Abstract
Glioblastoma (GBM) is the most aggressive primary brain tumor in adults, characterized by rapid growth and resistance to chemotherapy. Conventional treatments remain largely ineffective, with patient survival averaging around 18 months after diagnosis. Current diagnostic methods rely on invasive tissue biopsies and imaging tests. While traditional biopsies involve extracting tissue samples, their routine use is often limited by surgical risks and the challenge of accessing sensitive brain regions. Liquid biopsy has emerged as a promising noninvasive alternative, analyzing circulating tumor components—such as DNA, RNA, extracellular vesicles, and microRNAs—found in body fluids. This approach enables initial diagnosis and continuous disease monitoring, offering a significant advantage over traditional biopsies, which are impractical for frequent repetition during treatment follow-up. This review highlights recent advances in liquid biopsy-derived biomarkers for the clinical management of GBM. The discussion includes the advantages, limitations, and potential of these biomarkers as tools for early diagnosis and disease monitoring. A narrative review of the literature published over the last decade (2014–2024) was conducted using major health-focused scientific databases. The analysis focuses on evaluating the clinical relevance and applicability of liquid biopsy in GBM, offering insights into its potential as a minimally invasive and effective tool for improving glioblastoma management.
1. Introduction
1.1. Glioblastoma Overview
Glioblastoma (GBM) is the most aggressive and prevalent primary brain tumor in adults, with a poor prognosis and a median survival of 14 to 20 months after diagnosis, even with aggressive multimodal treatments [1]. GBM is highly invasive and characterized by rapid proliferation and resistance to conventional therapies, including surgery, radiation, and chemotherapy [2]. One of the main challenges in treating GBM is its intrinsic heterogeneity, both within the tumor (molecular and cellular levels) and between patients, leading to variable responses to treatment and frequent therapeutic resistance [3]. This heterogeneity complicates the development of universal therapeutic strategies, as distinct tumor regions can evolve independently and foster diverse resistance mechanisms.
Histopathological and molecular analysis of tissue obtained via biopsy or resection remains the gold standard for GBM diagnosis. Additionally, surgery is necessary to relieve mass effect, delay recurrence, and enable molecular profiling [1]. Current clinical methods for monitoring GBM progression rely primarily on imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI). However, these approaches have limitations, particularly in distinguishing true tumor progression from pseudoprogression, a phenomenon where treatment-induced tissue changes, such as necrosis and inflammation, mimic tumor growth but do not represent actual tumor activity [4]. In contrast, true progression involves active tumor proliferation and growth. Advanced techniques, such as perfusion MRI and PET scans, may provide more specific insights into tissue dynamics [5], but are not broadly available in the clinical setting. Additionally, liquid biopsy biomarkers, including circulating tumor DNA (cfDNA) and extracellular vesicles (EVs), show promise in overcoming these limitations by offering real-time, minimally invasive tools to monitor tumor progression and therapeutic response [6], as well as in distinguishing true progression from pseudoprogression [7]. These advancements highlight the potential to improve GBM management, where current methods remain insufficient [8].
In this context, liquid biopsy has emerged as a promising method with reduced invasiveness for capturing tumor-derived biomarkers in body fluids like blood, plasma, or cerebrospinal fluid (CSF). Liquid biopsy analyzes tumor-derived biomarkers, including extracellular vesicles (EVs), such as exosomes, which carry DNA, RNA, and proteins that reflect the molecular characteristics of the tumor [9]. Liquid biopsy enables the detection of tumor-derived molecules circulating in body fluids, offering a minimally invasive strategy that may complement imaging and tissue biopsies, particularly in the longitudinal monitoring of tumor progression and recurrence. It is hypothesized that liquid biopsy could capture molecular signals from a broader array of tumor regions than localized tissue biopsy, potentially offering insights into spatial and temporal heterogeneity. This may be especially valuable in clinical scenarios where conventional biopsy is limited, such as tumors located in deep or eloquent brain areas.
Moreover, emerging data suggest that certain biomarkers—such as circulating tumor cells, extracellular vesicles, or circulating macrophages—may provide noninvasive molecular information that could support clinical decision-making, particularly when imaging findings are inconclusive [10]. Despite this promise, the approach remains largely investigational due to key limitations, including the presence of the blood–brain barrier (BBB) and the typically low abundance of tumor biomarkers in GBM circulation, which hinder consistent detection. Additionally, while liquid biopsy may offer a route to assess tumor heterogeneity, since analytes from different tumor areas could theoretically reach the bloodstream, this potential still requires robust clinical validation. This remains to be confirmed; however, one study has shown that mutations absent from tumor specimens were detected in the plasma of several glioblastoma patients [11]. Although false-positive cases cannot be ruled out, the fact that patients with mutations detected in plasma displayed reduced survival argues against this hypothesis.
For now, liquid biopsy should be regarded as an investigational and complementary tool to histopathology, which remains the gold standard for diagnosis due to its direct visualization of tumor tissue and established diagnostic accuracy. Even so, the ability of liquid biopsy to access molecular information from otherwise inaccessible tumor regions underscores its promise and fully justifies continued efforts toward its clinical development and integration [12].
1.2. Liquid Biopsy Overview
Liquid biopsy refers to the detection and quantification of tumor-derived elements (tumor biomarkers), such as cell-free nucleic acids (DNA/RNA), circulating tumor cells (CTCs), extracellular vesicles (EVs), proteins, and metabolites found in biological fluids [6]. Its primary advantage lies in the ability to provide real-time insights into tumor dynamics, including genetic alterations, tumor burden, clonal fitness or acquisition, and resistance mechanisms, without requiring highly invasive procedures [9]. This capability has positioned liquid biopsy as a key tool in precision oncology, enabling the capture of tumor heterogeneity and the detection of emerging resistant clones, which helps clinicians adapt treatment strategies more effectively [6,13].
In the case of GBM, liquid biopsy offers a less invasive strategy that may complement imaging and tissue biopsies, particularly in longitudinal monitoring of tumor progression and recurrence. While imaging techniques such as amino acid PET and MRI perfusion have demonstrated high sensitivity and specificity in distinguishing true progression from pseudoprogression and can even provide insights into molecular characteristics [14,15], their broader application remains limited by the availability of specialized equipment and trained personnel. Traditional MRI continues to serve as the clinical cornerstone, yet emerging evidence suggests that liquid biopsy markers—such as circulating tumor cells, extracellular vesicles, or circulating macrophages—may offer additional, noninvasive insights that support and enrich current diagnostic approaches [10]. Although still in an investigational stage, liquid biopsy holds strong potential as a valuable adjunct to imaging, contributing complementary molecular information that may enhance the accuracy and timing of clinical decision-making in glioblastoma care [6,10]. Despite challenges posed by the BBB and the typically low levels of cfDNA in the bloodstream, alternative approaches, such as CSF analysis, and the collection of larger blood volumes are being explored [7]. Successful integration of liquid biopsy in GBM could provide valuable insights into tumor evolution and therapeutic responses, ultimately contributing to improved patient outcomes.
2. Materials and Methods
This review explores recent advancements and challenges in liquid biopsy for glioblastoma, with a particular focus on technological innovations, clinical applications, and future perspectives. A comprehensive literature search was conducted using renowned scientific databases, including PubMed-MEDLINE, Scopus, Web of Science, and Google Scholar. The keywords used were ‘glioblastoma’, ‘liquid biopsy’, and ‘biomarker’. Articles published within the last 10 years (2014–2024) were selected, specifically those addressing the clinical application of liquid biopsy-derived tumor biomarkers in glioblastoma.
The selection process prioritized studies based on their originality and relevance to glioblastoma and liquid biopsy, with emphasis on research presenting clinical data, technological advancements, or field-specific challenges. Studies focusing on other tumor types or unrelated to liquid biopsy applications were excluded.
Data analysis involved extracting information on recent advances, advantages, limitations, and the clinical potential of biomarkers derived from liquid biopsy in the context of glioblastoma. Any discrepancies in article selection were resolved through discussion and consensus among the reviewers.
3. Selected Papers and the Clinical Applicability of Biomarkers Derived from Liquid Biopsy in Glioblastoma
3.1. Panorama of the Selected Papers
When adopting the described methodology, 89 articles were identified, of which only 73 had the full text available for access. After screening titles and abstracts for their meaningful contribution to the liquid biopsy field on GBM, 64 articles were selected for discussion in this review (Figure 1).
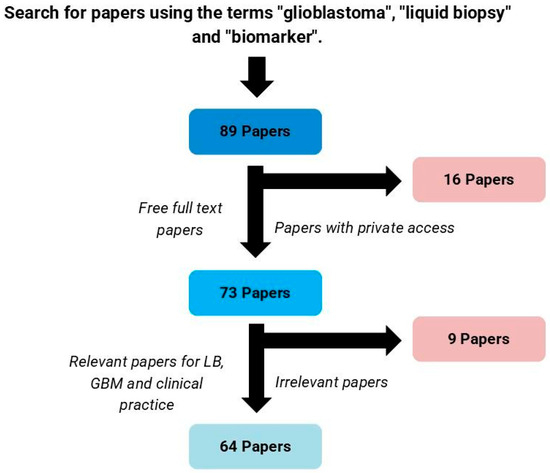
Figure 1.
Process for selection of articles. LB: liquid biopsy; GBM: glioblastoma.
The 64 studies were then classified according to the biological matrix used, the sample size, the type of biomarker, and the analysis technique employed (Table 1).

Table 1.
Classification of scientific studies included in review. cfDNA: cell-free deoxyribonucleic acid; CGGA: Chinese glioma genome atlas; circRNA: circular ribonucleic acid; CSF: cerebrospinal fluid; CTC: circulating tumor cell; ctDNA: circulating tumor deoxyribonucleic acid; ddPCR: droplet digital polymerase chain reaction; ELISA: enzyme-linked immunosorbent assay; EV: extracellular vesicle; FFPE: formalin-fixed paraffin-embedded; GBM: glioblastoma; IDH: isocitrate dehydrogenase; IHC: immunohistochemistry; LC-MS: liquid chromatography–mass spectrometry; lncRNA: long noncoding ribonucleic acid; mRNA: messenger ribonucleic acid; miRNA: micro ribonucleic acid; NA: not available; NGS: next-generation sequencing; PBMC: peripheral blood mononuclear cell; PCR: polymerase chain reaction; piRNA: PIWI-interacting RNA; qPCR: quantitative (real-time) polymerase chain reaction; qRT-PCR: quantitative reverse-transcribed PCR; WB: western blot; WT: wild-type. * Only IDH wild-type (true GBM according to the WHO 2021 classification) were considered; ▲ No information on IDH status or GBM classification version. Summary data based on information from [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].
