Plasma Exosomal Proteomics Identifies Differentially Expressed Proteins as Biomarkers for Acute Myocardial Infarction
Abstract
1. Introduction
2. Material and Methods
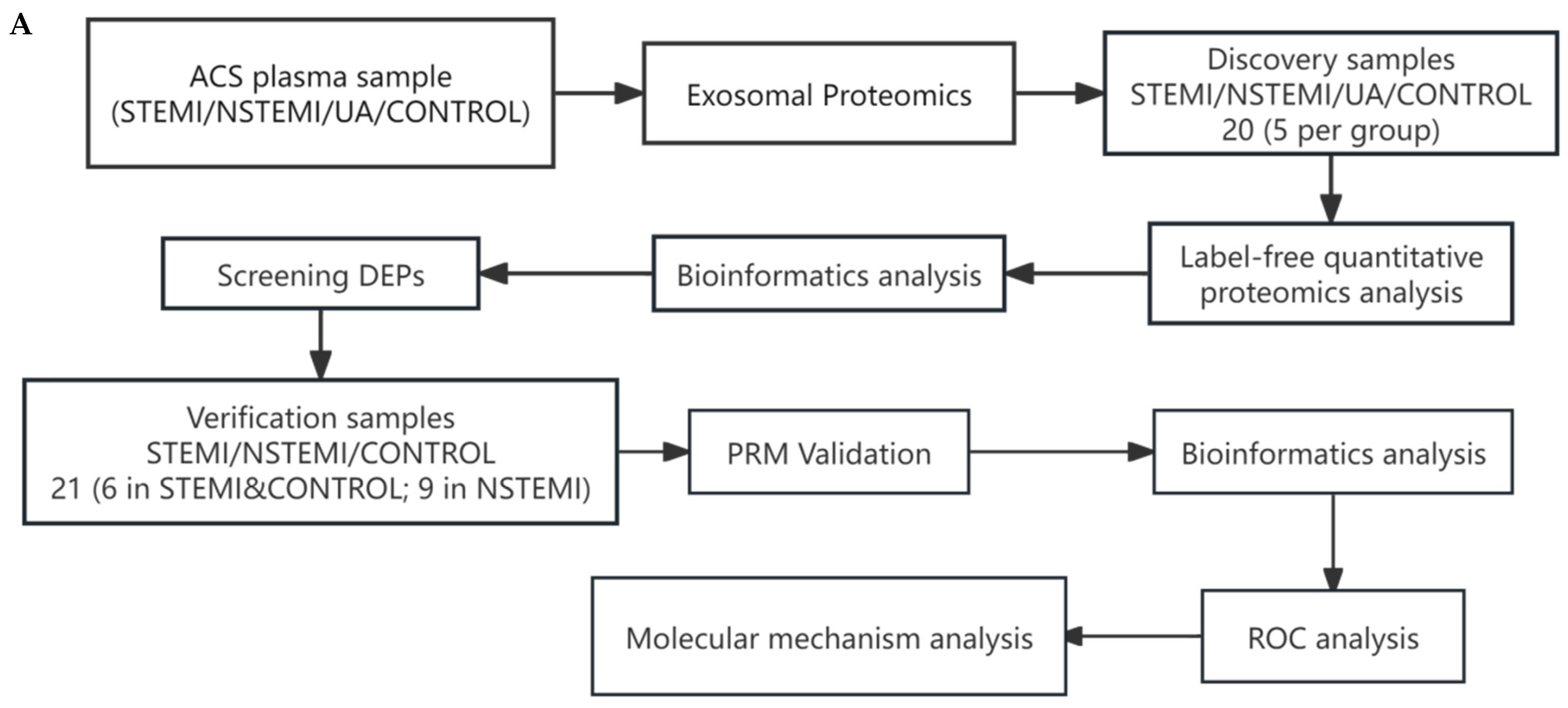
2.1. Experimental Design
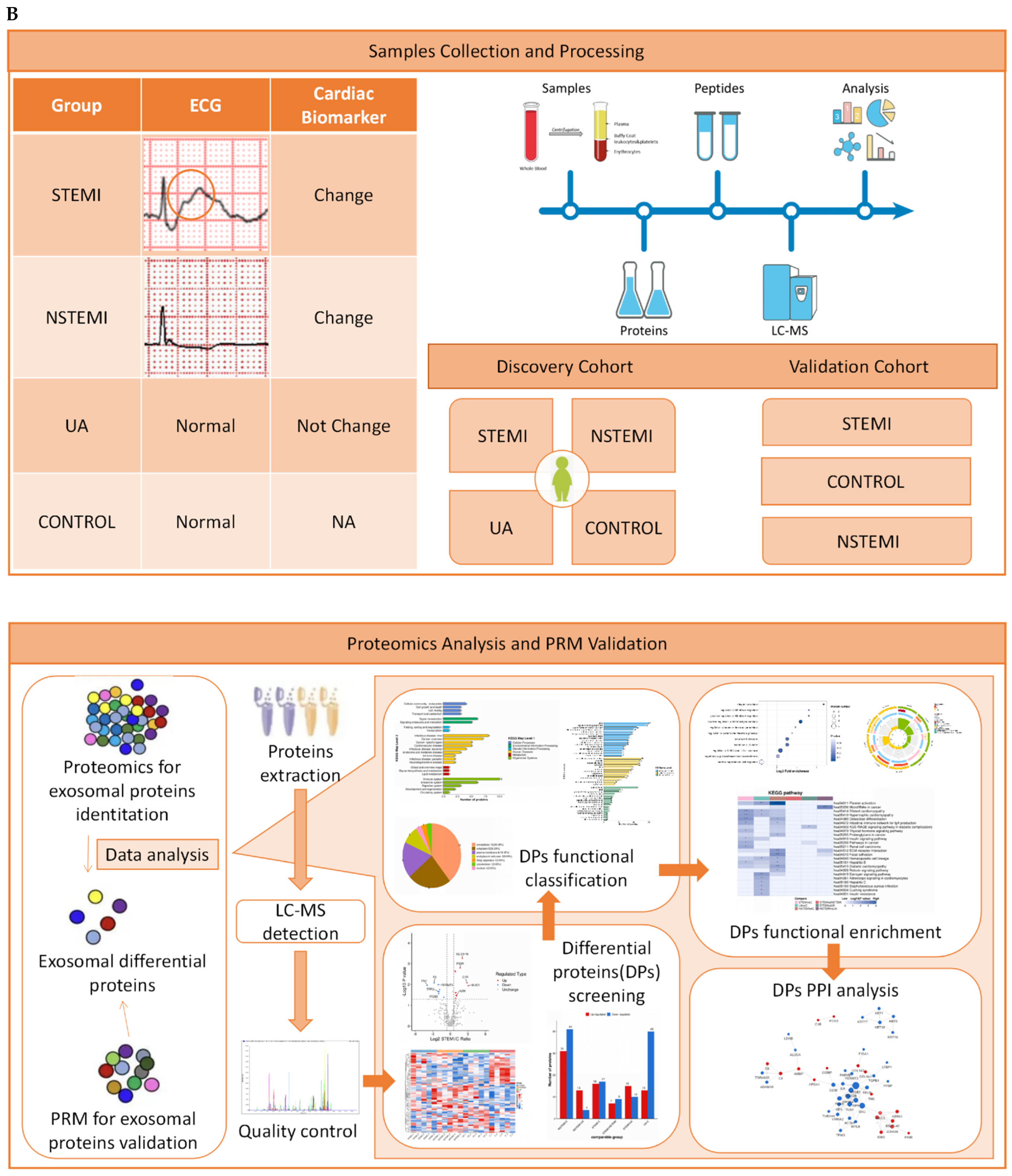
2.2. Study Design and Population
2.3. Samples Collection
