-J.; Chen, Y.-T.; Tang, H.-W. [The Mechanisms of Piceatannol in Inhibiting the Malignant Biological Characteristics of Acute Myeloid Leukemia Cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 985–991. (In Chinese) [Google Scholar]
- Claudia, K.; Simón, M.F. Myeloid malignancies and the microenvironment. Blood 2017, 129, 811–822. [Google Scholar]
- Karantanou, C.; Godavarthy, P.S.; Krause, D.S. Targeting the bone marrow microenvironment in acute leukemia. Leuk. Lymphoma 2018, 59, 2535–2545. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, M.; Yin, Y.; Shen, J.; Tang, H. Abnormal expression and clinical significance of CXC chemokine receptor 1/2 in acute leukemia. Acad. J. Chin. Pla Med. Sch. 2023, 44, 213–219. [Google Scholar]
- Korbecki, J.; Bosiacki, M.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. CXCR4 as a therapeutic target in acute myeloid leukemia. Leukemia 2024, 38, 2303–2317. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Su, J.; Wang, D.; Feng, W.; Wang, X.; Lu, H.; Wang, A.; Liu, M.; Xia, G. A Dual-Function LipoAraN-E5 Coloaded with N4-Myristyloxycarbonyl-1-β-d-arabinofuranosylcytosine (AraN) and a CXCR4 Antagonistic Peptide (E5) for Blocking the Dissemination of Acute Myeloid Leukemia. ACS Nano 2024, 18, 27917–27932. [Google Scholar] [CrossRef]
- Braitsch, K.; Lorenzini, T.; Hefter, M.; Koch, K.; Nickel, K.; Peeken, J.C.; Götze, K.S.; Weber, W.; Allmann, A.; D’Alessandria, C.; et al. CXCR4-directed endoradiotherapy with [177Lu]Pentixather added to total body irradiation for myeloablative conditioning in patients with relapsed/refractory acute myeloid leukemia. Theranostics 2025, 15, 19–29. [Google Scholar] [CrossRef]
- Hou, Y.; Chen, Y.; Zhang, Y.; Li, M.; Chen, J. Prognostic role of chemokine-related genes in acute myeloid leukemia. PeerJ 2024, 12, e17862. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Shen, J.Z.; Yin, Y.; Chen, Y.T.; Yang, H.; Tang, H.W. [The Effects and Regulatory Mechanism of Targeting CXC Chemokine Receptor 1/2 Combined with Ara-C on the Malignant Biological Behaviors of U937 Cells of Acute Myeloid Leu-kemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 364–376 (In Chinese). (In Chinese) [Google Scholar]
- Verachi, P.; Gobbo, F.; Martelli, F.; Martinelli, A.; Sarli, G.; Dunbar, A.; Levine, R.L.; Hoffman, R.; Massucci, M.T.; Brandolini, L.; et al. The CXCR1/CXCR2 inhibitor Reparixin alters the development of myelofibrosis in the Gata1low mice. Front. Oncol. 2022, 12, 853484. [Google Scholar] [CrossRef] [PubMed]
- Maganti, H.; Visram, A.; Shorr, R.; Fulcher, J.; Sabloff, M.; Allan, D.S. Plerixafor in combination with chemotherapy and/or hematopoietic cell transplantation to treat acute leukemia: A systematic review and metanalysis of preclinical and clinical studies. Leuk. Res. 2020, 97, 106442. [Google Scholar] [CrossRef]
- Hematology Oncology Committee, Chinese Anti-Cancer Association; Leukemia & Lymphoma Group, Chinese Society of Hematology, Chinese Medical Association. [Chinese guideline for diagnosis and treatment of adult acute lymphoblastic leu-kemia (2024)]. Zhonghua Xue Ye Xue Za Zhi 2024, 45, 417–429. (In Chinese) [Google Scholar]
- Pagliaro, L.; Chen, S.J.; Herranz, D.; Mecucci, C.; Harrison, C.J.; Mullighan, C.G.; Zhang, M.; Chen, Z.; Boissel, N.; Winter, S.S.; et al. Acute lymphoblastic leukaemia. Nat. Rev. Dis. Primers 2024, 10, 41. [Google Scholar] [CrossRef]
- Zanetti, C.; Kumar, R.; Ender, J.; Godavarthy, P.S.; Hartmann, M.; Hey, J.; Breuer, K.; Weissenberger, E.S.; Minciacchi, V.R.; Karantanou, C.; et al. The age of the bone marrow microenvironment influences B-cell acute lymphoblastic leukemia progression via CXCR5-CXCL13. Blood 2021, 138, 1870–1884. [Google Scholar] [CrossRef]
- Tsaouli, G.; Ferretti, E.; Bellavia, D.; Vacca, A.; Felli, M.P. Notch/CXCR4 Partnership in Acute Lymphoblastic Leukemia Progression. J. Immunol. Res. 2019, 2019, 5601396. [Google Scholar] [CrossRef]
- Su, L.; Hu, Z.; Yang, Y. Role of CXCR4 in the progression and therapy of acute leukaemia. Cell Prolif. 2021, 54, e13076. [Google Scholar] [CrossRef]
- Pohl, J.; Litz, A.; El Ayoubi, O.; Rodríguez-Alfonso, A.; Ständker, L.; Harms, M.; Münch, J.; Jumaa, H.; Datta, M. An Optimized Peptide Antagonist of CXCR4 Limits Survival of BCR–ABL1-Transformed Cells in Philadelphia-Chromosome-Positive B-Cell Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2024, 25, 8306. [Google Scholar] [CrossRef]
- Abdoul-Azize, S.; Hami, R.; Riou, G.; Derambure, C.; Charbonnier, C.; Vannier, J.P.; Guzman, M.L.; Schneider, P.; Boyer, O. Glucocorticoids paradoxically promote steroid resistance in B cell acute lympho-blastic leukemia through CXCR4/PLC signaling. Nat. Commun. 2024, 15, 4557. [Google Scholar] [CrossRef]
- Jonart, L.M.; Ostergaard, J.; Brooks, A.; Fitzpatrick, G.; Chen, L.; Gordon, P.M. CXCR4 antagonists disrupt leukaemia-meningeal cell adhesion and attenuate chemoresistance. Br. J. Haematol. 2022, 201, 459–469. [Google Scholar] [CrossRef]
- Ma, W.; Wan, Y.; Zhang, J.; Yao, J.; Wang, Y.; Lu, J.; Liu, H.; Huang, X.; Zhang, X.; Zhou, H.; et al. Growth arrest-specific protein 2 (GAS2) interacts with CXCR4 to promote T-cell leukemo-genesis partially via c-MYC. Mol. Oncol. 2022, 16, 3720–3734. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Gonzalez-Anton, S.; Li, X.; Mi, E.; Wu, L.; Zhao, H.; Zhang, G.; Lu, A.; Celso, C.L.; Ma, D. General anaesthetics reduce acute lymphoblastic leukaemia malignancies in vitro and in vivovia CXCR4 and osteopontin mediated mechanisms. F1000Research 2024, 11, 1491. [Google Scholar] [CrossRef]