3.2. Circulating Tumor Cells (CTCs)
Circulating tumor cells (CTCs) are shed into the bloodstream from primary or metastatic tumors and serve as a valuable tool for tumor characterization when tissue biopsies are not feasible [80]. However, detecting CTCs is challenging due to their rarity, found at approximately one cell per billion blood cells [81]. The quality and quantity of isolated CTC samples can vary depending on the isolation method, impacting the sensitivity and specificity of detection [82].
Standard techniques for CTC detection include qPCR (both DNA and cDNA), although these approaches may generate false positives. The CellSearch® System, validated by the FDA, is a robust method but has limited applicability in glioblastoma because it primarily targets epithelial markers, such as EpCAM, which are often absent in tumors of the central nervous system (CNS), including GBM. Furthermore, the significant cellular heterogeneity in GBM makes single-marker detection difficult [83,84,85].
Advanced methods like the CTC chip and EPISPOT enable the isolation of viable tumor cells for more detailed analyses [86]. Lynch et al. challenged the assumption that brain tumor cells do not cross the BBB by isolating GBM-derived CTCs using immunomagnetic enrichment and identifying specific markers like GLAST to distinguish GBM-CTCs from lymphocytes [17]. Similarly, Bark et al. used microfluidic technology to isolate CTCs from newly diagnosed GBM patients (18/20 IDH wild-type), reporting that patients with zero CTC counts post-surgery experienced longer recurrence-free survival [18].
Kolostova et al. analyzed peripheral blood from GBM patients after tumor resection, finding a high concordance between the molecular profiles of CTCs and their respective primary tumors. Interestingly, they identified more mutations in CTCs than in the primary tumors [19]. Recent findings have shown that CTCs in glioblastoma can display mesenchymal and stem-like features, contributing to their invasive potential and ability to migrate through the bloodstream. Although extracranial metastases are extremely rare in GBM due to the restrictive nature of the brain environment, the blood–brain barrier and the restricted survival time of GBM patients, they have been reported in isolated cases involving organs such as the lungs and bones. Śledzińska et al. highlighted that CTCs may harbor distinct molecular alterations compared to the primary tumor, potentially contributing to metastatic spread. Mutations in EGFR, RB1, and SETD2, for example, have been identified in metastatic sites despite being absent in the corresponding primary tumor [82]. These rare occurrences highlight the complex biology of GBM and underscore the need for further investigation into CTCs and their role in tumor spread. Additionally, Gao et al. reported that CTCs showed potential in monitoring treatment response and distinguishing radionecrosis from glioma recurrence, although the study was limited to a small patient cohort, warranting further validation [16].
In summary, despite the technical challenges associated with CTC isolation and detection, these cells hold significant potential for prognosis and patient monitoring in GBM. They allow molecular assessments of the disease and reveal new aspects, such as distinct GBM cell populations circulating in the bloodstream with potentially unique properties. However, further studies are required to validate and integrate these approaches into clinical practice.
3.3. Extracellular Vesicles (EVs)
Extracellular vesicles (EVs) are lipid membrane-bound nanoparticles of various sizes, classified based on their cellular origin and biogenesis mechanisms [87,88]. Exosomes, a specific subtype of EVs, are extensively studied in glioblastoma liquid biopsy due to their abundance in bodily fluids and their ability to transport biomolecules, including genetic and protein material that reflect the tumor’s status and can be useful for diagnosis and monitoring [89,90,91].
Recent studies have emphasized the potential of EVs as tumor biomarkers, particularly when combined with conventional tumor markers. Yang et al. investigated gene expression in brain and blood exosomes using an orthotopic xenograft mouse model. In primary tumors and blood exosomes, DNM3, p65, and CD117 expression increased, while PTEN and p53 decreased (both at the mRNA and protein levels). In recurrent tumors, DNM3 and p65 remained elevated, whereas ST14 and p53 decreased, suggesting that serum exosomes could distinguish primary from recurrent GBM based on gene/protein transcription/expression profiles [20].
Hallal et al. identified 4054 proteins in EVs isolated from plasma samples, among which a subset was found to correlate with glioma histological grades. EVs from aggressive tumors exhibited elevated levels of proteins such as AIDA, BNIP3L, CETN3, FYB1, and POLR2D [21]. Similarly, Cilibrasi et al. highlighted the potential of inflammatory proteins, including VWF, FCGBP, C3, PROS1, and SERPINA1, in EVs derived from IDH wild-type GBM patients, reinforcing their role in disease monitoring [23]. Dobra et al. demonstrated that small extracellular vesicles (sEVs) were more effective than whole serum in differentiating GBM from brain metastasis, meningioma, and disc herniation [22].
Lennartz et al. investigated vesicular heat shock protein 70 (Hsp70) and its effect on the immunophenotypic profile of lymphocytes in glioma patients. Elevated Hsp70 levels in GBM patients were associated with a decrease in CD3+/CD4+ T cells and a worse prognosis [27]. Conversely, in grade 3 gliomas, elevated Hsp70 levels and activated NK cells correlated with better survival. Similar findings on increased Hsp70 levels in GBM were reported by Werner et al. [28].
Nucleic acids, particularly miRNAs and piRNAs, have also been studied as EVs cargo in the liquid biopsy context. MicroRNAs (miRNAs) are small, non-coding RNA molecules (18–25 nucleotides) that regulate gene expression post-transcriptionally by targeting mRNAs for degradation or translational inhibition, while PIWI-interacting RNAs (piRNAs) are longer non-coding RNAs (24–31 nucleotides) primarily involved in silencing transposable elements and maintaining genomic stability, especially in germ cells. Akers et al. profiled EV-derived miRNAs from tumor tissue and CSF, identifying a GBM-specific signature of nine miRNAs, including miR-21, miR-218, and miR-193b. The signature showed higher sensitivity in cisternal CSF compared to lumbar CSF, confirming that proximity to the tumor enhances detection [36]. Shi et al. further demonstrated elevated levels of miR-21 in CSF-derived EVs from glioma patients, with no significant differences in serum-derived EVs [37].
Hallal et al. identified piRNAs, including piR_016658, piR_016659, and piR_020829, in EVs derived from ultrasonic aspirates and serum, distinguishing GBM from lower-grade gliomas and healthy controls [32]. Stella et al. found decreased levels of CircSMARCA5 and circHIPK3—circular RNAs (circRNAs), a class of stable, non-coding RNAs formed by back-splicing, which regulate gene expression and are often dysregulated in cancer—in serum EVs from GBM patients compared to lower-grade gliomas and controls. CircSMARCA5 and circHIPK3 have been implicated in glioma progression and tumorigenesis, highlighting their potential as diagnostic markers [62]. Larger studies have identified additional circRNAs, such as hsa_circ_0055202, hsa_circ_0074920, and hsa_circ_0024108, as promising diagnostic and prognostic markers [65,92].
Genetic alterations detected in EVs have demonstrated significant clinical utility. Rosas-Alonso et al. showed that MGMT methylation analysis in EVs correlates with improved responses to temozolomide chemotherapy and better prognostic outcomes, often surpassing tissue biopsies due to sampling limitations [38]. Similarly, Ricklefs et al. reported that elevated EV plasma levels in GBM patients were associated with shorter overall survival (OS) and progression-free survival (PFS), allowing tumor recurrence to be predicted months before MRI detection [30]. Expanding on this, Aibaidula et al. utilized spectral flow cytometry to identify non-neoplastic EVs in GBM patient plasma, highlighting new possibilities for non-invasive biomarker development [29]. Hallal et al. further demonstrated that urine-derived EVs from IDH wild-type GBM patients carried molecular signatures indicative of glioblastoma, representing a promising, fully non-invasive approach for tumor detection and monitoring [24]. However, limitations such as small cohort sizes and the absence of validated common EV markers for GBM underscore the need for further research. In conclusion, although several studies suggest the promise of EV-based liquid biopsy in the context of GBM, its clinical applicability for disease monitoring has yet to be firmly established.
3.4. Circulating Tumor Nucleic Acids (ctNAs)
Circulating tumor DNA (ctDNA) has emerged as a promising biomarker in GBM liquid biopsy, offering a non-invasive approach to monitor tumor evolution and guide treatment decisions. Despite the low concentration of ctDNA in the plasma of glioma patients, advances in next-generation sequencing (NGS) and PCR platforms have significantly improved its detection and clinical relevance. CtDNA can be identified through molecular markers such as mutations, rearrangements, copy number variations, epigenetic modifications, and abnormal fragmentation patterns. Mattos-Arruda et al. demonstrated that CSF-derived ctDNA is more representative of brain tumor genomic alterations than plasma ctDNA, making it particularly useful for guiding treatment in recurrent GBM cases [45]. Similarly, Miller et al. detected CSF ctDNA in 49.4% of cases, correlating its presence with disease burden and adverse outcomes. The genetic alterations identified in CSF closely mirrored those found in tumor biopsies, including mutations in IDH1/2 and the 1p/19q codeletion, facilitating the development of targeted therapies for glioma [40]. Although IDH1/2-mutated tumors and those with 1p/19q codeletion are no longer classified as GBM, these findings highlight the potential of CSF for detecting tumor-related DNA alterations and improving differential diagnosis.
Dai et al. established CSF ctDNA methylation profiles in recurrent GBM patients using data from the China glioma genome atlas (CGGA) and the Gene expression omnibus (GEO). Using Lasso and Cox multiplex analyses, they identified eight core genes (FLRT2, NKD1, GNB5, NTRK3, COMMD1, C1orf226, CHI3L2, and ETV1) for diagnostic and prognostic models, demonstrating high predictive accuracy [48]. Bagley et al. investigated the association between cfDNA concentration and survival in GBM patients, finding that higher preoperative cfDNA levels correlated with shorter progression-free survival (PFS) and overall survival (OS). Moreover, an increase in cfDNA concentration following chemoradiotherapy, compared to preoperative baseline levels, was associated with worse survival projections, reinforcing its potential as a prognostic biomarker [46].
