The Insertion Domain of Mti2 Facilitates the Association of Mitochondrial Initiation Factors with Mitoribosomes in Schizosaccharomyces pombe
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Media
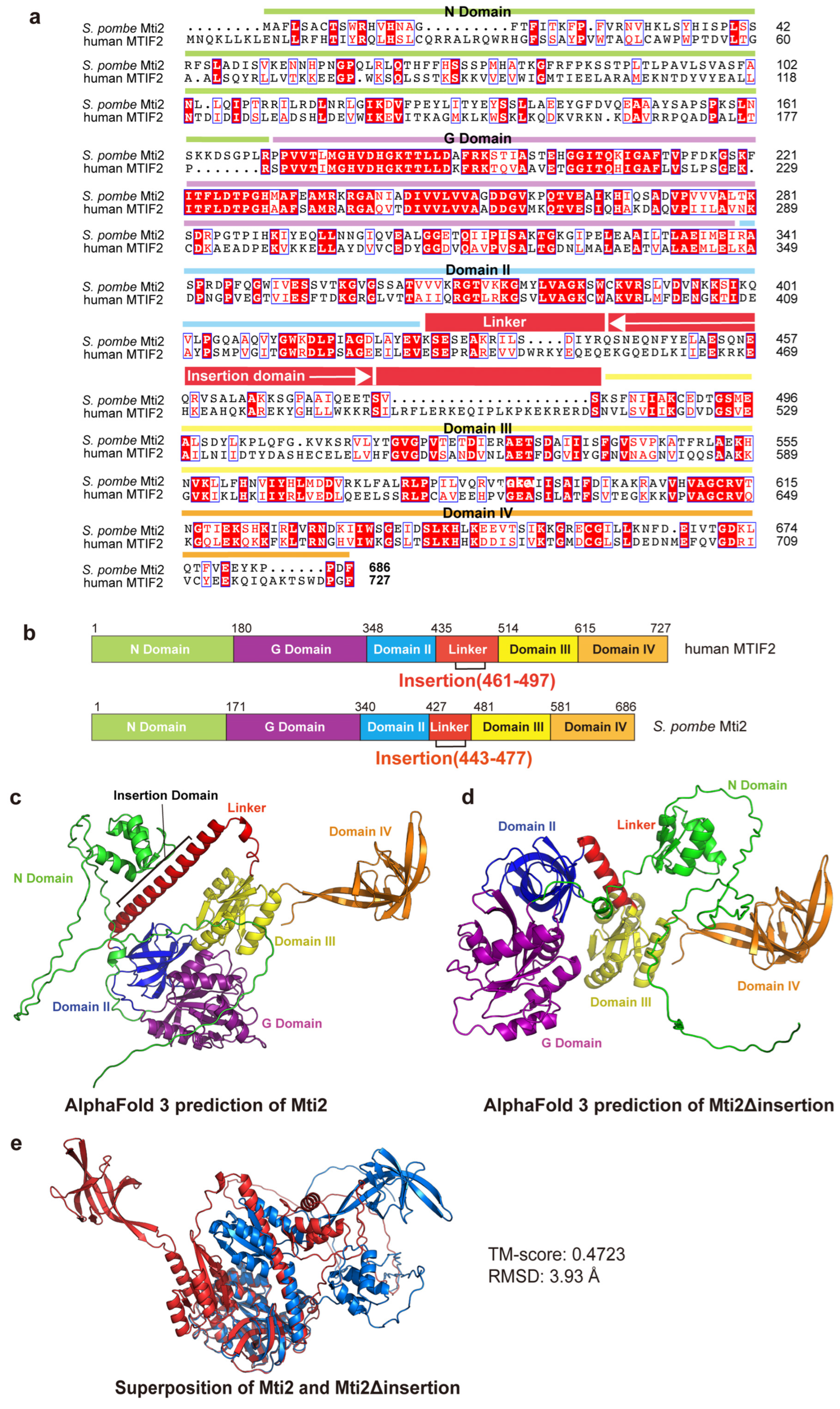
2.2. Prediction and Alignment of Protein Structure
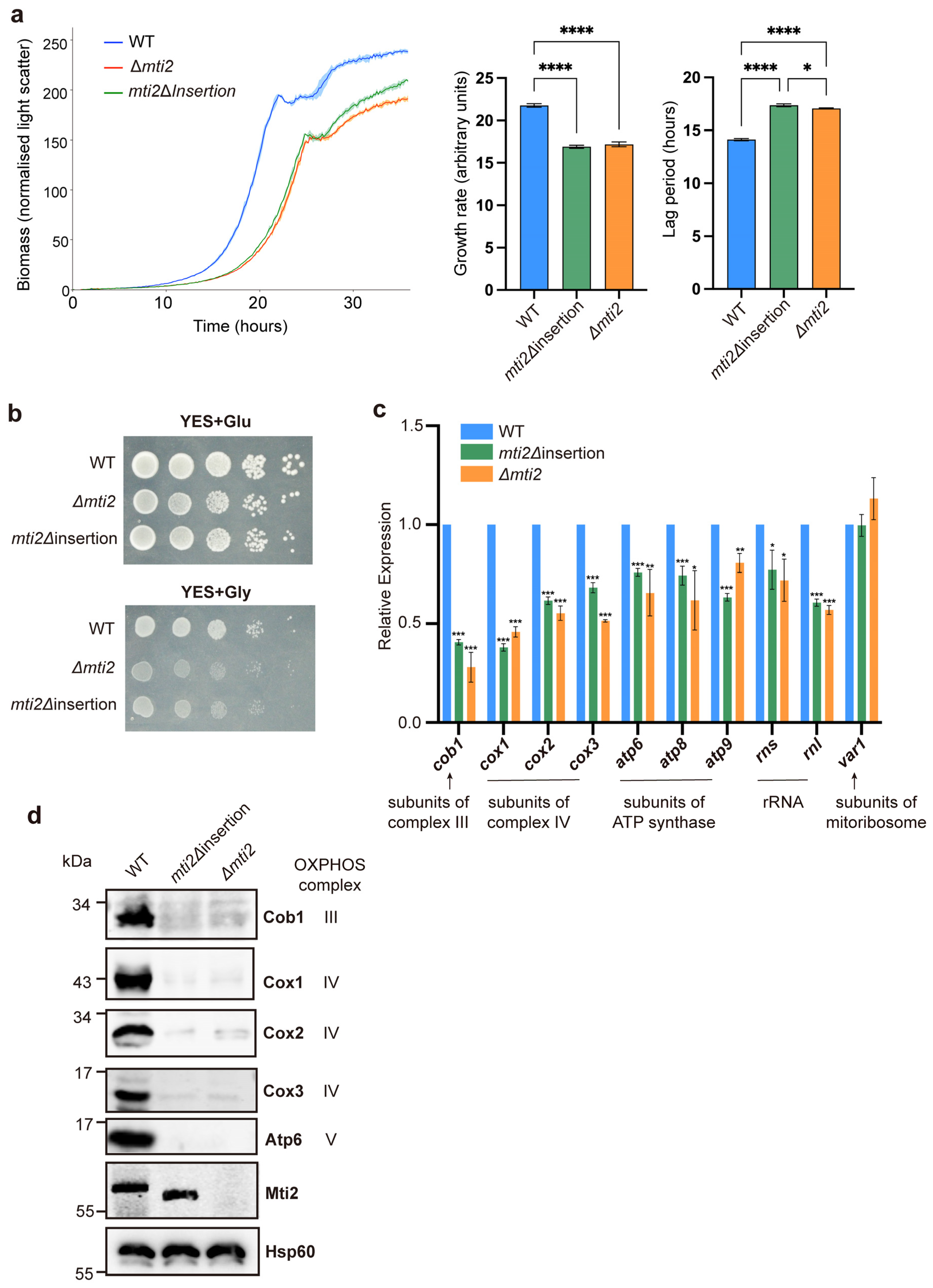
2.3. Real-Time Quantitative Yeast Growth Assays
2.4. Quantitative Real-Time RT-PCR
2.5. Isolation of Mitochondria and Western Blot Analysis
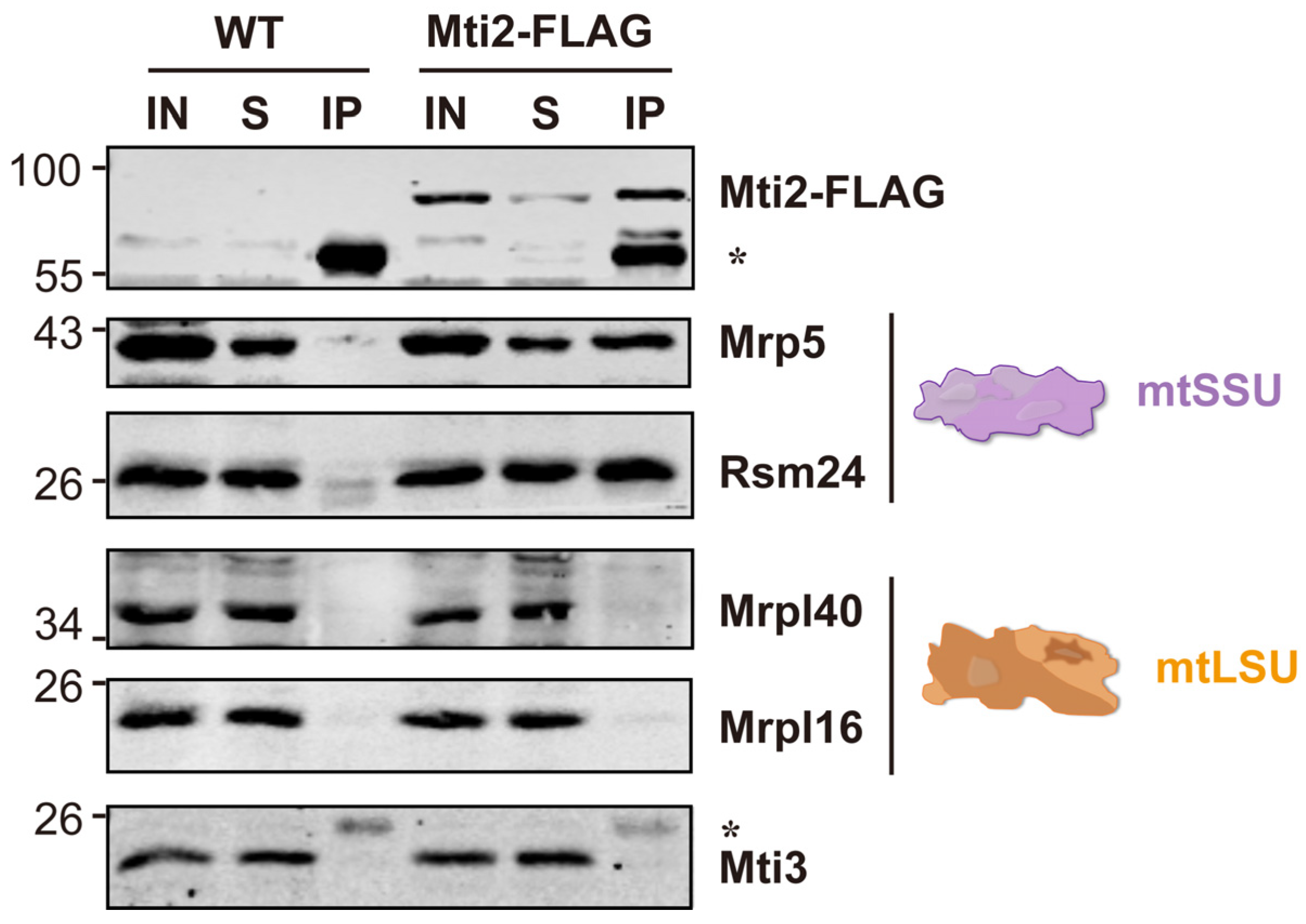
2.6. Immunoprecipitation Assay
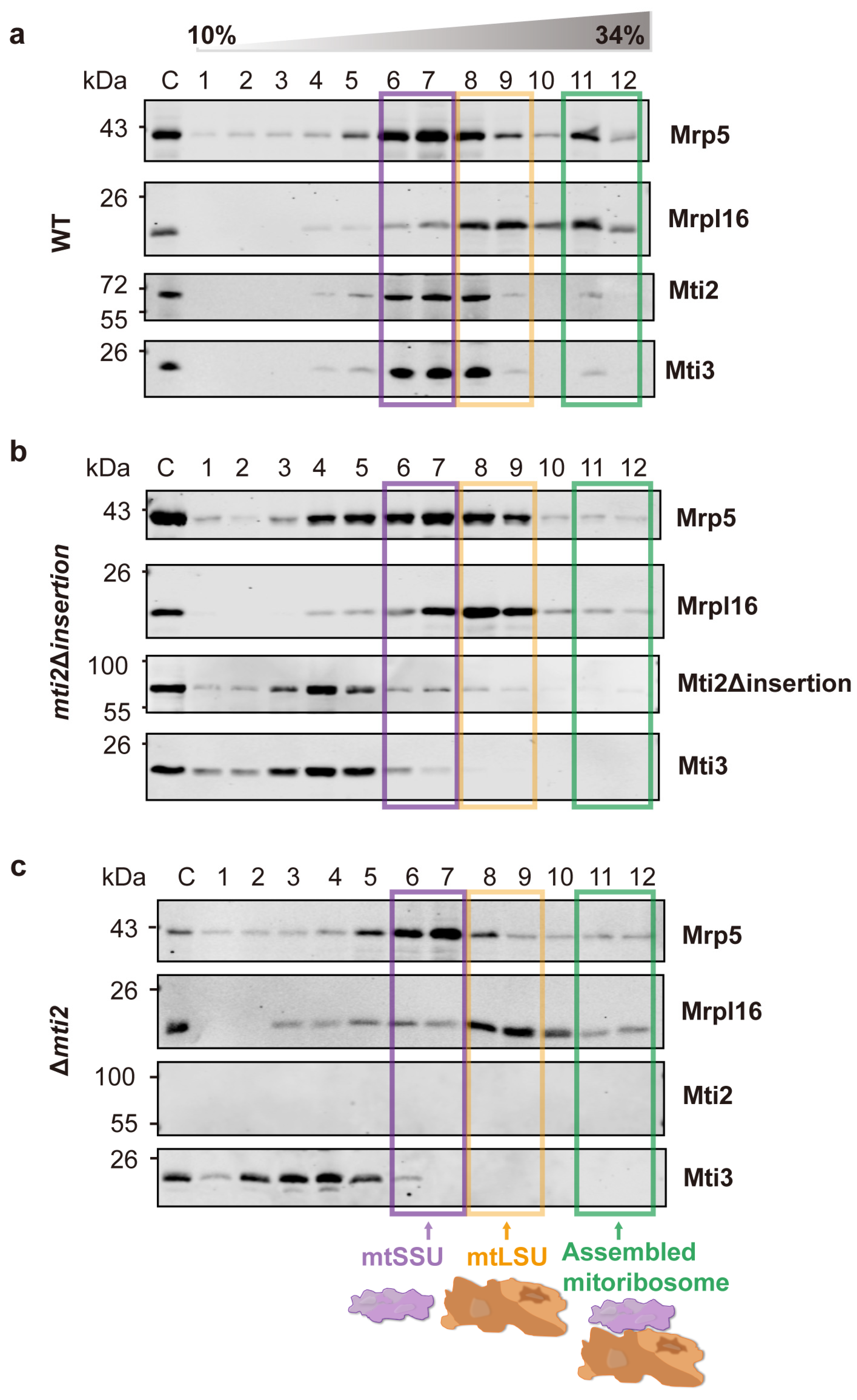
2.7. Sucrose Gradient Sedimentation Analysis
3. Results
3.1. The Insertion Domain Is Required for the Proper Folding of Mti2
3.2. Deletion of the Insertion Domain Impairs Mti2 Function
3.3. Mti2 Physically Interacts with the Small Mitoribosome Subunit
3.4. Deletion of the Insertion Domain Reduces the Affinity Between Mti2 and Mti3 and the mtSSU
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mtSSU | The small subunits of mitoribosomes |
| mtLSU | The large subunits of mitoribosomes |
| mtDNA | Mitochondrial genome |
| IF | Initiation factors |
| qRT-PCR | Quantitative real-time PCR |
| CoIP | Co-immunoprecipitation |
References
- Rutter, J.; Hughes, A.L. Power(2): The power of yeast genetics applied to the powerhouse of the cell. Trends Endocrinol. Metab. 2015, 26, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, I.; Jain, S.M.; Blot-Chabaud, M.; Pathak, S.; Banerjee, A.; Rawat, S.; Sharma, N.R.; Duttaroy, A.K. Mitochondrial dysfunction and its association with age-related disorders. Front. Physiol. 2024, 15, 1384966. [Google Scholar] [CrossRef]
