Biophysical Characterization of Shrimp Hemocyanins: Stability and Emerging Biotechnological Applications
Abstract
1. Introduction
1.1. General Overview of Hemocyanins in Crustaceans and Mollusks
1.2. Essential Biological Functions, Emphasizing Oxygen Transport and Immune Defense
Molecular Structure of Hemocyanins
1.3. Context of the Emerging Biotechnological Importance of Hemocyanins
Biotechnological Applications
1.4. Justification for Choosing Macrobachium Acanthurus as the Species of Study
2. Materials and Methods
2.1. HcMac Extraction and Purification
2.2. One-Dimensional SDS-PAGE Electrophoresis Experiments
2.3. MALDI-TOF-MS Mass Spectrometry Experiments
2.4. Analytical Ultracentrifugation Analysis (AUC)
2.5. Spectroscopic Analysis
3. Results
3.1. Purification
3.2. One-Dimensional SDS-PAGE Electrophoresis
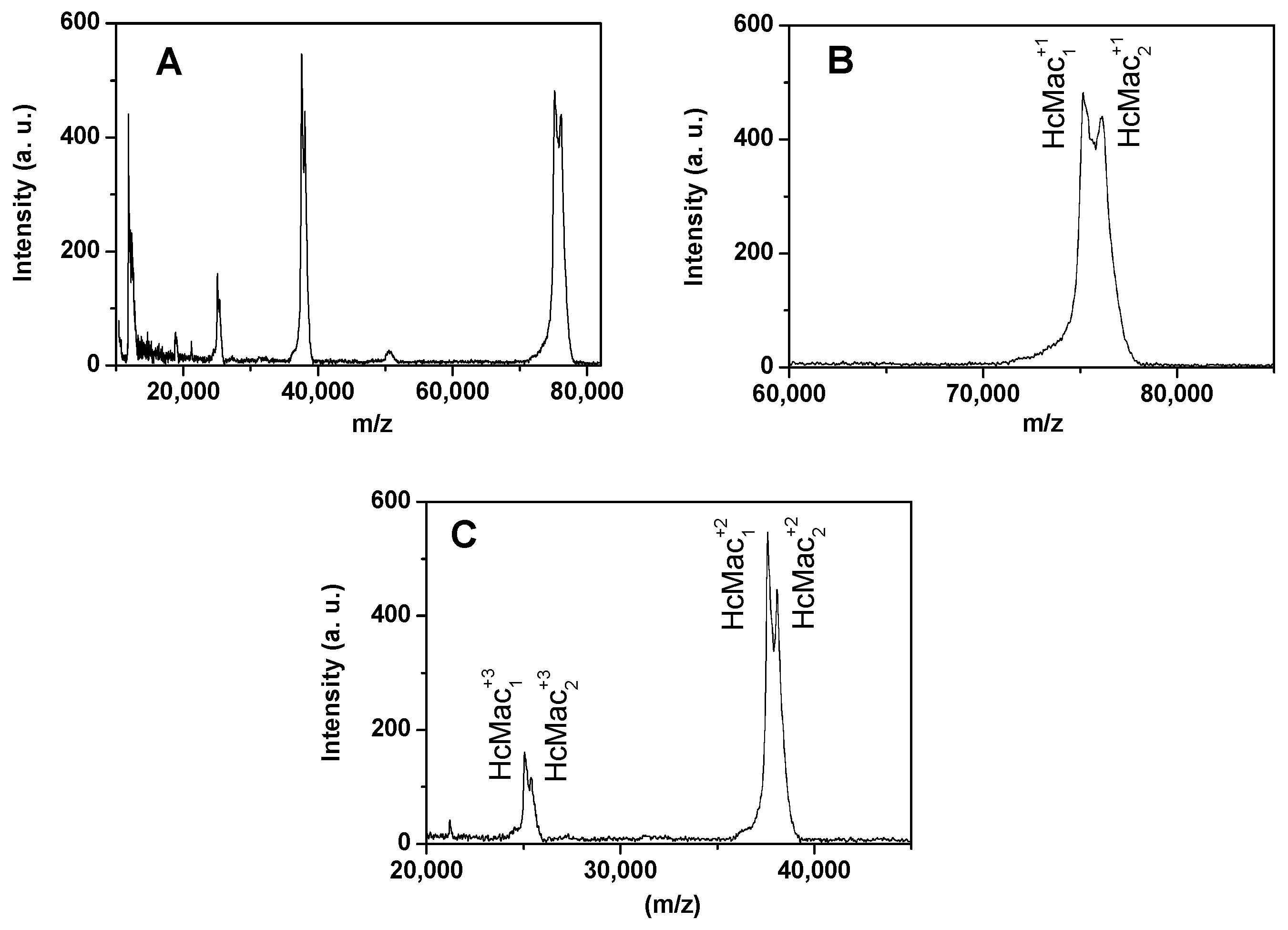
3.3. Analysis of MALDI-TOF-MS Spectra
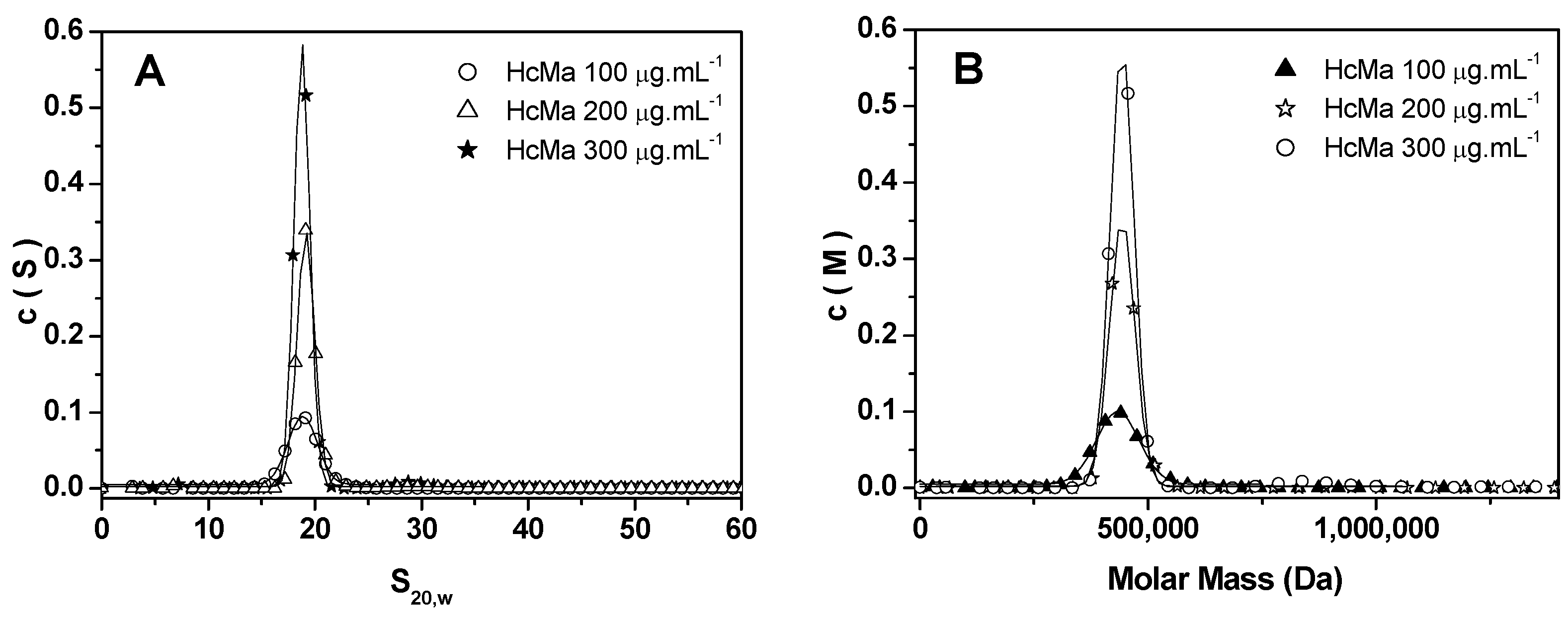
3.4. Analysis of AUC Data
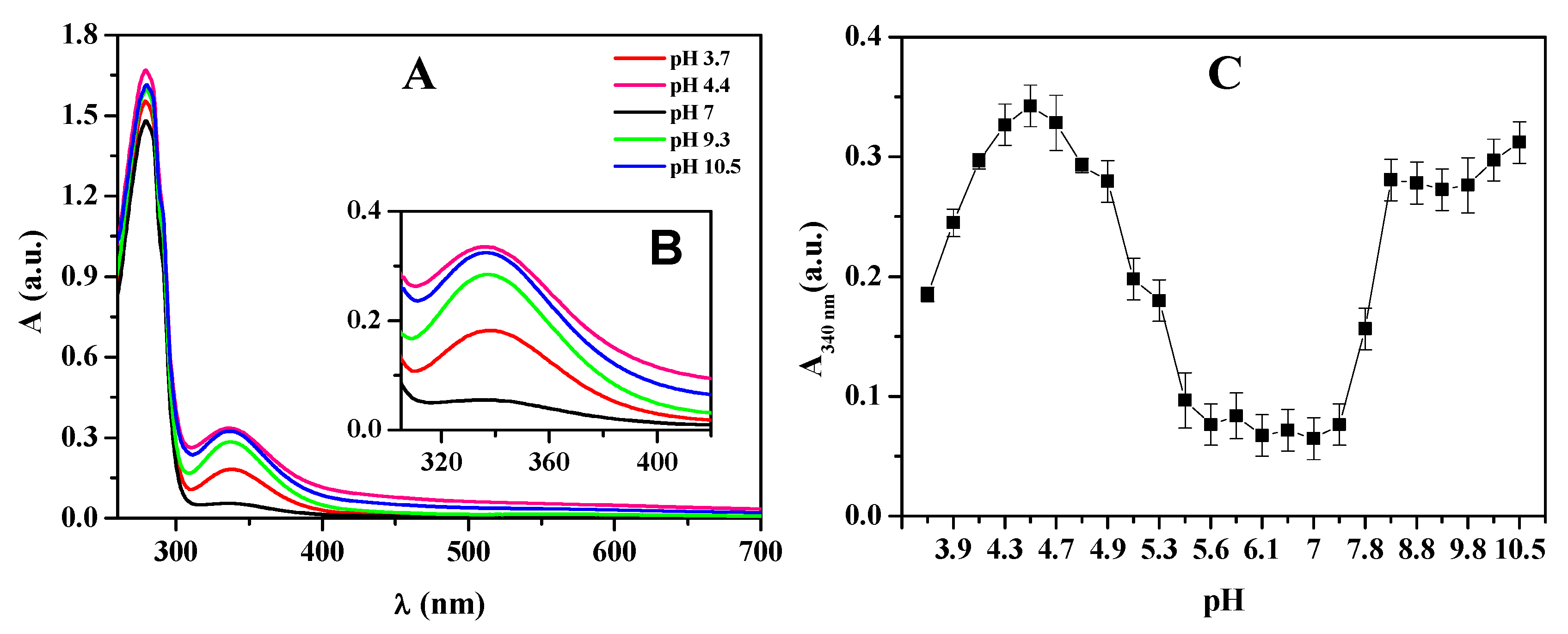
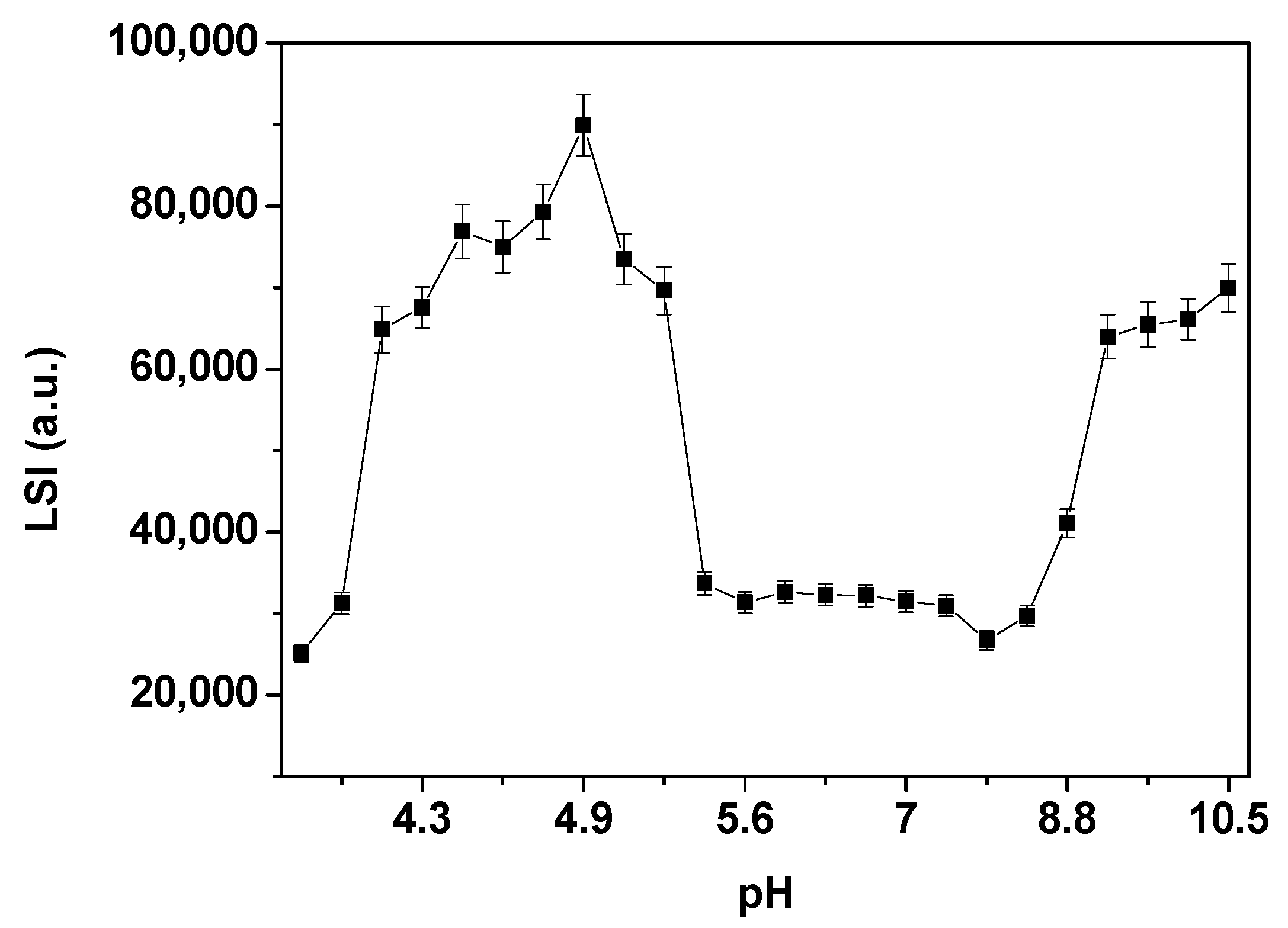
3.5. Spectroscopic Analysis of Native Hemocyanin from Macrobrachium Acanthurus (HcMac) as a Function of pH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Ren, Q. Innate immune responses against viral pathogens in Macrobrachium. Dev. Comp. Immunol. 2021, 117, 103966. [Google Scholar] [PubMed]
- Coates, C.J.; Nairn, J. Diverse immune functions of hemocyanins. Dev. Comp. Immunol. 2014, 45, 43–55. [Google Scholar]
- Todorovska, E.; Ivanov, M.; Radkova, M.; Dolashki, A.; Dolashka, P. Molecular Cloning, Structure and Phylogenetic Analysis of a Hemocyanin Subunit from the Black Sea Crustacean Eriphia verrucosa (Crustacea, Malacostraca). Genes 2021, 12, 93. [Google Scholar] [CrossRef]
- Adachi, K.; Hirata, T.; Nishioka, T.; Sakaguchi, M. Hemocyte components in crustaceans convert hemocyanin into a phenoloxidase-like enzyme. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 134, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Gianazza, E.; Eberini, I.; Palazzolo, L.; Miller, I. Hemolymph proteins: An overview across marine arthropods and molluscs. J. Proteom. 2021, 245, 104294. [Google Scholar]
