Tannic Acid and Ethacridine Lactate Attenuate Markers of Stress-Induced Intestinal Barrier Dysfunctions in Murine Small Intestinal Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Organoid Cell Culture
2.1.1. Isolation and Cultivation
2.1.2. Medium Change and Splitting
2.2. Determination of Tannic Acid (TA) and Ethacridine Lactate (Eta) Concentration
2.3. Establishing Methods for Organoid Stress
2.4. Exposure to Pharmacologic Molecules
2.5. RNA Isolation, Generation of Standard Plasmids and RT-PCR
2.6. Statistical Analysis
3. Results
3.1. Dose-Finding Studies
3.2. GFRed and LPS Exposure Induce Proinflammatory Cytokine Transcripts Expression in Small Intestinal Organoids
3.3. TA and Eta Stimulation Improve Stress-Induced Myd88 and Cytokine Transcript Expression
3.4. TA and Eta Attenuate GFRed- and LPS-Induced Gut Barrier Dysfunctions
3.4.1. Stimulation with TA and Eta Improve TJ and Muc Gene Expression in Stressed Organoids
3.4.2. TA and Eta Regulate Antimicrobial Peptide Gene Expression in GFRed- and LPS-Treated Organoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Eta | Ethacridine lactate |
| TA | Tannic acid |
| LPS | Lipopolysaccharide |
| GFRed | CCM with reduced growth factors |
| GI | Gastrointestinal |
| TJ | Tight junction |
| AJ | Adherent junction |
| IBD | Inflammatory bowel disease |
| CD | Crohn’s disease |
| Ocln | Occludin |
| ZO-1 | Zonula occludens 1 |
| Cldn | Claudin |
| AMP | Antimicrobial Peptide |
| TLR | Toll-like receptor |
| SCFA | Short-chain fatty acid |
| CIB | Crypt isolation buffer |
| CCM | Cell culture medium |
| IL | Interleukin |
| Defa | α-defensin |
| mBD1 | Murine β-defensin 1 |
| Lyz1 | Lysozyme |
| Reg3γ | Regenerating islet-derived protein 3 gamma |
| Mmp7 | Matrix metalloproteinase-7 |
| JAM-A | Junctional adhesion molecule A |
| Muc | Mucin |
| Myd88 | Myeloid differentiation primary response 88 |
| Tnfα | Tumor necrosis factor α |
| ANOVA | One-way analysis of variance |
| SEM | Standard error of the mean |
| Ctrl | PBSO control |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B-cells () |
| CXCL12 | CXC motif chemokine 12 |
| NO | Nitric oxide |
| PGE2 | Prostaglandin 2 |
| OA | Osteoarthritis |
| MAPK | Mitogen-activated protein kinases |
| INF-γ | Interferon-γ |
| HCN | Hepatitis C virus |
