Pancreatic MicroRNAs in Ictidomys tridecemlineatus Associated with Metabolic Diseases: Nature’s Insights into Important Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatments and Tissues
2.2. RNA Isolation and Small RNA Sequencing
2.3. Data Processing
2.4. Differential Expression Analysis
3. Results
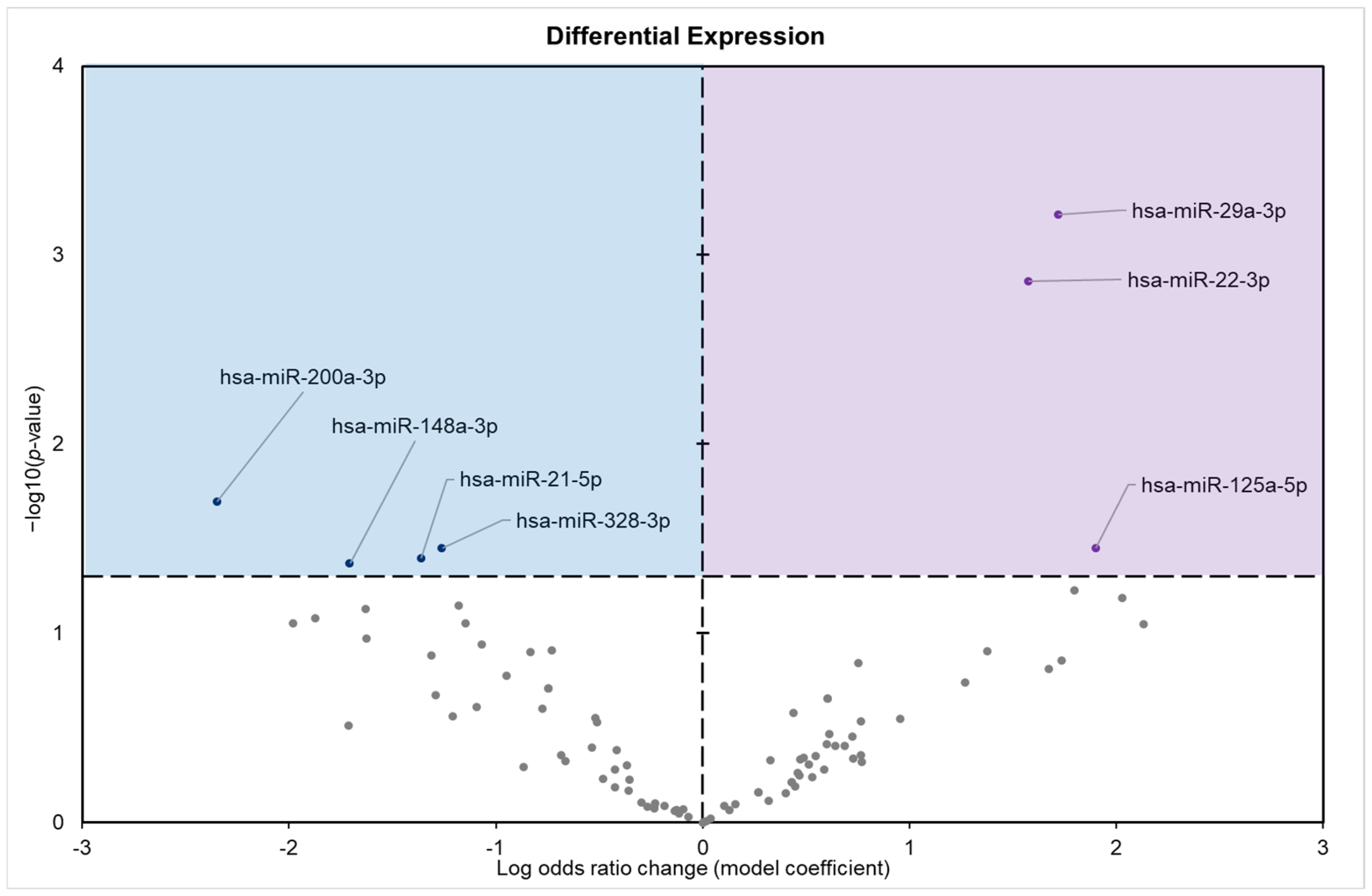
3.1. Analysis of Differentially Expressed miRNAs
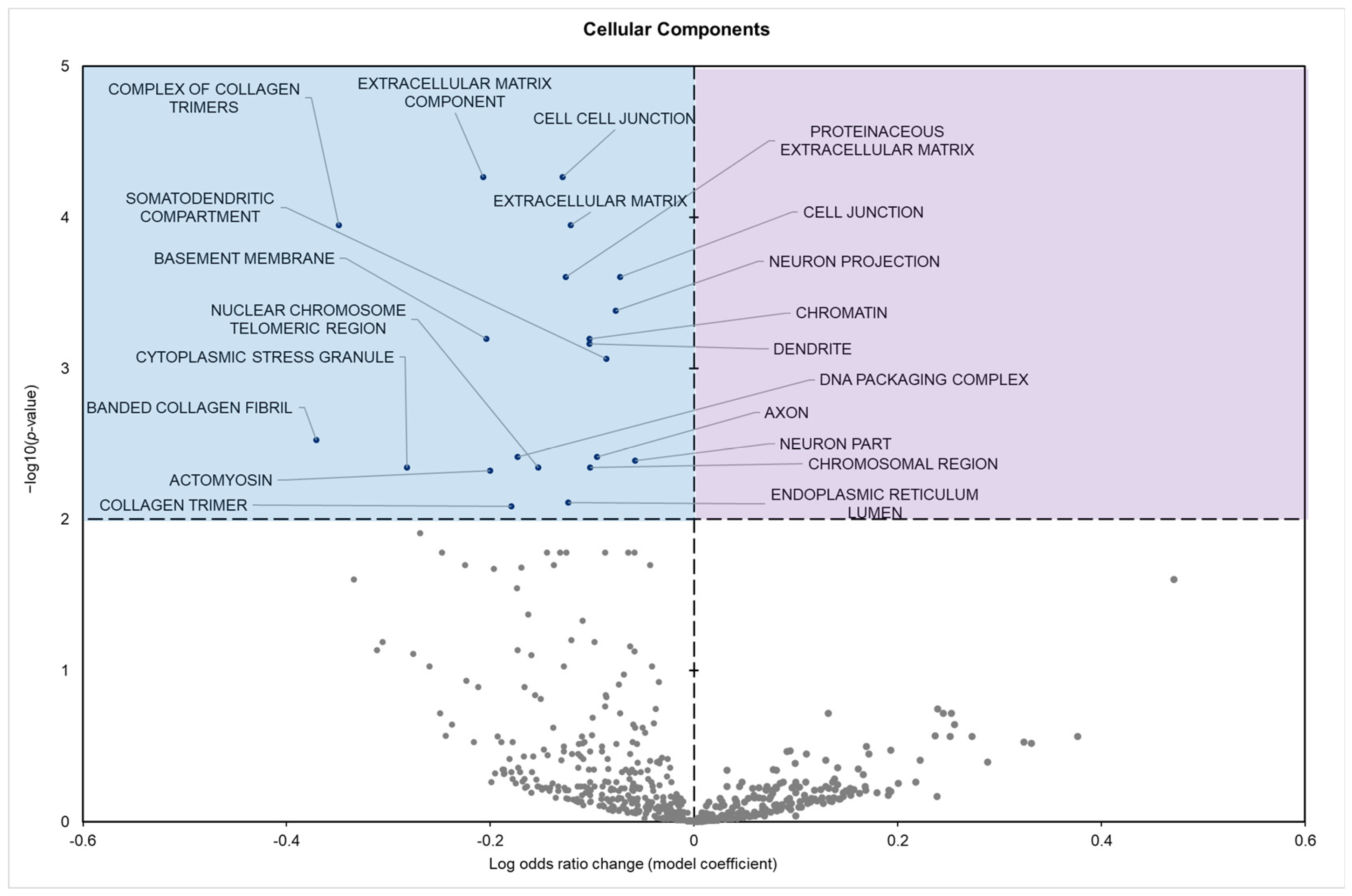
3.2. Gene Set Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Category | GO Term | Coefficient | FDR-Adjusted p-Value |
|---|---|---|---|
| Molecular Functions | Platelet-derived growth factor binding | −0.5504746 | 3.142731917 × 10−15 |
| Protein complex binding | −0.0942946 | 4.3737568447164 × 10−6 | |
| Macromolecular complex binding | −0.0795339 | 4.373756844716 × 10−6 | |
| Biological Processes | Blood vessel morphogenesis | −0.1572424 | 1.711962697235 × 10−7 |
| Vasculature development | −0.1410867 | 1.711962697235 × 10−7 | |
| Positive regulation of cell morphogenesis involved in differentiation | −0.1999583 | 6.1046556730018 × 10−6 | |
| Cellular Components | Extracellular matrix component | −0.2068074 | 5.424849580565 × 10−5 |
| Cell–cell junction | −0.1291901 | 5.424849580565 × 10−5 | |
| Extracellular matrix | −0.1210426 | 0.00011302 |
References
- Carey, H.V.; Andrews, M.T.; Martin, S.L. Mammalian Hibernation: Cellular and Molecular Responses to Depressed Metabolism and Low Temperature. Physiol. Rev. 2003, 83, 1153–1181. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.; Storey, K.B. Mammalian Hibernation: Differential Gene Expression and Novel Application of Epigenetic Controls. Int. J. Dev. Biol. 2009, 53, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Sonsalla, M.M.; Love, S.L.; Hoh, L.J.; Summers, L.N.; Follett, H.M.; Bojang, A.; Duddleston, K.N.; Kurtz, C.C. Development of Metabolic Inflammation during Pre-Hibernation Fattening in 13-Lined Ground Squirrels (Ictidomys Tridecemlineatus). J. Comp. Physiol. B 2021, 191, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.H.; Wolowyk, M.W. Torpor in Mammals and Birds. Can. J. Zoöl. 2011, 66, 133–137. [Google Scholar] [CrossRef]