- Boczonadi, V.; Horvath, R. Mitochondria: Impaired mitochondrial translation in human disease. Int. J. Biochem. Cell Biol. 2014, 48, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Dinh, N.; Bonnefoy, N. Schizosaccharomyces pombe as a fundamental model for research on mitochondrial gene expression: Progress, achievements and outlooks. IUBMB Life 2024, 76, 397–419. [Google Scholar] [CrossRef]
- Kuzmenko, A.; Atkinson, G.C.; Levitskii, S.; Zenkin, N.; Tenson, T.; Hauryliuk, V.; Kamenski, P. Mitochondrial translation initiation machinery: Conservation and diversification. Biochimie 2014, 100, 132–140. [Google Scholar] [CrossRef]
- Lightowlers, R.N.; Rozanska, A.; Chrzanowska-Lightowlers, Z.M. Mitochondrial protein synthesis: Figuring the fundamentals, complexities and complications, of mammalian mitochondrial translation. FEBS Lett. 2014, 588, 2496–2503. [Google Scholar] [CrossRef]
- Agirrezabala, X.; Frank, J. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q. Rev. Biophys. 2009, 42, 159–200. [Google Scholar] [CrossRef] [PubMed]
- Christian, B.E.; Spremulli, L.L. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta 2012, 1819, 1035–1054. [Google Scholar] [CrossRef]
- Kummer, E.; Ban, N. Mechanisms and regulation of protein synthesis in mitochondria. Nat. Rev. Mol. Cell Biol. 2021, 22, 307–325. [Google Scholar] [CrossRef]