Juratli et al. evaluated the detection of TERT promoter (TERTp) mutations in ctDNA from CSF and plasma in GBM patients, detecting mutations in 38 of 60 cases. The sensitivity of TERTp mutation detection in CSF (92.1%) was significantly higher than in plasma (7.9%). Additionally, a higher variant allele frequency (VAF) of TERTp mutations in CSF was linked to poor survival, indicating its potential as a prognostic biomarker [41].
Meng et al. explored magnetic resonance imaging-guided focused ultrasound (MRgFUS) as a non-invasive technique to improve access to circulating brain biomarkers by temporarily disrupting the BBB. Their study demonstrated that MRgFUS, combined with temozolomide (TMZ) treatment, effectively increased plasma levels of cfDNA, neuron-derived EVs, and the glial injury marker S100b, highlighting its potential in enhancing liquid biopsy sensitivity for central nervous system tumors [47].
Zhao et al. analyzed ctDNA mutations in various glioma subtypes, finding a high concordance between CSF ctDNA mutations and tumor DNA mutations in 17 paired samples. Mutations in PTEN and TP53 were frequently detected in recurrent gliomas, while IDH1/2 mutations were predominant in astrocytomas, and RB1 and EGFR mutations were identified in IDH wild-type GBM. These results underscore the potential of CSF ctDNA in distinguishing glioma subtypes [42]. These observations were supported by Miller et al., who demonstrated that CSF ctDNA is useful for monitoring tumor evolution and serves as a valuable prognostic tool [40]. Contrary to most studies, Piccioni et al. detected ctDNA alterations in 55% of GBM patients (n = 222) using the Guardant360 assay, with significant implications for personalized targeted therapy and treatment monitoring [44].
Cell-free RNAs (cfRNAs) are another important class of circulating biomarkers, released into biological fluids through passive secretion from necrotic or apoptotic cells, as well as active secretion via membranous vesicles. These molecules include messenger RNAs (mRNAs) and non-coding RNAs (ncRNAs), the latter subdivided into small (e.g., microRNAs, miRNAs) and long (lncRNAs) forms. Studies indicate that while less than 2% of the human genome is transcribed into protein-coding RNAs, the majority of the transcriptome consists of ncRNAs, increasing interest in their viability as cancer biomarkers [12,93].
MiRNAs play a crucial role in gene expression regulation and are actively released into the CSF and bloodstream, acting as both biomarkers and therapeutic targets in GBM. They influence tumorigenesis and cancer progression, affecting key processes such as cell proliferation, invasion, and resistance to treatment. Several studies have linked miRNA profiling to disease staging, prognosis, and treatment response assessment [6,94].
Lucero et al. identified eight miRNAs (miR-148a-3p, miR-9-5p, miR-9-3p, miR-22-3p, miR-182-5p, miR-186-5p, miR-16-2-3p, and miR-378e) associated with angiogenesis and poor survival in endothelial cells exposed to glioblastoma stem cell-derived EVs (GSC-EVs). Notably, silencing miR-148a normalized tumor vasculature in murine GBM models, suggesting that miRNA export by GSCs may contribute to therapy resistance [49].
Drusco et al. identified a CSF miRNA signature that differentiates GBM from other CNS malignancies. Their analysis revealed upregulation of miR-451, miR-223, and miR-125b in GBM compared to normal individuals, miR-711 upregulation in GBM versus lymphoma and medulloblastoma, and downregulation of miR-125b in GBM versus medulloblastoma. Notably, GBM lacks miR-935 expression, distinguishing it from other CNS tumors [50]. These findings provide insights into GBM molecular characteristics, aiding diagnosis and potentially informing treatment strategies.
Additional studies support miRNA expression as a diagnostic and prognostic tool. D’Urso et al. identified increased miR-21 and miR-15b levels in glioma patient serum, with miR-16 downregulation distinguishing GBM from lower-grade gliomas [52]. Qu et al. also observed higher miR-21 levels in CSF than in tumor tissue, reinforcing its value as a prognostic indicator [51]. Lai et al. reported that serum miRNA-210 levels were significantly elevated in GBM patients, correlating with tumor grade and poor outcomes [56]. Other studies demonstrated that serum miR-221 and miR-222 levels were associated with poor survival [60], while miR-100 upregulation post-treatment correlated with improved prognosis [57].
LncRNAs have also been implicated in GBM. Amer et al. identified increased lncRNA565 and lncRNA641 expression in serum samples, correlating with worse PFS and OS, suggesting their role as prognostic biomarkers and therapeutic targets [64]. Chen et al. identified lncRNA MALAT1 as a prognostic marker, with increased expression correlating with TMZ resistance and reduced survival [66].
Taken together, these findings underscore the growing potential of ctDNA, cfRNAs, and extracellular vesicle-derived markers in glioblastoma liquid biopsy. While ctDNA in CSF has demonstrated greater sensitivity and informativeness for brain tumor analysis, ctDNA detection in peripheral blood—although less invasive—still lacks sufficient sensitivity for clinical application. Nonetheless, circulating biomarkers may offer a promising avenue for future approaches aiming to classify gliomas, capture tumor heterogeneity, inform treatment strategies, and support disease monitoring within the framework of precision oncology. As research continues, addressing challenges related to detection sensitivity, standardization, and clinical validation will be crucial to fully integrate liquid biopsy into routine GBM management. Moreover, despite advances in the process of CSF acquisition, careful consideration regarding the benefits of CSF-based liquid biopsy is needed given the risks associated with repetitive lumbar puncture [95].
3.5. Circulating Proteins and Metabolites
Metabolomics encompasses the investigation of small molecules present in biofluids, cells, tissues, or organs, enabling the study of metabolic pathways within the organism [96]. Metabolites represent the final products of protein translation and gene transcription, as well as cellular modifications affecting the proteome, genome, and transcriptome. The metabolome serves as the interface between the external environment and the genome, comprising bioactive small molecules such as nucleotides, carbohydrates, amino acids, and fatty acids. Identifying metabolites and alterations in metabolic pathways is crucial for understanding disease pathophysiology and discovering potential therapeutic targets [97]. Additionally, circulating proteins in biofluids provide a broader molecular profile of tumor heterogeneity. These proteins often include membrane receptors, receptor ligands, growth factors, and cytokines. Given the relatively low cost of isolation techniques, these approaches have been widely explored [98,99]. Research involving these proteins primarily focuses on evaluating their altered levels in tumor cells due to the lack of glioma-specific proteins [12]. Consequently, circulating proteins and metabolites are emerging as promising tumor biomarkers derived from liquid biopsy. These components reflect not only the molecular characteristics of the tumor but also its interactions with the surrounding microenvironment [94,95]. The integration of proteomic and metabolic data with genomic and clinical information has the potential to enhance the identification of predictive, prognostic, and therapeutic response biomarkers, thus advancing precision medicine [100].
Dufrusine et al. demonstrated that galectin-3, encoded by the LGALS3BP gene, is overexpressed in EVs derived from the serum of GBM patients and correlates with tumor volume. Their findings suggest that tumor cells increase the release of EVs expressing LGALS3BP, making galectin-3 a promising biomarker for diagnosis and disease monitoring [26]. Likewise, Masood et al. reported that both protein and mRNA levels of PD-L1 were elevated in GBM patients’ plasma, with high levels correlating with poor overall survival, suggesting its potential as a prognostic marker and therapeutic target [68]. Soler et al. identified that the ratio between CD14+ blood cells with low levels or no HLA-DR ( HLA-DRneg/low) and those positive for VNN2 (VNN2⁺) can differentiate patients with recurrent GBM from those with radiation necrosis, highlighting its potential in challenging clinical cases [70]. Ghorbani et al. investigated four potential biomarkers—glial fibrillary acidic protein (GFAP), neurofilament light (NfL), matrix metalloproteinase 3 (MMP3), and fatty acid-binding protein type 4 (FABP4)—as non-invasive markers for glioblastoma. Their study suggested that these proteins, detectable in blood samples, could aid in GBM diagnosis and patient monitoring. However, they were unable to differentiate GBM from lymphomas [71].
Bark et al. applied metabolomics to analyze saliva and blood samples from 21 newly diagnosed GBM patients, 20 of whom were IDH wild-type. Among 151 metabolites and 197 lipids identified, cyclic AMP (adenosine monophosphate), 3-hydroxykynurenine, dihydroorotate, UDP (uridine diphosphate), and cis-aconitate were increased, while oxamic acid was decreased in patients with poor outcomes. Additionally, lipid profiles in these patients exhibited greater heterogeneity and fewer marker associations compared to patients with better prognosis [77]. Shen et al., in a larger cohort of newly diagnosed GBM patients, found that decreased plasma levels of arginine and methionine as well as increased levels of kynurenate were associated with poorer two-year OS and PFS [75]. Liu et al. conducted a pilot study on the metabolomic characterization of human glioblastomas and patient plasma samples to identify metabolic profiles suitable for diagnosis and monitoring. Their results revealed significant metabolic differences between GBM patients and healthy individuals, suggesting that metabolomics could provide valuable insights into tumor status and disease progression. The study proposes that metabolomics could enhance liquid biopsy approaches, improving early diagnosis and enabling personalized treatment strategies [76].
Further studies have demonstrated the potential of plasma metabolomics to classify glioma patients with high accuracy. Zhao et al. showed that metabolomic profiling could distinguish low- and high-grade gliomas with 91.1% accuracy using a panel of 18 metabolites and differentiate IDH-mutated from IDH wild-type patients through six specific metabolites. Notably, lower plasma levels of arginine (indicating increased tumor uptake) in high-grade gliomas suggest that patients could be stratified for arginine-deprivation therapy. Additionally, uridine and ornithine plasma levels could be used to distinguish GBM from malignant gliomas, aiding differential diagnosis [74]. Furthermore, 2-hydroxyglutarate (2-HG) has been proposed as a key metabolic marker for IDH-mutant gliomas [101], although these are now categorized separately from GBM according to the 2021 WHO classification. Björkblom et al. found that high serum levels of vitamin E in grade 4 glioma patients could predict disease years before diagnosis, aligning with studies linking elevated vitamin E levels to an increased risk of developing certain cancers and its role in tumor progression [72]. Supporting this, a study by Bao et al. also reported a significant association between elevated alpha-tocopherol levels and glioblastoma risk [73], reinforcing the potential relevance of this antioxidant in glioma pathogenesis. Lipidomics, another growing field, has also provided promising insights. Zhou et al. conducted a large-scale study incorporating machine learning and identified an 11-lipid plasma signature capable of differentiating malignant gliomas from healthy subjects with an accuracy of 0.9641 [78]. Other research groups have reported quantitative differences in various lipid classes, although these findings were based on smaller patient cohorts [79].