References
- Salama-Müller, A.; Roese, N. Antidiarrheal Properties of the Combination of Tannin Albuminate and Ethacridine Lactate—A Narrative Review. Nat. Prod. Commun. 2023, 18, 1934578X2311709. [Google Scholar] [CrossRef]
- Ziegenhagen, D.J.; Raedsch, R.; Kruis, W. Reisediarrhö in der Türkei. Prospektiv randomisierter Therapievergleich Kohle versus Tanninalbuminat/Ethacridinlactat. Med. Klin. 1992, 87, 637–639. [Google Scholar] [PubMed]
- Raedsch, R.; Walter-Sack, I.; Galle, P.R.; Kommerell, B. Prophylaxis of traveler’s diarrhea in Egypt: Results of a double blind controlled study. Klin. Wochenschr. 1991, 69, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Elham, A.; Arken, M.; Kalimanjan, G.; Arkin, A.; Iminjan, M. A review of the phytochemical, pharmacological, pharmacokinetic, and toxicological evaluation of Quercus Infectoria galls. J. Ethnopharmacol. 2021, 273, 113592. [Google Scholar] [CrossRef]
- Adamczyk, B.; Simon, J.; Kitunen, V.; Adamczyk, S.; Smolander, A. Tannins and Their Complex Interaction with Different Organic Nitrogen Compounds and Enzymes: Old Paradigms versus Recent Advances. ChemistryOpen 2017, 6, 610–614. [Google Scholar] [CrossRef]
- Hamiza, O.O.; Rehman, M.U.; Tahir, M.; Khan, R.; Khan, A.Q.; Lateef, A.; Ali, F.; Sultana, S. Amelioration of 1,2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac. J. Cancer Prev. 2012, 13, 4393–4402. [Google Scholar] [CrossRef]
- Jabri, T.; Khan, N.A.; Makhlouf, Z.; Akbar, N.; Gul, J.; Shah, M.R.; Siddiqui, R. Antibacterial Properties of Ethacridine Lactate and Sulfmethoxazole Loaded Functionalized Graphene Oxide Nanocomposites. Antibiotics 2023, 12, 755. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Liu, S.; Zhuang, Y.; Yang, H.; Li, Y.; Chen, S.; Wang, L.; Yin, L.; Yao, Y.; et al. Tannic acid modulates intestinal barrier functions associated with intestinal morphology, antioxidative activity, and intestinal tight junction in a diquat-induced mouse model. RSC Adv. 2019, 9, 31988–31998. [Google Scholar] [CrossRef]
- Wang, K.; Ding, Y.; Xu, C.; Hao, M.; Li, H.; Ding, L. Cldn-7 deficiency promotes experimental colitis and associated carcinogenesis by regulating intestinal epithelial integrity. Oncoimmunology 2021, 10, 1923910. [Google Scholar] [CrossRef]