- Hussain, T.; Llácer, J.L.; Fernández, I.S.; Munoz, A.; Martin-Marcos, P.; Savva, C.G.; Lorsch, J.R.; Hinnebusch, A.G.; Ramakrishnan, V. Structural changes enable start codon recognition by the eukaryotic translation initiation complex. Cell 2014, 159, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, A.; Itoh, Y.; Remes, C.; Spåhr, H.; Yukhnovets, O.; Höfig, H.; Amunts, A.; Rorbach, J. Distinct pre-initiation steps in human mitochondrial translation. Nat. Commun. 2020, 11, 2932. [Google Scholar] [CrossRef]
- Garofalo, C.; Trinko, R.; Kramer, G.; Appling, D.R.; Hardesty, B. Purification and characterization of yeast mitochondrial initiation factor 2. Arch. Biochem. Biophys. 2003, 413, 243–252. [Google Scholar] [CrossRef]
- Herbert, C.J.; Labarre-Mariotte, S.; Cornu, D.; Sophie, C.; Panozzo, C.; Michel, T.; Dujardin, G.; Bonnefoy, N. Translational activators and mitoribosomal isoforms cooperate to mediate mRNA-specific translation in Schizosaccharomyces pombe mitochondria. Nucleic Acids Res. 2021, 49, 11145–11166. [Google Scholar] [CrossRef]
- Kummer, E.; Leibundgut, M.; Rackham, O.; Lee, R.G.; Boehringer, D.; Filipovska, A.; Ban, N. Unique features of mammalian mitochondrial translation initiation revealed by cryo-EM. Nature 2018, 560, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Yassin, A.S.; Haque, M.E.; Datta, P.P.; Elmore, K.; Banavali, N.K.; Spremulli, L.L.; Agrawal, R.K. Insertion domain within mammalian mitochondrial translation initiation factor 2 serves the role of eubacterial initiation factor 1. Proc. Natl. Acad. Sci. USA 2011, 108, 3918–3923. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Grasso, D.; Datta, P.P.; Krishna, P.D.; Das, G.; Spencer, A.; Agrawal, R.K.; Spremulli, L.; Varshney, U. A single mammalian mitochondrial translation initiation factor functionally replaces two bacterial factors. Mol. Cell 2008, 29, 180–190. [Google Scholar] [CrossRef]
- Spencer, A.C.; Spremulli, L.L. The interaction of mitochondrial translational initiation factor 2 with the small ribosomal subunit. Biochim. Biophys. Acta 2005, 1750, 69–81. [Google Scholar] [CrossRef]
- Ma, J.; Farwell, M.A.; Burkhart, W.A.; Spremulli, L.L. Cloning and sequence analysis of the cDNA for bovine mitochondrial translational initiation factor 2. Biochim. Biophys. Acta 1995, 1261, 321–324. [Google Scholar] [CrossRef]
- Atkinson, G.C.; Kuzmenko, A.; Kamenski, P.; Vysokikh, M.Y.; Lakunina, V.; Tankov, S.; Smirnova, E.; Soosaar, A.; Tenson, T.; Hauryliuk, V. Evolutionary and genetic analyses of mitochondrial translation initiation factors identify the missing mitochondrial IF3 in S. cerevisiae. Nucleic Acids Res. 2012, 40, 6122–6134. [Google Scholar] [CrossRef]
- Bähler, J.; Wu, J.Q.; Longtine, M.S.; Shah, N.G.; McKenzie, A., 3rd; Steever, A.B.; Wach, A.; Philippsen, P.; Pringle, J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 1998, 14, 943–951. [Google Scholar] [CrossRef]
- Keeney, J.B.; Boeke, J.D. Efficient targeted integration at leu1-32 and ura4-294 in Schizosaccharomyces pombe. Genetics 1994, 136, 849–856. [Google Scholar] [CrossRef]
