AMPK Signaling Axis-Mediated Regulation of Lipid Metabolism: Ameliorative Effects of Sodium Octanoate on Intestinal Dysfunction in Hu Sheep
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals and Experimental Design
2.3. H&E Staining
2.4. Enzyme-Linked Immunosorbent Assay
2.5. Immunofluorescence Staining
2.6. Immunohistochemical Staining
2.7. Quantitative RT-PCR Analysis
2.8. RNA-Seq Analysis
2.9. Broadly Targeted Lipidomics
2.10. Bioinformatics Analysis
2.11. KEGG Annotation and Enrichment Analysis
2.12. Statistical Analysis
3. Results
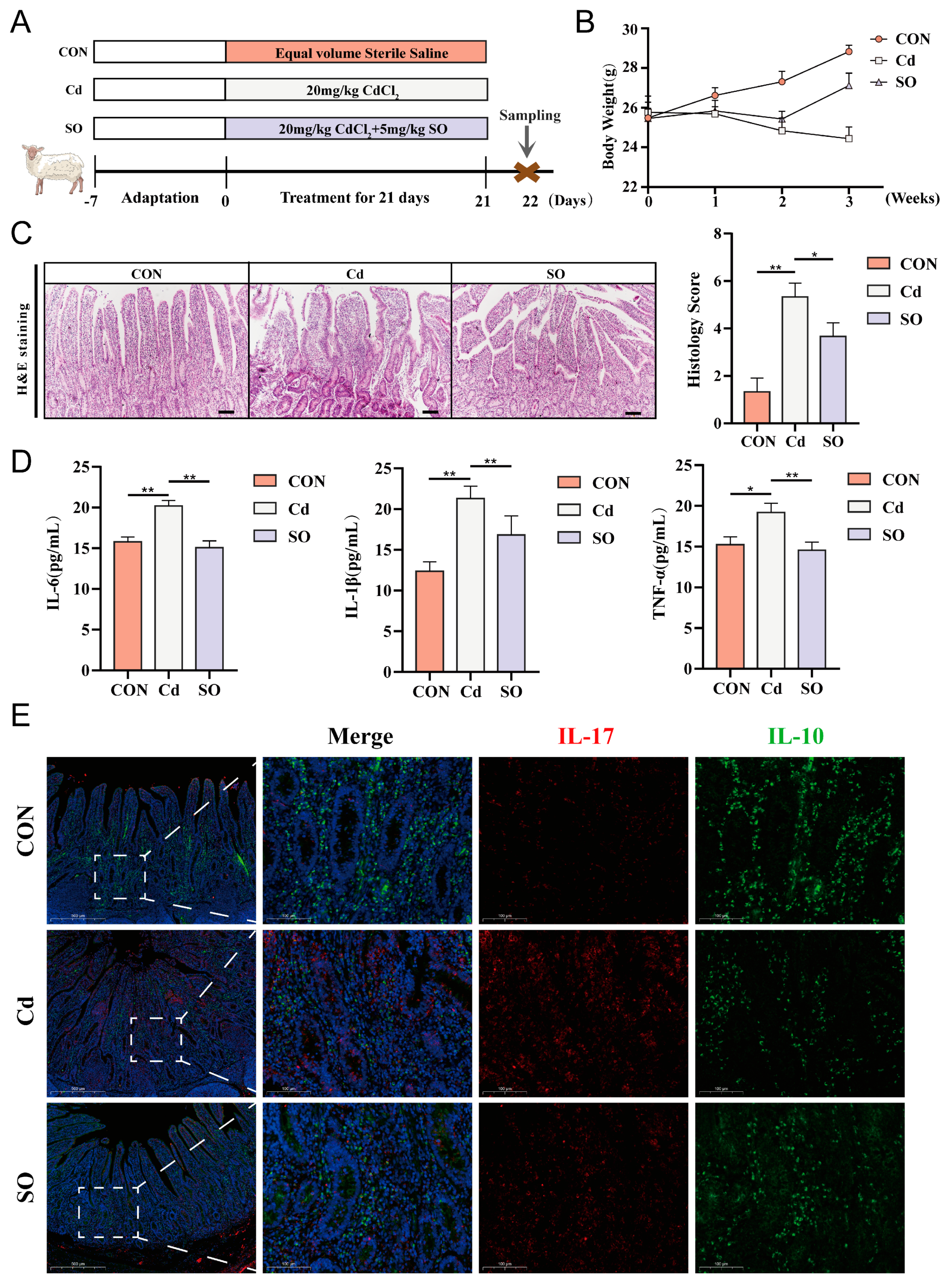
3.1. Sodium Octanoate Ameliorates Cadmium-Induced Intestinal Inflammation in Hu Sheep
3.2. Cadmium-Induced the Oxidative Stress Disrupts Intestinal Barrier Integrity and the Reparative Effect of Sodium Octanoate
3.3. Sodium Octanoate Attenuates Cadmium-Induced Disruption of Immune Homeostasis
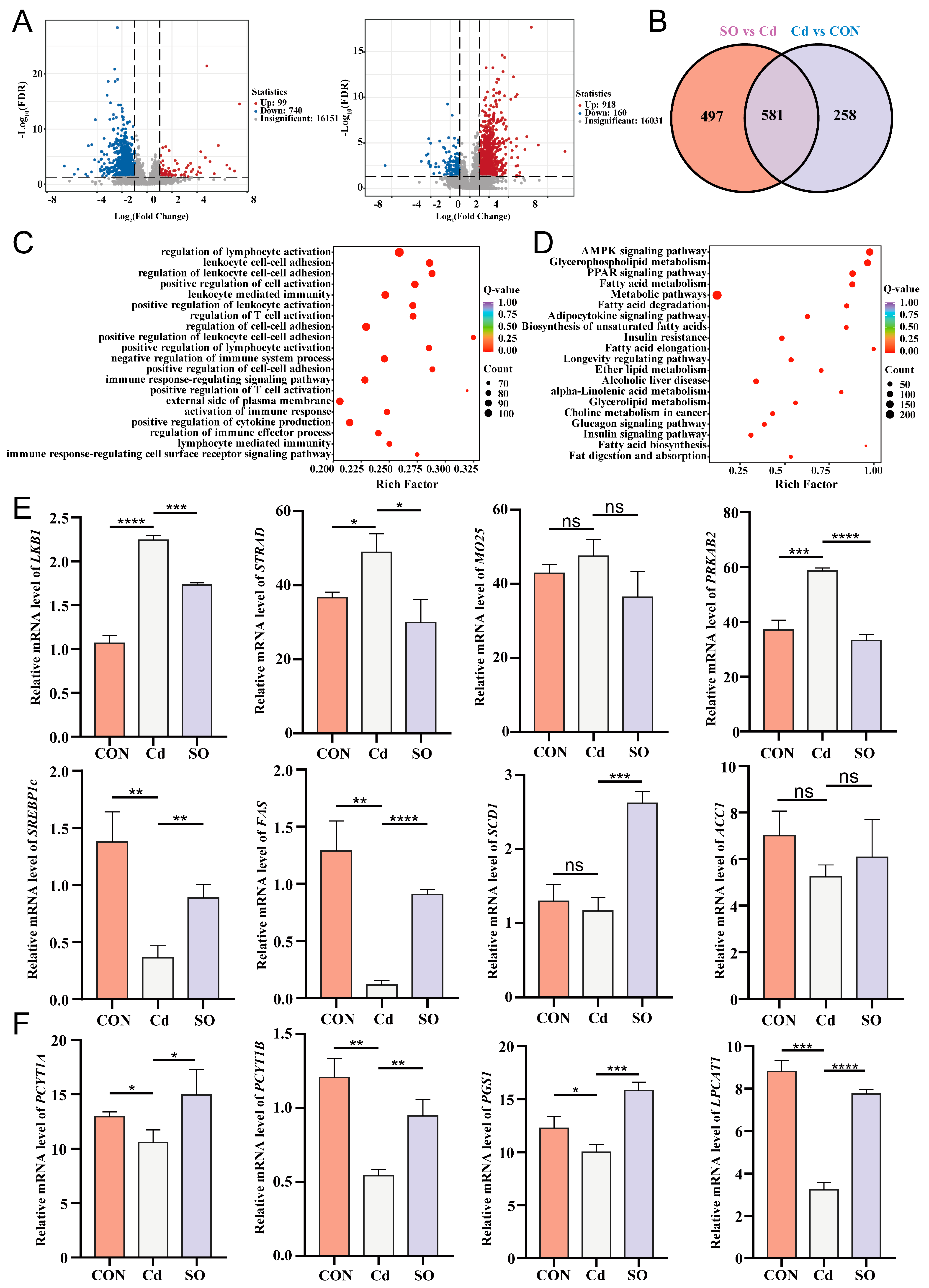
3.4. Sodium Octanoate Alleviates Lipid Metabolism Disorders Induced by Cadmium Exposure
3.5. Sodium Octanoate Broadly Modulates the Jejunal Transcriptional Profile
3.6. A Potential Link Between Lipid Metabolism and Transcriptional Regulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gyamfi, D.; Ofori Awuah, E.; Owusu, S. Chapter 2—Lipid Metabolism: An Overview. In The Molecular Nutrition of Fats; Patel, V.B., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 17–32. [Google Scholar]
- Yin, D.; Zhong, Y.; Liu, H.; Hu, J. Lipid metabolism regulation by dietary polysaccharides with different structural properties. Int. J. Biol. Macromol. 2024, 270, 132253. [Google Scholar] [CrossRef] [PubMed]
- Raal, F.; Cuchel, M. Lipid metabolism. Curr. Opin. Lipidol. 2023, 34, 93–95. [Google Scholar] [CrossRef] [PubMed]
- Bauchart, D. Lipid Absorption and Transport in Ruminants. J. Dairy Sci. 1993, 76, 3864–3881. [Google Scholar] [CrossRef] [PubMed]
- Bauchart, D.; Gruffat, D.; Durand, D. Lipid absorption and hepatic metabolism in ruminants. Proc. Nutr. Soc. 1996, 55, 39–47. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Q.; Zhou, J. Mechanisms of Abnormal Lipid Metabolism in the Pathogenesis of Disease. Int. J. Mol. Sci. 2024, 25, 8465. [Google Scholar] [CrossRef]
