Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone
Abstract
1. Introduction
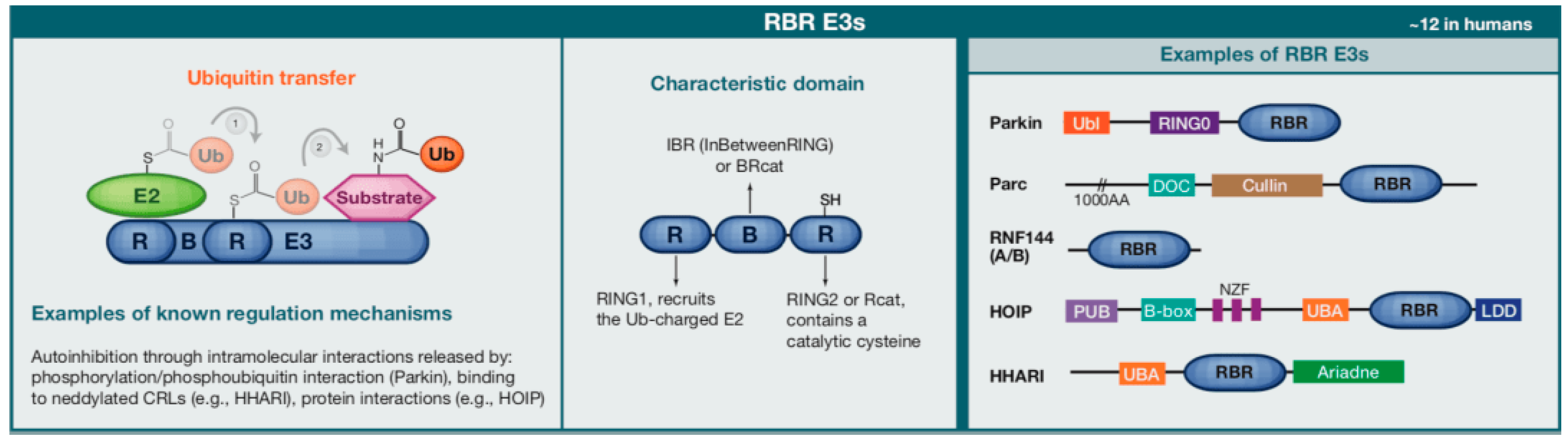
2. The Role of E3 Ubiquitin Ligases in Bone Homeostasis
2.1. Effects of E3 Ubiquitin Ligases on Osteoblast Differentiation
2.2. Effects of E3 Ubiquitin Ligases on Osteoblast Proliferation
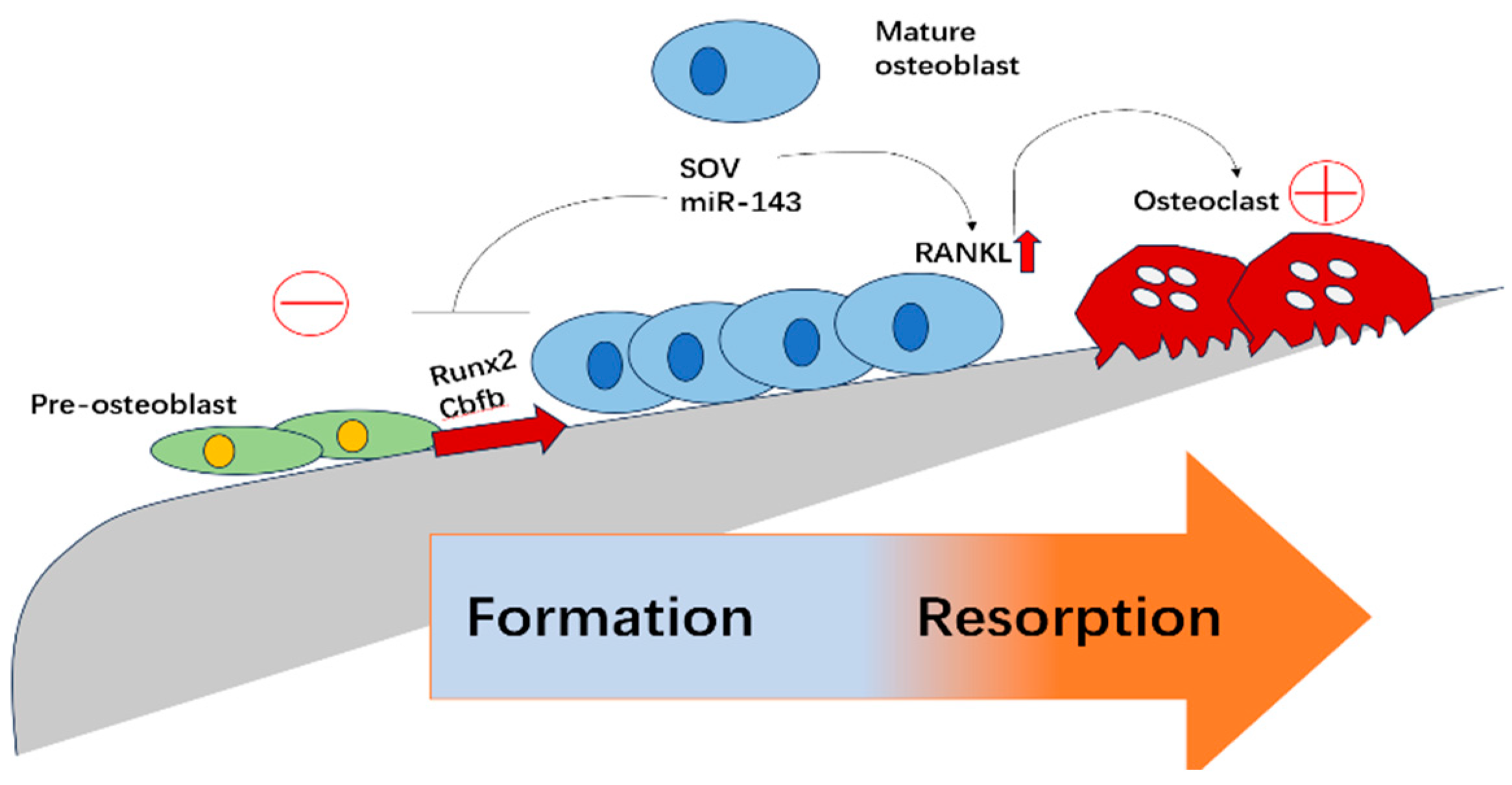
2.3. The Role of E3 Ubiquitin Ligases in Osteoclasts
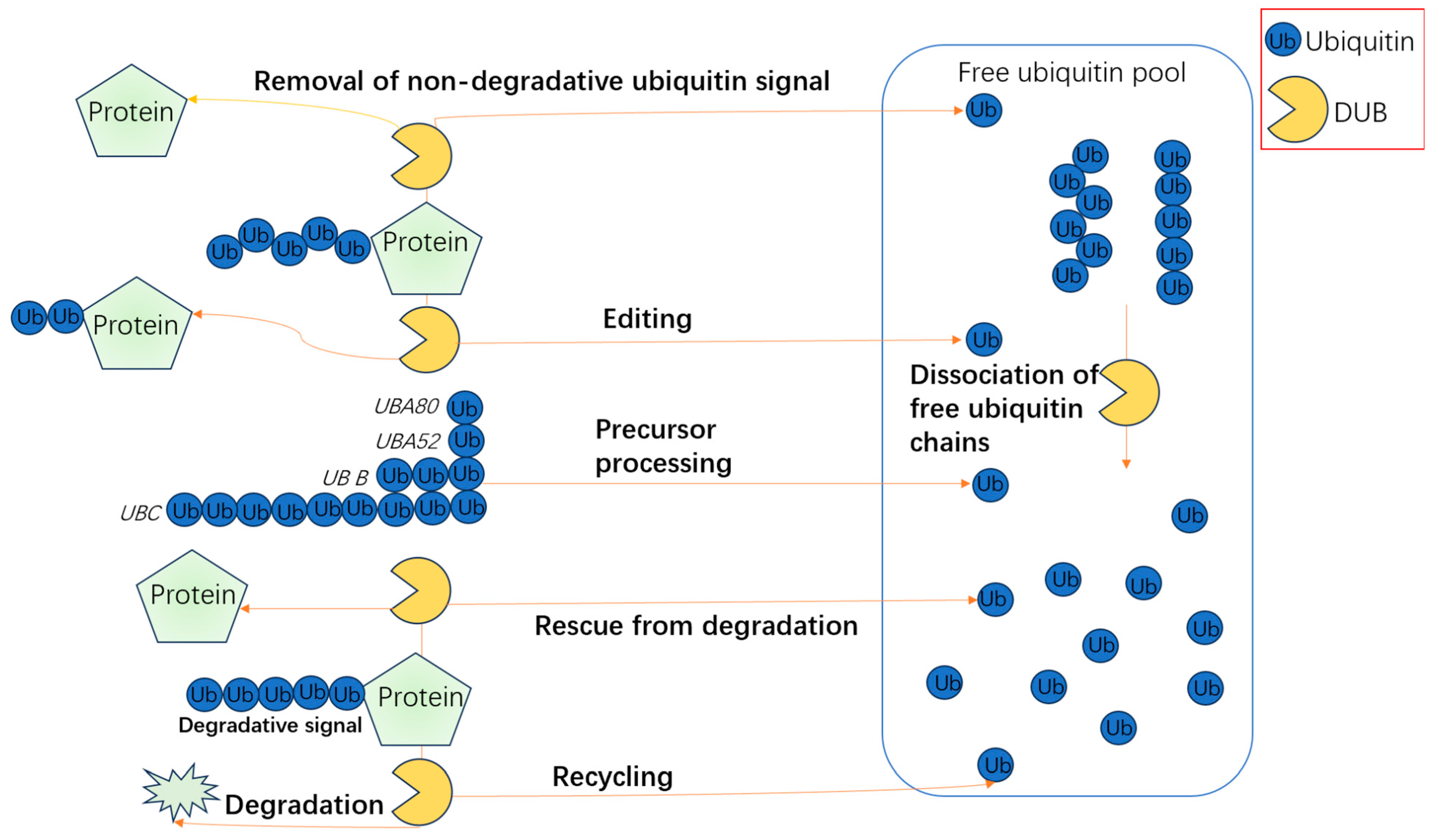
3. The Role of Deubiquitinating Enzymes in Bone Formation
3.1. Effects of Deubiquitinating Enzymes on Osteoblast Differentiation
3.1.1. The USP Family Plays a Key Role in Osteoblast Differentiation
3.1.2. Non-USP DUB Plays an Important Role in Osteoblast Differentiation
3.2. Effects of Deubiquitinating Enzymes on Osteoblast Proliferation
3.3. Role of Deubiquitinating Enzymes in Osteoclasts
4. The Role of Molecules with Deubiquitinating Activity in Bone Homeostasis
4.1. Non-Canonical Deubiquitinating Enzymes
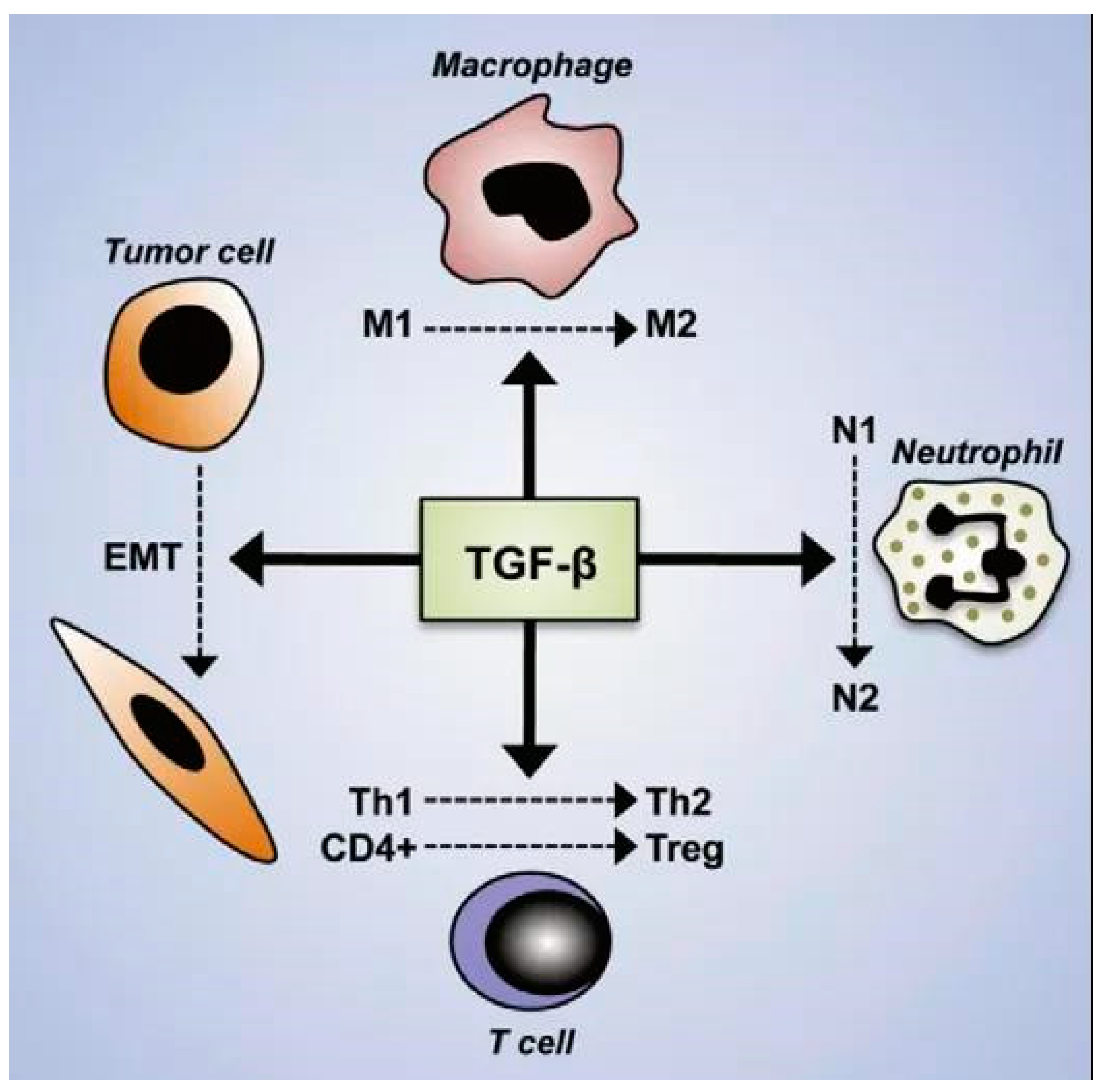
4.2. Cytokines and Signaling Molecules
4.3. Post-Transcriptional Regulation by miRNAs and lncRNAs
4.4. Environmental Factors
5. Discussion and Perspectives
5.1. Complexity and Diversity of E3 Ligases and DUBs in Bone Homeostasis
5.2. Future Research Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef] [PubMed]
- Herhaus, L.; Al-Salihi, M.A.; Dingwell, K.S.; Cummins, T.D.; Wasmus, L.; Vogt, J.; Ewan, R.; Bruce, D.; Macartney, T.; Weidlich, S.; et al. USP15 targets ALK3/BMPR1A for deubiquitylation to enhance bone morphogenetic protein signalling. Open Biol. 2014, 4, 140065. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 2015, 16, 322–324. [Google Scholar] [CrossRef]
- Mevissen, T.E.T.; Komander, D. Mechanisms of Deubiquitinase Specificity and Regulation. Annu. Rev. Biochem. 2017, 86, 159–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, H.; He, L.; Zhong, L.; Zhou, D.; Yin, Z. The promotive role of USP1 inhibition in coordinating osteogenic differentiation and fracture healing during nonunion. J. Orthop. Surg. Res. 2023, 18, 152. [Google Scholar] [CrossRef]
- Snyder, N.A.; Silva, G.M. Deubiquitinating enzymes (DUBs): Regulation, homeostasis, and oxidative stress response. J. Biol. Chem. 2021, 297, 101077. [Google Scholar] [CrossRef]
- Murakami, G.; Watabe, T.; Takaoka, K.; Miyazono, K.; Imamura, T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol. Biol. Cell 2003, 14, 2809–2817. [Google Scholar] [CrossRef]
- Lin, H.; Ying, Y.; Wang, Y.Y.; Wang, G.; Jiang, S.S.; Huang, D.; Luo, L.; Chen, Y.G.; Gerstenfeld, L.C.; Luo, Z. AMPK downregulates ALK2 via increasing the interaction between Smurf1 and Smad6, leading to inhibition of osteogenic differentiation. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2369–2377. [Google Scholar] [CrossRef]
- Ji, L.; Lu, B.; Zamponi, R.; Charlat, O.; Aversa, R.; Yang, Z.E.; Sigoillot, F.; Zhu, X.; Hu, T.; Reece-Hoyes, J.S.; et al. USP7 inhibits Wnt/β-catenin signaling through promoting stabilization of Axin. Nat. Commun. 2019, 10, 14. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Niu, D.; Li, H.; Han, Y.; Peng, J.; Qian, Q. Carbamazepine regulates USP10 through miR-20a-5p to affect the deubiquitination of SKP2 and inhibit osteogenic differentiation. J. Orthop. Surg. Res. 2023, 18, 820. [Google Scholar] [CrossRef]