- Moreno, S.; Klar, A.; Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991, 194, 795–823. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef]
- Anver, S.; Sumit, A.F.; Sun, X.M.; Hatimy, A.; Thalassinos, K.; Marguerat, S.; Alic, N.; Bähler, J. Ageing-associated long non-coding RNA extends lifespan and reduces translation in non-dividing cells. EMBO Rep. 2024, 25, 4921–4949. [Google Scholar] [CrossRef]
- Kahm, M.; Hasenbrink, G.; Lichtenberg-Fraté, H.; Ludwig, J.; Kschischo, M. grofit: Fitting Biological Growth Curves with R. J. Stat. Softw. 2010, 33, 1–21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Meisinger, C.; Pfanner, N.; Truscott, K.N. Isolation of yeast mitochondria. Methods Mol. Biol. 2006, 313, 33–39. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Wang, Y.; Xie, W.; Liu, Z.; Huang, Y. Schizosaccharomyces pombe Mti2 and Mti3 act in conjunction during mitochondrial translation initiation. FEBS J. 2019, 286, 4542–4553. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, Y.; Ahmad, F.; Feng, G.; Huang, Y. Characterization of Shy1, the Schizosaccharomyces pombe homolog of human SURF1. Sci. Rep. 2024, 14, 21678. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Huang, Y. Schizosaccharomyces pombe Ppr10 and Mpa1 together mediate mitochondrial translational initiation. J. Biol. Chem. 2021, 297, 100869. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Barrientos, A. Sucrose Gradient Sedimentation Analysis of Mitochondrial Ribosomes. Methods Mol. Biol. 2021, 2192, 211–226. [Google Scholar] [CrossRef]