- Yin, W.; Liu, M.; Jin, Z.; Hao, Z.; Liu, C.; Liu, J.; Liu, H.; Zheng, M.; Cai, D. Ameliorative effects of insoluble dietary fiber and its bound polyphenols from adzuki bean seed coat on acute murine colitis induced by DSS: The inflammatory response, intestinal barrier and gut microbiota. Int. J. Biol. Macromol. 2025, 286, 138343. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.H.; Oh, T.Y.; Hong, S.P.; Jeon, T.J.; Kim, C.H.; Park, S.W.; Chung, J.B.; Song, S.Y.; Bang, S. Up-regulated claudin 7 expression in intestinal-type gastric carcinoma. Oncol. Rep. 2007, 18, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, J.; Xia, Y.; Wang, J.; Xia, L.-N. Mechanistic study of the effect of a high-salt diet on the intestinal barrier. Sci. Rep. 2025, 15, 3826. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Filipe Rosa, L.; Kaden-Volynets, V.; Stolzer, I.; Günther, C.; Bischoff, S.C. Prebiotic Inulin and Sodium Butyrate Attenuate Obesity-Induced Intestinal Barrier Dysfunction by Induction of Antimicrobial Peptides. Front. Immunol. 2021, 12, 678360. [Google Scholar] [CrossRef] [PubMed]
- Reichold, A.; Brenner, S.A.; Förster-Fromme, K.; Bergheim, I.; Mollenhauer, J.; Bischoff, S.C. Dmbt1 does not affect a Western style diet-induced liver damage in mice. J. Clin. Biochem. Nutr. 2013, 53, 145–149. [Google Scholar] [CrossRef]
- Thuy, S.; Ladurner, R.; Volynets, V.; Wagner, S.; Strahl, S.; Königsrainer, A.; Maier, K.-P.; Bischoff, S.C.; Bergheim, I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J. Nutr. 2008, 138, 1452–1455. [Google Scholar] [CrossRef]
- Spruss, A.; Kanuri, G.; Wagnerberger, S.; Haub, S.; Bischoff, S.C.; Bergheim, I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009, 50, 1094–1104. [Google Scholar] [CrossRef]
- Xing, T.; Benderman, L.J.; Sabu, S.; Parker, J.; Yang, J.; Lu, Q.; Ding, L.; Chen, Y.-H. Tight Junction Protein Claudin-7 Is Essential for Intestinal Epithelial Stem Cell Self-Renewal and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 641–659. [Google Scholar] [CrossRef]
- Lueschow-Guijosa, S.R.; Stanford, A.H.; Amy, H.; Berger, J.N.; Gong, H.; Boly, T.J.; Jensen, B.A.H.; Nordkild, P.; Leegwater, A.J.; Wehkamp, J.; et al. Host defense peptides human β defensin 2 and LL-37 ameliorate murine necrotizing enterocolitis. iScience 2024, 27, 109993. [Google Scholar] [CrossRef]