- Watanabe, M.; Hatakeyama, S. TRIM proteins and diseases. J. Biochem. 2017, 161, 135–144. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, Y.; Zheng, Y.; Chen, B. The miRNA-15b/USP7/KDM6B axis engages in the initiation of osteoporosis by modulating osteoblast differentiation and autophagy. J. Cell Mol. Med. 2021, 25, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bengtson, M.H.; Ulbrich, A.; Matsuda, A.; Reddy, V.A.; Orth, A.; Chanda, S.K.; Batalov, S.; Joazeiro, C.A. Genome-wide and functional annotation of human E3 ubiquitin ligases identifies MULAN, a mitochondrial E3 that regulates the organelle’s dynamics and signaling. PLoS ONE 2008, 3, e1487. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.Y.; Sun, C.S.; Han, X.; Fan, M.Y.; Qiao, W.J. NEDD4L affects stability of the CHEK2/TP53 axis through ubiquitination modification to enhance osteogenic differentiation of periodontal ligament stem cells. Connect. Tissue Res. 2024, 65, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, V.; Singh, A.K.; Sharma, S.; Sethi, A.; Srivastava, S.; Chowdhury, S.; Siddiqui, S.; Chattopadhyay, N.; Trivedi, A.K. RING finger E3 ligase, RNF138 inhibits osteoblast differentiation by negatively regulating Runx2 protein turnover. J. Cell Physiol. 2024, 239, 17. [Google Scholar] [CrossRef]
- Liu, J.; Han, L.; Li, B.; Yang, J.; Huen, M.S.; Pan, X.; Tsao, S.W.; Cheung, A.L. F-box only protein 31 (FBXO31) negatively regulates p38 mitogen-activated protein kinase (MAPK) signaling by mediating lysine 48-linked ubiquitination and degradation of mitogen-activated protein kinase kinase 6 (MKK6). J. Biol. Chem. 2014, 289, 21508–21518. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Kim, I.; Seong, S.; Kim, N. TRIM38 regulates NF-κB activation through TAB2 degradation in osteoclast and osteoblast differentiation. Bone 2018, 113, 17–28. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Q.L.; Liu, H.; Xi, X.; Chen, S.; Liu, D.X. TRIM16 Promotes Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Modulating CHIP-Mediated Degradation of RUNX2. Front. Cell Dev. Biol. 2021, 8, 14. [Google Scholar] [CrossRef]
- Qu, M.Y.; Gong, Y.; Jin, Y.Y.; Gao, R.B.; He, Q.Q.; Xu, Y.A.; Shen, T.; Mei, L.; Xu, C.; Hussain, M.; et al. HSP90β chaperoning SMURF1-mediated LATS proteasomal degradation in the regulation of bone formation. Cell Signal. 2023, 102, 12. [Google Scholar] [CrossRef]
- Winter, J.; Jung, S.; Keller, S.; Gregory, R.I.; Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009, 11, 228–234. [Google Scholar] [CrossRef]
- Metzger, M.B.; Hristova, V.A.; Weissman, A.M. HECT and RING finger families of E3 ubiquitin ligases at a glance. J. Cell Sci. 2012, 125, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Budhidarmo, R.; Nakatani, Y.; Day, C.L. RINGs hold the key to ubiquitin transfer. Trends Biochem. Sci. 2012, 37, 58–65. [Google Scholar] [CrossRef]
- Pao, K.C.; Wood, N.T.; Knebel, A.; Rafie, K.; Stanley, M.; Mabbitt, P.D.; Sundaramoorthy, R.; Hofmann, K.; van Aalten, D.M.F.; Virdee, S. Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity. Nature 2018, 556, 381–385. [Google Scholar] [CrossRef]
- Wenzel, D.M.; Klevit, R.E. Following Ariadne’s thread: A new perspective on RBR ubiquitin ligases. BMC Biol. 2012, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Sangadala, S.; Rao Metpally, R.P.; Reddy, B.V.B. Molecular Interaction Between Smurfl WW2 Domain and PPXY Motifs of Smadl, Smad5, and Smad6-Modeling and Analysis. J. Biomol. Struct. Dyn. 2007, 25, 11–23. [Google Scholar] [CrossRef]