- Rocco, S.; Maglione, A.; Schiavo, V.; Ferrando, A.; Fava, C.; Cilloni, D.; Pergolizzi, B.; Panuzzo, C. Tyrosine Kinase Inhibitor Therapy Enhances Stem Cells Profile and May Contribute to Survival of Chronic Myeloid Leukemiastem Cells. J. Clin. Med. 2025, 14, 392. [Google Scholar] [CrossRef]
- Laganà, A.; Scalzulli, E.; Bisegna, M.L.; Ielo, C.; Martelli, M.; Breccia, M. Understanding and overcoming resistance to tyrosine kinase inhibitors (TKIs) in Chronic myeloid leukemia (CML). Expert Rev. Hematol. 2024, 18, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, K.-W.; Park, Y.; Kim, B.S. CXCR2 inhibition overcomes ponatinib intolerance by eradicating chronic myeloid leukemic stem cells through PI3K/Akt/mTOR and dipeptidylpeptidase Ⅳ (CD26). Heliyon 2023, 9, e22091. [Google Scholar] [CrossRef]
- Agarwal, P.; Li, H.; Choi, K.; Hueneman, K.; He, J.; Welner, R.S.; Starczynowski, D.T.; Bhatia, R. TNF-α-induced alterations in stromal progenitors enhance leukemic stem cell growth via CXCR2 signaling. Cell Rep. 2021, 36, 109386. [Google Scholar] [CrossRef]
- Herrmann, A.B.; Müller, M.; Orth, M.F.; Müller, J.P.; Zernecke, A.; Hochhaus, A.; Ernst, T.; Butt, E.; Frietsch, J.J. Knockout of LASP1 in CXCR4 expressing CML cells promotes cell persistence, proliferation and TKI resistance. J. Cell. Mol. Med. 2020, 24, 2942–2955. [Google Scholar] [CrossRef]
- Hallek, M.; Al-Sawaf, O. Chronic lymphocytic leukemia: 2022 update on diagnostic and therapeutic procedures. Am. J. Hematol. 2021, 96, 1679–1705. [Google Scholar] [CrossRef] [PubMed]
- Hematology Committee of Chinese Medical Association; Hematological Oncology Committee of China Anti-Cancer Associa-tion; Chinese Working Group for Chronic Lymphocytic Leukemia. [The guidelines for diagnosis and treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma in China (2018 edition)]. Zhonghua Xue Ye Xue Za Zhi 2018, 39, 353–358. (In Chinese) [Google Scholar]
- Nabhan, C.; Raca, G.; Wang, Y.L. Predicting Prognosis in Chronic Lymphocytic Leukemia in the Contemporary Era. JAMA Oncol. 2015, 1, 965–974. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, J.; Awal Issah, M.; Liu, T.; Zhou, H.; Fu, H. CD56-positive diffuse large B-cell lymphoma/leukemia with BCL6/MYC double-hit and multiple gene mutations: An indicator of poor prognosis? J. Int. Med. Res. 2020, 48, 300060520918087. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, J.; Liu, Y.; Lin, S.; Shen, J.; Yin, Y.; Wang, Y. Primary intestinal diffuse large B-cell lymphoma: Novel insights and clinical perception. Front. Oncol. 2024, 14, 1404298. [Google Scholar] [CrossRef]
- George, D.M.; Lakshmanan, A. Lymphomas with Primary Gastrointestinal Presentation: A Retrospective Study Covering a Five-Year Period at a Quaternary Care Center in Southern India. Cureus 2024, 16, e75161. [Google Scholar] [CrossRef] [PubMed]
- Rahimzada, M.; Nahavandi, M.; Saffari, M.; Shafaei, A.; Mosavat, A.; Gezeldasht, S.A.; Ariaee, N.; Valizadeh, N.; Rahimi, H.; Rezaee, S.A.; et al. Gene expression study of host-human T-cell leukaemia virus type 1 (HTLV-1) interactions: Adult T-cell leukaemia/lymphoma (ATLL). Mol. Biol. Rep. 2023, 50, 7479–7487. [Google Scholar] [CrossRef]
- Antonello, P.; Pizzagalli, D.U.; Foglierini, M.; Melgrati, S.; Radice, E.; Thelen, S.; Thelen, M. ACKR3 promotes CXCL12/CXCR4-mediated cell-to-cell-induced lymphoma migration through LTB4 production. Front. Immunol. 2023, 13, 1067885. [Google Scholar] [CrossRef]
- Kosmala, A.; Seifert, S.; Schneid, S.; Dreher, N.; Higuchi, T.; Weich, A.; Serfling, S.E.; Hartrampf, P.E.; Einsele, H.; Buck, A.K.; et al. Lymphoma-Sink Effect in Marginal Zone Lymphoma Based on CXCR4-Targeted Molecular Imaging. Mol. Imaging Biol. 2023, 25, 758–764. [Google Scholar] [CrossRef]
- Nugent, A.; Proia, R.L. The role of G protein-coupled receptors in lymphoid malignancies. Cell. Signal. 2017, 39, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Treon, S.P.; Cao, Y.; Xu, L.; Yang, G.; Liu, X.; Hunter, Z.R. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenström macroglobulinemia. Blood 2014, 123, 2791–2796. [Google Scholar] [CrossRef]
- Lewis, R.; Maurer, H.C.; Singh, N.; Gonzalez-Menendez, I.; Wirth, M.; Schick, M.; Zhang, L.; Isaakidis, K.; Scherger, A.K.; Schulze, V.; et al. CXCR4 hyperactivation cooperates with TCL1 in CLL development and aggressiveness. Leukemia 2021, 35, 2895–2905. [Google Scholar] [CrossRef]
- Pansy, K.; Feichtinger, J.; Ehall, B.; Uhl, B.; Sedej, M.; Roula, D.; Pursche, B.; Wolf, A.; Zoidl, M.; Steinbauer, E.; et al. The CXCR4–CXCL12-Axis Is of Prognostic Relevance in DLBCL and Its Antagonists Exert Pro-Apoptotic Effects In Vitro. Int. J. Mol. Sci. 2019, 20, 4740. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Tang, H.W.; Chen, Y.T. Analysis of the Status of Traditional Chinese Medicine in the Diagnosis and Treatment of Multiple Myeloma. J. Oncol. Chin. Med. 2022, 4, 46–52. [Google Scholar]
- Liu, Y.-Q.; Yin, Y.; Chen, Y.-T.; Shen, J.-Z.; Tang, H.-W. [Experimental Study on the Mechanism of Mangiferin Inhibiting Malignant Biological Characteristics of Multiple Myeloma and Exerting Anticancer Effect]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2023, 31, 794–800. (In Chinese) [Google Scholar] [PubMed]
- Muylaert, C.; Van Hemelrijck, L.A.; Van der Vreken, A.; Heestermans, R.; Satilmis, H.; Verheye, E.; Alaterre, E.; Olsen, C.; De Beule, N.; De Veirman, K.; et al. The de novo DNA methyltransferase 3B is a novel epigenetic regulator of MYC in multiple myeloma, representing a promising therapeutic target to counter relapse. J. Exp. Clin. Cancer Res. 2025, 44, 125. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-T.; Wu, Y.-P.U.; Li, Y.-Q.; Chen, Q.; Yao, L.-Y.; Lin, L.U.; Gao, G.-Y. Comprehensive bioinformatics analysis reveals key hub genes linked to prognosis in multiple myeloma with drug resistance. Medicine 2025, 104, e41707. [Google Scholar] [CrossRef]