- Ouellette, A.J. Defensin-mediated innate immunity in the small intestine. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 405–419. [Google Scholar] [CrossRef]
- George, M.D.; Wehkamp, J.; Kays, R.J.; Leutenegger, C.M.; Sabir, S.; Grishina, I.; Dandekar, S.; Bevins, C.L. In vivo gene expression profiling of human intestinal epithelial cells: Analysis by laser microdissection of formalin fixed tissues. BMC Genom. 2008, 9, 209. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Koeninger, L.; Armbruster, N.S.; Brinch, K.S.; Kjaerulf, S.; Andersen, B.; Langnau, C.; Autenrieth, S.E.; Schneidawind, D.; Stange, E.F.; Malek, N.P.; et al. Human β-Defensin 2 Mediated Immune Modulation as Treatment for Experimental Colitis. Front. Immunol. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Beisner, J.; Teltschik, Z.; Ostaff, M.J.; Tiemessen, M.M.; Staal, F.J.T.; Wang, G.; Gersemann, M.; Perminow, G.; Vatn, M.H.; Schwab, M.; et al. TCF-1-mediated Wnt signaling regulates Paneth cell innate immune defense effectors HD-5 and -6: Implications for Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G487–G498. [Google Scholar] [CrossRef]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Shen, B.; Schaeffeler, E.; Schwab, M.; Linzmeier, R.; et al. Reduced Paneth cell alpha-defensins in ileal Crohn’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 18129–18134. [Google Scholar] [CrossRef]
- Palrasu, M.; Kakar, K.; Marudamuthu, A.; Hamida, H.; Thada, S.; Zhong, Y.; Staley, S.; Busbee, P.B.; Li, J.; Garcia-Buitrago, M.; et al. AhR Activation Transcriptionally Induces Anti-Microbial Peptide Alpha-Defensin 1 Leading to Reversal of Gut Microbiota Dysbiosis and Colitis. Gut Microbes 2025, 17, 2460538. [Google Scholar] [CrossRef]
- Hrdý, J.; Couturier-Maillard, A.; Boutillier, D.; Lapadatescu, C.; Blanc, P.; Procházka, J.; Pot, B.; Ryffel, B.; Grangette, C.; Chamaillard, M. Oral supplementation with selected Lactobacillus acidophilus triggers IL-17-dependent innate defense response, activation of innate lymphoid cells type 3 and improves colitis. Sci. Rep. 2022, 12, 17591. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. BioMed Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef]