- Dilna, C.; Prasanth, G.K.; Ghufran, M.S.; Soni, P.; Kanade, S.R.; Duddukuri, G.R. Purification and characterization of a hemocyanin with lectin-like activity isolated from the hemolymph of speckled shrimp, Metapenaeus monoceros. Biochimie 2023, 206, 36–48. [Google Scholar]
- García-Carreño, F.L.; Cota, K.; Navarrete Del Toro, M.A. Phenoloxidase Activity of Hemocyanin in Whiteleg Shrimp Penaeus vannamei: Conversion, Characterization of Catalytic Properties, and Role in Postmortem Melanosis. J. Agric. Food Chem. 2008, 56, 6454–6459. [Google Scholar]
- Mendoza-Porras, O.; Kamath, S.; Harris, J.O.; Colgrave, M.L.; Huerlimann, R.; Lopata, A.L.; Wade, N.M. Resolving hemocyanin isoform complexity in haemolymph of black tiger shrimp Penaeus monodon—Implications in aquaculture, medicine and food safety. J. Proteom. 2020, 218, 103689. [Google Scholar]
- Li, H.; Xia, X.; Wang, Z.; Zang, J.; Du, M. Oyster (Crassostrea gigas) ferritin should be a promising Fe2+ nanocarrier. Food Chem. 2023, 404, 134586. [Google Scholar]
- Coates, C.J.; Decker, H. Immunological properties of oxygen-transport proteins: Hemoglobin, hemocyanin and hemerythrin. Cell. Mol. Life Sci. 2017, 74, 293–317. [Google Scholar] [CrossRef] [PubMed]
- Marxen, J.C.; Pick, C.; Kwiatkowski, M.; Burmester, T. Molecular characterization and evolution of haemocyanin from the two freshwater shrimps Caridina multidentata (Stimpson, 1860) and Atyopsis moluccensis (De Haan, 1849). J. Comp. Physiol. B 2013, 183, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Nairn, J. Hemocyanin-derived phenoloxidase activity: A contributing factor to hyperpigmentation in Nephrops norvegicus. Food Chem. 2013, 140, 361–369. [Google Scholar] [PubMed]