2.4. Isolation and Identification of Exosomes
2.4.1. Extraction and Purification of Exosomes
2.4.2. Identification of Exosomes
2.5. Proteomics Analysis and Validation
2.5.1. Identification of Exosomal Differentially Expressed Proteins (DEPs)
2.5.2. Validation of Exosomal DEPs
2.5.3. Bioinformatics Analysis
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients
3.2. Characterization of Exosomes
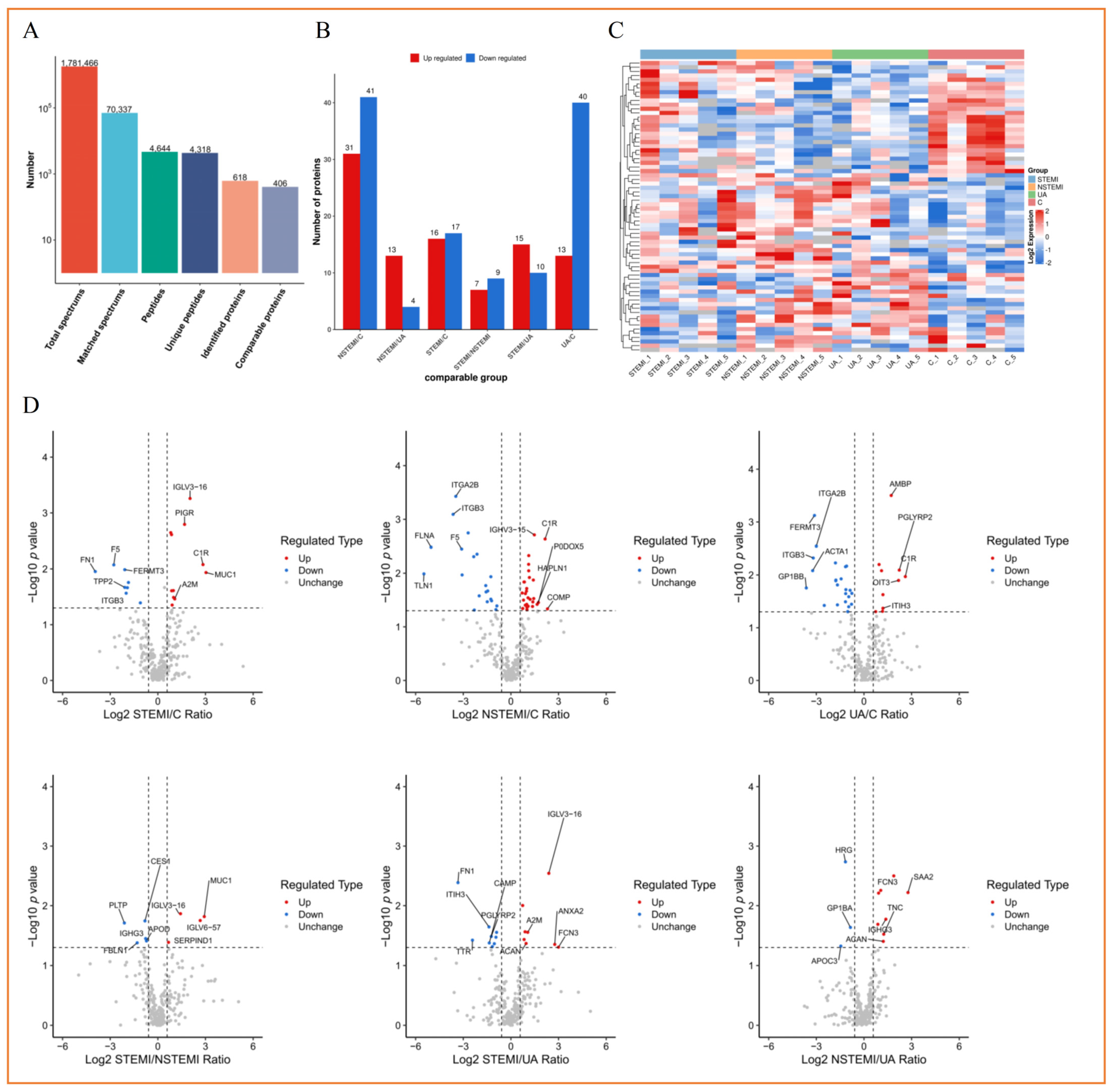
3.3. Label-Free Quantitative Proteomics Profiling of Plasma Exosomes from Discovery Phase
3.3.1. Screening of Exosomal DEPs
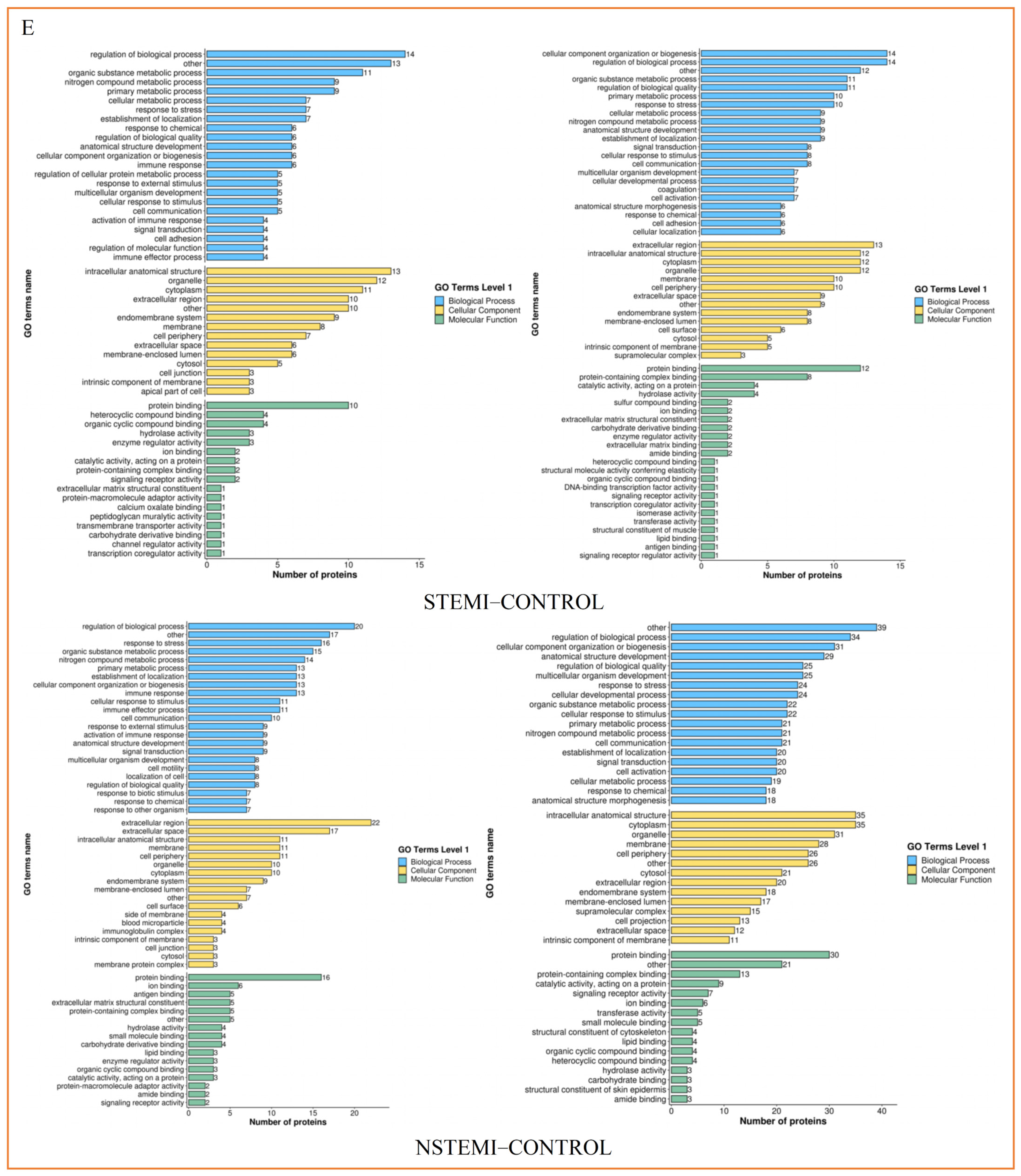
3.3.2. Functional Classification of Exosomal DEPs
3.3.3. Functional Enrichment Analysis of Exosomal DEPs