- Sawasdee, N.; Thepmalee, C.; Junking, M.; Okada, S.; Panya, A.; Yenchitsomanus, P.-T. Enhancing T cell cytotoxicity in multiple myeloma with bispecific αPD-L1 × αCD3 T cell engager-armed T cells and low-dose bortezomib therapy. Biomed. Pharmacother. 2025, 184, 117878. [Google Scholar] [CrossRef]
- Ito, S.; Sato, T.; Maeta, T. Role and Therapeutic Targeting of SDF-1α/CXCR4 Axis in Multiple Myeloma. Cancers 2021, 13, 1793. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, J.; Li, X.-M.; Huang, C.-L. [Correlation between Expression of CXCL12, CXCR4, VEGF, MVD and Prognosis in Patients with Multiple Myeloma]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 1962–1971. (In Chinese) [Google Scholar]
- Mouhieddine, T.H.; Ahmad, Y.; Barlogie, B.; Jagannath, S.; Teruya-Feldstein, J.; Richter, J. Increased Muscle CXCR4 Expression in the Setting of Rare Muscle-invasive Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, E341–E344. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, H.M.; Lv, Y.Q.; Tang, S.M.; Cheng, P. Blockade of SDF-1/CXCR4 reduces adhesion-mediated chemoresistance of multiple mye-loma cells via interacting with interleukin-6. J. Cell. Physiol. 2019, 234, 19702–19714. [Google Scholar] [CrossRef]
- Yang, X.; Wan, M.; Yu, F.; Wang, Z. Efficacy and safety of plerixafor for hematopoietic stem cell mobilization for autologous transplantation in patients with non-Hodgkin lymphoma and multiple myeloma: A systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 1141–1148. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Liu, C.; Zavidij, O.; Azab, A.K.; Baz, R.; Laubach, J.P.; Mishima, Y.; Armand, P.; Munshi, N.C.; Basile, F.; et al. Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. Am. J. Hematol. 2019, 94, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Makishima, H.; Ito, T.; Asano, N.; Nakazawa, H.; Shimodaira, S.; Kamijo, Y.; Nakazawa, Y.; Suzuki, T.; Kobayashi, H.; Kiyosawa, K.; et al. Significance of chemokine receptor expression in aggressive NK cell leukemia. Leukemia 2005, 19, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Rodrigues, J.; Santos, A.H.; Teixeira Mdos, A.; Queirós, M.L.; Santos, M.; Gonçalves, M.; Fonseca, S.; Laranjeira, C.; Rodrigues, A.S.; et al. Chemokine receptor repertoire reflects mature T-cell lymphoproliferative disorder clinical presentation. Blood Cells Mol. Dis. 2009, 42, 57–63. [Google Scholar] [CrossRef]
- Trentin, L.; Miorin, M.; Facco, M.; Baesso, I.; Carraro, S.; Cabrelle, A.; Maschio, N.; Bortoli, M.; Binotto, G.; Piazza, F.; et al. Multiple myeloma plasma cells show different chemokine receptor profiles at sites of disease activity. Br. J. Haematol. 2007, 138, 594–602. [Google Scholar] [CrossRef]
- Arciprete, F.; Verachi, P.; Martelli, F.; Valeri, M.; Balliu, M.; Guglielmelli, P.; Vannucchi, A.M.; Migliaccio, A.R.; Zingariello, M. Inhibition of CXCR1/2 reduces the emperipolesis between neutrophils and megakaryocytes in the Gata1low model of myelofibrosis. Exp. Hematol. 2023, 121, 30–37. [Google Scholar] [CrossRef]
- Levidou, G.; Sachanas, S.; Pangalis, G.A.; Kalpadakis, C.; Yiakoumis, X.; Moschogiannis, M.; Sepsa, A.; Lakiotaki, E.; Milionis, V.; Kyrtsonis, M.C.; et al. Immunohistochemical analysis of IL-6, IL-8/CXCR2 axis, Tyr p-STAT-3, and SOCS-3 in lymph nodes from patients with chronic lymphocytic leukemia: Correlation between microvascular characteristics and prognostic significance. Biomed. Res. Int. 2014, 2014, 251479. [Google Scholar] [CrossRef]
- Tensen, C.P.; Vermeer, M.H.; van der Stoop, P.M.; van Beek, P.; Scheper, R.J.; Boorsma, D.M.; Willemze, R. Epidermal interferon-gamma inducible protein-10 (IP-10) and monokine induced by gamma-interferon (Mig) but not IL-8 mRNA expression is associated with epidermotropism in cutaneous T cell lymphomas. J. Investig. Dermatol. 1998, 111, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, G.; Papajik, T.; Mikulkova, Z.; Urbanova, R.; Kraiczova, V.S.; Savara, J.; Kudelka, M.; Turcsanyi, P.; Kriegova, E. High CXCR3 on Leukemic Cells Distinguishes IgHVmut from IgHVunmut in Chronic Lymphocytic Leukemia: Evidence from CD5high and CD5low Clones. J. Immunol. Res. 2020, 2020, 7084268. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Hamzic, E.; Willis, F.; Pettengell, R. Differences between chronic lymphocytic leukaemia and small lymphocytic lymphoma cells by proteomic profiling and SNP microarray analysis. Cancer Genet. 2017, 218–219, 20–38. [Google Scholar] [CrossRef]
- Han, B.; Lim, S.; Yim, J.; Song, Y.K.; Koh, J.; Kim, S.; Lee, C.; Kim, Y.A.; Jeon, Y.K. Clinicopathological implications of immunohistochemical expression of TBX21, CXCR3, GATA3, CCR4, and TCF1 in nodal follicular helper T-cell lymphoma and peripheral T-cell lymphoma, not otherwise specified. J. Pathol. Transl. Med. 2024, 58, 59–71. [Google Scholar] [CrossRef]
- Saber, M.M. Coexpression of PD-L1/PD-1 with CXCR3/CD36 and IL-19 Increase in Extranodal Lymphoma. J. Immunol. Res. 2023, 2023, 4556586. [Google Scholar] [CrossRef]
- Takashima, Y.; Hamano, M.; Yoshii, K.; Hayano, A.; Fukai, J.; Iwadate, Y.; Kajiwara, K.; Hondoh, H.; Yamanaka, R. Reciprocal expression of the immune response genes CXCR3 and IFI44L as module hubs are associated with patient survivals in primary central nervous system lymphoma. Int. J. Clin. Oncol. 2023, 28, 468–481. [Google Scholar] [CrossRef]