- Shimazu, J.; Wei, J.; Karsenty, G. Smurf1 Inhibits Osteoblast Differentiation, Bone Formation, and Glucose Homeostasis through Serine 148. Cell Rep. 2016, 15, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dang, Y.M.; Liu, M.C.; Gao, L.; Guan, T.; Hu, A.; Xiong, L.; Lin, H. AMPK induces PIAS3 mediated SUMOylation of E3 ubiquitin ligase Smurf1 impairing osteogenic differentiation and traumatic heterotopic ossification. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119771. [Google Scholar] [CrossRef]
- Rotin, D.; Kumar, S. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2009, 10, 398–409. [Google Scholar] [CrossRef]
- Ganji, A.; Roshan, H.M.; Varasteh, A.; Moghadam, M.; Sankian, M. The effects of WW2/WW3 domains of Smurf2 molecule on TGF-β signaling and arginase I gene expression. Cell Biol. Int. 2015, 39, 690–695. [Google Scholar] [CrossRef]
- Horie, T.; Fukasawa, K.; Yamada, T.; Mizuno, S.; Iezaki, T.; Tokumura, K.; Iwahashi, S.; Sakai, S.; Suzuki, A.; Kubo, T.; et al. Erk5 in Bone Marrow Mesenchymal Stem Cells Regulates Bone Homeostasis by Preventing Osteogenesis in Adulthood. Stem Cells 2022, 40, 411–422. [Google Scholar] [CrossRef]
- Xian, J.; Liang, D.; Zhao, C.; Chen, Y.; Zhu, Q. TRIM21 inhibits the osteogenic differentiation of mesenchymal stem cells by facilitating K48 ubiquitination-mediated degradation of Akt. Exp. Cell Res. 2022, 412, 113034. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Tena, S.; Cubillos-Rojas, M.; Schneider, T.; Rosa, J.L. Functional and pathological relevance of HERC family proteins: A decade later. Cell Mol. Life Sci. 2016, 73, 1955–1968. [Google Scholar] [CrossRef]
- Pedrazza, L.; Martinez-Martinez, A.; Sánchez-de-Diego, C.; Valer, J.A.; Pimenta-Lopes, C.; Sala-Gaston, J.; Szpak, M.; Tyler-Smith, C.; Ventura, F.; Rosa, J.L. HERC1 deficiency causes osteopenia through transcriptional program dysregulation during bone remodeling. Cell Death Dis. 2023, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Therrien, M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 2015, 16, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.X.; Liang, G.Y.; Zheng, X.Q.; Huang, Y.X.; Huang, S.H.; Yin, D. RSP5 Positively Regulates the Osteogenic Differentiation of Mesenchymal Stem Cells by Activating the K63-Linked Ubiquitination of Akt. Stem Cells Int. 2020, 2020, 13. [Google Scholar]
- Li, Q.; Li, Y.; Li, J.; Ma, Y.; Dai, W.; Mo, S.; Xu, Y.; Li, X.; Cai, S. FBW7 suppresses metastasis of colorectal cancer by inhibiting HIF1α/CEACAM5 functional axis. Int. J. Biol. Sci. 2018, 14, 726–735. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ma, Y.; Du, W.; Feng, K.; Wang, S. miR-101-loaded exosomes secreted by bone marrow mesenchymal stem cells requires the FBXW7/HIF1α/FOXP3 axis, facilitating osteogenic differentiation. J. Cell Physiol. 2021, 236, 4258–4272. [Google Scholar] [CrossRef]
- Li, X.; Huang, M.; Zheng, H.; Wang, Y.; Ren, F.; Shang, Y.; Zhai, Y.; Irwin, D.M.; Shi, Y.; Chen, D.; et al. CHIP promotes Runx2 degradation and negatively regulates osteoblast differentiation. J. Cell Biol. 2008, 181, 959–972. [Google Scholar] [CrossRef]
- Thacker, G.; Kumar, Y.; Khan, M.P.; Shukla, N.; Kapoor, I.; Kanaujiya, J.K.; Lochab, S.; Ahmed, S.; Sanyal, S.; Chattopadhyay, N.; et al. Skp2 inhibits osteogenesis by promoting ubiquitin-proteasome degradation of Runx2. Biochim. Biophys. Acta. 2016, 1863, 510–519. [Google Scholar] [CrossRef]
- Jeon, S.A.; Lee, J.H.; Kim, D.W.; Cho, J.Y. E3-ubiquitin ligase NEDD4 enhances bone formation by removing TGFβ1-induced pSMAD1 in immature osteoblast. Bone 2018, 116, 248–258. [Google Scholar] [CrossRef]