3.3.4. Cluster Analysis of Exosomal DEPs
3.3.5. Analysis of PPI Network of Exosomal DEPs
3.4. PRM Analysis Revealed the Potential Biomarkers of STEMI and NSTEMI Patients in the Validation Phase
4. Discussion
4.1. The Key Findings
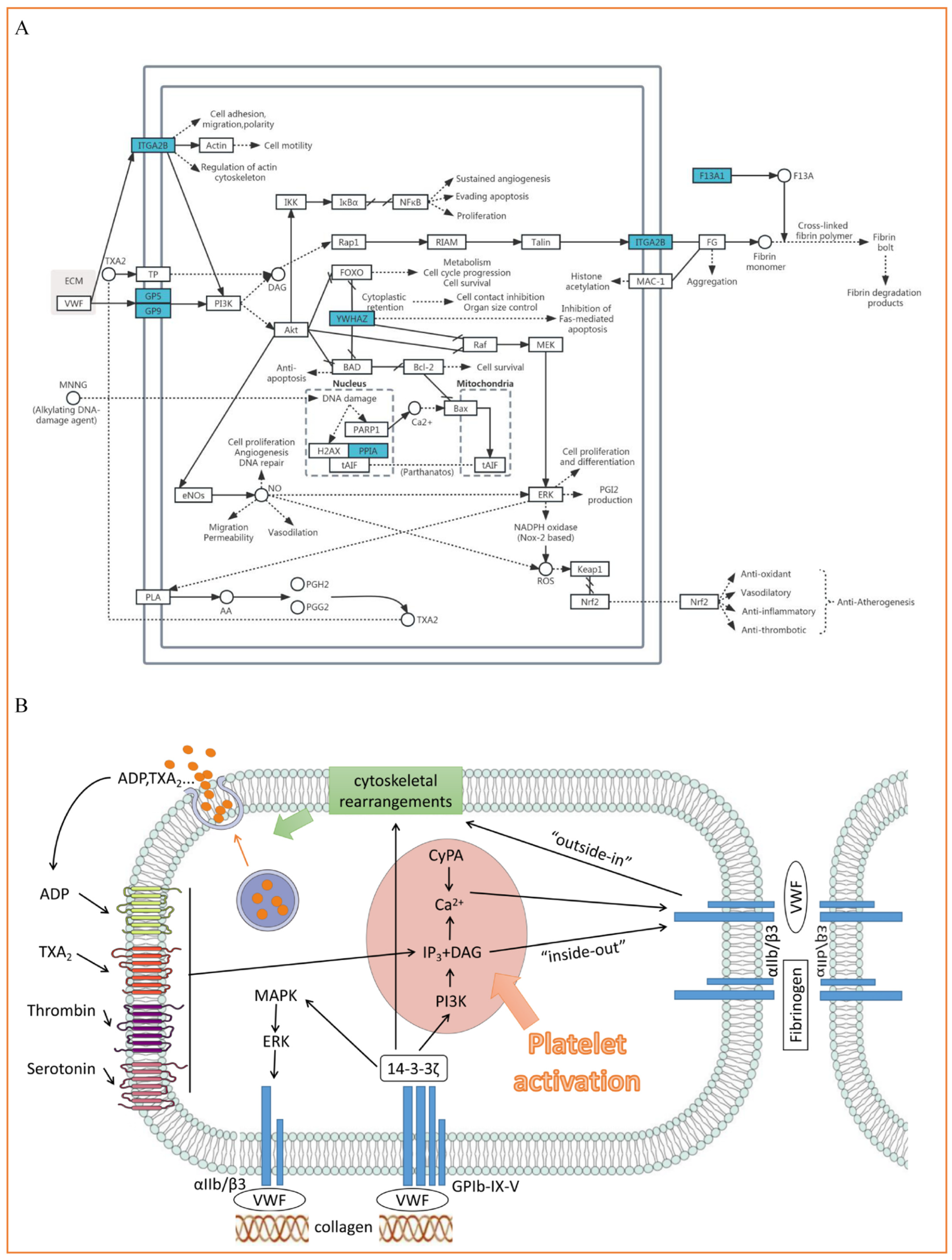
4.2. ITGA2B(αIIb), YWHAZ(14-3-3ζ), GP5(V) and GP9(IX) Participate in the Process of Platelet Adhesion, Activation and Aggregation
4.3. PPIA (Cypa) Is Involved in Inflammation and Thrombosis
4.4. F13A1 (FXIII) Is Involved in Thrombosis and Causes Poor Prognosis
4.5. TSPAN33 (Tetraspanin-33)
4.6. Influence of Cofounders of MI
4.7. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 24 August 2023).