- Wang, C.; Mu, T.; Feng, X.; Zhang, J.; Gu, Y. Study on fatty acid binding protein in lipid metabolism of livestock and poultry. Res. Vet. Sci. 2023, 158, 185–195. [Google Scholar] [CrossRef]
- Natesan, V.; Kim, S.J. Lipid Metabolism, Disorders and Therapeutic Drugs—Review. Biomol. Ther. 2021, 29, 596–604. [Google Scholar] [CrossRef]
- Taroni, F.; Gellera, C. Chapter 54—Disorders of lipid metabolism. In Rosenberg’s Molecular and Genetic Basis of Neurological and Psychiatric Disease, 6th ed.; Rosenberg, R.N., Pascual, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 731–753. [Google Scholar]
- Zhu, L.; Tang, L.; Zhang, K.; Nie, H.; Gou, X.; Kong, X.; Deng, W. Genetic and Epigenetic Adaptation Mechanisms of Sheep Under Multi-Environmental Stress Environment. Int. J. Mol. Sci. 2025, 26, 3261. [Google Scholar] [CrossRef]
- Burfening, P.J.; Carpio, M.P. Genetic and environmental factors affecting growth rate and survival of Junin sheep in the central highlands of Peru. Small Rumin. Res. 1993, 11, 275–287. [Google Scholar] [CrossRef]
- Rasin, P.; Ashwathi, V.A.; Basheer, S.M.; Haribabu, J.; Santibanez, J.F.; Garrote, C.A.; Arulraj, A.; Mangalaraja, R.V. Exposure to cadmium and its impacts on human health: A short review. J. Hazard. Mater. Adv. 2025, 17, 100608. [Google Scholar] [CrossRef]
- Saini, S.; Dhania, G. Cadmium as an Environmental Pollutant: Ecotoxicological Effects, Health Hazards, and Bioremediation Approaches for Its Detoxification from Contaminated Sites. In Bioremediation of Industrial Waste for Environmental Safety: Volume II: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer: Singapore, 2020; pp. 357–387. [Google Scholar]
- Liu, H.; Fu, M.; Ren, Z.; Liu, Z.; Cao, X.; Chen, J.; Pang, Y.; Liu, J. Cadmium exposure induces inflammation, oxidative stress and DNA damage in HUVEC and promotes THP-1 adhesion: A possible mechanism on the formation of atherosclerotic plaque. Toxicology 2025, 511, 154046. [Google Scholar] [CrossRef]