- Ji, R.; Guan, L.; Hu, Z.; Cheng, Y.; Cai, M.; Zhao, G.; Zang, J. A comprehensive review on hemocyanin from marine products: Structure, functions, its implications for the food industry and beyond. Int. J. Biol. Macromol. 2024, 269, 132041. [Google Scholar]
- Masuda, T.; Baba, S.; Matsuo, K.; Ito, S.; Mikami, B. The high-resolution crystal structure of lobster hemocyanin shows its enzymatic capability as a phenoloxidase. Arch. Biochem. Biophys. 2020, 688, 108370. [Google Scholar] [CrossRef]
- Markl, J. Evolution of molluscan hemocyanin structures. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2013, 1834, 1840–1852. [Google Scholar]
- Lieb, B.; Dimitrova, K.; Kang, H.S.; Braun, S.; Gebauer, W.; Martin, A.; Hanelt, B.; Saenz, S.A.; Adema, C.M.; Mark, J. Red blood with blue-blood ancestry: Intriguing structure of a snail hemoglobin. Proc. Natl. Acad. Sci. USA 2006, 103, 12011–12016. [Google Scholar] [CrossRef]
- Van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and Invertebrate Evolution. J. Biol. Chem. 2001, 276, 15563–15566. [Google Scholar]
- Zhao, M.; Zheng, Z.; Wang, C.; Yao, D.; Lin, Z.; Zhao, Y.; Chen, X.; Li, S.; Aweya, J.J.; Zhang, Y. Penaeid shrimp counteract high ammonia stress by generating and using functional peptides from hemocyanin, such as HMCs27. Sci. Total Environ. 2023, 905, 167073. [Google Scholar] [CrossRef]
- Tassanakajon, A.; Rimphanitchayakit, V.; Visetnan, S.; Amparyup, P.; Somboonwiwat, K.; Charoensapsri, W.; Tang, S. Shrimp humoral responses against pathogens: Antimicrobial peptides and melanization. Dev. Comp. Immunol. 2018, 80, 81–93. [Google Scholar]
- Cerenius, L.; Söderhäll, K. Immune properties of invertebrate phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [PubMed]
- Liu, S.; Aweya, J.J.; Zheng, L.; Wang, F.; Zheng, Z.; Zhong, M.; Lun, J.; Zhang, Y. A Litopenaeus vannamei Hemocyanin-Derived Antimicrobial Peptide (Peptide B11) Attenuates Cancer Cells’ Proliferation. Molecules 2018, 23, 3202. [Google Scholar] [CrossRef]