- Narahara, S.; Sakai, E.; Kadowaki, T.; Yamaguchi, Y.; Narahara, H.; Okamoto, K.; Asahina, I.; Tsukuba, T. KBTBD11, a novel BTB-Kelch protein, is a negative regulator of osteoclastogenesis through controlling Cullin3-mediated ubiquitination of NFATc1. Sci. Rep. 2019, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qian, W.; Qian, Y.; Giltiay, N.V.; Lu, Y.; Swaidani, S.; Misra, S.; Deng, L.; Chen, Z.J.; Li, X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2009, 2, ra63. [Google Scholar] [CrossRef]
- Chen, Y.; Evankovich, J.W.; Lear, T.B.; Tuncer, F.; Kennerdell, J.R.; Camarco, D.P.; Shishido, M.S.; Liu, Y.; Chen, B.B. A small molecule NRF2 activator BC-1901S ameliorates inflammation through DCAF1/NRF2 axis. Redox Biol. 2020, 32, 101485. [Google Scholar] [CrossRef]
- Hirata, H.; Xu, X.H.; Nishioka, K.; Matsuhisa, F.; Kitajima, S.; Kukita, T.; Murayama, M.; Urano, Y.; Miyamoto, H.; Mawatari, M.; et al. PMEPA1 and NEDD4 control the proton production of osteoclasts by regulating vesicular trafficking. Faseb J. 2021, 35, 21. [Google Scholar] [CrossRef]
- Sun, X.W.; Xie, Z.; Hu, B.; Zhang, B.Y.; Ma, Y.; Pan, X.; Huang, H.; Wang, J.; Zhao, X.; Jie, Z.; et al. The Nrf2 activator RTA-408 attenuates osteoclastogenesis by inhibiting STING dependent NF-κb signaling. Redox Biol. 2020, 28, 14. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Nagai-Yoshioka, Y.; Yamasaki, R.; Kawamoto, T.; Nishihara, T.; Ariyoshi, W. Mechanisms involved in suppression of osteoclast supportive activity by transforming growth factor-β1 via the ubiquitin-proteasome system. PLoS ONE 2022, 17, 14. [Google Scholar] [CrossRef]
- Chen, J.; Liang, J.Q.; Zhen, Y.F.; Chang, L.; Zhou, Z.T.; Shen, X.J. DCAF1-targeting microRNA-3175 activates Nrf2 signaling and inhibits dexamethasone-induced oxidative injury in human osteoblasts. Cell Death Dis. 2021, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Lee, Y.N.; Su, C.H.; Shu, K.T.; Liu, W.T.; Hsieh, C.L.; Yeh, H.I.; Wu, Y.J. S-Phase Kinase-associated Protein-2 Rejuvenates Senescent Endothelial Progenitor Cells and Induces Angiogenesis in Vivo. Sci. Rep. 2020, 10, 6646. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.S.; Oh, S.E.; Lee, K.M.; Jung, S.J.; Ko, E.A.; Kim, T.G.; Park, K.H.; Lee, J.W. Age-Related Decrease in Pellino-1 Expression Contributes to Osteoclast-Mediated Bone Loss. Adv. Biol. 2024, 8, 6. [Google Scholar] [CrossRef]
- Dunphy, G.; Flannery, S.M.; Almine, J.F.; Connolly, D.J.; Paulus, C.; Jønsson, K.L.; Jakobsen, M.R.; Nevels, M.M.; Bowie, A.G.; Unterholzner, L. Non-canonical Activation of the DNA Sensing Adaptor STING by ATM and IFI16 Mediates NF-κB Signaling after Nuclear DNA Damage. Mol. Cell 2018, 71, 745–760.e5. [Google Scholar] [CrossRef]
- Clague, M.J.; Urbé, S.; Komander, D. Breaking the chains: Deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 2019, 20, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, N.; Wang, Z.; Chen, H.; Sun, J.; Yao, C.; Zhang, Y. LncRNA USP2-AS1 facilitates the osteogenic differentiation of bone marrow mesenchymal stem cells by targeting KDM3A/ETS1/USP2 to activate the Wnt/β-catenin signaling pathway. RNA Biol. 2024, 21, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hang, K.; Ye, C.; Xu, J.; Chen, E.; Wang, C.; Zhang, W.; Ni, L.; Kuang, Z.; Ying, L.; Xue, D.; et al. Apelin enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly through Wnt/β-catenin signaling pathway. Stem Cell Res. Ther. 2019, 10, 189. [Google Scholar] [CrossRef]