- Jiaxin, S.; Shengchen, W.; Yirong, C.; Shuting, W.; Shu, L. Cadmium exposure induces apoptosis, inflammation and immunosuppression through CYPs activation and antioxidant dysfunction in common carp neutrophils. Fish Shellfish Immunol 2020, 99, 284–290. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yang, J.; Cai, J.; Zhang, H.; Zhu, Y.; Zheng, Y.; Liu, Q.; Shi, G.; Zhang, Z. Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J. Hazard. Mater. 2022, 421, 126704. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fang, F.; Wu, K.; Song, J.; Li, Y.; Lu, X.; Liu, J.; Zhou, L.; Yu, W.; Yu, F.; et al. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome 2023, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yan, S.; Du, R.; Xue, Y.; Yao, W.; Teligun; Zhao, Y.; Li, Y.; Bao, H.; Cao, S.; et al. Cadmium exposure promotes inflammation through the PPAR signaling pathway in the small intestine and colon of Hu Sheep. Ecotoxicol. Environ. Saf. 2024, 284, 117004. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hong, Y.; Yao, C.; Zhang, C.; Zhao, Z.; Zhang, W.; Lai, W.; Zhang, J.; Li, Y.; Mai, K.; et al. Role of sodium octanoate in regulating survival, growth, intestinal development, digestive and absorptive capacities, antioxidant capacity and innate immunity of large yellow croaker (Larimichthys crocea) larvae. Aquaculture 2024, 582, 740476. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, J.; Ma, X. Sodium caprylate improves intestinal mucosal barrier function and antioxidant capacity by altering gut microbial metabolism. Food Funct. 2021, 12, 9750–9762. [Google Scholar] [CrossRef]
- Jackman, J.A.; Boyd, R.D.; Elrod, C.C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: Towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 2020, 11, 44. [Google Scholar] [CrossRef]
- Tachibana, S.; Sato, K.; Cho, Y.; Chiba, T.; Schneider, W.J.; Akiba, Y. Octanoate reduces very low-density lipoprotein secretion by decreasing the synthesis of apolipoprotein B in primary cultures of chicken hepatocytes. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2005, 1737, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Cai, G.; Chen, J.; Yang, X.; Hua, G.; Han, D.; Li, X.; Feng, D.; Deng, X. Combined transcriptome and metabolome analysis reveals breed-specific regulatory mechanisms in Dorper and Tan sheep. BMC Genom. 2024, 25, 70. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Luo, Q.; Qin, X.; Guo, Y. Genome-wide analysis of microRNAs identifies the lipid metabolism pathway to be a defining factor in adipose tissue from different sheep. Sci. Rep. 2015, 5, 18470. [Google Scholar] [CrossRef]