- Tessier, S.N.; Wu, C.W.; Storey, K.B. Molecular Control of Protein Synthesis, Glucose Metabolism, and Apoptosis in the Brain of Hibernating Thirteen-Lined Ground Squirrels. Biochem. Cell Biol. 2019, 97, 536–544. [Google Scholar] [CrossRef]
- Rouble, A.N.; Hefler, J.; Mamady, H.; Storey, K.B.; Tessier, S.N. Anti-Apoptotic Signaling as a Cytoprotective Mechanism in Mammalian Hibernation. PeerJ 2013, 2013, e29. [Google Scholar] [CrossRef]
- Biggar, K.K.; Wu, C.W.; Tessier, S.N.; Zhang, J.; Pifferi, F.; Perret, M.; Storey, K.B. Primate Torpor: Regulation of Stress-Activated Protein Kinases during Daily Torpor in the Gray Mouse Lemur, Microcebus Murinus. Genom. Proteom. Bioinform. 2015, 13, 81–90. [Google Scholar] [CrossRef]
- Capraro, A.; O‘Meally, D.; Waters, S.A.; Patel, H.R.; Georges, A.; Waters, P.D. MicroRNA Dynamics during Hibernation of the Australian Central Bearded Dragon (Pogona vitticeps). Sci. Rep. 2020, 10, 17854. [Google Scholar] [CrossRef]
- Davis-Dusenbery, B.N.; Hata, A. Mechanisms of Control of MicroRNA Biogenesis. J. Biochem. 2010, 148, 381–392. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Tüfekci, K.U.; Meuwissen, R.L.J.; Genç, Ş. The Role of MicroRNAs in Biological Processes. Methods Mol. Biol. 2014, 1107, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Ingelson-Filpula, W.A.; Storey, K.B. Hibernation-Induced MicroRNA Expression Promotes Signaling Pathways and Cell Cycle Dysregulation in Ictidomys Tridecemlineatus Cardiac Tissue. Metabolites 2023, 13, 1096. [Google Scholar] [CrossRef] [PubMed]
- Logan, S.M.; Storey, K.B. MicroRNA Expression Patterns in the Brown Fat of Hibernating 13-Lined Ground Squirrels. Genomics 2021, 113, 769–781. [Google Scholar] [CrossRef]
- Rehman, S.; Storey, K.B. Small RNA and Freeze Survival: The Cryoprotective Functions of MicroRNA in the Frozen Muscle Tissue of the Grey Tree Frog. Metabolites 2024, 14, 387. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic Regulation of Glucose Homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Wu, C.W.; Biggar, K.K.; Storey, K.B. Biochemical Adaptations of Mammalian Hibernation: Exploring Squirrels as a Perspective Model for Naturally Induced Reversible Insulin Resistance. Braz. J. Med. Biol. Res. 2013, 46, 1. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Li, H.; Wang, D.W.; Chen, C. Roles of MicroRNAs in Glucose and Lipid Metabolism in the Heart. Front. Cardiovasc. Med. 2021, 8, 716213. [Google Scholar] [CrossRef]