- Volynets, V.; Louis, S.; Pretz, D.; Lang, L.; Ostaff, M.J.; Wehkamp, J.; Bischoff, S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017, 147, 770–780. [Google Scholar] [CrossRef]
- Courth, L.F.; Ostaff, M.J.; Mailänder-Sánchez, D.; Malek, N.P.; Stange, E.F.; Wehkamp, J. Crohn’s disease-derived monocytes fail to induce Paneth cell defensins. Proc. Natl. Acad. Sci. USA 2015, 112, 14000–14005. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Liang, S.; Guo, X.-K.; Hu, X. α-Defensins Promote Bacteroides Colonization on Mucosal Reservoir to Prevent Antibiotic-Induced Dysbiosis. Front. Immunol. 2020, 11, 2065. [Google Scholar] [CrossRef] [PubMed]
- Shawky, L.M.; Abo El Wafa, S.M.; Behery, M.; Bahr, M.H.; Abu Alnasr, M.T.; Morsi, A.A. Lactobacillus rhamnosus GG and Tannic Acid Synergistically Promote the Gut Barrier Integrity in a Rat Model of Experimental Diarrhea via Selective Immunomodulatory Cytokine Targeting. Mol. Nutr. Food Res. 2024, 68, e2400295. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Lerma-Aguilera, A.; Jiménez-Hernández, N.; Gosalbes, M.J.; Rufián-Henares, J.Á.; Francino, M.P. Enrichment of Food With Tannin Extracts Promotes Healthy Changes in the Human Gut Microbiota. Front. Microbiol. 2021, 12, 625782. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Constabel, C.P.; Yoshida, K.; Walker, V. Diverse Ecological Roles of Plant Tannins: Plant Defense and Beyond. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 115–142. ISBN 9781118329672. [Google Scholar]
- Ashok, P.K.; Upadhyaya, K. Tannins are Astringent. J. Pharmacogn. Phytochem. 2012, 1, 45–50. [Google Scholar]
- Frasca, G.; Cardile, V.; Puglia, C.; Bonina, C.; Bonina, F. Gelatin tannate reduces the proinflammatory effects of lipopolysaccharide in human intestinal epithelial cells. Clin. Exp. Gastroenterol. 2012, 5, 61–67. [Google Scholar]
- Wu, Y.; Zhong, L.; Yu, Z.; Qi, J. Anti-neuroinflammatory effects of tannic acid against lipopolysaccharide-induced BV2 microglial cells via inhibition of NF-κB activation. Drug Dev. Res. 2019, 80, 262–268. [Google Scholar] [CrossRef]
- Chen, X.; Beutler, J.A.; McCloud, T.G.; Loehfelm, A.; Yang, L.; Dong, H.-F.; Chertov, O.Y.; Salcedo, R.; Oppenheim, J.J.; Howard, O.M.Z. Tannic Acid Is an Inhibitor of CXCL12 (SDF-1α)/CXCR4 with Antiangiogenic Activity1. Clin. Cancer Res. 2003, 9, 3115–3123. [Google Scholar]