- Kizheva, Y.K.; Rasheva, I.K.; Petrova, M.N.; Milosheva-Ivanova, A.V.; Velkova, L.G.; Dolashka, P.A.; Dolashki, A.K.; Hristova, P.K. Antibacterial activity of crab haemocyanin against clinical pathogens. Biotechnol. Biotechnol. Equip. 2019, 33, 873–880. [Google Scholar]
- Petrova, M.; Vlahova, Z.; Schröder, M.; Todorova, J.; Tzintzarov, A.; Gospodinov, A.; Velkova, L.; Kaynarov, D.; Dolashki, A.; Dolashka, P.; et al. Antitumor Activity of Bioactive Compounds from Rapana venosa against Human Breast Cell Lines. Pharmaceuticals 2023, 16, 181. [Google Scholar] [CrossRef]
- Dolashki, A.; Velkova, L.; Voelter, W.; Dolashka, P. Structural and conformational stability of hemocyanin from the garden snail Cornu aspersum. Z. Für Naturforschung C 2019, 74, 113–123. [Google Scholar]
- Lyu, X.; Tsui, Y.M.; Ho, D.W.H.; Ng, I.O.L. Liquid Biopsy Using Cell-Free or Circulating Tumor DNA in the Management of Hepatocellular Carcinoma. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1611–1624. [Google Scholar] [PubMed]
- Alinejad, T.; Bin, K.Q.; Vejayan, J.; Othman, R.Y.; Bhassu, S. Proteomic analysis of differentially expressed protein in hemocytes of wild giant freshwater prawn Macrobrachium rosenbergii infected with infectious hypodermal and hematopoietic necrosis virus (IHHNV). Meta Gene 2015, 5, 55–67. [Google Scholar]
- Engel, D.; Brouwer, M.; McKenna, S. Hemocyanin concentrations in marine crustaceans as a function of environmental conditions. Mar. Ecol. Prog. Ser. 1993, 93, 235–244. [Google Scholar]
- Toon, A.; Finley, M.; Staples, J.; Crandall, K. Decapod Phylogenetics and Molecular Evolution. In Decapod Crustacean Phylogenetics; Martin, J., Crandall, K., Felder, D., Eds.; CRC Press: Boca Raton, FL, USA, 2009; Volume 20090863, pp. 15–29. [Google Scholar]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Dolashki, A.; Velkova, L.; Dolashka, P.; Toshkova, R. Assessment of the In Vitro and In Vivo Antitumor Activity of Hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi Myeloid Tumor Model. Biomedicines 2023, 11, 1545. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar]