- Pellegrino, A.; Antonaci, F.; Russo, F.; Merchionne, F.; Ribatti, D.; Vacca, A.; Dammacco, F. CXCR3-binding chemokines in multiple myeloma. Cancer Lett. 2004, 207, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Ye, Y.; Liao, H.; Shuai, X.; Jin, Y.; Su, J.; Zheng, Q. Relationship between CXC chemokine receptor 4 expression and prognostic significance in acute myeloid leukemia. Medicine 2019, 98, e15948. [Google Scholar] [CrossRef] [PubMed]
- Rady, A.S.; Badawy, R.H.; Gamal, B.M.E.; Darwish, A.D.; Aziz, R.S.A.; Gammal, M.E.; Goweda, R.A. Association of CXCR4 Expression and Clinical Outcome in Different Subsets of De Novo Acute Myeloid Leukemia Patients. Clin. Lab. 2020, 66, 401–408. [Google Scholar] [CrossRef]
- Crazzolara, R.; Kreczy, A.; Mann, G.; Heitger, A.; Eibl, G.; Fink, F.M.; Möhle, R.; Meister, B. High expression of the chemokine receptor CXCR4 predicts extramedullary organ infiltration in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2001, 115, 545–553. [Google Scholar] [CrossRef]
- Ko, S.Y.; Park, C.J.; Park, S.H.; Cho, Y.U.; Jang, S.; Seo, E.J.; Kim, N.; Kim, D.Y.; Koh, K.N.; Im, H.J.; et al. High CXCR4 and low VLA-4 expression predicts poor survival in adults with acute lymphoblastic leukemia. Leuk. Res. 2014, 38, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Lu, C.; Zhu, X.; Hu, D.; Chen, X.; Li, J.; Liu, W.; Zhu, J.; He, Y.; Yao, J. Prognostic significance of CXCR4 expression in acute myeloid leukemia. Cancer Med. 2019, 8, 6595–6603. [Google Scholar] [CrossRef]
- El-Sherif, W.T.; Salah El-Din, E.M.; Aly, M.M.; Said, M.G.; Nigm, D.A. CXCR4 as a prognostic marker in Egyptian chronic lymphocytic leukemia patients. Egypt. J. Immunol. 2021, 28, 114–126. [Google Scholar] [CrossRef]
- Chen, J.; Xu-Monette, Z.Y.; Deng, L.; Shen, Q.; Manyam, G.C.; Martinez-Lopez, A.; Zhang, L.; Montes-Moreno, S.; Visco, C.; Tzankov, A.; et al. Dysregulated CXCR4 expression promotes lymphoma cell survival and independently predicts disease progression in germinal center B-cell-like diffuse large B-cell lymphoma. Oncotarget 2015, 6, 5597–5614. [Google Scholar] [CrossRef]
- Du, H.; Zhang, L.; Li, G.; Liu, W.; Tang, W.; Zhang, H.; Luan, J.; Gao, L.; Wang, X. CXCR4 and CCR7 Expression in Primary Nodal Diffuse Large B-Cell Lymphoma-A Clinical and Immunohistochemical Study. Am. J. Med. Sci. 2019, 357, 302–310. [Google Scholar] [CrossRef]
- Zhang, Y.A.; Yang, X.; Yao, J.; Ren, Y.; Liu, P. Identification of CXCR4 Upregulation in Diffuse Large B-Cell Lymphoma Associated with Prognostic Significance and Clinicopathological Characteristics. Dis. Markers 2022, 2022, 3276925. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Takata, K.; Zhang, Z.; Chong, L.; Fraser, B.; Zeisler, J.; Miyata-Takata, T.; Merkens, H.; Rousseau, J.; Aoki, T.; et al. Targeting Refractory Mantle Cell Lymphoma for Imaging and Therapy Using C-X-C Chemokine Receptor Type 4 Radioligands. Clin. Cancer Res. 2022, 28, 1628–1639. [Google Scholar] [CrossRef]
- Mazur, G.; Butrym, A.; Kryczek, I.; Dlubek, D.; Jaskula, E.; Lange, A.; Kuliczkowski, K.; Jelen, M. Decreased expression of CXCR4 chemokine receptor in bone marrow after chemotherapy in patients with non-Hodgkin lymphomas is a good prognostic factor. PLoS ONE 2014, 9, e98194. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Lai, Y.; Liu, Y.; Qin, Y.; Zhao, X.; Lu, X.; Jiang, Q.; Lu, J.; Huang, X. CXCR4 is a good survival prognostic indicator in multiple myeloma patients. Leuk. Res. 2013, 37, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhan, Y.; Yan, H.; Liang, H.; Yao, F.; Xu, H. Reduced CXCR4 expression in associated with extramedullary and predicts poor survival in newly diagnosed multiple myeloma. Expert. Rev. Hematol. 2022, 15, 1017–1021. [Google Scholar] [CrossRef]
- Rosti, V.; Massa, M.; Vannucchi, A.M.; Bergamaschi, G.; Campanelli, R.; Pecci, A.; Viarengo, G.; Meli, V.; Marchetti, M.; Guglielmelli, P.; et al. The expression of CXCR4 is down-regulated on the CD34+ cells of patients with myelofibrosis with myeloid metaplasia. Blood Cells Mol. Dis. 2007, 38, 280–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Zhao, H.; Zhao, D.; Wu, X.; Zhao, W.; Wang, Y.; Xia, B.; Da, W. Expression of CXCR4 is an independent prognostic factor for overall survival and progression-free survival in patients with myelodysplastic syndrome. Med. Oncol. 2013, 30, 341. [Google Scholar] [CrossRef]
- Guo, X.Z.; Guo, Y.F.; Wu, S.X. Expression and Clinical Significance of CXCR5 and LAG-3 on Peripheral Blood CD8+ T Cells in Patients with Diffuse Large B-Cell Lymphoma. Kaohsiung J. Med. Sci. 2025, 17, e70005. [Google Scholar] [CrossRef]
- Charbonneau, B.; Wang, A.H.; Maurer, M.J.; Asmann, Y.W.; Zent, C.S.; Link, B.K.; Ansell, S.M.; Weiner, G.J.; Ozsan, N.; Feldman, A.L.; et al. CXCR5 polymorphisms in non-Hodgkin lymphoma risk and prognosis. Cancer Immunol. Immunother. 2013, 62, 1475–1484. [Google Scholar] [CrossRef][Green Version]
- Hu, Y.; Li, J.; Wang, L.H.; Xu, Q.F.; Xia, R.X.; Zeng, Q.S.; Liu, X.Y.; Ge, J. [Expression of CXCR7 in Acute Monocytic Leukemia and Its Effects on THP-1 Cell Functions]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2017, 25, 1300–1306. (In Chinese) [Google Scholar] [PubMed]