- Li, K.; Zhao, B.; Wei, D.; Wang, W.; Cui, Y.; Qian, L.; Liu, G. MiR-146a Improves Hepatic Lipid and Glucose Metabolism by Targeting MED1. Int. J. Mol. Med. 2020, 45, 543–555. [Google Scholar] [CrossRef]
- Pheiffer, C.; Dias, S.; Rheeder, P.; Adam, S. Decreased Expression of Circulating MiR-20a-5p in South African Women with Gestational Diabetes Mellitus. Mol. Diagn. Ther. 2018, 22, 345–352. [Google Scholar] [CrossRef]
- Dinesen, S.; El-Faitarouni, A.; Dalgaard, L.T. Circulating MicroRNAs Associated with Gestational Diabetes Mellitus: Useful Biomarkers? J. Endocrinol. 2022, 256, e220170. [Google Scholar] [CrossRef]
- Frerichs, K.U.; Kennedy, C.; Sokoloff, L.; Hallenbeck, J.M. Local Cerebral Blood Flow during Hibernation, a Model of Natural Tolerance to “Cerebral Ischemia”. J. Cereb. Blood Flow Metab. 1994, 14, 193–205. [Google Scholar] [CrossRef] [PubMed]
- McMullen, D.C.; Hallenbeck, J.M. Regulation of Akt during Torpor in the Hibernating Ground Squirrel, Ictidomys Tridecemlineatus. J. Comp. Physiol. B 2010, 180, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hadj-Moussa, H.; Storey, K.B. Current Progress of High-Throughput MicroRNA Differential Expression Analysis and Random Forest Gene Selection for Model and Non-Model Systems: An R Implementation. J. Integr. Bioinform. 2016, 13, 35–46. [Google Scholar] [CrossRef]
- Sai Lakshmi, S.; Agrawal, S. PiRNABank: A Web Resource on Classified and Clustered Piwi-Interacting RNAs. Nucleic Acids Res. 2008, 36, D173–D177. [Google Scholar] [CrossRef]
- Kalvari, I.; Nawrocki, E.P.; Argasinska, J.; Quinones-Olvera, N.; Finn, R.D.; Bateman, A.; Petrov, A.I. Non-Coding RNA Analysis Using the Rfam Database. Curr. Protoc. Bioinform. 2018, 62, e51. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map Format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma Powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Massart, J.; Sjögren, R.J.O.; Lundell, L.S.; Mudry, J.M.; Franck, N.; O’Gorman, D.J.; Egan, B.; Zierath, J.R.; Krook, A. Altered MiR-29 Expression in Type 2 Diabetes Influences Glucose and Lipid Metabolism in Skeletal Muscle. Diabetes 2017, 66, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kotru, S.; Singh, S.; Behera, B.S.; Munshi, A. Role of MiRNAs in the Pathogenesis of T2DM, Insulin Secretion, Insulin Resistance, and β Cell Dysfunction: The Story so Far. J. Physiol. Biochem. 2020, 76, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Kwon, J.J.; Liu, S.; Hodge, G.A.; Taleb, S.; Zimmers, T.A.; Wan, J.; Kota, J. MiR-29a Is Repressed by MYC in Pancreatic Cancer and Its Restoration Drives Tumor Suppressive Effects via Downregulation of LOXL2. Mol. Cancer Res. 2019, 18, 311. [Google Scholar] [CrossRef]
- Dooley, J.; Garcia-Perez, J.E.; Sreenivasan, J.; Schlenner, S.M.; Vangoitsenhoven, R.; Papadopoulou, A.S.; Tian, L.; Schonefeldt, S.; Serneels, L.; Deroose, C.; et al. The MicroRNA-29 Family Dictates the Balance Between Homeostatic and Pathological Glucose Handling in Diabetes and Obesity. Diabetes 2016, 65, 53–61. [Google Scholar] [CrossRef]
- Vo, D.T.; Karanam, N.K.; Ding, L.; Saha, D.; Yordy, J.S.; Giri, U.; Heymach, J.V.; Story, M.D. MiR-125a-5p Functions as Tumor Suppressor MicroRNA And Is a Marker of Locoregional Recurrence And Poor Prognosis in Head And Neck Cancer. Neoplasia 2019, 21, 849–862. [Google Scholar] [CrossRef]
- Dakir, E.-H.; Mollinedo, F.; Dakir, E.-H.; Mollinedo, F. Genome-Wide MiRNA Profiling and Pivotal Roles of MiRs 125a-5p and 17-92 Cluster in Human Neutrophil Maturation and Differentiation of Acute Myeloid Leukemia Cells. Oncotarget 2019, 10, 5313–5331. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Z.; Fang, L. MiR-22-3p/PGC1β Suppresses Breast Cancer Cell Tumorigenesis via PPARγ. PPAR Res. 2021, 2021, 6661828. [Google Scholar] [CrossRef]
- Hu, Y.; Setayesh, T.; Vaziri, F.; Wu, X.; Hwang, S.T.; Chen, X.; Yvonne Wan, Y.J. MiR-22 Gene Therapy Treats HCC by Promoting Anti-Tumor Immunity and Enhancing Metabolism. Mol. Ther. 2023, 31, 1829–1845. [Google Scholar] [CrossRef]
- You, Y.; Tan, J.-X.; Dai, H.-S.; Chen, H.-W.; Xu, X.-J.; Yang, A.-G.; Zhang, Y.-J.; Bai, L.-H.; Bie, P.; You, Y.; et al. MiRNA-22 Inhibits Oncogene Galectin-1 in Hepatocellular Carcinoma. Oncotarget 2016, 7, 57099–57116. [Google Scholar] [CrossRef]