- de Veras, B.O.; da Silva, M.V.; Ribeiro, P.P.C. Tannic acid is a gastroprotective that regulates inflammation and oxidative stress. Food Chem. Toxicol. 2021, 156, 112482. [Google Scholar] [CrossRef]
- Lee, H.-R.; Jeong, Y.-J.; Lee, J.-W.; Jhun, J.; Na, H.S.; Cho, K.-H.; Kim, S.J.; Cho, M.-L.; Heo, T.-H. Tannic acid, an IL-1β-direct binding compound, ameliorates IL-1β-induced inflammation and cartilage degradation by hindering IL-1β-IL-1R1 interaction. PLoS ONE 2023, 18, e0281834. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M.; Korbecka-Paczkowska, M.; Stasiewicz, M.; Mrozikiewicz, A.E.; Włodkowic, D.; Cielecka-Piontek, J. Activity of Antiseptics Against Pseudomonas aeruginosa and Its Adaptation Potential. Antibiotics 2025, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lidsky, P.V.; Xiao, Y.; Wu, C.-T.; Garcia-Knight, M.; Yang, J.; Nakayama, T.; Nayak, J.V.; Jackson, P.K.; Andino, R.; et al. Ethacridine inhibits SARS-CoV-2 by inactivating viral particles. PLoS Pathog. 2021, 17, e1009898. [Google Scholar] [CrossRef] [PubMed]
- van Ampting, M.T.J.; Schonewille, A.J.; Vink, C.; Brummer, R.J.M.; van der Meer, R.; Bovee-Oudenhoven, I.M.J. Damage to the intestinal epithelial barrier by antibiotic pretreatment of salmonella-infected rats is lessened by dietary calcium or tannic acid. J. Nutr. 2010, 140, 2167–2172. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Wang, L.; Yin, L.; Yang, H.; Chen, C.; Zheng, Q.; He, S. Tannic acid attenuates intestinal oxidative damage by improving antioxidant capacity and intestinal barrier in weaned piglets and IPEC-J2 cells. Front. Nutr. 2022, 9, 1012207. [Google Scholar] [CrossRef]
- Kakogiannos, N.; Ferrari, L.; Giampietro, C.; Scalise, A.A.; Maderna, C.; Ravà, M.; Taddei, A.; Lampugnani, M.G.; Pisati, F.; Malinverno, M.; et al. JAM-A Acts via C/EBP-α to Promote Claudin-5 Expression and Enhance Endothelial Barrier Function. Circ. Res. 2020, 127, 1056–1073. [Google Scholar] [CrossRef]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Tsai, P.-Y.; Zhang, B.; He, W.-Q.; Zha, J.-M.; Odenwald, M.A.; Singh, G.; Tamura, A.; Shen, L.; Sailer, A.; Yeruva, S.; et al. IL-22 Upregulates Epithelial Claudin-2 to Drive Diarrhea and Enteric Pathogen Clearance. Cell Host Microbe 2017, 21, 671–681.e4. [Google Scholar] [CrossRef]