- World Health Organization. Global Health Estimates: Leading Causes of Death. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death (accessed on 28 August 2023).
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 28 August 2023).
- Li, W.; Li, Y.; Zhi, W.; Liu, C.; Fan, W.; Miao, Q.; Gu, X. Diagnostic value of using exosome-derived cysteine-rich protein 61 as biomarkers for acute coronary syndrome. Exp. Ther. Med. 2021, 22, 1437. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2023 Update: A Report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Little, K.M.; Smalley, D.M.; Harthun, N.L.; Ley, K. The plasma microparticle proteome. Semin Thromb. Hemost. 2010, 36, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Majka, M.; Kleibert, M.; Wojciechowska, M. Impact of the Main Cardiovascular Risk Factors on Plasma Extracellular Vesicles and Their Influence on the Heart’s Vulnerability to Ischemia-Reperfusion Injury. Cells 2021, 10, 3331. [Google Scholar] [CrossRef] [PubMed]
- Zarà, M.; Campodonico, J.; Cosentino, N.; Biondi, M.L.; Amadio, P.; Milanesi, G.; Assanelli, E.; Cerri, S.; Biggiogera, M.; Sandrini, L.; et al. Plasma Exosome Profile in ST-Elevation Myocardial Infarction Patients with and without Out-of-Hospital Cardiac Arrest. Int. J. Mol. Sci. 2021, 22, 8065. [Google Scholar] [CrossRef]
- Barrachina, M.N.; Calderón-Cruz, B.; Fernandez-Rocca, L.; García, Á. Application of Extracellular Vesicles Proteomics to Cardiovascular Disease: Guidelines, Data Analysis, and Future Perspectives. Proteomics 2019, 19, e1800247. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, L.; Wang, Z.; Yan, K.; Zhao, L.; Xiao, W. Research Progress on Transorgan Regulation of the Cardiovascular and Motor System through Cardiogenic Exosomes. Int. J. Mol. Sci. 2022, 23, 5765. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, M.; Zheng, X.; He, Y.; Ma, X.; Li, J.; Pu, K. Application of exosome engineering modification in targeted delivery of therapeutic drugs. Biochem. Pharmacol. 2023, 215, 115691. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, X.; Guo, J.; Cao, C.; Zhang, Z.; Wang, B.; Zhang, L.; Shen, D.; Lim, K.; Woodfield, T.; et al. Small but significant: Insights and new perspectives of exosomes in cardiovascular disease. J. Cell Mol. Med. 2020, 24, 8291–8303. [Google Scholar] [CrossRef]
- Gidlöf, O.; Evander, M.; Rezeli, M.; Marko-Varga, G.; Laurell, T.; Erlinge, D. Proteomic profiling of extracellular vesicles reveals additional diagnostic biomarkers for myocardial infarction compared to plasma alone. Sci. Rep. 2019, 9, 8991. [Google Scholar] [CrossRef]
- Foglio, E.; Puddighinu, G.; Fasanaro, P.; D’Arcangelo, D.; Perrone, G.A.; Mocini, D.; Campanella, C.; Coppola, L.; Logozzi, M.; Azzarito, T.; et al. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int. J. Cardiol. 2015, 197, 333–347. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Costa, L.; Barros, A.S.; Lourenço, A.P.; Leite-Moreira, A.F.; Nogueira-Ferreira, R.; Thongboonkerd, V.; Vitorino, R. Exosome-Derived Mediators as Potential Biomarkers for Cardiovascular Diseases: A Network Approach. Proteomes 2021, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Adamiak, M.; Mathiyalagan, P.; Kenneweg, F.; Kafert-Kasting, S.; Thum, T. Therapeutic and Diagnostic Translation of Extracellular Vesicles in Cardiovascular Diseases: Roadmap to the Clinic. Circulation 2021, 143, 1426–1449. [Google Scholar] [CrossRef]