- Fan, Q.; Li, Y.; Sun, Q.; Jia, Y.; He, C.; Sun, T. miR-532-3p inhibits osteogenic differentiation in MC3T3-E1 cells by downregulating ETS1. Biochem. Biophys. Res. Commun. 2020, 525, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, K.H.; Lee, K.M.; Chun, Y.M.; Lee, J.W. Deubiquitinating Enzyme USP7 Is Required for Self-Renewal and Multipotency of Human Bone Marrow-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 23, 8674. [Google Scholar] [CrossRef]
- Wang, X.; Zou, C.; Hou, C.; Bian, Z.; Jiang, W.; Li, M.; Zhu, L. Extracellular vesicles from bone marrow mesenchymal stem cells alleviate osteoporosis in mice through USP7-mediated YAP1 protein stability and the Wnt/β-catenin pathway. Biochem. Pharmacol. 2023, 217, 115829. [Google Scholar] [CrossRef]
- Sun, X.; Ding, Y.; Zhan, M.; Li, Y.; Gao, D.; Wang, G.; Gao, Y.; Li, Y.; Wu, S.; Lu, L.; et al. Usp7 regulates Hippo pathway through deubiquitinating the transcriptional coactivator Yorkie. Nat. Commun. 2019, 10, 411. [Google Scholar] [CrossRef]
- Chaugule, S.; Kim, J.M.; Yang, Y.S.; Knobeloch, K.P.; He, X.; Shim, J.H. Deubiquitinating Enzyme USP8 Is Essential for Skeletogenesis by Regulating Wnt Signaling. Int. J. Mol. Sci. 2021, 22, 13. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, B.K.; Chang, M.L.; Zhang, X.C.; Zou, H.; Zhang, Z.; Han, G. USP12 regulates ER stress-associated osteogenesis in human periodontal ligament cells under tension stress. Cell Signal. 2024, 114, 14. [Google Scholar] [CrossRef]
- Kim, M.J.; Piao, M.; Li, Y.; Lee, S.H.; Lee, K.Y. Deubiquitinase USP17 Regulates Osteoblast Differentiation by Increasing Osterix Protein Stability. Int. J. Mol. Sci. 2023, 24, 12. [Google Scholar] [CrossRef]
- Li, C.W.; Qiu, M.L.; Chang, L.L.; Qi, J.; Zhang, L.F.; Ryffel, B.; Deng, L. The osteoprotective role of USP26 in coordinating bone formation and resorption. Cell Death Differ. 2022, 29, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.C.; Wang, M.Y.; Zhang, S.W.; Wu, Y.S.; Zhou, C.C.; Zheng, R.X.; Shao, B.; Wang, Y.; Xie, L.; Liu, W.Q.; et al. Ubiquitin-specific protease USP34 controls osteogenic differentiation and bone formation by regulating BMP2 signaling. Embo J. 2018, 37, e99398. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.F.; Gu, X.F.; Gao, X.L.; Shao, Y.; Ji, M.H. USP36 regulates the proliferation, survival, and differentiation of hFOB1.19 osteoblast. J. Orthop. Surg. Res. 2024, 19, 11. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, Y.; Wang, P. Icariin promotes osteogenic differentiation of human bone marrow mesenchymal stem cells by regulating USP47/SIRT1/Wnt/β-catenin. Chem. Biol. Drug Des. 2024, 103, e14431. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yang, Y.S.; Park, K.H.; Ge, X.; Xu, R.; Li, N.; Song, M.; Chun, H.; Bok, S.; Charles, J.F.; et al. A RUNX2 stabilization pathway mediates physiologic and pathologic bone formation. Nat. Commun. 2020, 11, 2289. [Google Scholar] [CrossRef]
- Li, J.T.; Wang, P.; Xie, Z.Y.; Wang, S.; Cen, S.Z.; Li, M.; Liu, W.; Tang, S.; Ye, G.; Zheng, G.; et al. TRAF4 positively regulates the osteogenic differentiation of mesenchymal stem cells by acting as an E3 ubiquitin ligase to degrade Smurf2. Cell Death Differ. 2019, 26, 2652–2666. [Google Scholar] [CrossRef]
- Upadhyay, V.; Sharma, S.; Sethi, A.; Singh, A.K.; Chowdhury, S.; Srivastava, S.; Mishra, S.; Singh, S.; Chattopadhyay, N.; Trivedi, A.K. Hakai, a novel Runx2 interacting protein, augments osteoblast differentiation by rescuing Runx2 from Smurf2-mediated proteasome degradation. J. Cell Physiol. 2024, 19, e31388. [Google Scholar] [CrossRef]
- Huang, H.; Lu, J.R.; Aukhil, I.; Yu, C.; Bhut, B.; Marchesan, J.; Nieto, M.A. FBXO11 regulates bone development. Bone 2023, 170, 9. [Google Scholar] [CrossRef]