These findings underscore the potential of metabolomics, proteomics, and lipidomics in glioblastoma liquid biopsy. Integrating these molecular signatures with genomic and transcriptomic data enhances tumor characterization, enabling earlier diagnosis, personalized treatment, and better patient monitoring. Advancing standardization, expanding cohort studies, and improving technologies will be key to fully implementing these approaches in precision oncology, ultimately refining GBM diagnostics and patient management.
4. Technical Challenges and Future Directions
The low concentration of certain biomarkers, such as ctDNA and CTCs, reduces the sensitivity of isolation techniques and the detection of genetic alterations in liquid biopsy analyses. Additionally, biological variability among patients and tumor heterogeneity can influence the quantity and quality of detected biomarkers. Furthermore, biological barriers like the BBB can limit the availability of biomarkers in peripheral blood, further decreasing the sensitivity of these tests [12].
Sensitivity and specificity vary significantly across different studies and techniques. While some studies report high sensitivity and specificity for certain biomarkers, such as ctDNA in CSF, others show more modest results for plasma-derived biomarkers [102]. Mattos-Arruda et al. and Miller et al. demonstrated that CSF-derived ctDNA more accurately represents brain tumor genomic alterations than plasma ctDNA [40,45], suggesting that CSF may be a more suitable choice for liquid biopsy in GBM, particularly for patient follow-up [37]. Having said so, although uncommon, complications related to repeated lumbar punctures [95,103,104,105] might limit their practical use in routine clinical follow-up. Thus, enhancing the sensitivity and robustness of liquid biopsy analyses from peripheral blood—an easier and safer source—would be highly preferable.
Traditional biopsy remains the gold standard for glioblastoma diagnosis, with high diagnostic accuracy ranging from 90–96% in clinical studies [106]. In contrast, liquid biopsy offers a non- or minimally invasive alternative that enables real-time monitoring of disease dynamics, although its diagnostic accuracy is generally lower than that of tissue-based methods. Table 2 summarizes the diagnostic performance of different liquid biopsy strategies in glioblastoma, comparing their sensitivity and specificity to matched tumor tissue. Among the fluids analyzed, CSF consistently outperformed plasma and serum, particularly in ctDNA-based assays, reaching up to 95% sensitivity for TERT promoter mutations. EVs biomarkers also showed encouraging results, with EV-RNA from serum achieving 81.6% sensitivity and EV-miRNA panels from CSF demonstrating specificity as high as 95%. While performance varies depending on the biomarker type, fluid source, and analytical platform, these findings support the role of liquid biopsy—especially CSF-based approaches—as a valuable complementary tool. Its utility may be particularly relevant in cases where traditional biopsy poses increased risk or is not feasible.

Table 2.
Diagnostic performance of liquid biopsy approaches compared to tissue biopsy in glioblastoma. Table summarizes sensitivity and specificity for each biomarker studied, using tumor tissue as reference when applicable. Summary data based on information from [31,35,36,38,39,40,41,42,44,45].
In addition, liquid biopsy enables molecular monitoring through the detection of tumor-specific alterations in circulating biomarkers. A 2021 study introduced the GeLB score, a serum-based DNA methylation signature that accurately distinguishes glioma patients from controls (sensitivity: 100%, specificity: 97.8%). Importantly, the GeLB score was able to differentiate true progression from pseudoprogression, decreasing in cases with histologically confirmed pseudoprogression despite suspicious MRI findings [107]. Although highly promising, it is important to note that this study analyzed gliomas of various types together, and the performance of the GeLB score specifically in glioblastoma remains to be confirmed in larger, subtype-specific cohorts.
In a recent paper published in Clinical Chemistry (2025), Iorgulescu et al. demonstrated that analyzing larger volumes of plasma using a personalized ctDNA assay called MAESTRO-Pool significantly improves the sensitivity of liquid biopsy for glioblastoma [7]. MAESTRO-Pool is a mutation-enrichment method that uses patient-specific tumor “fingerprints” to selectively capture and sequence rare ctDNA fragments from the bloodstream with high precision. A key finding was the assay’s ability to help differentiate true tumor progression from pseudoprogression, an ongoing clinical challenge in neuro-oncology. In cases where MRI results were indeterminate, ctDNA detection strongly correlated with histologically confirmed true progression, while its absence aligned with cases of pseudoprogression confirmed by pathology. These results suggest that high-yield, mutation-informed liquid biopsy can offer a valuable, noninvasive tool to support clinical decision-making in glioblastoma management by providing early molecular evidence to complement imaging and guide treatment response assessment.
Combining liquid biopsy data with imaging may thus improve diagnostic precision and guide clinical decisions. Among the available techniques, CSF-derived ctDNA analyzed by NGS and ddPCR currently appears to be one of the most promising approaches, offering higher sensitivity and specificity in detecting tumor-specific alterations [40,41,42,44]. Despite these advances, liquid biopsy is better positioned as a complementary tool, particularly useful in cases where surgical procedures present high risks, such as in deep-seated or brainstem lesions, and in therapeutic monitoring, where early detection of molecular changes may precede radiological progression.
Additionally, studies comparing different analytes compartments, such as exosomes, plasma, serum, and whole blood, are necessary to determine whether exosomes offer advantages for biomarker detection. Exosomes are particularly promising due to their abundance in blood, resistance to degradation, and their demonstrated superiority over whole blood in protein-based liquid biopsies [22,26].
A lack of concordance is observed among studies analyzing the same biomarker type (e.g., circulating microRNAs, circular RNAs), which may be attributed to variations in sample size, GBM classification criteria, patient cohorts (e.g., recurrent vs. newly diagnosed, treated vs. treatment-naïve, different therapies), selected biological matrices (e.g., serum vs. extracellular vesicles), and methodological differences in sample isolation and analysis. Indeed, 70% of the studies cited in this review analyzed fewer than 50 patients and employed diverse reagents, isolation methods, analytical platforms, and statistical approaches, often using heterogeneous or poorly defined cohorts. This highlights the urgent need for standardization in liquid biopsy methodologies.
Regarding specificity, the detection of non-tumor-specific genetic alterations, such as clonal hematopoiesis of indeterminate potential (CHIP), can lead to false positives and complicate diagnosis. However, parallel whole-blood analysis can help distinguish true tumor-derived alterations from CHIP-related findings. Currently, eight registered clinical trials (www.clinicaltrial.org, accessed on 15 December 2024) are dedicated to evaluating liquid biopsy in GBM patients, which may help address some of these challenges. The PLANET (NCT05099068) and GRETeL (NCT05695976) trials focus on validating ctDNA as a marker for therapy response, survival, recurrence, and its ability to reflect tumor alterations. Another trial (NCT05934630) is assessing cfDNA in CSF from pediatric and young adult with primary brain tumors. A novel approach involves the collection of tear fluid as a liquid biopsy matrix to evaluate the effects of tumor-treating fields (TTFields) in GBM patients (NCT06136611), which could provide a less invasive alternative for biomarker analysis.
In parallel, researchers are actively developing new techniques to improve access to certain biomarkers [108]. For example, Lynch et al. established an immunomagnetic enrichment method targeting MCAM (melanoma cell adhesion molecule) and MCSP (melanoma-associated chondroitin sulfate proteoglycan), followed by the use of GLAST (glutamate aspartate transporter) and GFAP (glial fibrillary acidic protein) to differentiate CTCs from lymphocytes, thereby improving the identification of GBM-derived CTCs [17].
Another approach to enhance liquid biopsy sensitivity involves increasing BBB permeability, as demonstrated by Meng et al. [47]. Two ongoing clinical trials (NCT05383872, NCT05281731) are currently validating the impact of ultrasound-based transient BBB permeabilization on the detection of GBM ctDNA.
Additionally, advances in detection techniques are further improving the sensitivity and specificity of liquid biopsy-based analyses. Digital droplet PCR (ddPCR) and next-generation sequencing (NGS) methodologies are being refined to enhance the detection of cfDNA and other biomarkers, reducing false negatives. The integration of bioinformatics tools is also significantly improving the accuracy of liquid biopsy-based NGS [109]. These advancements hold great promise for enhancing the early detection and monitoring of glioblastoma.
In summary, liquid biopsy represents a promising non-invasive tool for helping the clinical evaluation of glioblastoma. In this review, we highlighted the increasing importance of various biomarkers, including ctDNA, cfRNAs, EVs, CTCs, proteins, and metabolites, in characterizing tumors, detecting mutations, identifying methylation and genome structural alterations, assessing recurrence risk, predicting prognosis, and monitoring therapeutic responses (Table 3, Figure 2).

Table 3.
Overview of main findings and potential clinical application of biomarkers found. Summary data based on information from [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].
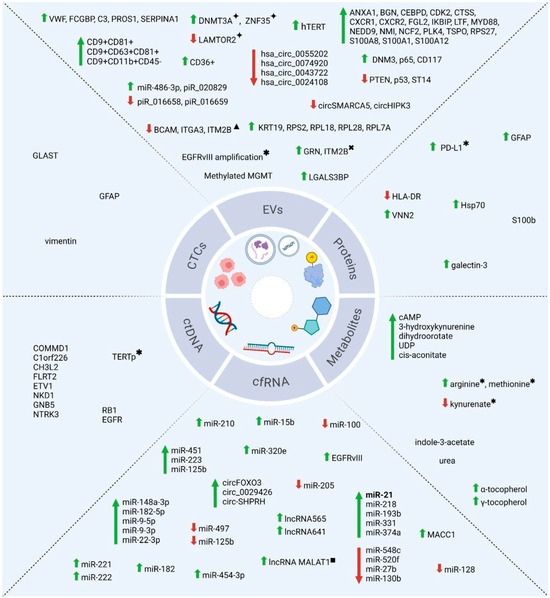
Figure 2.
Key liquid biopsy biomarkers for glioblastoma. Schematic overview of main biomarkers discussed in the study. Green arrows indicate increased analyte levels; red arrows indicate decreased levels. Biomarkers shown without arrows were reported as simply detected in GBM liquid biopsy. Biomarkers identified in multiple studies (2014–2024) are highlighted in bold (e.g., miR-21). ✦ in responders to dacomitinib; ✱ in patients with poor survival; ✖ in recurrent patients; ▲ in resected patients; ■ in non-responders to temozolomide. Image created with BioRender (https://BioRender.com/s28x312).