- Moreno, M.J.; Gallardo, A.; Novelli, S.; Mozos, A.; Aragó, M.; Pavón, M.Á.; Céspedes, M.V.; Pallarès, V.; Falgàs, A.; Alcoceba, M.; et al. CXCR7 expression in diffuse large B-cell lymphoma identifies a subgroup of CXCR4+ patients with good prognosis. PLoS ONE 2018, 13, e0198789. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcellos, J.F.; Laranjeira, A.B.; Leal, P.C.; Bhasin, M.K.; Zenatti, P.P.; Nunes, R.J.; Yunes, R.A.; Nowill, A.E.; Libermann, T.A.; Zerbini, L.F.; et al. SB225002 Induces Cell Death and Cell Cycle Arrest in Acute Lymphoblastic Leukemia Cells through the Activation of GLIPR1. PLoS ONE 2015, 10, e0134783. [Google Scholar] [CrossRef]
- Cooper, T.M.; Sison, E.A.R.; Baker, S.D.; Li, L.; Ahmed, A.; Trippett, T.; Gore, L.; Macy, M.E.; Narendran, A.; August, K.; et al. A phase 1 study of the CXCR4 antagonist plerixafor in combination with high-dose cytarabine and etoposide in children with relapsed or refractory acute leukemias or myelodysplastic syndrome: A Pediatric Oncology Experimental Therapeutics Investigators’ Consortium study (POE 10-03). Pediatr. Blood Cancer 2017, 64, e26414. [Google Scholar] [CrossRef]
- Huselton, E.; Rettig, M.P.; Fletcher, T.; Ritchey, J.; Gehrs, L.; McFarland, K.; Christ, S.; Eades, W.C.; Trinkaus, K.; Romee, R.; et al. A phase I trial evaluating the effects of plerixafor, G-CSF, and azacitidine for the treatment of myelodysplastic syndromes. Leuk. Lymphoma 2021, 62, 1441–1449. [Google Scholar] [CrossRef]
- Andritsos, L.A.; Byrd, J.C.; Cheverton, P.; Wu, J.; Sivina, M.; Kipps, T.J.; Burger, J.A. A multicenter phase 1 study of plerixafor and rituximab in patients with chronic lymphocytic leukemia. Leuk. Lymphoma 2019, 60, 3461–3469. [Google Scholar] [CrossRef]
- Michelis, F.V.; Hedley, D.W.; Malhotra, S.; Chow, S.; Loach, D.; Gupta, V.; Kim, D.D.; Kuruvilla, J.; Lipton, J.H.; Viswabandya, A.; et al. Mobilization of Leukemic Cells Using Plerixafor as Part of a Myeloablative Preparative Regimen for Patients with Acute Myelogenous Leukemia Undergoing Allografting: Assessment of Safety and Tolerability. Biol. Blood Marrow Transplant. 2019, 25, 1158–1163. [Google Scholar] [CrossRef]
- Roboz, G.J.; Ritchie, E.K.; Dault, Y.; Lam, L.; Marshall, D.C.; Cruz, N.M.; Hsu, H.C.; Hassane, D.C.; Christos, P.J.; Ippoliti, C.; et al. Phase I trial of plerixafor combined with decitabine in newly diagnosed older patients with acute myeloid leukemia. Haematologica 2018, 103, 1308–1316. [Google Scholar] [CrossRef]
- Sison, E.A.; Magoon, D.; Li, L.; Annesley, C.E.; Rau, R.E.; Small, D.; Brown, P. Plerixafor as a chemosensitizing agent in pediatric acute lymphoblastic leukemia: Efficacy and potential mechanisms of resistance to CXCR4 inhibition. Oncotarget 2014, 5, 8947–8958. [Google Scholar] [CrossRef]
- Welschinger, R.; Liedtke, F.; Basnett, J.; Dela Pena, A.; Juarez, J.G.; Bradstock, K.F.; Bendall, L.J. Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp. Hematol. 2013, 41, 293–302.e1. [Google Scholar] [CrossRef]
- Agarwal, A.; Fleischman, A.G.; Petersen, C.L.; MacKenzie, R.; Luty, S.; Loriaux, M.; Druker, B.J.; Woltjer, R.L.; Deininger, M.W. Effects of plerixafor in combination with BCR-ABL kinase inhibition in a murine model of CML. Blood 2012, 120, 2658–2668. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, F.; Veldwijk, M.R.; Laufs, S.; Sperandio, M.; Calandra, G.; Wenz, F.; Zeller, J.; Fruehauf, S. Plerixafor inhibits chemotaxis toward SDF-1 and CXCR4-mediated stroma contact in a dose-dependent manner resulting in increased susceptibility of BCR-ABL+ cell to Imatinib and Nilotinib. Leuk. Lymphoma 2009, 50, 1676–1686. [Google Scholar] [CrossRef]
- Stamatopoulos, B.; Meuleman, N.; De Bruyn, C.; Pieters, K.; Mineur, P.; Le Roy, C.; Saint-Georges, S.; Varin-Blank, N.; Cymbalista, F.; Bron, D.; et al. AMD3100 disrupts the cross-talk between chronic lymphocytic leukemia cells and a mesenchymal stromal or nurse-like cell-based microenvironment: Pre-clinical evidence for its association with chronic lymphocytic leukemia treatments. Haematologica 2012, 97, 608–615. [Google Scholar] [CrossRef]
- Gaudio, F.; Mele, A.; Prete, E.; Laddaga, F.E.; Maggi, A.; Di Renzo, N.; Milone, G.; Ostuni, A.; Pavone, V. Plerixafor in association with R-DHAP and G-CSF to mobilize a large number of CD34 + cells in patients with relapsed-refractory diffuse large B-cell lymphomas. Ann. Hematol. 2024, 103, 5799–5805. [Google Scholar] [CrossRef]
- Montesinos-Rongen, M.; Sanchez-Ruiz, M.; Siebert, S.; Winter, C.; Siebert, R.; Brunn, A.; Deckert, M. AMD3100-Mediated CXCR4 Inhibition Impairs Development of Primary Lymphoma of the Central Nervous System. Am. J. Pathol. 2023, 193, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Shin, S.H.; Lee, J.Y.; Jeon, Y.W.; Yhang, S.A.; Min, C.K. Prospective Comparative Study of Etoposide plus G-CSF versus G-CSF Alone, Followed by Risk-Adapted Plerixafor for Peripheral Blood Stem Cell Mobilization in Patients with Newly Diagnosed Multiple Myeloma: CAtholic REsearch Network for Multiple Myeloma Study (CAREMM-2001). Cancers 2023, 15, 4783. [Google Scholar] [PubMed]
- van de Wyngaert, Z.; Malard, F.; Hulin, C.; Caillot, D.; Mariette, C.; Facon, T.; Touzeau, C.; Perrot, A.; Moreau, P.; Hebraud, B.; et al. Non-interventional Study Evaluating the Mobilization of Stem Cells by Plerixafor Before Salvage Autologous Stem Cell Transplant in Relapsed Multiple Myeloma (IFM-2015-03). Clin. Hematol. Int. 2023, 5, 38–42. [Google Scholar] [CrossRef]
- He, D.; Zhu, C.; Guo, X.; Huang, X.; Han, X.; Zheng, G.; Zhao, Y.; Yang, Y.; Wu, W.; Ge, J.; et al. The efficacy of residual plerixafor for second-day stem cell mobilization in multiple myeloma patients. Transfus. Apher. Sci. 2023, 62, 103618. [Google Scholar] [CrossRef]
- Maechler, M.; Bacher, U.; Daskalakis, M.; Nilius, H.; Nagler, M.; Taleghani, B.M.; Jeker, B.; Pabst, T. Long-term safety of the stem cell releasing compound plerixafor for peripheral stem cell collection in myeloma patients. Hematol. Oncol. 2023, 41, 583–586. [Google Scholar] [CrossRef]