- Leipe, D.D.; Wolf, Y.I.; Koonin, E.V.; Aravind, L. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 2002, 317, 41–72. [Google Scholar] [CrossRef] [PubMed]
- Laalami, S.; Sacerdot, C.; Vachon, G.; Mortensen, K.; Sperling-Petersen, H.U.; Cenatiempo, Y.; Grunberg-Manago, M. Structural and functional domains of E coli initiation factor IF2. Biochimie 1991, 73, 1557–1566. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S.K.; Roll-Mecak, A.; Burley, S.K.; Dever, T.E. Universal conservation in translation initiation revealed by human and archaeal homologs of bacterial translation initiation factor IF2. Proc. Natl. Acad. Sci. USA 1999, 96, 4342–4347. [Google Scholar] [CrossRef] [PubMed]
- Roll-Mecak, A.; Cao, C.; Dever, T.E.; Burley, S.K. X-Ray structures of the universal translation initiation factor IF2/eIF5B: Conformational changes on GDP and GTP binding. Cell 2000, 103, 781–792. [Google Scholar] [CrossRef]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]
- Zuin, A.; Gabrielli, N.; Calvo, I.A.; García-Santamarina, S.; Hoe, K.L.; Kim, D.U.; Park, H.O.; Hayles, J.; Ayté, J.; Hidalgo, E. Mitochondrial dysfunction increases oxidative stress and decreases chronological life span in fission yeast. PLoS ONE 2008, 3, e2842. [Google Scholar] [CrossRef]
- Chen, H.; Bjerknes, M.; Kumar, R.; Jay, E. Determination of the optimal aligned spacing between the Shine-Dalgarno sequence and the translation initiation codon of Escherichia coli mRNAs. Nucleic Acids Res. 1994, 22, 4953–4957. [Google Scholar] [CrossRef] [PubMed]