5. Final Remarks
Despite its potential, several challenges remain, particularly the low levels of cfDNA in the bloodstream due to limited tumor DNA release, rapid clearance, and the restrictive nature of the BBB, as well as the invasive nature of CSF collection. However, technological advancements in ddPCR, NGS, multi-omics integration and biomarker enrichment techniques offer promising solutions to enhance detection sensitivity and specificity. Future innovations, including more sensitive assays and less invasive sampling methods, could significantly improve the clinical applicability of liquid biopsy in GBM, ultimately leading to better patient outcomes.
Nonetheless, before liquid biopsy can be fully integrated into clinical practice, rigorously designed clinical trials using standardized methodologies are essential to validate the reproducibility, specificity, and prognostic value of liquid biopsy biomarkers. These trials should follow the WHO 2021 guidelines and involve large, well-defined patient cohorts to ensure clinical reliability.
Although liquid biopsy offers a minimally invasive alternative for obtaining molecular information in GBM, it is important to emphasize that, at present; this method does not achieve the same diagnostic accuracy or therapeutic value as tissue biopsy or surgical resection. In addition, surgery may be required at different stages of the disease for various purposes, including symptom relief, tumor debulking, and molecular profiling. The true promise of liquid biopsy lies in its potential to complement existing tools—rather than replace them—offering a valuable option for continuous disease monitoring and for patients in whom surgical access to the tumor poses significant risk.
In addition, the current concentration of studies on specific analytes, such as vesicular or free ctDNA, miRNAs, and mRNAs, combined with small sample sizes, heterogeneous cohorts, and variations in analytical methods, underscores the urgent need for standardization, careful patient selection, and diversification of biomarkers and biological matrices. While challenges remain, the results so far are promising, and it is expected that at least some liquid biopsy biomarkers will be validated in the near future for GBM diagnosis, monitoring, and personalized treatment strategies. Notably, the field of fragmentomics and strategies such as small fragment enrichment remain unexplored for GBM in this new exciting field and warrants future investigation in the search for more sensitive liquid biopsy approaches.
Author Contributions
B.P.d.L. analyzed the papers, wrote the first version, and edited the manuscript. L.S.F. helped with the analysis, revised and edited the manuscript, and created the figures. S.D. helped with the analysis, and revised and edited the manuscript. H.L.B.: acquired funding for the study, and revised and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq—Process 310652/2021-9), the Brazilian Federal Agency for Support and Evaluation of Higher Education (CAPES—Finance Code 001), the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ—Processes E-26/201.201/2022 (273290) and E-26/210.626/2023 (286130)), and the National Program to Support Oncological Care (PRONON—Process 25000.016131/2018-77).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no conflicts of interest to declare. All authors have reviewed and approved the final version of the manuscript.
Abbreviations
| GBM | Glioblastoma |
| IDH | Isocitrate dehydrogenase |
| TMZ | Temozolomide |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| EVs | Extracellular vesicles |
| CTCs | Circulating tumor cells |
| miRNA | microRNA |
| ctNAs | Circulating tumor nucleic acids |
| cfDNA | Circulating free DNA |
| cfRNA | Circulating free RNA |
| lncRNA | Long non-coding RNA |
| circRNA | Circular ribonucleic acid |
| mRNA | Messenger ribonucleic acid |
| piRNA | PIWI-interacting RNA |
| sEVs | Small extracellular vesicles |
| MRI | Magnetic resonance imaging |
| MRgFUS | Magnetic resonance imaging-guided focused ultrasound |
| MR | Magnetic resonance |
| CT | Computerized tomography |
| PET | Positron emission tomography |
| GeLB | Epigenetic Glioma Liquid Biopsy |
| LB | Liquid biopsy |
| NGS | Next-generation sequencing |
| n | Sample size |
| PFS | Progression-free survival |
| PBMCs | Peripheral blood mononuclear cells |
| GSC-EV | Glioblastoma stemcell–extracellular vesicles |
| qPCR | Quantitative polymerase chain reaction |
| ddPCR | Droplet digital polymerase chain reaction |
| qRT-PCR | Quantitative reverse-transcribed PCR |
| TERT | Telomerase reverse transcriptase |
| hTERT | Human telomerase reverse transcriptase |
| TERTp | Telomerase reverse transcriptase promoter |
| EGFR | Epidermal growth factor receptor |
| MGMT | O6-methylguanine-DNA methyltransferase |
| PTEN | Phosphatase and tensin homolog |
| GFAP | Glial fibrillar acid protein |
| WT | Wild-type |
| IHC | Immunohistochemistry |
| FDA | Food and drugaAdministration |
| WHO | World health organization |
| CGGA | Chinese glioma genome atlas |
| FFPE | Formalin-fixed paraffin-embedded |
| ELISA | Enzyme-linked immunosorbent assay |
| LC-MS | Liquid chromatography–mass spectrometry |
| WB | Western blot |
| NA | Not available |
| OS | Overall survival |
| PFS | Progression-free survival |
References
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2021, 10, 574012. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma Hijacks Neuronal Mechanisms for Brain Invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef]
- Davis, M. Glioblastoma: Overview of Disease and Treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Young, R.M.; Jamshidi, A.; Davis, G.; Sherman, J.H. Current Trends in the Surgical Management and Treatment of Adult Glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Lara-Velazquez, M.; Al-Kharboosh, R.; Jeanneret, S.; Vazquez-Ramos, C.; Mahato, D.; Tavanaiepour, D.; Rahmathulla, G.; Quinones-Hinojosa, A. Advances in Brain Tumor Surgery for Glioblastoma in Adults. Brain Sci. 2017, 7, 166. [Google Scholar] [CrossRef]
- Eibl, R.H.; Schneemann, M. Liquid Biopsy and Glioblastoma. Explor. Target. Anti-Tumor Ther. 2023, 4, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Iorgulescu, J.B.; Blewett, T.; Xiong, K.; Crnjac, A.; Liu, R.; Sridhar, S.; Braun, D.A.; Sellars, M.C.; Cheng, J.; Rhoades, J.; et al. Impact of Higher Cell-Free DNA Yields on Liquid Biopsy Testing in Glioblastoma Patients. Clin. Chem. 2025, 71, 215–225. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Galanis, E.; Weller, M. Glioblastoma. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 381–397. ISBN 978-0-12-802997-8. [Google Scholar]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid Biopsy: A Step Closer to Transform Diagnosis, Prognosis and Future of Cancer Treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Giordano, C.; Sabatino, G.; Romano, S.; Della Pepa, G.M.; Tufano, M.; D’Alessandris, Q.G.; Cottonaro, S.; Gessi, M.; Balducci, M.; Romano, M.F.; et al. Combining Magnetic Resonance Imaging with Systemic Monocyte Evaluation for the Implementation of GBM Management. Int. J. Mol. Sci. 2021, 22, 3797. [Google Scholar] [CrossRef]
- Bagley, S.J.; Nabavizadeh, S.A.; Mays, J.J.; Till, J.E.; Ware, J.B.; Levy, S.; Sarchiapone, W.; Hussain, J.; Prior, T.; Guiry, S.; et al. Clinical Utility of Plasma Cell-Free DNA in Adult Patients with Newly Diagnosed Glioblastoma: A Pilot Prospective Study. Clin. Cancer Res. 2020, 26, 397–407. [Google Scholar] [CrossRef]
- Senhaji, N.; Squalli Houssaini, A.; Lamrabet, S.; Louati, S.; Bennis, S. Molecular and Circulating Biomarkers in Patients with Glioblastoma. Int. J. Mol. Sci. 2022, 23, 7474. [Google Scholar] [CrossRef] [PubMed]
- Nikanjam, M.; Kato, S.; Kurzrock, R. Liquid Biopsy: Current Technology and Clinical Applications. J. Hematol. Oncol. 2022, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, Q.; Yang, J.; Zheng, Y.; Du, S.; Song, M.; Peng, Q.; Yang, R.; Liu, Y.; Qi, L. Imaging and Liquid Biopsy for Distinguishing True Progression From Pseudoprogression in Gliomas, Current Advances and Challenges. Acad. Radiol. 2024, 31, 3366–3383. [Google Scholar] [CrossRef] [PubMed]