- Wei, K.; Yang, J. Oxidative damage of hepatopancreas induced by pollution depresses humoral immunity response in the freshwater crayfish Procambarus clarkii. Fish Shellfish. Immunol. 2015, 43, 510–519. [Google Scholar] [PubMed]
- Wang, Q.; Liu, N.; Wang, J.X.; Wu, Y.L.; Wang, L. Physiological changes and acetylcholinesterase activity in the cladoceran Moina macrocopa (Straus, 1820) exposed to mercury and sodium dodecyl sulfate. Crustaceana 2014, 87, 1678–1690. [Google Scholar]
- Anger, K. Neotropical Macrobrachium (Caridea: Palaemonidae): On the biology, origin, and radiation of freshwater-invading shrimp. J. Crustac. Biol. 2013, 33, 151–183. [Google Scholar]
- Pileggi, L.; Rossi, N.; Wehrtmann, I.; Mantelatto, F. Molecular perspective on the American transisthmian species of Macrobrachium (Caridea, Palaemonidae). ZooKeys 2014, 457, 109–131. [Google Scholar]
- Huang, Y.; Tan, D.; Chen, X.; Xia, B.; Zhao, Y.; Chen, X.; Zhang, Y.; Zheng, Z. Function of hemocyanin-mediated succinate dehydrogenase in glucose metabolism and immunity of Penaeus vannamei. Fish Shellfish. Immunol. 2024, 151, 109689. [Google Scholar]
- Paoli, M.; Giomi, F.; Hellmann, N.; Jaenicke, E.; Decker, H.; Di Muro, P.; Beltramini, M. The molecular heterogeneity of hemocyanin: Structural and functional properties of the 4 × 6-meric protein of Upogebia pusilla (Crustacea). Gene 2007, 398, 177–182. [Google Scholar] [PubMed]
- Carvalho, F.A.O.; Carvalho, J.W.P.; Santiago, P.S.; Tabak, M. Further characterization of the subunits of the giant extracellular hemoglobin of Glossoscolex paulistus (HbGp) by SDS-PAGE electrophoresis and MALDI-TOF-MS. Process Biochem. 2011, 46, 2144–2151. [Google Scholar]
- Schuck, P. On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 2003, 320, 104–124. [Google Scholar]