- de Frutos, C.A.; Dacquin, R.; Vega, S.; Jurdic, P.; Machuca-Gayet, I.; Nieto, M.A. Snail1 controls bone mass by regulating Runx2 and VDR expression during osteoblast differentiation. Embo J. 2009, 28, 686–696. [Google Scholar] [CrossRef]
- Jin, Y.; Shenoy, A.K.; Doernberg, S.; Chen, H.; Luo, H.; Shen, H.; Lin, T.; Tarrash, M.; Cai, Q.; Hu, X.; et al. FBXO11 promotes ubiquitination of the Snail family of transcription factors in cancer progression and epidermal development. Cancer Lett. 2015, 362, 70–82. [Google Scholar] [CrossRef]
- Hariri, H.; Addison, W.N.; St-Arnaud, R. Ubiquitin specific peptidase Usp53 regulates osteoblast versus adipocyte lineage commitment. Sci. Rep. 2021, 11, 18. [Google Scholar] [CrossRef]
- Hariri, H.; Kose, O.; Bezdjian, A.; Daniel, S.J.; St-Arnaud, R. USP53 Regulates Bone Homeostasis by Controlling Rankl Expression in Osteoblasts and Bone Marrow Adipocytes. J. Bone Miner. Res. 2023, 38, 578–596. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.; Park, K.H.; Lee, K.M.; Jung, S.; Joung, S.; Kim, J.; Lee, J.W. Ubiquitin-specific protease 53 promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Death Dis. 2021, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Fu, Y.S.; Cui, C.P.; Ding, Y.; Deng, Z.K.; Ning, C.; Hu, F.; Qiu, C.; Yu, B.; Zhou, X.; et al. OTUB1 promotes osteoblastic bone formation through stabilizing FGFR2. Signal Transduct. Target. Ther. 2023, 8, 13. [Google Scholar] [CrossRef]
- Nguyen, J.; Massoumi, R.; Alliston, T. CYLD, a mechanosensitive deubiquitinase, regulates TGFβ signaling in load-induced bone formation. Bone 2020, 131, 115148. [Google Scholar] [CrossRef]
- Zhang, H.; Du, Y.; Lu, D.; Wang, X.; Li, Y.; Qing, J.; Zhang, Y.; Liu, H.; Lv, L.; Zhang, X.; et al. UBE2C orchestrates bone formation through stabilization of SMAD1/5. Bone 2024, 187, 117175. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.Y.; Xia, D.M.; Wang, Y.; Lv, H.D.; Wang, Z.Q.; Xing, M.; Zhao, Q.; Xu, S. Matrine derivate MASM protects murine MC3T3-E1 osteoblastic cells against dexamethasone-induced apoptosis via the regulation of USP14/p53. Artif. Cell Nanomed. Biotechnol. 2019, 47, 3720–3728. [Google Scholar] [CrossRef]
- Lin, Y.C.; Zheng, G.; Liu, H.T.; Wang, P.; Yuan, W.Q.; Zhang, Y.H.; Peng, X.S.; Li, G.J.; Wu, Y.F.; Shen, H.Y. USP7 promotes the osteoclast differentiation of CD14+ human peripheral blood monocytes in osteoporosis via HMGB1 deubiquitination. J. Orthop. Transl. 2023, 40, 80–91. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, W.; Zhong, Z.; Qiu, R.; Chen, G.; Zhang, P. High-mobility group box chromosomal protein-1 deletion alleviates osteoporosis in OVX rat model via suppressing the osteoclastogenesis and inflammation. J. Orthop. Surg. Res. 2022, 17, 232. [Google Scholar] [CrossRef]
- Xie, Z.A.; Wu, Y.Z.; Shen, Y.; Guo, J.D.; Yuan, P.T.; Ma, Q.L.; Wang, S.; Jie, Z.; Zhou, H.; Fan, S.; et al. USP7 Inhibits Osteoclastogenesis via Dual Effects of Attenuating TRAF6/TAK1 Axis and Stimulating STING Signaling. Aging Dis. 2023, 14, 2267–2283. [Google Scholar] [CrossRef]
- Shrestha, R.K.; Ronau, J.A.; Davies, C.W.; Guenette, R.G.; Strieter, E.R.; Paul, L.N.; Das, C. Insights into the mechanism of deubiquitination by JAMM deubiquitinases from cocrystal structures of the enzyme with the substrate and product. Biochemistry 2014, 53, 3199–3217. [Google Scholar] [CrossRef]