- Balana, C.; Castañer, S.; Carrato, C.; Moran, T.; Lopez-Paradís, A.; Domenech, M.; Hernandez, A.; Puig, J. Preoperative Diagnosis and Molecular Characterization of Gliomas With Liquid Biopsy and Radiogenomics. Front. Neurol. 2022, 13, 865171. [Google Scholar] [CrossRef]
- Gao, F.; Cui, Y.; Jiang, H.; Sui, D.; Wang, Y.; Jiang, Z.; Zhao, J.; Lin, S. Circulating Tumor Cell Is a Common Property of Brain Glioma and Promotes the Monitoring System. Oncotarget 2016, 7, 71330–71340. [Google Scholar] [CrossRef]
- Lynch, D.; Powter, B.; Po, J.W.; Cooper, A.; Garrett, C.; Koh, E.-S.; Sheridan, M.; Van Gelder, J.; Darwish, B.; Mckechnie, S.; et al. Isolation of Circulating Tumor Cells from Glioblastoma Patients by Direct Immunomagnetic Targeting. Appl. Sci. 2020, 10, 3338. [Google Scholar] [CrossRef]
- Müller Bark, J.; Kulasinghe, A.; Hartel, G.; Leo, P.; Warkiani, M.E.; Jeffree, R.L.; Chua, B.; Day, B.W.; Punyadeera, C. Isolation of Circulating Tumour Cells in Patients With Glioblastoma Using Spiral Microfluidic Technology–A Pilot Study. Front. Oncol. 2021, 11, 681130. [Google Scholar] [CrossRef]
- Kolostova, K.; Pospisilova, E.; Pavlickova, V.; Bartos, R.; Sames, M.; Pawlak, I.; Bobek, V. Next Generation Sequencing of Glioblastoma Circulating Tumor Cells: Non-Invasive Solution for Disease Monitoring. Am. J. Transl. Res. 2021, 13, 4489–4499. [Google Scholar]
- Yang, J.; Song, J.; Huo, H.; Zhao, Y.; Zhang, G.; Zhao, Z.; Sun, G.; Jiao, B. DNM3, P65 and P53 from Exosomes Represent Potential Clinical Diagnosis Markers for Glioblastoma Multiforme. Ther. Adv. Med. Oncol. 2017, 9, 741–754. [Google Scholar] [CrossRef]
- Hallal, S.; Azimi, A.; Wei, H.; Ho, N.; Lee, M.Y.T.; Sim, H.-W.; Sy, J.; Shivalingam, B.; Buckland, M.E.; Alexander-Kaufman, K.L. A Comprehensive Proteomic SWATH-MS Workflow for Profiling Blood Extracellular Vesicles: A New Avenue for Glioma Tumour Surveillance. Int. J. Mol. Sci. 2020, 21, 4754. [Google Scholar] [CrossRef]
- Dobra, G.; Bukva, M.; Szabo, Z.; Bruszel, B.; Harmati, M.; Gyukity-Sebestyen, E.; Jenei, A.; Szucs, M.; Horvath, P.; Biro, T.; et al. Small Extracellular Vesicles Isolated from Serum May Serve as Signal-Enhancers for the Monitoring of CNS Tumors. Int. J. Mol. Sci. 2020, 21, 5359. [Google Scholar] [CrossRef] [PubMed]
- Cilibrasi, C.; Simon, T.; Vintu, M.; Tolias, C.; Samuels, M.; Mazarakis, N.K.; Eravci, M.; Stewart, N.; Critchley, G.; Giamas, G. Definition of an Inflammatory Biomarker Signature in Plasma-Derived Extracellular Vesicles of Glioblastoma Patients. Biomedicines 2022, 10, 125. [Google Scholar] [CrossRef]
- Hallal, S.M.; Tűzesi, Á.; Sida, L.A.; Xian, E.; Madani, D.; Muralidharan, K.; Shivalingam, B.; Buckland, M.E.; Satgunaseelan, L.; Alexander, K.L. Glioblastoma Biomarkers in Urinary Extracellular Vesicles Reveal the Potential for a ‘Liquid Gold’ Biopsy. Br. J. Cancer 2024, 130, 836–851. [Google Scholar] [CrossRef]
- Brahmer, A.; Geiß, C.; Lygeraki, A.; Neuberger, E.; Tzaridis, T.; Nguyen, T.T.; Luessi, F.; Régnier-Vigouroux, A.; Hartmann, G.; Simon, P.; et al. Assessment of Technical and Clinical Utility of a Bead-Based Flow Cytometry Platform for Multiparametric Phenotyping of CNS-Derived Extracellular Vesicles. Cell Commun. Signal. 2023, 21, 276. [Google Scholar] [CrossRef]
- Dufrusine, B.; Capone, E.; Ponziani, S.; Lattanzio, R.; Lanuti, P.; Giansanti, F.; De Laurenzi, V.; Iacobelli, S.; Ippoliti, R.; Mangiola, A.; et al. Extracellular LGALS3BP: A Potential Disease Marker and Actionable Target for Antibody–Drug Conjugate Therapy in Glioblastoma. Mol. Oncol. 2023, 17, 1460–1473. [Google Scholar] [CrossRef] [PubMed]
- Lennartz, P.; Thölke, D.; Bashiri Dezfouli, A.; Pilz, M.; Lobinger, D.; Messner, V.; Zanth, H.; Ainslie, K.; Kafshgari, M.H.; Rammes, G.; et al. Biomarkers in Adult-Type Diffuse Gliomas: Elevated Levels of Circulating Vesicular Heat Shock Protein 70 Serve as a Biomarker in Grade 4 Glioblastoma and Increase NK Cell Frequencies in Grade 3 Glioma. Biomedicines 2023, 11, 3235. [Google Scholar] [CrossRef]
- Werner, C.; Stangl, S.; Salvermoser, L.; Schwab, M.; Shevtsov, M.; Xanthopoulos, A.; Wang, F.; Dezfouli, A.B.; Thölke, D.; Ostheimer, C.; et al. Hsp70 in Liquid Biopsies-A Tumor-Specific Biomarker for Detection and Response Monitoring in Cancer. Cancers 2021, 13, 3706. [Google Scholar] [CrossRef] [PubMed]
- Aibaidula, A.; Fain, C.E.; Garcia, L.C.; Wier, A.; Bouchal, S.M.; Bauman, M.M.; Jung, M.-Y.; Sarkaria, J.N.; Johnson, A.J.; Parney, I.F. Spectral Flow Cytometry Identifies Distinct Nonneoplastic Plasma Extracellular Vesicle Phenotype in Glioblastoma Patients. Neuro-Oncol. Adv. 2023, 5, vdad082. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Wollmann, K.; Salviano-Silva, A.; Drexler, R.; Maire, C.L.; Kaul, M.G.; Reimer, R.; Schüller, U.; Heinemann, S.; Kolbe, K.; et al. Circulating Extracellular Vesicles as Biomarker for Diagnosis, Prognosis, and Monitoring in Glioblastoma Patients. Neuro-Oncol. 2024, 26, 1280–1291. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Skog, J.; Akers, J.; Li, H.; Komotar, R.; Jensen, R.; Ringel, F.; Yang, I.; Kalkanis, S.; Thompson, R.; et al. Detection of Wild-Type EGFR Amplification and EGFRvIII Mutation in CSF-Derived Extracellular Vesicles of Glioblastoma Patients. Neuro-Oncology 2017, 19, 1494–1502. [Google Scholar] [CrossRef]
- Hallal, S.; Ebrahim Khani, S.; Wei, H.; Lee, M.Y.T.; Sim, H.-W.; Sy, J.; Shivalingam, B.; Buckland, M.E.; Alexander-Kaufman, K.L. Deep Sequencing of Small RNAs from Neurosurgical Extracellular Vesicles Substantiates miR-486-3p as a Circulating Biomarker That Distinguishes Glioblastoma from Lower-Grade Astrocytoma Patients. Int. J. Mol. Sci. 2020, 21, 4954. [Google Scholar] [CrossRef] [PubMed]
- Yekula, A.; Hsia, T.; Kitchen, R.R.; Chakrabortty, S.K.; Yu, W.; Batool, S.M.; Lewis, B.; Szeglowski, A.J.; Weissleder, R.; Lee, H.; et al. Longitudinal Analysis of Serum-Derived Extracellular Vesicle RNA to Monitor Dacomitinib Treatment Response in EGFR-Amplified Recurrent Glioblastoma Patients. Neuro-Oncol. Adv. 2023, 5, vdad104. [Google Scholar] [CrossRef]
- Uziel, O.; Kanner, A.A.; Beery, E.; Lev, S.; Lahav, M.; Horn-Fichman, S.; Nof, S.H.; Laviv, Y.; Yust-Katz, S.; Amiel, A.; et al. Is Serum-derived Exosomal hTERT Transcript a Marker of Oncogenic Activity in Primary Brain Tumors? An Exploratory Study. Cancer Med. 2024, 13, e6784. [Google Scholar] [CrossRef] [PubMed]
- Manda, S.V.; Kataria, Y.; Tatireddy, B.R.; Ramakrishnan, B.; Ratnam, B.G.; Lath, R.; Ranjan, A.; Ray, A. Exosomes as a Biomarker Platform for Detecting Epidermal Growth Factor Receptor–Positive High-Grade Gliomas. J. Neurosurg. 2018, 128, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Hua, W.; Li, H.; Ramakrishnan, V.; Yang, Z.; Quan, K.; Zhu, W.; Li, J.; Figueroa, J.; Hirshman, B.R.; et al. A Cerebrospinal Fluid microRNA Signature as Biomarker for Glioblastoma. Oncotarget 2017, 8, 68769–68779. [Google Scholar] [CrossRef]
- Shi, R.; Wang, P.-Y.; Li, X.-Y.; Chen, J.-X.; Li, Y.; Zhang, X.-Z.; Zhang, C.-G.; Jiang, T.; Li, W.-B.; Ding, W.; et al. Exosomal Levels of miRNA-21 from Cerebrospinal Fluids Associated with Poor Prognosis and Tumor Recurrence of Glioma Patients. Oncotarget 2015, 6, 26971–26981. [Google Scholar] [CrossRef]
- Rosas-Alonso, R.; Colmenarejo-Fernández, J.; Pernía, O.; Burdiel, M.; Rodríguez-Antolín, C.; Losantos-García, I.; Rubio, T.; Moreno-Velasco, R.; Esteban-Rodríguez, I.; Martínez-Marín, V.; et al. Evaluation of the Clinical Use of MGMT Methylation in Extracellular Vesicle-Based Liquid Biopsy as a Tool for Glioblastoma Patient Management. Sci. Rep. 2024, 14, 11398. [Google Scholar] [CrossRef]
- Wang, Y.; Springer, S.; Zhang, M.; McMahon, K.W.; Kinde, I.; Dobbyn, L.; Ptak, J.; Brem, H.; Chaichana, K.; Gallia, G.L.; et al. Detection of Tumor-Derived DNA in Cerebrospinal Fluid of Patients with Primary Tumors of the Brain and Spinal Cord. Proc. Natl. Acad. Sci. USA 2015, 112, 9704–9709. [Google Scholar] [CrossRef]
- Miller, A.M.; Shah, R.H.; Pentsova, E.I.; Pourmaleki, M.; Briggs, S.; Distefano, N.; Zheng, Y.; Skakodub, A.; Mehta, S.A.; Campos, C.; et al. Tracking Tumour Evolution in Glioma through Liquid Biopsies of Cerebrospinal Fluid. Nature 2019, 565, 654–658. [Google Scholar] [CrossRef]