- Grieco, G.E.; Cataldo, D.; Ceccarelli, E.; Nigi, L.; Catalano, G.; Brusco, N.; Mancarella, F.; Ventriglia, G.; Fondelli, C.; Guarino, E.; et al. Serum Levels of MiR-148a and MiR-21-5p Are Increased in Type 1 Diabetic Patients and Correlated with Markers of Bone Strength and Metabolism. Noncoding RNA 2018, 4, 37. [Google Scholar] [CrossRef]
- Zhang, D.; Ran, J.; Li, J.; Yu, C.; Cui, Z.; Amevor, F.K.; Wang, Y.; Jiang, X.; Qiu, M.; Du, H.; et al. MiR-21-5p Regulates the Proliferation and Differentiation of Skeletal Muscle Satellite Cells by Targeting KLF3 in Chicken. Genes 2021, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Gou, D.; Turaka, P.; Viktorova, E.; Ramchandran, R.; Raj, J.U. MicroRNA-21 Plays a Role in Hypoxia-Mediated Pulmonary Artery Smooth Muscle Cell Proliferation and Migration. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L861–L871. [Google Scholar] [CrossRef] [PubMed]
- Zeboudj, L.; Sideris-Lampretsas, G.; Silva, R.; Al-Mudaris, S.; Picco, F.; Fox, S.; Chambers, D.; Malcangio, M. Silencing MiR-21-5p in Sensory Neurons Reverses Neuropathic Allodynia via Activation of TGF-β–Related Pathway in Macrophages. J. Clin. Investig. 2023, 133, e164472. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.S.; Anderson, N.N.; Wagschal, A.; De Cabo, R.; et al. Identification of MiR-148a as a Novel Regulator of Cholesterol Metabolism. Nat. Med. 2015, 21, 1280. [Google Scholar] [CrossRef]
- Sun, G.; Qi, M.; Kim, A.S.; Lizhar, E.M.; Sun, O.W.; Al-Abdullah, I.H.; Riggs, A.D. Reassessing the Abundance of MiRNAs in the Human Pancreas and Rodent Cell Lines and Its Implication. Noncoding RNA 2023, 9, 20. [Google Scholar] [CrossRef]
- Bracken, C.P.; Gregory, P.A.; Kolesnikoff, N.; Bert, A.G.; Wang, J.; Shannon, M.F.; Goodall, G.J. A Double-Negative Feedback Loop between ZEB1-SIP1 and the MicroRNA-200 Family Regulates Epithelial-Mesenchymal Transition. Cancer Res. 2008, 68, 7846–7854. [Google Scholar] [CrossRef]
- Gregory, P.A.; Bert, A.G.; Paterson, E.L.; Barry, S.C.; Tsykin, A.; Farshid, G.; Vadas, M.A.; Khew-Goodall, Y.; Goodall, G.J. The MiR-200 Family and MiR-205 Regulate Epithelial to Mesenchymal Transition by Targeting ZEB1 and SIP1. Nat. Cell Biol. 2008, 10, 593–601. [Google Scholar] [CrossRef]