- Prakash, V.S.; Malik, P.S.; Sahoo, R.K.; Pramanik, R.; Choudhary, P.; Varshney, A.N.; Kumar, L. Multiple Myeloma: Risk Adapted Use of Plerixafor for Stem Cell Mobilization Prior to Autologous Stem Cell Transplantation is Effective and Cost Efficient. Clin. Lymphoma Myeloma Leuk. 2022, 22, 44–51. [Google Scholar] [CrossRef]
- Kovacsovics, T.J.; Mims, A.; Salama, M.E.; Pantin, J.; Rao, N.; Kosak, K.M.; Ahorukomeye, P.; Glenn, M.J.; Deininger, M.W.N.; Boucher, K.M.; et al. Combination of the low anticoagulant heparin CX-01 with chemotherapy for the treatment of acute myeloid leukemia. Blood Adv. 2018, 2, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.; Montillo, M.; Scarfò, L.; Mauro, F.R.; Andel, J.; Wildner, S.; Trentin, L.; Janssens, A.; Burgstaller, S.; Frömming, A.; et al. Olaptesed pegol (NOX-A12) with bendamustine and rituximab: A phase IIa study in patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica 2019, 104, 2053–2060. [Google Scholar] [CrossRef]
- Crees, Z.D.; Rettig, M.P.; Bashey, A.; Devine, S.M.; Jaglowski, S.; Wan, F.; Zhou, A.; Harding, M.; Vainstein-Haras, A.; Sorani, E.; et al. Hematopoietic stem cell mobilization for allogeneic stem cell transplantation by motixafortide, a novel CXCR4 inhibitor. Blood Adv. 2023, 7, 5210–5214. [Google Scholar] [CrossRef]
- Du, J.; Lin, Z.; Fu, X.H.; Gu, X.R.; Lu, G.; Hou, J. Research progress of the chemokine/chemokine receptor axes in the oncobiology of multiple myeloma (MM). Cell. Commun. Signal. 2024, 22, 177. [Google Scholar] [CrossRef]
- Dietz, L.; Marini, J.; Hashmi, H.; McGann, M. A comparative review of Aphexda and Mozobil for mobilization prior to stem cell collection. J. Oncol. Pharm. Pract. 2024, 30, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Liu, C.J.; Redd, R.A.; Perez, R.P.; Baz, R.; Zavidij, O.; Sklavenitis-Pistofidis, R.; Richardson, P.G.; Anderson, K.C.; Laubach, J.; et al. A Phase Ib/II Trial of the First-in-Class Anti-CXCR4 Antibody Ulocuplumab in Combination with Lenalidomide or Bortezomib Plus Dexamethasone in Relapsed Multiple Myeloma. Clin. Cancer Res. 2020, 26, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.K.; Kumar, D.; Jones, H.; Amaya-Chanaga, C.I.; Choi, M.Y.; Melo-Cardenas, J.; Ale-Ali, A.; Kuhne, M.R.; Sabbatini, P.; Cohen, L.J.; et al. Ulocuplumab (BMS-936564 / MDX1338): A fully human anti-CXCR4 antibody induces cell death in chronic lymphocytic leukemia mediated through a reactive oxygen species-dependent pathway. Oncotarget 2016, 7, 2809–2822. [Google Scholar] [CrossRef]
- Kuhne, M.R.; Mulvey, T.; Belanger, B.; Chen, S.; Pan, C.; Chong, C.; Cao, F.; Niekro, W.; Kempe, T.; Henning, K.A.; et al. BMS-936564/MDX-1338: A fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin. Cancer Res. 2013, 19, 357–366. [Google Scholar] [CrossRef]
| Category | Specific Ligands | Biological Function in Hematological Malignancies | Involved Signal Pathways | References |
|---|---|---|---|---|
| CXCR1 | CXCL3, CXCL5, CXCL6, CXCL8 | Regulate processes such as the functionality of leukemia stem cells (LSCs), tumor microenvironment interactions, and drug resistance mechanisms | PI3K/AKT/mTOR; JAK/STAT3; MAPK/ERK; NF-κB | [11,12,13,14] |
| Regulate the proliferation and invasion of lymphoma cells within the tumor microenvironment | NF-κB | [15] | ||
| Mediate the progression of myeloma by influencing cellular signaling pathways | NF-κB | [16] | ||
| Promote occurrence and development of primary myelofibrosis | JAK2/STAT/MAPK; TPO/MPL/JAK2;AKT/NF-κB | [17,18,19] | ||
| CXCR2 | CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, CXCL8 | Promote occurrence and development of primary myelofibrosis | JAK2/STAT/MAPK; TPO/MPL/JAK2; AKT/NF-κB | [17,18,19] |
| Promote the survival and the generation of chemotherapy resistance of AML cells | NF-κB2/MIF | [20,21] | ||
| Inhibit the proliferation of CML cells, promote apoptosis, and overcome drug resistance | AKT/mTOR/c-Myc | [22] | ||
| Promote the proliferation of MM cells, activate osteoclast signaling pathways, and mediate disease progression | JAK/STAT3 | [23] | ||
| Facilitate the development of myeloproliferative neoplasms, including myelofibrosis | JAK/STAT/NF-κB | [24] | ||
| CXCR3 | CXCL4, CXCL9, CXCL10, CXCL11, CXCL13 | Enhance the malignant biological characteristics of mantle cell lymphoma (MCL) cells, including proliferation, migration, invasion, and inhibition of apoptosis | JAK1/STAT1; β-catenin/c-Myc | [25] |
| Promote the malignant proliferation and immune dysfunction associated with Epstein–Barr virus (EBV)-positive lymphomas | NF-κB/p38 MAPK | [26] | ||
| Enhance the efficacy of adoptive metastasized activated natural killer (NK) cells against multiple myeloma | IL-12/15/18 | [27] | ||
| CXCR4 | CXCL3, CXCL12 | Promote the survival, proliferation, migration, and drug resistance of AML cells | PI3K/AKT/mTOR; MAPK/ERK; Wnt/β-catenin; VLA-4/CAM-DR | [28,29,30,31,32] |
| Regulate the migration, homing, survival, and drug resistance of ALL cells, thereby affecting disease progression and treatment response | PI3K/AKT/mTOR; JAK/STAT; Rac1/RhoA | [33,34,35] | ||
| Participate in the microenvironmental dependence, cell migration, survival, and drug resistance of CLL cells | JAK/STAT; VLA-4/CAM-DR; NF-κB; PI3K/AKT/mTOR; MAPK/ERK; Rac1/RhoA | [36,37,38,39,40,41,42] | ||
| Maintain the self-renewal capacity of CML LSCs, bone marrow microenvironment dependence, drug resistance, and disease progression | PI3K/AKT/mTOR; MAPK/ERK; JAK/STAT; NF-κB; Wnt/β-catenin | [43,44,45,46,47,48] | ||