- Juratli, T.A.; Stasik, S.; Zolal, A.; Schuster, C.; Richter, S.; Daubner, D.; Juratli, M.A.; Thowe, R.; Hennig, S.; Makina, M.; et al. TERT Promoter Mutation Detection in Cell-Free Tumor-Derived DNA in Patients with IDH Wild-Type Glioblastomas: A Pilot Prospective Study. Clin. Cancer Res. 2018, 24, 5282–5291. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, C.; Li, M.; Shen, Y.; Feng, S.; Liu, J.; Li, F.; Hou, L.; Chen, Z.; Jiang, J.; et al. Applications of Cerebrospinal Fluid Circulating Tumor DNA in the Diagnosis of Gliomas. Jpn. J. Clin. Oncol. 2020, 50, 325–332. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, Q.; Wei, W.; Niu, W.; Liang, C.; Wang, X.; Wang, X.; Pan, H. Concordance Analysis of Cerebrospinal Fluid with the Tumor Tissue for Integrated Diagnosis in Gliomas Based on Next-Generation Sequencing. Pathol. Oncol. Res. 2023, 29, 1611391. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, D.E.; Achrol, A.S.; Kiedrowski, L.A.; Banks, K.C.; Boucher, N.; Barkhoudarian, G.; Kelly, D.F.; Juarez, T.; Lanman, R.B.; Raymond, V.M.; et al. Analysis of Cell-Free Circulating Tumor DNA in 419 Patients with Glioblastoma and Other Primary Brain Tumors. CNS Oncol. 2019, 8, CNS34. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal Fluid-Derived Circulating Tumour DNA Better Represents the Genomic Alterations of Brain Tumours than Plasma. Nat. Commun. 2015, 6, 8839. [Google Scholar] [CrossRef] [PubMed]
- Bagley, S.J.; Till, J.; Abdalla, A.; Sangha, H.K.; Yee, S.S.; Freedman, J.; Black, T.A.; Hussain, J.; Binder, Z.A.; Brem, S.; et al. Association of Plasma Cell-Free DNA with Survival in Patients with IDH Wild-Type Glioblastoma. Neuro-Oncol. Adv. 2021, 3, vdab011. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Suppiah, S.; Llinas, M.; Huang, Y.; Sahgal, A.; Perry, J.; Keith, J.; Davidson, B.; Hamani, C.; et al. MR-Guided Focused Ultrasound Liquid Biopsy Enriches Circulating Biomarkers in Patients with Brain Tumors. Neuro-Oncology 2021, 23, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, Z.; Zhu, Y.; Ma, L. Genome-Wide Methylation Analysis of Circulating Tumor DNA: A New Biomarker for Recurrent Glioblastom. Heliyon 2023, 9, e14339. [Google Scholar] [CrossRef]
- Lucero, R.; Zappulli, V.; Sammarco, A.; Murillo, O.D.; Cheah, P.S.; Srinivasan, S.; Tai, E.; Ting, D.T.; Wei, Z.; Roth, M.E.; et al. Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep. 2020, 30, 2065–2074.e4. [Google Scholar] [CrossRef]
- Drusco, A.; Bottoni, A.; Laganà, A.; Acunzo, M.; Fassan, M.; Cascione, L.; Antenucci, A.; Kumchala, P.; Vicentini, C.; Gardiman, M.P.; et al. A Differentially Expressed Set of microRNAs in Cerebro-Spinal Fluid (CSF) Can Diagnose CNS Malignancies. Oncotarget 2015, 6, 20829–20839. [Google Scholar] [CrossRef]
- Qu, K.; Lin, T.; Pang, Q.; Liu, T.; Wang, Z.; Tai, M.; Meng, F.; Zhang, J.; Wan, Y.; Mao, P.; et al. Extracellular miRNA-21 as a Novel Biomarker in Glioma: Evidence from Meta-Analysis, Clinical Validation and Experimental Investigations. Oncotarget 2016, 7, 33994–34010. [Google Scholar] [CrossRef]
- Ivo D’Urso, P.; Fernando D’Urso, O.; Damiano Gianfreda, C.; Mezzolla, V.; Storelli, C.; Marsigliante, S. miR-15b and miR-21 as Circulating Biomarkers for Diagnosis of Glioma. Curr. Genom. 2015, 16, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liao, K.; Wu, X.; Huang, J.; Zhang, S.; Lu, X. Serum microRNA-128 as a Biomarker for Diagnosis of Glioma. Int. J. Clin. Exp. Med. 2015, 8, 456–463. [Google Scholar] [PubMed]
- Regazzo, G.; Terrenato, I.; Spagnuolo, M.; Carosi, M.; Cognetti, G.; Cicchillitti, L.; Sperati, F.; Villani, V.; Carapella, C.; Piaggio, G.; et al. A Restricted Signature of Serum miRNAs Distinguishes Glioblastoma from Lower Grade Gliomas. J. Exp. Clin. Cancer Res. 2016, 35, 124. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Song, Z.; Guo, C.; Zhu, J.; Li, Z.; Zhu, S. Potential Diagnostic and Prognostic Value of Plasma Circulating MicroRNA-182 in Human Glioma. Med. Sci. Monit. 2016, 22, 855–862. [Google Scholar] [CrossRef]
- Lai, N.; Wu, D.; Fang, X.; Lin, Y.; Chen, S.; Li, Z.; Xu, S. Serum microRNA-210 as a Potential Noninvasive Biomarker for the Diagnosis and Prognosis of Glioma. Br. J. Cancer 2015, 112, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Wang, Z.; Ruan, C.; Wang, L.; Guo, H. Serum miR-100 Is a Potential Biomarker for Detection and Outcome Prediction of Glioblastoma Patients. Cancer Biomark. 2019, 24, 43–49. [Google Scholar] [CrossRef]
- Morokoff, A.; Jones, J.; Nguyen, H.; Ma, C.; Lasocki, A.; Gaillard, F.; Bennett, I.; Luwor, R.; Stylli, S.; Paradiso, L.; et al. Serum microRNA Is a Biomarker for Post-Operative Monitoring in Glioma. J. Neurooncol. 2020, 149, 391–400. [Google Scholar] [CrossRef]
- Yue, X.; Lan, F.; Hu, M.; Pan, Q.; Wang, Q.; Wang, J. Downregulation of Serum microRNA-205 as a Potential Diagnostic and Prognostic Biomarker for Human Glioma. J. Neurosurg. 2016, 124, 122–128. [Google Scholar] [CrossRef]
- Swellam, M.; Ezz El Arab, L.; Al-Posttany, A.S.; Said, S.B. Clinical Impact of Circulating Oncogenic MiRNA-221 and MiRNA-222 in Glioblastoma Multiform. J. Neurooncol. 2019, 144, 545–551. [Google Scholar] [CrossRef]
- Shao, N.; Wang, L.; Xue, L.; Wang, R.; Lan, Q. Plasma miR-454-3p as a Potential Prognostic Indicator in Human Glioma. Neurol. Sci. 2015, 36, 309–313. [Google Scholar] [CrossRef]
- Stella, M.; Falzone, L.; Caponnetto, A.; Gattuso, G.; Barbagallo, C.; Battaglia, R.; Mirabella, F.; Broggi, G.; Altieri, R.; Certo, F.; et al. Serum Extracellular Vesicle-Derived circHIPK3 and circSMARCA5 Are Two Novel Diagnostic Biomarkers for Glioblastoma Multiforme. Pharmaceuticals 2021, 14, 618. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhong, L.; Ju, K.; Lu, T.; Lv, J.; Cao, H. Plasmatic circRNA Predicting the Occurrence of Human Glioblastoma. Cancer Manag. Res. 2020, 12, 2917–2923. [Google Scholar] [CrossRef]
- Amer, R.G.; Ezz El Arab, L.R.; Abd El Ghany, D.; Saad, A.S.; Bahie-Eldin, N.; Swellam, M. Prognostic Utility of lncRNAs (LINC00565 and LINC00641) as Molecular Markers in Glioblastoma Multiforme (GBM). J. Neurooncol. 2022, 158, 435–444. [Google Scholar] [CrossRef]
- Xia, D.; Gu, X. Plasmatic Exosome-Derived circRNAs Panel Act as Fingerprint for Glioblastoma. Aging 2021, 13, 19575–19586. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, X.-K.; Li, J.-L.; Kong, K.-K.; Li, H.; Chen, C.; He, J.; Wang, F.; Li, P.; Ge, X.-S.; et al. MALAT1 Is a Prognostic Factor in Glioblastoma Multiforme and Induces Chemoresistance to Temozolomide through Suppressing miR-203 and Promoting Thymidylate Synthase Expression. Oncotarget 2017, 8, 22783–22799. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.; Neuhaus, N.; Dahlmann, M.; Kessler, A.; Kobelt, D.; Herrmann, P.; Eyrich, M.; Freitag, B.; Linsenmann, T.; Monoranu, C.; et al. Circulating MACC1 Transcripts in Glioblastoma Patients Predict Prognosis and Treatment Response. Cancers 2019, 11, 825. [Google Scholar] [CrossRef]
- Masood, A.B.; Batool, S.; Bhatti, S.N.; Ali, A.; Valko, M.; Jomova, K.; Kuca, K. Plasma PD-L1 as a Biomarker in the Clinical Management of Glioblastoma Multiforme—A Retrospective Cohort Study. Front. Immunol. 2023, 14, 1202098. [Google Scholar] [CrossRef]
- Tsvetkov, P.O.; Eyraud, R.; Ayache, S.; Bougaev, A.A.; Malesinski, S.; Benazha, H.; Gorokhova, S.; Buffat, C.; Dehais, C.; Sanson, M.; et al. An AI-Powered Blood Test to Detect Cancer Using NanoDSF. Cancers 2021, 13, 1294. [Google Scholar] [CrossRef]
- Soler, D.C.; Young, A.B.; Cooper, K.D.; Kerstetter-Fogle, A.; Barnholtz-Sloan, J.S.; Gittleman, H.; McCormick, T.S.; Sloan, A.E. The Ratio of HLA-DR and VNN2+ Expression on CD14+ Myeloid Derived Suppressor Cells Can Distinguish Glioblastoma from Radiation Necrosis Patients. J. Neurooncol. 2017, 134, 189–196. [Google Scholar] [CrossRef]
- Ghorbani, A.; Chatanaka, M.K.; Avery, L.M.; Wang, M.; Brown, J.; Cohen, R.; Gorham, T.; Misaghian, S.; Padmanabhan, N.; Romero, D.; et al. Glial Fibrillary Acidic Protein, Neurofilament Light, Matrix Metalloprotease 3 and Fatty Acid Binding Protein 4 as Non-Invasive Brain Tumor Biomarkers. Clin. Proteom. 2024, 21, 41. [Google Scholar] [CrossRef]