- Figueroa-Soto, C.G.; De La Barca, A.M.C.; Vazquez-Moreno, L.; Higuera-Ciapara, I.; Yepiz-Plascencia, G. Purification of Hemocyanin from White Shrimp (Penaeus vannamei Boone) by Immobilized Metal Affinity Chromatography. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 117, 203–208. [Google Scholar]
- Mullaivanam Ramasamy, S.; Denis, M.; Sivakumar, S.; Munusamy, A. Phenoloxidase activity in humoral plasma, hemocyanin and hemocyanin separated proteins of the giant freshwater prawn Macrobrachium rosenbergii. Int. J. Biol. Macromol. 2017, 102, 977–985. [Google Scholar]
- De Oliveira, A.M.; Malunga, L.N.; Perussello, C.A.; Beta, T.; Ribani, R.H. Phenolic acids from fruits of Physalis angulata L. in two stages of maturation. South Afr. J. Bot. 2020, 131, 448–453. [Google Scholar]
- Jaenicke, E.; Decker, H. Tyrosinases from crustaceans form hexamers. Biochem. J. 2003, 371, 515–523. [Google Scholar] [PubMed]
- Lee, S.Y.; Lee, B.L.; Söderhäll, K. Processing of crayfish hemocyanin subunits into phenoloxidase. Biochem. Biophys. Res. Commun. 2004, 322, 490–496. [Google Scholar] [PubMed]
- Wright, J.; Clark, W.M.; Cain, J.A.; Patterson, A.; Coates, C.J.; Nairn, J. Effects of known phenoloxidase inhibitors on hemocyanin-derived phenoloxidase from Limulus polyphemus. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 303–308. [Google Scholar]
- Taylor, A.C.; Astall, C.M.; Atkinson, R.J.A. A comparative study of the oxygen transporting properties of the haemocyanin of five species of thalassinidean mud-shrimps. J. Exp. Mar. Biol. Ecol. 2000, 244, 265–283. [Google Scholar]
- Baird, S.; Kelly, S.M.; Price, N.C.; Jaenicke, E.; Meesters, C.; Nillius, D.; Decker, H.; Nairn, J. Hemocyanin conformational changes associated with SDS-induced phenol oxidase activation. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2007, 1774, 1380–1394. [Google Scholar]
- Arisaka, F.; Van Holde, K.E. Allosteric properties and the association equilibria of hemocyanin from Callianassa californiensis. J. Mol. Biol. 1979, 134, 41–73. [Google Scholar]