- Aujla, S.J.; Chan, Y.R.; Zheng, M.; Fei, M.; Askew, D.J.; Pociask, D.A.; Reinhart, T.A.; McAllister, F.; Edeal, J.; Gaus, K.; et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008, 14, 275–281. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Nunes, D.P.; Troxler, R.F.; Offner, G.D. Pro-inflammatory cytokines up-regulate MUC1 gene expression in oral epithelial cells. J. Dent. Res. 2003, 82, 883–887. [Google Scholar] [CrossRef]
- Wang, W.; Cao, J.; Yang, J.; Niu, X.; Liu, X.; Zhai, Y.; Qiang, C.; Niu, Y.; Li, Z.; Dong, N.; et al. Antimicrobial Activity of Tannic Acid In Vitro and Its Protective Effect on Mice against Clostridioides difficile. Microbiol. Spectr. 2023, 11, e0261822. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-T.; Lu, Z.; Chou, M.W. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Dai, Y.-C.; Zhong, W.; Tan, M.; Lv, Z.-P.; Zhou, Y.-C.; Jiang, X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 Chinese medicinal herbs. Bioorg. Med. Chem. 2012, 20, 1616–1623. [Google Scholar] [CrossRef]
- Wang, S.-C.; Chen, Y.; Wang, Y.-C.; Wang, W.-J.; Yang, C.-S.; Tsai, C.-L.; Hou, M.-H.; Chen, H.-F.; Shen, Y.-C.; Hung, M.-C. Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am. J. Cancer Res. 2020, 10, 4538–4546. [Google Scholar]
- Tintino, S.R.; Morais-Tintino, C.D.; Campina, F.F.; Costa, M.d.S.; Menezes, I.R.A.; de Matos, Y.M.L.S.; Calixto-Júnior, J.T.; Pereira, P.S.; Siqueira-Junior, J.P.; Leal-Balbino, T.C.; et al. Tannic acid affects the phenotype of Staphylococcus aureus resistant to tetracycline and erythromycin by inhibition of efflux pumps. Bioorg. Chem. 2017, 74, 197–200. [Google Scholar] [CrossRef]
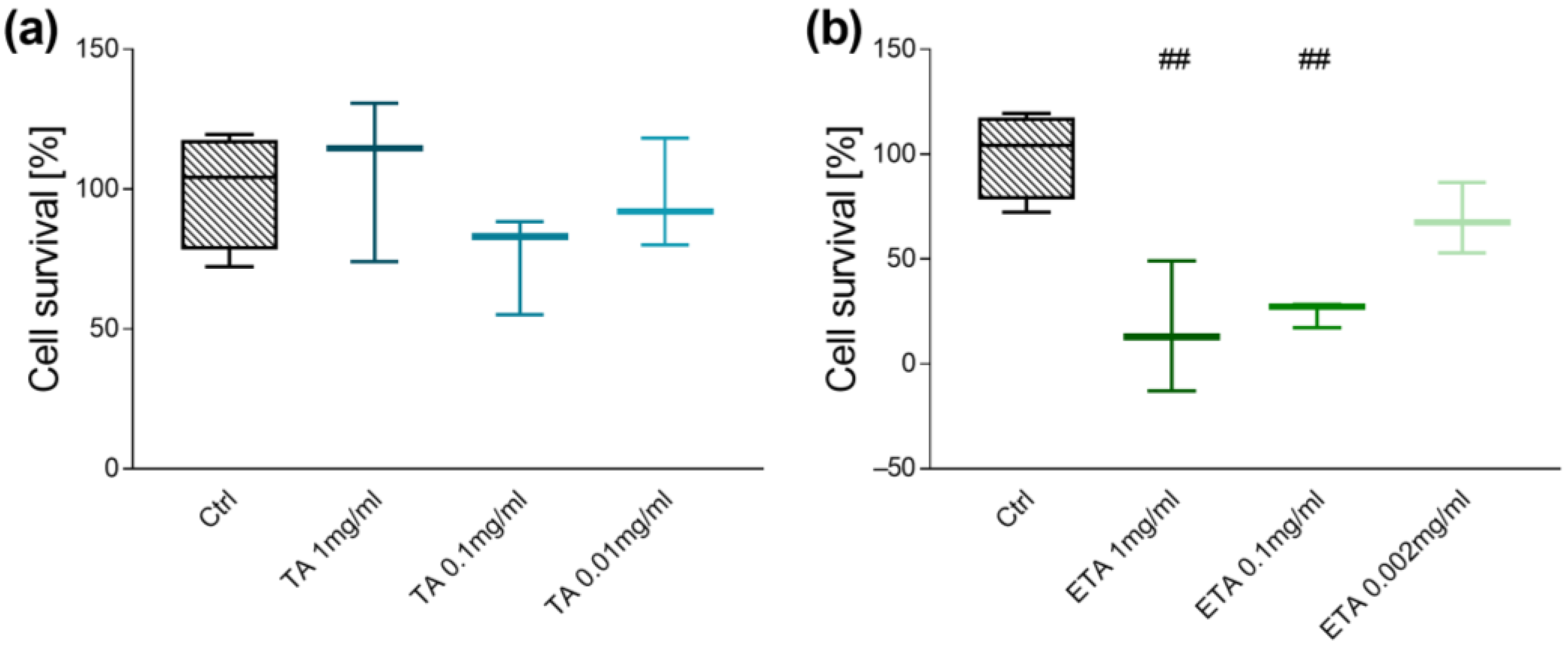
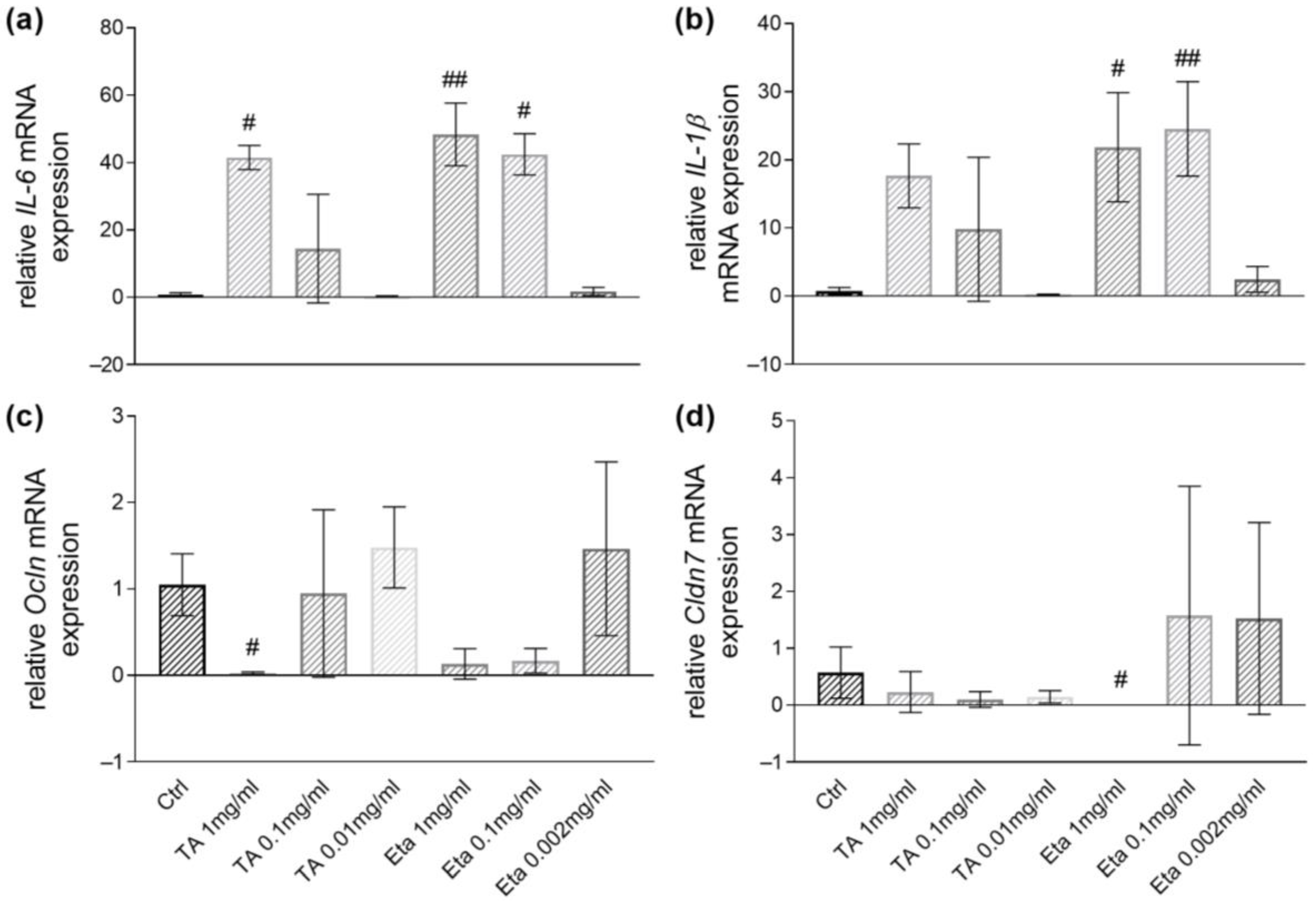
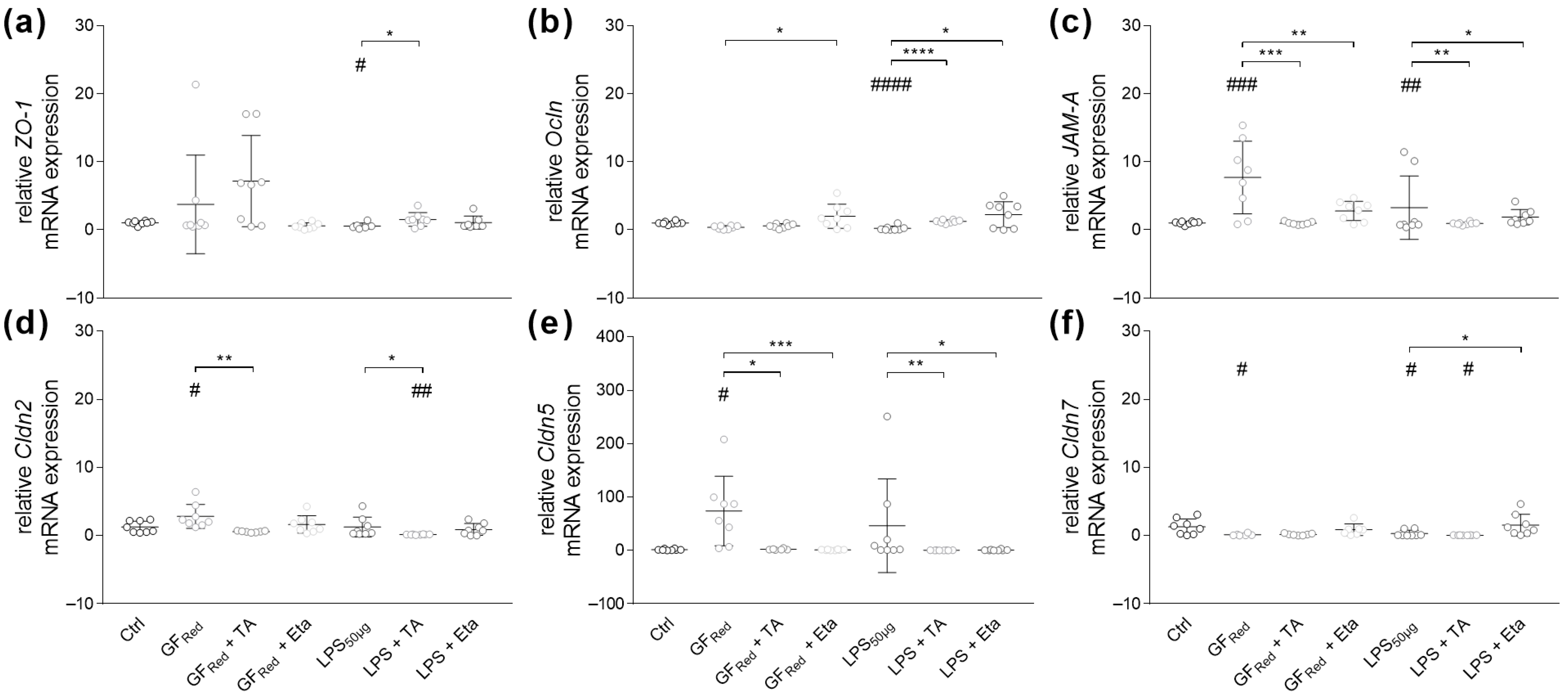
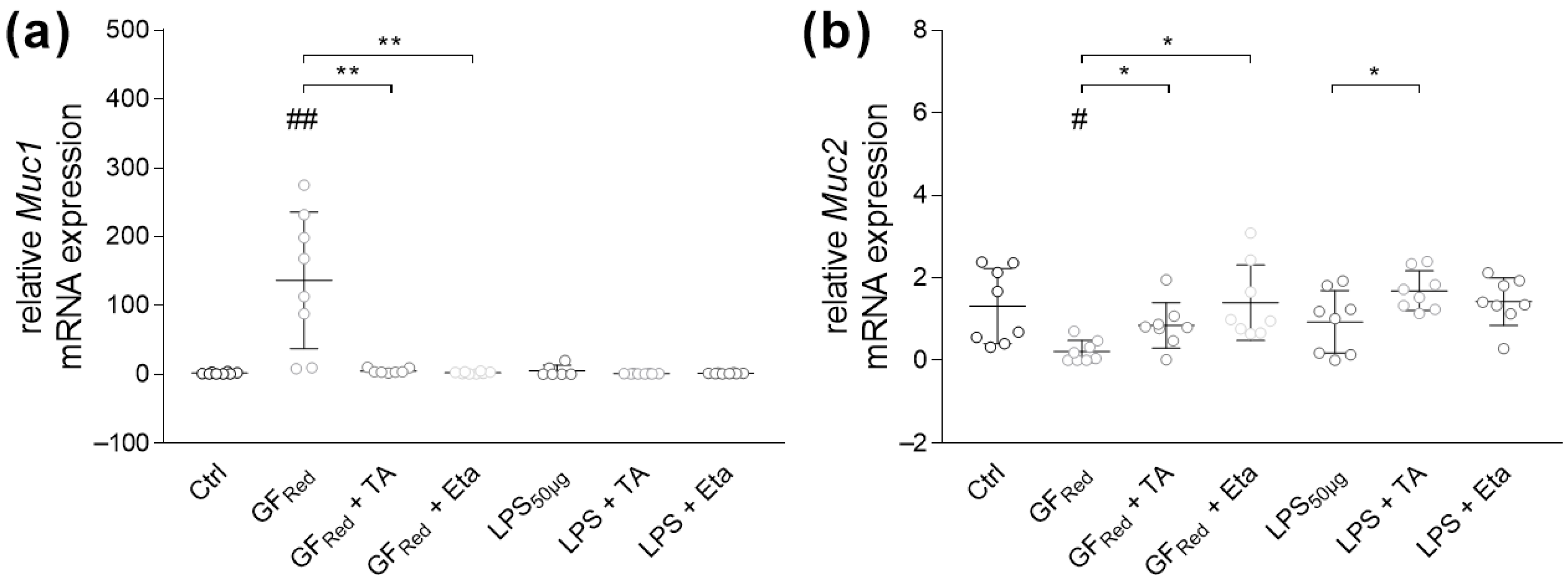
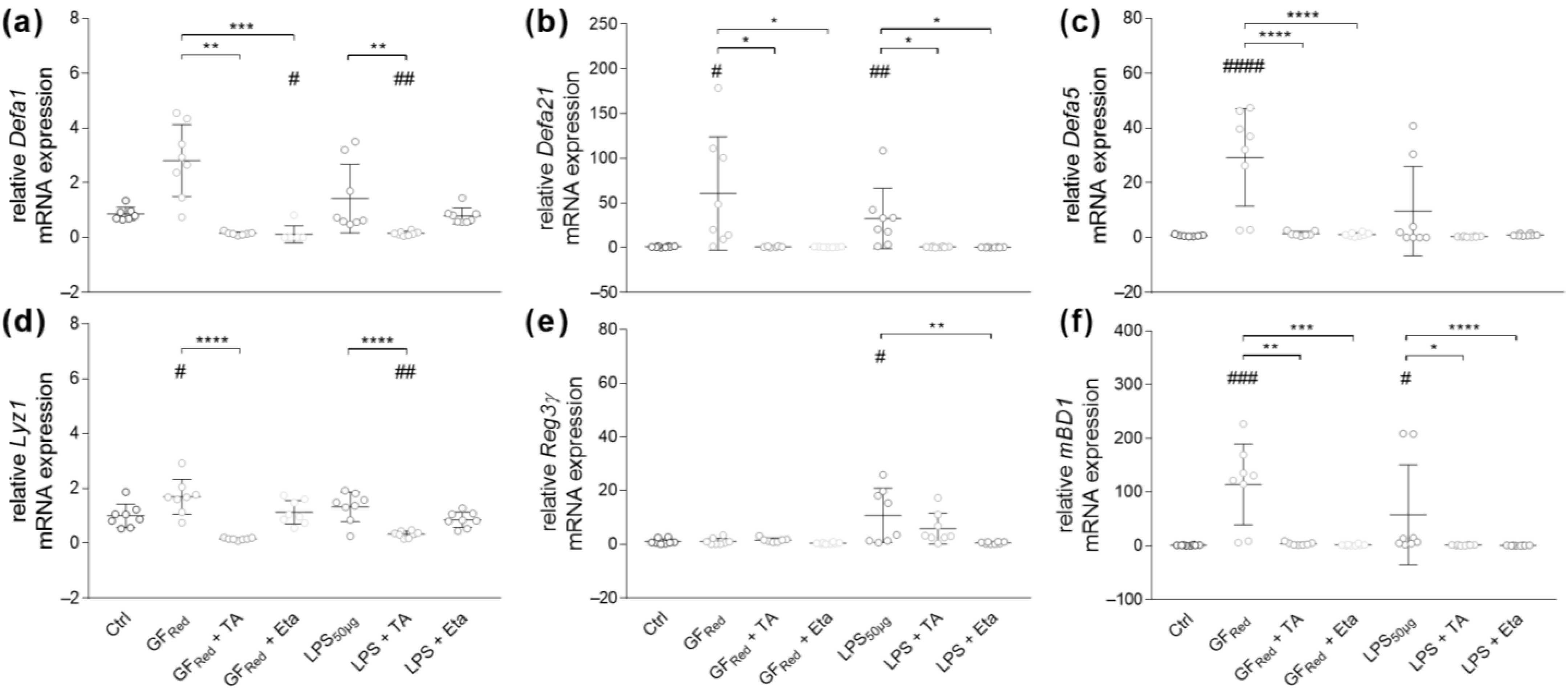
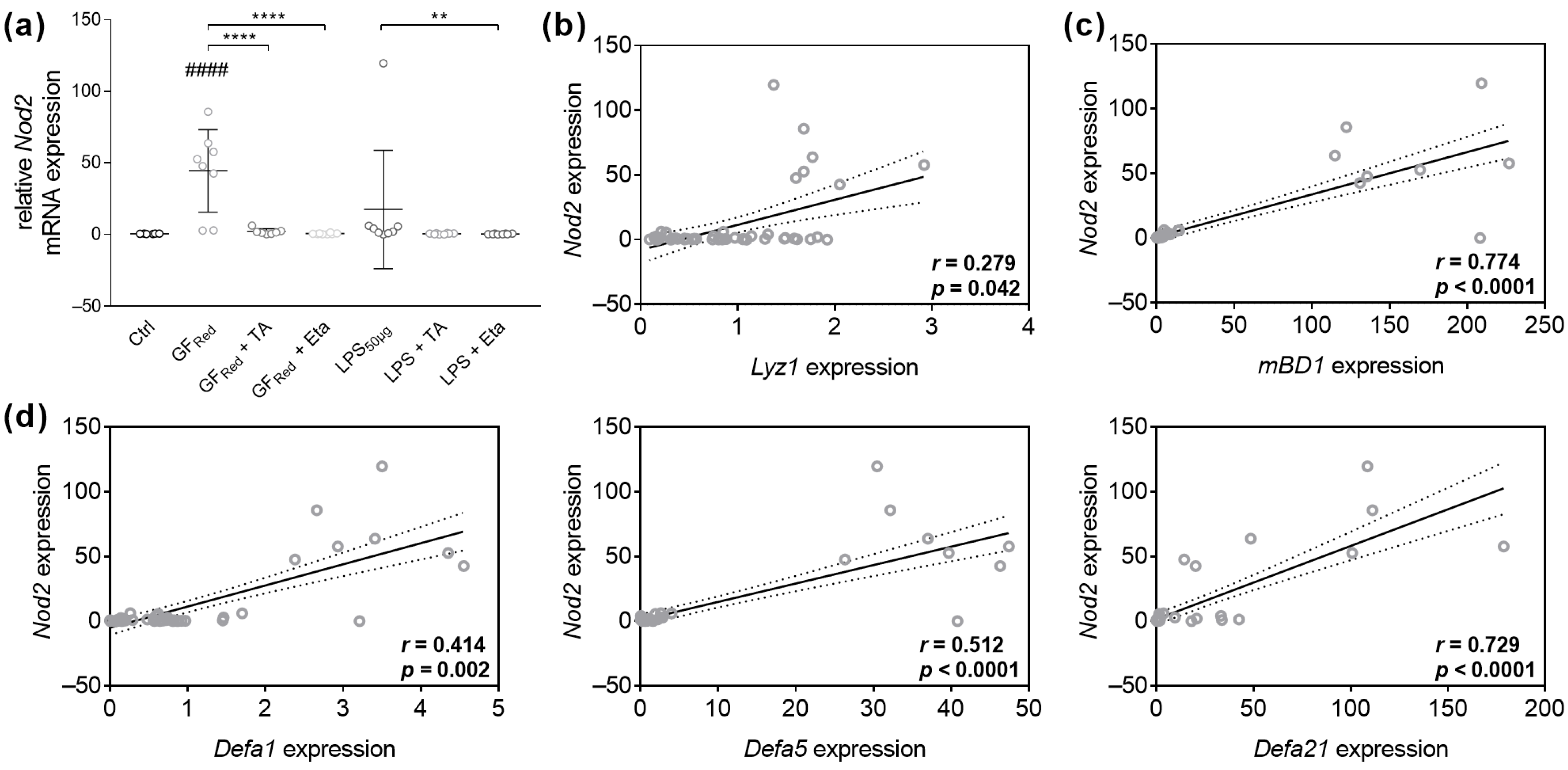
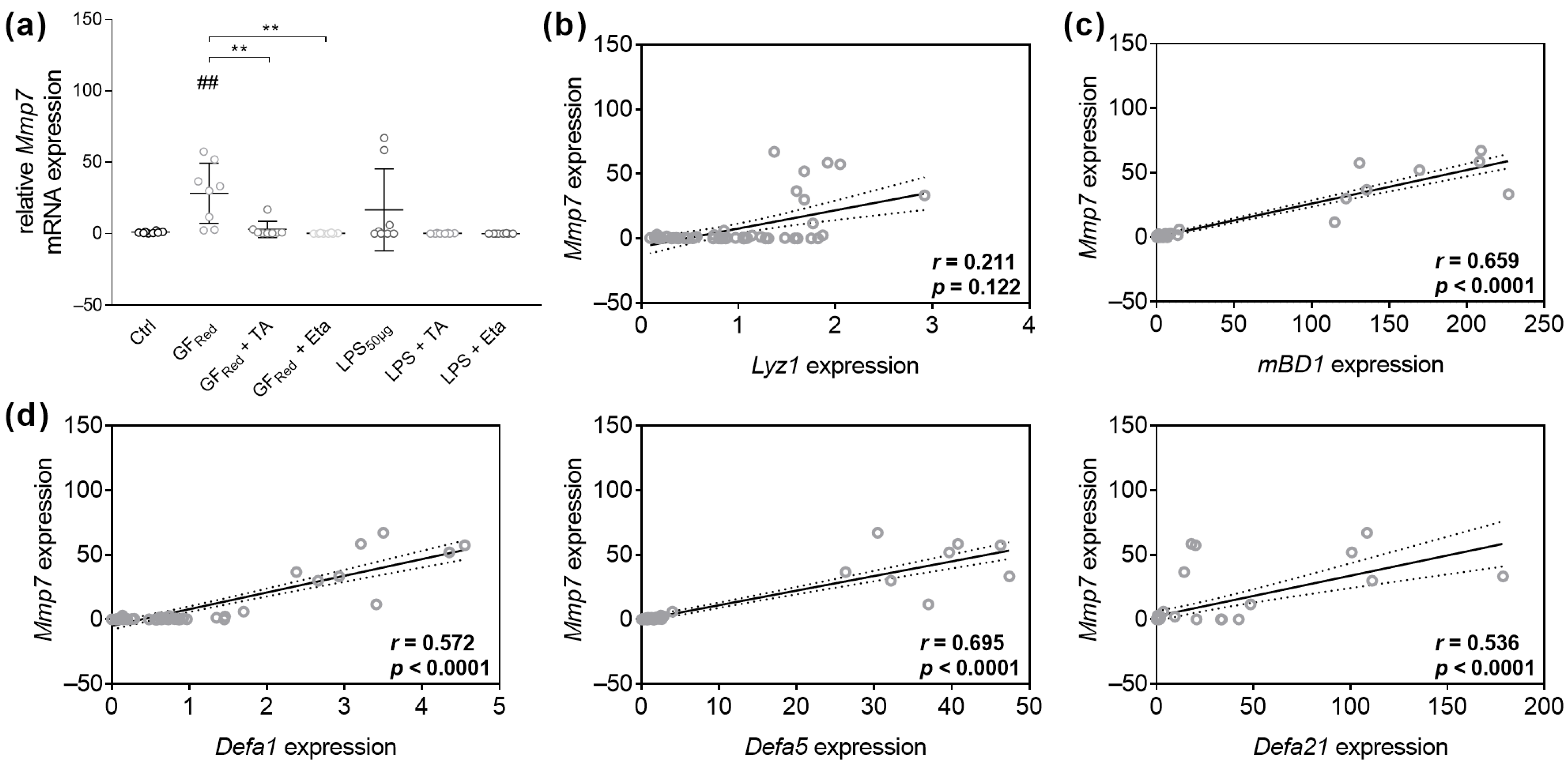
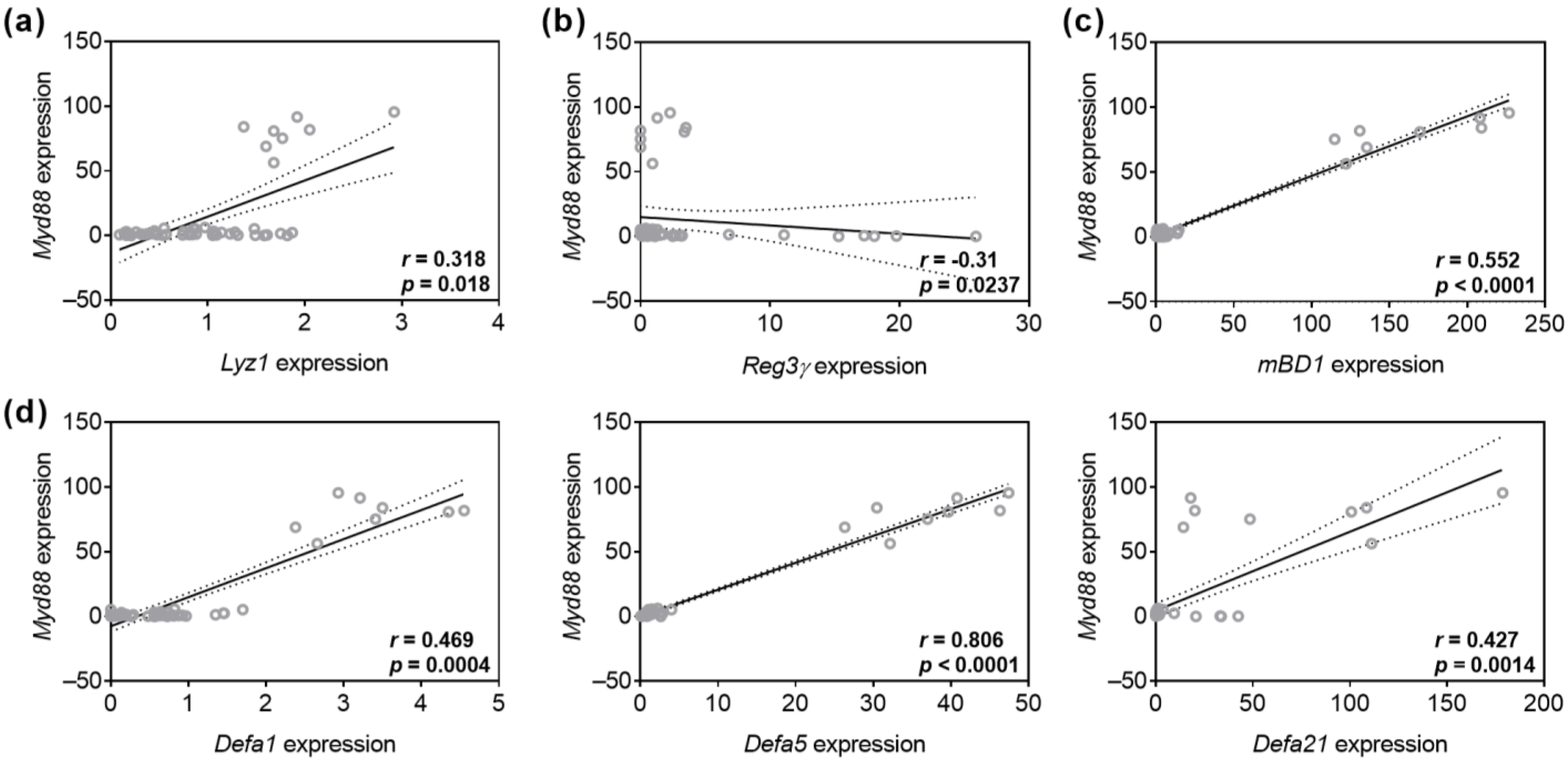
| CCM | GFRed | |
|---|---|---|
| GlutaMaxTM | 2 mM | 2 mM |
| Hepes | 10 mM | 10 mM |
| R-Spondin | 1 µg/mL | 0.5 µg/mL |
| Noggin | 100 ng/µL | 50 ng/µL |
| B-27™ supplement | 20 µL/mL | 20 µL/mL |
| N-Acetylcysteine | 1.63 mg/mL | 1.63 mg/mL |
| Primocin | 0.1 mg/mL | 0.1 mg/mL |