- Yan, S.; Du, R.; Yao, W.; Zhang, H.; Xue, Y.; Teligun; Li, Y.; Bao, H.; Zhao, Y.; Cao, S.; et al. Host–microbe interaction-mediated resistance to DSS-induced inflammatory enteritis in sheep. Microbiome 2024, 12, 208. [Google Scholar] [CrossRef]
- Begg, D.J.; Purdie, A.C.; de Silva, K.; Dhand, N.K.; Plain, K.M.; Whittington, R.J. Variation in susceptibility of different breeds of sheep to Mycobacterium avium subspecies paratuberculosis following experimental inoculation. Vet. Res. 2017, 48, 36. [Google Scholar] [CrossRef]
- Li, Y.; Shen, X. Effects of Cadmium on Liver Function and Its Metabolomics Profile in the Guizhou Black Goat. Metabolites 2023, 13, 268. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Kou, Z.; Tran, F.; Dai, W. Heavy metals, oxidative stress, and the role of AhR signaling. Toxicol. Appl. Pharmacol. 2024, 482, 116769. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Yao, Y.; Shang, W.; Bao, L.; Peng, Z.; Wu, C. Epithelial-immune cell crosstalk for intestinal barrier homeostasis. Eur. J. Immunol. 2024, 54, 2350631. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ding, Y.; Kong, Y.; Wang, G.; Wang, S.; Cheng, D. Purslane (Portulacae oleracea L.) attenuates cadmium-induced hepatorenal and colonic damage in mice: Role of chelation, antioxidant and intestinal microecological regulation. Phytomedicine 2021, 92, 153716. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, X.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int. J. Biol. Macromol. 2020, 164, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef]
- Yang, J.; Chen, W.; Sun, Y.; Liu, J.; Zhang, W. Effects of cadmium on organ function, gut microbiota and its metabolomics profile in adolescent rats. Ecotoxicol. Environ. Saf. 2021, 222, 112501. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2022, 8, 350–360. [Google Scholar] [CrossRef]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-associated metabolic pathways affected by heavy metals and metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef]
- Ahmad, S.; Single, S.; Liu, Y.; Hough, K.P.; Wang, Y.; Thannickal, V.J.; Athar, M.; Goliwas, K.F.; Deshane, J.S. Heavy Metal Exposure-Mediated Dysregulation of Sphingolipid Metabolism. Antioxidants 2024, 13, 978. [Google Scholar] [CrossRef]