- Luo, X.; Yang, S.; Zhou, C.; Pan, F.; Li, Q.; Ma, S. MicroRNA-328 Enhances Cellular Motility through Posttranscriptional Regulation of PTPRJ in Human Hepatocellular Carcinoma. Onco Targets Ther. 2015, 8, 3159. [Google Scholar] [CrossRef][Green Version]
- Hynes, R.O. Integrins: Bidirectional, Allosteric Signaling Machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Legate, K.R.; Wickström, S.A.; Fässler, R. Genetic and Cell Biological Analysis of Integrin Outside-in Signaling. Genes. Dev. 2009, 23, 397–418. [Google Scholar] [CrossRef]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The Extracellular Matrix and Insulin Resistance. Trends Endocrinol. Metab. 2015, 26, 357. [Google Scholar] [CrossRef] [PubMed]
- Storey, K.B.; Storey, J.M. Metabolic Rate Depression in Animals: Transcriptional and Translational Controls. Biol. Rev. Camb. Philos. Soc. 2004, 79, 207–233. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Storey, K.B. Dynamics of Epigenetic Regulation in Dryophytes Versicolor Skeletal Muscle: Lysine Methylation and Acetylation Involvement in Metabolic Rate Depression. J. Therm. Biol. 2024, 122, 103865. [Google Scholar] [CrossRef]
- Lant, B.; Storey, K.B. An Overview of Stress Response and Hypometabolic Strategies in Caenorhabditis Elegans: Conserved and Contrasting Signals with the Mammalian System. Int. J. Biol. Sci. 2010, 6, 9. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.L. Focal Adhesion Kinase and Its Signaling Pathways in Cell Migration and Angiogenesis. Adv. Drug Deliv. Rev. 2010, 63, 610. [Google Scholar] [CrossRef]
- Meyts, P.D. The Insulin Receptor and Its Signal Transduction Network. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2016. [Google Scholar]
- Huang, D.; Cheung, A.T.; Thomas Parsons, J.; Bryer-Ash, M. Focal Adhesion Kinase (FAK) Regulates Insulin-Stimulated Glycogen Synthesis in Hepatocytes. J. Biol. Chem. 2002, 277, 18151–18160. [Google Scholar] [CrossRef]