- Miller, K.I.; Eldred, N.W.; Arisaka, F.; Van Holde, K.E. Structure and function of hemocyanin from thalassinid shrimp. J. Comp. Physiol. B 1977, 115, 171–184. [Google Scholar]
- Santiago, P.S.; Moura, F.; Moreira, L.M.; Domingues, M.M.; Santos, N.C.; Tabak, M. Dynamic Light Scattering and Optical Absorption Spectroscopy Study of pH and Temperature Stabilities of the Extracellular Hemoglobin of Glossoscolex paulistus. Biophys. J. 2008, 94, 2228–2240. [Google Scholar]
- Raynova, Y.; Angelov, I.; Idakieva, K. Fluorescence Properties and Conformational Stability of Hemocyanin Isolated from Snails Helix Aspersa Maxima. J. Chem. Technol. Metall. 2020, 55, 948. [Google Scholar]
- Sanders, N.K.; Childress, J.J. Specific effects of thiosulphate and L-lactate on hemocyanin-O2 affinity in a brachyuran hydrothermal vent crab. Mar. Biol. 1992, 113, 175–180. [Google Scholar]
- Varshney, A.; Ahmad, B.; Rabbani, G.; Kumar, V.; Yadav, S.; Khan, R.H. Acid-induced unfolding of didecameric keyhole limpet hemocyanin: Detection and characterizations of decameric and tetrameric intermediate states. Amino Acids 2010, 39, 899–910. [Google Scholar] [PubMed]
- Idakieva, K.; Siddiqui, N.I.; Parvanova, K.; Nikolov, P.; Gielens, C. Fluorescence properties and conformational stability of the β-hemocyanin of Helix pomatia. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2006, 1764, 807–814. [Google Scholar]
- Velkova, L.; Dimitrov, I.; Schwarz, H.; Stevanovic, S.; Voelter, W.; Salvato, B.; Dolashka-Angelova, P. Structure of hemocyanin from garden snail Helix lucorum. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 157, 16–25. [Google Scholar]
- Makino, N.; Kimura, S. Subunits of Panulirus japonicus hemocyanin: 1. Isolation and properties. Eur. J. Biochem. 1988, 173, 423–430. [Google Scholar]
- Noel, P.Y.; Martin, M. Comparative Study of Hemocyanins of Decapoda Using Isoelectric Focusing. J. Crustac. Biol. 1995, 15, 418. [Google Scholar]





| Summarizes Investigations for | ||
|---|---|---|