- Cockcroft, S. Mammalian lipids: Structure, synthesis and function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef]
- Ertunc, M.E.; Hotamisligil, G.S. Lipid signaling and lipotoxicity in metaflammation: Indications for metabolic disease pathogenesis and treatment. J. Lipid Res. 2016, 57, 2099–2114. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-Mediated Regulation of Lipid Metabolism by Phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wang, S.; Wang, H.; Liu, S.; Liu, G.; Chen, H.; Kang, J.; Wang, H. Naringenin inhibits lipid accumulation by activating the AMPK pathway in vivo and vitro. Food Sci. Hum. Wellness 2023, 12, 1174–1183. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, Y.; Yang, D.; Liang, Y.; Yang, L.; Hu, S.; Liu, Z.; Fang, Q.; Tian, S.; Ding, Y. Gastrodin Improves Nonalcoholic Fatty Liver Disease Through Activation of the Adenosine Monophosphate-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 3074–3090. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yan, S.; Ma, Z.; Du, R.; Li, X.; Bao, S.; Song, Y. AMPK Signaling Axis-Mediated Regulation of Lipid Metabolism: Ameliorative Effects of Sodium Octanoate on Intestinal Dysfunction in Hu Sheep. Biomolecules 2025, 15, 707. https://doi.org/10.3390/biom15050707
Zhang H, Yan S, Ma Z, Du R, Li X, Bao S, Song Y. AMPK Signaling Axis-Mediated Regulation of Lipid Metabolism: Ameliorative Effects of Sodium Octanoate on Intestinal Dysfunction in Hu Sheep. Biomolecules. 2025; 15(5):707. https://doi.org/10.3390/biom15050707
Chicago/Turabian StyleZhang, Huimin, Shuo Yan, Zimeng Ma, Ruilin Du, Xihe Li, Siqin Bao, and Yongli Song. 2025. "AMPK Signaling Axis-Mediated Regulation of Lipid Metabolism: Ameliorative Effects of Sodium Octanoate on Intestinal Dysfunction in Hu Sheep" Biomolecules 15, no. 5: 707. https://doi.org/10.3390/biom15050707
APA StyleZhang, H., Yan, S., Ma, Z., Du, R., Li, X., Bao, S., & Song, Y. (2025). AMPK Signaling Axis-Mediated Regulation of Lipid Metabolism: Ameliorative Effects of Sodium Octanoate on Intestinal Dysfunction in Hu Sheep. Biomolecules, 15(5), 707. https://doi.org/10.3390/biom15050707