- Huang, J.M.; Ye, Z.Y.; Wang, J.; Chen, Q.C.; Huang, D.L.; Liu, H.Y. USP13 mediates PTEN to ameliorate osteoarthritis by restraining oxidative stress, apoptosis and inflammation via AKT-dependent manner. Biomed. Pharmacother. 2021, 133, 17. [Google Scholar] [CrossRef] [PubMed]
- Mennerich, D.; Kubaichuk, K.; Kietzmann, T. DUBs, Hypoxia, and Cancer. Trends Cancer 2019, 5, 632–653. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Fu, B.; Wu, Y.; Yang, Y.; Lin, X.; Lin, H.; Lin, H.; Liu, H.; Huang, W. USP25 Expression in Peripheral Blood Mononuclear Cells Is Associated With Bone Mineral Density in Women. Front. Cell Dev. Biol. 2021, 9, 811611. [Google Scholar] [CrossRef]
- Li, Q.W.; Wang, M.Y.; Xue, H.X.; Liu, W.Q.; Guo, Y.C.; Xu, R.S.; Shao, B.; Yuan, Q. Ubiquitin-Specific Protease 34 Inhibits Osteoclast Differentiation by RegulatingNF-κBSignaling. J. Bone Miner. Res. 2020, 35, 1597–1608. [Google Scholar] [CrossRef]
- Feng, Z.H.; Tao, S.Y.; Huang, Z.B.; Zheng, B.J.; Kong, X.X.; Xiang, Y.F.; Zhang, Q.; Song, H.; Xu, Z.; Wei, X.; et al. The deubiquitinase UCHL1 negatively controls osteoclastogenesis by regulating TAZ/NFATC1 signalling. Int. J. Biol. Sci. 2023, 19, 2319–2332. [Google Scholar] [CrossRef]
- Shalev, M.; Arman, E.; Stein, M.; Cohen-Sharir, Y.; Brumfeld, V.; Kapishnikov, S.; Royal, I.; Tuckermann, J.; Elson, A. PTPRJ promotes osteoclast maturation and activity by inhibiting Cbl-mediated ubiquitination of NFATc1 in late osteoclastogenesis. Febs J. 2021, 288, 4702–4723. [Google Scholar] [CrossRef]
- You, J.; Xu, D.; Zhang, C.; Chen, Y.; Huang, S.; Bian, H.; Lv, J.; Chen, D.; Su, L.; Yin, H.; et al. Koumine inhibits RANKL-induced ubiquitination and NF-κB activation to prevent ovariectomy and aging-induced bone loss. J. Cell Biochem. 2024, 125, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Y.; Wang, Y.N.; Chen, Y.T.; Wu, M.F.; Wang, Z.J.; Song, M.; Lu, F.; Lu, X.; Dong, Z. TGF-β prevents the denervation-induced reduction of bone formation and promotes the bone regeneration through inhibiting ubiquitin-proteasome pathway. Biosci. Rep. 2019, 39, 12. [Google Scholar] [CrossRef]
- Nam, B.; Park, H.; Lee, Y.L.; Oh, Y.; Park, J.; Kim, S.Y.; Weon, S.; Choi, S.H.; Yang, J.H.; Jo, S.; et al. TGFβ1 Suppressed Matrix Mineralization of Osteoblasts Differentiation by Regulating SMURF1-C/EBPβ-DKK1 Axis. Int. J. Mol. Sci. 2020, 21, 15. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Z.; Ma, Y.; Pan, X.; Wang, J.; Chen, Z.; Shi, P. TGF-β inhibits osteogenesis by upregulating the expression of ubiquitin ligase SMURF1 via MAPK-ERK signaling. J. Cell Physiol. 2018, 233, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Y.; Huang, N.; Zhao, Q.; Yuan, Q.; Shao, B. γ-Aminobutyric Acid Promotes Osteogenic Differentiation of Mesenchymal Stem Cells by Inducing TNFAIP3. Curr. Gene Ther. 2020, 20, 152–161. [Google Scholar] [CrossRef]
- Zhai, Q.L.; Zhao, Y.; Wang, L.P.; Dai, Y.; Zhao, P.Q.; Xiang, X.X.; Liu, K.; Du, W.; Tian, W.; Yang, B.; et al. CircRNA hsa_circ_0008500 Acts as a miR-1301-3p Sponge to Promote Osteoblast Mineralization by Upregulating PADI4. Front. Cell Dev. Biol. 2020, 8, 14. [Google Scholar] [CrossRef]
- Mishima, K.; Kitoh, H.; Ohkawara, B.; Okuno, T.; Ito, M.; Masuda, A.; Ishiguro, N.; Ohno, K. Lansoprazole Upregulates Polyubiquitination of the TNF Receptor-Associated Factor 6 and Facilitates Runx2-mediated Osteoblastogenesis. EBioMedicine 2015, 2, 2046–2061. [Google Scholar] [CrossRef]
- He, Q.; Liu, Z.B.R.; Xia, X.; Zeng, J.; Liu, Y.L.; Xun, J.Q.; Liu, M.; Mei, Y.; Dai, R. Amlexanox Enforces Osteogenic Differentiation and Bone Homeostasis Through Inhibiting Ubiquitin-Dependent Degradation of β-Catenin. Int. J. Biol. Sci. 2024, 20, 5254–5271. [Google Scholar] [CrossRef]