| Applications | Study Animal Species | References |
| Biomarkers of environmental pollution | Red swamp crayfish (Procambarus clarki); European green crab (Carcinus maenas); American lobster (Homarus americanus); Shrimp (Pandalus platyceros). | [27,31] |
| Molecular evolution research | European lobster (Homarus gammarus); Green crab (Carcinus maenas); Giant tiger shrimp (Penaeus monodon). | [28] |
| Immunological adjuvants | White-legged shrimp (Litopenaeus vannamei) | [32] |
| Cancer treatment | Common snail (Helix aspersa); Turkish snail (Helix lucorum); Rapana shell (Rapana venosa). | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, L.; Souza, C.O.; Sebastião, Í.; Bertini, G.; Carvalho, F.A.d.O.; da Silva, R.M.G.; Vilanculo, E.M.; Pereira, J.S.; Santiago, P.S. Biophysical Characterization of Shrimp Hemocyanins: Stability and Emerging Biotechnological Applications. Biomolecules 2025, 15, 675. https://doi.org/10.3390/biom15050675
Ramos L, Souza CO, Sebastião Í, Bertini G, Carvalho FAdO, da Silva RMG, Vilanculo EM, Pereira JS, Santiago PS. Biophysical Characterization of Shrimp Hemocyanins: Stability and Emerging Biotechnological Applications. Biomolecules. 2025; 15(5):675. https://doi.org/10.3390/biom15050675
Chicago/Turabian StyleRamos, Lierge, Claudemir O. Souza, Ísis Sebastião, Giovana Bertini, Francisco Adriano de Oliveira Carvalho, Regildo Márcio Gonçalves da Silva, Edson Miguel Vilanculo, Julianne Soares Pereira, and Patrícia Soares Santiago. 2025. "Biophysical Characterization of Shrimp Hemocyanins: Stability and Emerging Biotechnological Applications" Biomolecules 15, no. 5: 675. https://doi.org/10.3390/biom15050675
APA StyleRamos, L., Souza, C. O., Sebastião, Í., Bertini, G., Carvalho, F. A. d. O., da Silva, R. M. G., Vilanculo, E. M., Pereira, J. S., & Santiago, P. S. (2025). Biophysical Characterization of Shrimp Hemocyanins: Stability and Emerging Biotechnological Applications. Biomolecules, 15(5), 675. https://doi.org/10.3390/biom15050675