- Björkblom, B.; Wibom, C.; Jonsson, P.; Mörén, L.; Andersson, U.; Johannesen, T.B.; Langseth, H.; Antti, H.; Melin, B. Metabolomic Screening of Pre-Diagnostic Serum Samples Identifies Association between α- and γ-Tocopherols and Glioblastoma Risk. Oncotarget 2016, 7, 37043–37053. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Chen, Y.; Meng, Z.; Chu, Z. The Causal Relationship between CSF Metabolites and GBM: A Two-Sample Mendelian Randomization Analysis. BMC Cancer 2024, 24, 1119. [Google Scholar] [CrossRef]
- Zhao, H.; Heimberger, A.B.; Lu, Z.; Wu, X.; Hodges, T.R.; Song, R.; Shen, J. Metabolomics Profiling in Plasma Samples from Glioma Patients Correlates with Tumor Phenotypes. Oncotarget 2016, 7, 20486–20495. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Song, R.; Hodges, T.R.; Heimberger, A.B.; Zhao, H. Identification of Metabolites in Plasma for Predicting Survival in Glioblastoma. Mol. Carcinog. 2018, 57, 1078–1084. [Google Scholar] [CrossRef]
- Liu, Y.A.; Aboud, O.; Dahabiyeh, L.A.; Bloch, O.; Fiehn, O. Metabolomic Characterization of Human Glioblastomas and Patient Plasma: A Pilot Study. F1000Research 2024, 13, 98. [Google Scholar] [CrossRef]
- Muller Bark, J.; Karpe, A.V.; Doecke, J.D.; Leo, P.; Jeffree, R.L.; Chua, B.; Day, B.W.; Beale, D.J.; Punyadeera, C. A Pilot Study: Metabolic Profiling of Plasma and Saliva Samples from Newly Diagnosed Glioblastoma Patients. Cancer Med. 2023, 12, 11427–11437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ji, N.; Wang, G.; Zhang, Y.; Song, H.; Yuan, Y.; Yang, C.; Jin, Y.; Zhang, Z.; Zhang, L.; et al. Metabolic Detection of Malignant Brain Gliomas through Plasma Lipidomic Analysis and Support Vector Machine-Based Machine Learning. EBioMedicine 2022, 81, 104097. [Google Scholar] [CrossRef]
- Soylemez, B.; Bulut, Z.; Şahin-Bölükbaşı, S. Investigating the Potential of Lipids for Use as Biomarkers for Glioblastoma via an Untargeted Lipidomics Approach. J. Korean Neurosurg. Soc. 2023, 66, 133–143. [Google Scholar] [CrossRef]
- Sareen, H.; Garrett, C.; Lynch, D.; Powter, B.; Brungs, D.; Cooper, A.; Po, J.; Koh, E.-S.; Vessey, J.Y.; McKechnie, S.; et al. The Role of Liquid Biopsies in Detecting Molecular Tumor Biomarkers in Brain Cancer Patients. Cancers 2020, 12, 1831. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Lin, H.; Zhu, Y.; Huang, D.; Lai, M.; Xi, X.; Huang, J.; Zhang, W.; Zhong, T. Research Progress of CTC, ctDNA, and EVs in Cancer Liquid Biopsy. Front. Oncol. 2024, 14, 1303335. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef]
- Müller Bark, J.; Kulasinghe, A.; Chua, B.; Day, B.W.; Punyadeera, C. Circulating Biomarkers in Patients with Glioblastoma. Br. J. Cancer 2020, 122, 295–305. [Google Scholar] [CrossRef]
- Hassan, S. Applications of RNA from Circulating Tumor Cells. Front. Biosci. 2020, 25, 874–892. [Google Scholar] [CrossRef] [PubMed]
- Lessi, F.; Morelli, M.; Franceschi, S.; Aretini, P.; Menicagli, M.; Marranci, A.; Pasqualetti, F.; Gambacciani, C.; Pieri, F.; Grimod, G.; et al. Innovative Approach to Isolate and Characterize Glioblastoma Circulating Tumor Cells and Correlation with Tumor Mutational Status. Int. J. Mol. Sci. 2023, 24, 10147. [Google Scholar] [CrossRef]
- Adamczyk, L.A.; Williams, H.; Frankow, A.; Ellis, H.P.; Haynes, H.R.; Perks, C.; Holly, J.M.P.; Kurian, K.M. Current Understanding of Circulating Tumor Cells–Potential Value in Malignancies of the Central Nervous System. Front. Neurol. 2015, 6, 174. [Google Scholar] [CrossRef]
- Indira Chandran, V.; Gopala, S.; Venkat, E.H.; Kjolby, M.; Nejsum, P. Extracellular Vesicles in Glioblastoma: A Challenge and an Opportunity. Npj Precis. Oncol. 2024, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, M.; Osti, D.; Faletti, S.; Beznoussenko, G.V.; DiMeco, F.; Pelicci, G. Extracellular Vesicles: The Key for Precision Medicine in Glioblastoma. Neuro-Oncology 2022, 24, 184–196. [Google Scholar] [CrossRef]
- Hu, C.; Jiang, W.; Lv, M.; Fan, S.; Lu, Y.; Wu, Q.; Pi, J. Potentiality of Exosomal Proteins as Novel Cancer Biomarkers for Liquid Biopsy. Front. Immunol. 2022, 13, 792046. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a New Frontier of Cancer Liquid Biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Yin, K.; Liu, X. CircMMP1 Promotes the Progression of Glioma through miR-433/HMGB3 Axis in Vitro and in Vivo. IUBMB Life 2020, 72, 2508–2524. [Google Scholar] [CrossRef]
- Khristov, V.; Lin, A.; Freedman, Z.; Staub, J.; Shenoy, G.; Mrowczynski, O.; Rizk, E.; Zacharia, B.; Connor, J. Tumor-Derived Biomarkers in Liquid Biopsy of Glioblastoma. World Neurosurg. 2023, 170, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Mafi, A.; Rahmati, A.; Babaei Aghdam, Z.; Salami, R.; Salami, M.; Vakili, O.; Aghadavod, E. Recent Insights into the microRNA-Dependent Modulation of Gliomas from Pathogenesis to Diagnosis and Treatment. Cell. Mol. Biol. Lett. 2022, 27, 65. [Google Scholar] [CrossRef]
- Ali, H.; Harting, R.; De Vries, R.; Ali, M.; Wurdinger, T.; Best, M.G. Blood-Based Biomarkers for Glioma in the Context of Gliomagenesis: A Systematic Review. Front. Oncol. 2021, 11, 665235. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Vishnu, V.Y. Lumbar Puncture: Indications, Challenges and Recent Advances. Neurology 2021, 17, 23. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small Molecule Metabolites: Discovery of Biomarkers and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Adhit, K.K.; Wanjari, A.; Menon, S.; Siddhaarth, K. Liquid Biopsy: An Evolving Paradigm for Non-Invasive Disease Diagnosis and Monitoring in Medicine. Cureus 2023, 15, e50176. [Google Scholar] [CrossRef]
- Skouras, P.; Markouli, M.; Kalamatianos, T.; Stranjalis, G.; Korkolopoulou, P.; Piperi, C. Advances on Liquid Biopsy Analysis for Glioma Diagnosis. Biomedicines 2023, 11, 2371. [Google Scholar] [CrossRef]
- Donnelly, D.; Aung, P.P.; Jour, G. The “-OMICS” Facet of Melanoma: Heterogeneity of Genomic, Proteomic and Metabolomic Biomarkers. Semin. Cancer Biol. 2019, 59, 165–174. [Google Scholar] [CrossRef]
- Yaghoubi Naei, V.; Bordhan, P.; Mirakhorli, F.; Khorrami, M.; Shrestha, J.; Nazari, H.; Kulasinghe, A.; Ebrahimi Warkiani, M. Advances in Novel Strategies for Isolation, Characterization, and Analysis of CTCs and ctDNA. Ther. Adv. Med. Oncol. 2023, 15, 17588359231192401. [Google Scholar] [CrossRef]
- Fujita, Y.; Nunez-Rubiano, L.; Dono, A.; Bellman, A.; Shah, M.; Rodriguez, J.C.; Putluri, V.; Kamal, A.H.M.; Putluri, N.; Riascos, R.F.; et al. IDH1 p.R132H ctDNA and D-2-Hydroxyglutarate as CSF Biomarkers in Patients with IDH-Mutant Gliomas. J. Neurooncol. 2022, 159, 261–270. [Google Scholar] [CrossRef]
- Saenz-Antoñanzas, A.; Auzmendi-Iriarte, J.; Carrasco-Garcia, E.; Moreno-Cugnon, L.; Ruiz, I.; Villanua, J.; Egaña, L.; Otaegui, D.; Samprón, N.; Matheu, A. Liquid Biopsy in Glioblastoma: Opportunities, Applications and Challenges. Cancers 2019, 11, 950. [Google Scholar] [CrossRef] [PubMed]
- Sievänen, H.; Kari, J.; Eskola, V.; Huurre, A.; Soukka, H.; Palmu, S. Incidence of Traumatic Lumbar Punctures in Adults: The Impact of a Patient’s First Procedure. Clin. Med. 2023, 23, 31–37. [Google Scholar] [CrossRef]
- Kim, K.T. Lumbar Puncture: Considerations, Procedure, and Complications. Encephalitis 2022, 2, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.W. Complications of Lumbar Puncture. Neurol. Clin. 1998, 16, 83–105. [Google Scholar] [CrossRef]
- Katzendobler, S.; Do, A.; Weller, J.; Dorostkar, M.M.; Albert, N.L.; Forbrig, R.; Niyazi, M.; Egensperger, R.; Thon, N.; Tonn, J.C.; et al. Diagnostic Yield and Complication Rate of Stereotactic Biopsies in Precision Medicine of Gliomas. Front. Neurol. 2022, 13, 822362. [Google Scholar] [CrossRef] [PubMed]
- Sabedot, T.S.; Malta, T.M.; Snyder, J.; Nelson, K.; Wells, M.; deCarvalho, A.C.; Mukherjee, A.; Chitale, D.A.; Mosella, M.S.; Sokolov, A.; et al. A Serum-Based DNA Methylation Assay Provides Accurate Detection of Glioma. Neuro-Oncology 2021, 23, 1494–1508. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, X.; Xie, P.; Zhang, W. Liquid Biopsy: An Arsenal for Tumour Screening and Early Diagnosis. Cancer Treat. Rev. 2024, 129, 102774. [Google Scholar] [CrossRef]
- Gatto, L.; Franceschi, E.; Di Nunno, V.; Tosoni, A.; Lodi, R.; Brandes, A.A. Liquid Biopsy in Glioblastoma Management: From Current Research to Future Perspectives. Oncologist 2021, 26, 865–878. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).