| Regulate the interaction between the bone marrow microenvironment, tumor cell homing, drug resistance, and disease progression in patients with MM | PI3K/AKT/mTOR; MAPK/ERK; NF-κB; JAK/STAT3; RANK/RANKL/OPG | [49,50,51,52,53] | ||
| Regulate lymphoma cell migration, microenvironmental dependence, drug resistance, and immune escape to promote disease progression | PI3K/AKT/mTOR; MAPK/ERK; JAK/STAT; NF-κB | [54,55,56,57,58,59,60,61] | ||
| CXCR5 | CXCL13 | Regulate the proliferation, migration, survival of leukemia cells, and their interaction with the microenvironment to promote disease progression | MAPK/ERK JAK/STAT VLA-4; CAM-DR | [62,63,64,65,66] |
| Regulate the proliferation, migration, survival of lymphoma cells, and their interaction with the immune microenvironment to promote tumor progression | NF-κB; VLA-4 | [66,67,68] | ||
| Mediate the regulation of the tumor microenvironment (TME), cell proliferation, migration, and the development of drug resistance in multiple myeloma | NF-κB; p53 | [69,70] | ||
| CXCR6 | CXCL12, CXCL16 | Mediate the immune escape, proliferation, and interaction with the BME of leukemia cells | Not yet mentioned | [71] |
| Regulate the tumor microenvironment of lymphoma, promote cell migration, and enhance survival signals | death receptor-caspase, TNF, NF-κB | [72] | ||
| CXCR7 | CXCL12 | Participate in the maintenance of LSCs, bone marrow microenvironment interactions, and drug resistance | Wnt/β-catenin; STAT3; ERK/MAPK | [73,74,75,76,77,78,79] |
| Mediate the progression of lymphoma, microenvironmental adaptation, and drug resistance | CXCL12-CXCR4 | [80,81] | ||
| Play a critical role in bone marrow microenvironment colonization, treatment resistance, and disease progression in multiple myeloma | CAM-DR; PI3K/AKT; β-arrestin; ERK | [82,83] | ||
| CXCR8 (GPR35) | CXCL17 | Not yet clearly reported | Not yet clearly reported | - |
| Category | Prognostic Value for Different Hematological Malignancies | Country | Number of Patients Included | References |
|---|---|---|---|---|
| CXCR1 | High expression of CXCR1/2 in AL indicates a poor prognosis and a low long-term survival rate | China | 86 cases | [95] |
| A diagnostic marker for aggressive NK cell leukemia (ANKL) | Japan | 15 cases | [142] | |
| A diagnostic marker for T-cell large granular lymphoblastic leukemia | Portugal | 46 cases | [143] | |
| A molecular marker for the progression of MM | Italy | 44 cases | [144] | |
| As a diagnostic marker for patients with MPN | Italy | 9 cases | [145] | |
| A therapeutic target for myelofibrosis | France | 37 cases | [19] | |
| CXCR2 | High expression of CXCR1/2 in AL indicates a poor prognosis and a low long-term survival rate | China | 86 cases | [95] |
| High expression of CXCR2 in AML is an independent risk factor for poor prognosis | China | 83 cases | [10] | |
| As one of the co-diagnostic markers for patients with CLL | Greece | 62 cases | [146] | |
| A diagnostic marker for T-cell lymphoma | Netherlands | 17 cases | [147] | |
| CXCR3 | An evaluation marker for the diagnosis and prognosis of CLL | Czech Republic | 60 cases | [148] |
| A molecular marker for the diagnosis and differentiation of CLL/SLL | UK. | 78 cases | [149] | |
| A diagnostic marker and therapeutic target for mantle cell lymphoma (MCL) | China | 30 cases | [25] | |
| A diagnostic marker for angioimmunoblastic T-cell lymphomas (AITLs) | Korea | 82 cases | [150] | |
| An indicator for evaluating the adverse clinical characteristics and prognosis of extranodal lymphoma | Egypt | 78 cases | [151] | |
| It is related to the survival rate of patients with primary central nervous system lymphoma | Japan | 31 cases | [152] | |
| A molecular marker for distant dissemination and disease progression of MM | Italy | 20 cases | [153] | |
| CXCR4 | High expression of CXCR4 is an independent prognostic factor for recurrence and poor OS in AML patients | China | 134 cases | [154] |
| A marker of poor prognosis in AML patients | Egypt | 58 cases | [155] | |
| A marker of extramedullary invasion and poor prognosis in children with ALL | Austria | 73 cases | [156] | |
| CXCR4 is abnormally highly expressed in both childhood and adult ALL and is associated with shorter disease-free survival and overall survival | South Korea | 54 cases | [157] | |
| High expression of CXCR4 is associated with poor prognosis and is an independent predictor of survival in AML | China | 122 cases | [158] | |
| High expression of CXCR4 is associated with poor prognosis and chemotherapy resistance in patients with CLL, and it is a key molecular marker for disease prediction | Egypt | 30 cases | [159] | |
| High expression of CXCR4 is associated with poor clinical characteristics and poor laboratory indicators of lymphoma, and in germinal center B-cell-like (GCB)-DLBCL, CXCR4 is an independent factor for predicting poor PFS | China | 743 cases | [160] | |
| High expression of CXCR4 is positively correlated with brain metastasis of DLBCL and is associated with poor overall survival rate, and it is an independent prognostic factor for DLBCL | China | 61 cases | [161] | |
| The upregulation of CXCR4 expression in ABC-DLBCL suggests a poor prognosis | China | 103 cases | [162] | |
| CXCR4 is an independent risk factor for poor prognosis of MCL and is an ideal target for therapy | Canada | 146 cases | [163] | |
| The decreased expression of CXCR4 in the BM of NHL patients after chemotherapy suggests a good prognosis | Poland | 26 cases | [164] | |