- Zhu, B.; Xue, F.; Zhang, C.Q.; Li, G.Y. LMCD1 promotes osteogenic differentiation of human bone marrow stem cells by regulating BMP signaling. Cell Death Dis. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.Y.; Cai, Y.; Cheng, D.; Wang, H.; Deng, G.Y.; Xiang, D.Y. CYLD alleviates NLRP3 inflammasome-mediated pyroptosis in osteoporosis by deubiquitinating WNK1. J. Orthop. Surg. Res. 2024, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Q.; Wang, X.L.; Song, M.X.; Du, J.; Yu, J.L.; Zheng, W.Z.; Zhang, C.; Wang, Y. MiR-497-5p Regulates Osteo/Odontogenic Differentiation of Stem Cells From Apical Papilla via the Smad Signaling Pathway by Targeting Smurf2. Front. Genet. 2020, 11, 12. [Google Scholar] [CrossRef]
- Duan, G.; Lu, Y.F.; Chen, H.L.; Zhu, Z.Q.; Yang, S.; Wang, Y.Q.; Wang, J.Q.; Jia, X.H. Smurf1-targeting microRNA-136-5p-modified bone marrow mesenchymal stem cells combined with 3D-printed β-tricalcium phosphate scaffolds strengthen osteogenic activity and alleviate bone defects. Kaohsiung J. Med. Sci. 2024, 40, 621–630. [Google Scholar] [CrossRef]
- Wang, R.R.; Feng, Y.H.; Xu, H.Y.; Huang, H.R.; Zhao, S.; Wang, Y.H.; Li, H.; Cao, J.; Xu, G.; Huang, S. Synergistic effects of miR-708-5p and miR-708-3p accelerate the progression of osteoporosis. J. Int. Med. Res. 2020, 48, 20. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Y.; Feng, S.; He, P.; Sheng, B.; Ni, J. miR-19b enhances osteogenic differentiation of mesenchymal stem cells and promotes fracture healing through the WWP1/Smurf2-mediated KLF5/β-catenin signaling pathway. Exp. Mol. Med. 2021, 53, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.D.; Xu, W.C.; Cui, J.; Liang, Y.C.; Cheng, W.Q.; Xin, B.C.; Song, J. Long non-coding RNA maternally expressed gene 3 inhibits osteogenic differentiation of human dental pulp stem cells via microRNA-543/smad ubiquitin regulatory factor 1/runt-related transcription factor 2 axis. Arch. Oral. Biol. 2020, 118, 8. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.E.; Wu, B.; Liu, M.Q.; Cui, C.P. The Discovery of a Specific CKIP-1 Ligand for the Potential Treatment of Disuse Osteoporosis. Int. J. Mol. Sci. 2024, 25, 18. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Ying, S.X.; Zhang, G.M.; Li, C.; Cheng, S.Y.; Deng, C.X.; Zhang, Y.E. Ubiquitin ligase Smurf1 controls osteoblast activity and bone homeostasis by targeting MEKK2 for degradation. Cell 2005, 121, 101–113. [Google Scholar] [CrossRef]
- Lopez, V.A.; Park, B.C.; Nowak, D.; Sreelatha, A.; Zembek, P.; Fernandez, J.; Servage, K.A.; Gradowski, M.; Hennig, J.; Tomchick, D.R.; et al. A Bacterial Effector Mimics a Host HSP90 Client to Undermine Immunity. Cell 2019, 179, 205–218.e21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Wang, L.; Xian, B.; Xia, Y. Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone. Biomolecules 2025, 15, 679. https://doi.org/10.3390/biom15050679
He H, Wang L, Xian B, Xia Y. Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone. Biomolecules. 2025; 15(5):679. https://doi.org/10.3390/biom15050679
Chicago/Turabian StyleHe, Haotian, Lifei Wang, Bao Xian, and Yayi Xia. 2025. "Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone" Biomolecules 15, no. 5: 679. https://doi.org/10.3390/biom15050679
APA StyleHe, H., Wang, L., Xian, B., & Xia, Y. (2025). Regulatory Roles of E3 Ubiquitin Ligases and Deubiquitinases in Bone. Biomolecules, 15(5), 679. https://doi.org/10.3390/biom15050679