- Antoun, A.; Pavlov, M.Y.; Lovmar, M.; Ehrenberg, M. How initiation factors maximize the accuracy of tRNA selection in initiation of bacterial protein synthesis. Mol. Cell 2006, 23, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Spremulli, L.L.; Coursey, A.; Navratil, T.; Hunter, S.E. Initiation and elongation factors in mammalian mitochondrial protein biosynthesis. Prog. Nucleic Acid. Res. Mol. Biol. 2004, 77, 211–261. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Bähler, J.; Huang, Y. The Insertion Domain of Mti2 Facilitates the Association of Mitochondrial Initiation Factors with Mitoribosomes in Schizosaccharomyces pombe. Biomolecules 2025, 15, 695. https://doi.org/10.3390/biom15050695
Luo Y, Bähler J, Huang Y. The Insertion Domain of Mti2 Facilitates the Association of Mitochondrial Initiation Factors with Mitoribosomes in Schizosaccharomyces pombe. Biomolecules. 2025; 15(5):695. https://doi.org/10.3390/biom15050695
Chicago/Turabian StyleLuo, Ying, Jürg Bähler, and Ying Huang. 2025. "The Insertion Domain of Mti2 Facilitates the Association of Mitochondrial Initiation Factors with Mitoribosomes in Schizosaccharomyces pombe" Biomolecules 15, no. 5: 695. https://doi.org/10.3390/biom15050695
APA StyleLuo, Y., Bähler, J., & Huang, Y. (2025). The Insertion Domain of Mti2 Facilitates the Association of Mitochondrial Initiation Factors with Mitoribosomes in Schizosaccharomyces pombe. Biomolecules, 15(5), 695. https://doi.org/10.3390/biom15050695