| CXCR4 is an independent predictor of survival prognosis for patients with MM | China | 227 cases | [165] | |
| Low expression of CXCR4 indicates a poor prognosis in MM patients and a high incidence of extramedullary lesions | China | 48 cases | [166] | |
| Low expression of CXCR4 indicates poor clinical characteristics and indicators as well as disease progression in patients with MPN | Italy | 100 cases | [167] | |
| High expression of CXCR4 suggests a poor prognosis for patients with MDS | China | 81 cases | [168] | |
| CXCR5 | CXCR5 is highly expressed in patients with MDS and is an indicator for therapeutic effect evaluation | China | 42 cases | [89] |
| Abnormally high expression of CXCR5 indicates the disease progression and poor prognosis of DLBCL | China | 71 cases | [169] | |
| The CXCR5 polymorphism has a relatively low correlation with DLBCL and PTCL, and is not related to the risk of MCL or MZL. However, it has certain value for the diagnosis and prognosis of FL patients | USA | 2694 cases | [170] | |
| CXCR6 | Not yet clearly reported | - | - | - |
| CXCR7 | CXCR7 is highly expressed in acute monocytic leukemia and is a key molecular marker for disease prediction | China | 10 cases | [171] |
| CXCR7 is an independent prognostic factor for lymphoma and is associated with good clinical outcomes | Spain | 94 cases | [172] | |
| CXCR7 is abnormally highly expressed in MM patients, which may be related to the disease progression | USA | 20 cases | [83] | |
| CXCR8 (GPR35) | Not yet clearly reported | - | - | - |
| Category | Specific Inhibitors (Antagonists) | Application of the Types of Hematological Malignancies | Specific Therapeutic Effect | References |
|---|---|---|---|---|
| CXCR1 | Reparixin | AML | Inhibits the malignant biological behaviors of AML cells and induce autophagy and apoptosis | [100] |
| Reparixin | MPN (myelofibrosis) | Slows down the process of myelofibrosis | [145] | |
| CXCR2 | Reparixin | AML | Inhibits the malignant biological behaviors of AML cells and induce autophagy and apoptosis | [100] |
| SB225002 | CML | Overcomes TKI resistance in CML | [22] | |
| ALL | Induces the death of ALL cells and cell cycle arrest, and have an anti-leukemia effect | [173] | ||
| CXCR3 | Not yet clearly reported | - | - | - |
| CXCR4 | Plerixaflor (AMD3100) | AL, MDS | Improves the tolerance and remission rate of patients with relapsed/refractory acute leukemia and MDS | [174] |
| MDS | Enhances the sensitivity of MDS patients to azacitidine and increase the remission rate | [175] | ||
| CLL | Enhances the sensitivity and tolerance of chemotherapy drugs | [176] | ||
| AML | A BM clearance preparation protocol for allogeneic transplantation in AML patients | [177] | ||
| AML | The safety and efficacy of treating newly diagnosed elderly patients with acute myeloid leukemia are good | [178] | ||
| ALL | A chemotherapy sensitizer for children with ALL | [179] | ||
| ALL | Induces the long-term mobilization of mouse ALL cells, increase the proportion of circulating cells in the blood, enhance the efficacy of chemotherapy and improve survival | [180] | ||
| CML | Ineffective in reducing the burden of leukemia, and infiltration of the central nervous system may occur, and the beneficial effect is limited when it is combined with TKIs | [181] | ||
| CML | Enhances the sensitivity of BCR-ABL+ cells to imatinib and nilotinib | [182] | ||
| CLL | Improves the efficacy of chemotherapy and enhance chemotherapy-induced sensitization | [183] | ||
| R/R DLBCL | A salvage treatment method and improve the therapeutic effect | [184] | ||
| PCNSL | Improves the therapeutic effect and control the progression of lymphoma | [185] | ||
| MM | A bone-marrow-mobilizing agent for patients with MM to improve the success rate of bone marrow transplantation | [186,187,188,189,190] | ||
| DSTAT (CX-01) | AML, MDS | Improves the therapeutic effect and the effective remission rate | [90] | |
| AML | Enhances the efficacy of chemotherapy and improve tolerance | [191] | ||
| Olaptesed pegol (NOX-A12) | CLL | Improves the efficacy of chemotherapy and chemosensitization while enhancing the tolerance of patients | [192] | |
| Motixafortide | MM | Stem cell mobilization for MM | [193,194,195] | |
| Ulocuplumab (BMS-936564/MDX1338) | R/R MM | Chemotherapy sensitizes and improves therapeutic effects | [196] | |
| CLL | Induces the cell death of chronic lymphocytic leukemia through the reactive oxygen species dependent pathway | [197] | ||
| ML, NHL, CLL | Monotherapy can show anti-tumor activity against AML, NHL and MM xenotransplantation models | [198] | ||
| CXCR5 | Not yet clearly reported | - | - | - |
| CXCR6 | Not yet clearly reported | - | - | - |
| CXCR7 | CCX771 | CLL | Inhibits the trans-endothelial migration (TEM) of CLL cells | [74] |
| CXCR8 (GPR35) | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tang, H. CXCR Family and Hematologic Malignancies in the Bone Marrow Microenvironment. Biomolecules 2025, 15, 716. https://doi.org/10.3390/biom15050716
Liu Y, Tang H. CXCR Family and Hematologic Malignancies in the Bone Marrow Microenvironment. Biomolecules. 2025; 15(5):716. https://doi.org/10.3390/biom15050716
Chicago/Turabian StyleLiu, Yanquan, and Huanwen Tang. 2025. "CXCR Family and Hematologic Malignancies in the Bone Marrow Microenvironment" Biomolecules 15, no. 5: 716. https://doi.org/10.3390/biom15050716
APA StyleLiu, Y., & Tang, H. (2025). CXCR Family and Hematologic Malignancies in the Bone Marrow Microenvironment. Biomolecules, 15(5), 716. https://doi.org/10.3390/biom15050716