| mEGF | 50 ng/mL | 50 ng/mL |
| Ctrl | GFRed | LPS100µg | LPS50µg | |
|---|---|---|---|---|
| Myd88 | 0.49 ± 0.18 | 10.25 ± 2.54 ## | 10.13 ± 3.76 # | 13.15 ± 7.73 # |
| Tnfα | 0.53 ± 0.11 | 25.27 ± 6.52 ## | 13.39 ± 9.39 | 22.45 ± 11.3 # |
| IL-6 | 0.85 ± 0.29 | 42.8 ± 11.77 # | 8.34 ± 0.46 #### | 26.79 ± 14.15 # |
| IL-1β | 0.49 ± 0.18 | 10.25 ± 2.54 ## | 10.13 ± 3.76 # | 13.15 ± 7.73 # |
| Ocln | 1.14 ± 0.17 | 0.57 ± 0.14 # | 0.28 ± 0.1 # | 0.17 ± 0.09 ## |
| Cldn7 | 1.11 ± 0.33 | 0.16 ± 0.08 # | 0.18 ± 0.09 | 0.68 ± 0.34 |
| Myd88 | Tnfα | IL-6 | IL-1β | |
|---|---|---|---|---|
| Ctrl | 1.11 ± 0.19 | 1.07 ± 0.14 | 0.99 ± 0.28 | 1.64 ± 0.72 |
| GFRed | 58.05 ± 12.69 ### | 46.58 ± 12.02 ## | 134.4 ± 36.97 ## | 66.39 ± 16.45 ## |
| GFRed ± TA | 1.42 ± 0.37 ** | 1.8 ± 0.51 ** | 3.5 ± 0.94 * | 3.93 ± 1.41 ** |
| GFRed ± Eta | 3.17 ± 0.94 *** | 2.21 ± 0.75 ** | 1.67 ± 0.48 * | 4.7 ± 1.39 ** |
| LPS | 23.05 ± 14.18 | 20.87 ± 12.37 | 42.24 ± 25.97 | 42.8 ± 28.17 |
| LPS ± TA | 0.89 ± 0.15 | 1.03 ± 0.26 | 0.33 ± 0.08 $ | 0.93 ± 0.15 |
| LPS ± Eta | 1.82 ± 0.49 | 1.35 ± 0.4 | 2.24 ± 0.51 | 4.73 ± 1.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipe Rosa, L.; Gonda, S.; Roese, N.; Bischoff, S.C. Tannic Acid and Ethacridine Lactate Attenuate Markers of Stress-Induced Intestinal Barrier Dysfunctions in Murine Small Intestinal Organoids. Biomolecules 2025, 15, 650. https://doi.org/10.3390/biom15050650
Filipe Rosa L, Gonda S, Roese N, Bischoff SC. Tannic Acid and Ethacridine Lactate Attenuate Markers of Stress-Induced Intestinal Barrier Dysfunctions in Murine Small Intestinal Organoids. Biomolecules. 2025; 15(5):650. https://doi.org/10.3390/biom15050650
Chicago/Turabian StyleFilipe Rosa, Louisa, Steffen Gonda, Nadine Roese, and Stephan C. Bischoff. 2025. "Tannic Acid and Ethacridine Lactate Attenuate Markers of Stress-Induced Intestinal Barrier Dysfunctions in Murine Small Intestinal Organoids" Biomolecules 15, no. 5: 650. https://doi.org/10.3390/biom15050650
APA StyleFilipe Rosa, L., Gonda, S., Roese, N., & Bischoff, S. C. (2025). Tannic Acid and Ethacridine Lactate Attenuate Markers of Stress-Induced Intestinal Barrier Dysfunctions in Murine Small Intestinal Organoids. Biomolecules, 15(5), 650. https://doi.org/10.3390/biom15050650


_Kwok.png)