- Rondas, D.; Tomas, A.; Soto-Ribeiro, M.; Wehrle-Haller, B.; Halban, P.A. Novel Mechanistic Link between Focal Adhesion Remodeling and Glucose-Stimulated Insulin Secretion. J. Biol. Chem. 2011, 287, 2423. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, J.; Field, R.L.; Ye, D.; Hu, Z.; Xu, K.; Xu, L.; Gong, Y.; Yue, Y.; Kravitz, A.V.; et al. Induction of a Torpor-like Hypothermic and Hypometabolic State in Rodents by Ultrasound. Nat. Metab. 2023, 5, 789–803. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 1–38. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sun, M.M.; Zhang, G.G.; Yang, J.; Chen, K.S.; Xu, W.W.; Li, B. Targeting PI3K/Akt Signal Transduction for Cancer Therapy. Signal Transduct. Target. Ther. 2021, 6, 425. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.B.; Bouma, H.R.; Henning, R.H. Unraveling the Big Sleep: Molecular Aspects of Stem Cell Dormancy and Hibernation. Front. Physiol. 2021, 12, 624950. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, L.T.; Sørensen, A.E.; Hardikar, A.A.; Joglekar, M.V. The microRNA-29 family: Role in metabolism and metabolic disease. Am. J. Physiol. Cell Physiol. 2022, 2, 323. [Google Scholar] [CrossRef]
- Senese, R.; Cioffi, F.; Petito, G.; de Lange, P.; Russo, A.; Goglia, F.; Lanni, A.; Potenza, N. miR-22-3p is involved in gluconeogenic pathway modulated by 3,5-diiodo-L-thyronine (T2). Sci. Rep. 2019, 9, 16645. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiwo, O.O.; Rehman, S.; Storey, K.B. Pancreatic MicroRNAs in Ictidomys tridecemlineatus Associated with Metabolic Diseases: Nature’s Insights into Important Biomarkers. Biomolecules 2025, 15, 616. https://doi.org/10.3390/biom15050616
Taiwo OO, Rehman S, Storey KB. Pancreatic MicroRNAs in Ictidomys tridecemlineatus Associated with Metabolic Diseases: Nature’s Insights into Important Biomarkers. Biomolecules. 2025; 15(5):616. https://doi.org/10.3390/biom15050616
Chicago/Turabian StyleTaiwo, Olawale O., Saif Rehman, and Kenneth B. Storey. 2025. "Pancreatic MicroRNAs in Ictidomys tridecemlineatus Associated with Metabolic Diseases: Nature’s Insights into Important Biomarkers" Biomolecules 15, no. 5: 616. https://doi.org/10.3390/biom15050616
APA StyleTaiwo, O. O., Rehman, S., & Storey, K. B. (2025). Pancreatic MicroRNAs in Ictidomys tridecemlineatus Associated with Metabolic Diseases: Nature’s Insights into Important Biomarkers. Biomolecules, 15(5), 616. https://doi.org/10.3390/biom15050616