- Ku, W.C.; Sridharan, B.; Chen, J.Y.; Li, J.Y.; Yang, S.Y.; Lee, M.J. Kaempferitrin-Treated HepG2 Differentially Expressed Exosomal Markers and Affect Extracellular Vesicle Sizes in the Secretome. Biomolecules 2021, 11, 187. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Y.; Wang, Y.; Cai, X.; Fan, X. Cytokine Gene Variants as Predisposing Factors for the Development and Progression of Coronary Artery Disease: A Systematic Review. Biomolecules 2024, 14, 1631. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Barstow, C.; Rice, M.; McDivitt, J.D. Acute coronary syndrome: Diagnostic evaluation. Am. Fam. Physician 2017, 95, 170–177. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European society of cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Ahn, J.H.; Jung, M.H.; Kim, J.H.; Kang, M.G.; Kim, K.H.; Jang, J.Y.; Park, H.W.; Koh, J.S.; Hwang, S.J.; et al. Serum microRNA-185 levels and myocardial injury in patients with acute ST-segment elevation myocardial infarction. Intern Med. 2022, 61, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, H.; Umezu, T.; Ohyashiki, K.; Hirano, T.; Ohyashiki, J.H. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J. Biol. Chem. 2013, 288, 34343–34351. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- de Araujo Farias, V.; O’Valle, F.; Serrano-Saenz, S.; Anderson, P.; Andrés, E.; López-Peñalver, J.; Tovar, I.; Nieto, A.; Santos, A.; Martín, F.; et al. Exosomes derived from mesenchymal stem cells enhance radiotherapy-induced cell death in tumor and metastatic tumor foci. Mol. Cancer 2018, 17, 122. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Li, X.; Wang, X.; Lin, Y.; Wang, X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018, 435, 80–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Clay, D.; Mitjavila-Garcia, M.T.; Alama, A.; Mennesson, B.; Berseneff, H.; Louache, F.; Bennaceur-Griscelli, A.; Oberlin, E. VE-Cadherin and ACE Co-Expression Marks Highly Proliferative Hematopoietic Stem Cells in Human Embryonic Liver. Stem Cells Dev. 2019, 28, 165–185. [Google Scholar] [CrossRef] [PubMed]
- Vilahur, G.; Gutiérrez, M.; Arzanauskaite, M.; Mendieta, G.; Ben-Aicha, S.; Badimon, L. Intracellular platelet signalling as a target for drug development. Vasc. Pharmacol. 2018, 111, 22–25. [Google Scholar] [CrossRef]
- Barriuso, I.; Worner, F.; Vilahur, G. Novel Antithrombotic Agents in Ischemic Cardiovascular Disease: Progress in the Search for the Optimal Treatment. J. Cardiovasc. Dev. Dis. 2022, 9, 397. [Google Scholar] [CrossRef]
- Carresi, C.; Mollace, R.; Macrì, R.; Scicchitano, M.; Bosco, F.; Scarano, F.; Coppoletta, A.R.; Guarnieri, L.; Ruga, S.; Zito, M.C.; et al. Oxidative Stress Triggers Defective Autophagy in Endothelial Cells: Role in Atherothrombosis Development. Antioxidants 2021, 10, 387. [Google Scholar] [CrossRef]
- Andrews, R.K.; Gardiner, E.E.; Shen, Y.; Berndt, M.C. Platelet interactions in thrombosis. IUBMB Life 2004, 56, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Moliterno, D.J. The role of the platelet in the pathogenesis of atherothrombosis. Am. J. Cardiovasc. Drugs 2005, 5, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Berndt, M.C. Platelet physiology and thrombosis. Thromb. Res. 2004, 114, 447–453. [Google Scholar] [CrossRef]
- Kuijpers, M.J.; Schulte, V.; Bergmeier, W.; Lindhout, T.; Brakebusch, C.; Offermanns, S.; Fässler, R.; Heemskerk, J.W.; Nieswandt, B. Complementary roles of glycoprotein VI and alpha2beta1 integrin in collagen-induced thrombus formation in flowing whole blood ex vivo. FASEB J. 2003, 17, 685–687. [Google Scholar] [CrossRef]
- Shi, R.; Luo, Y.; Li, S.; Kong, M.; Liu, X.; Yu, M.; Wu, J.; Huang, L.; Yang, Z. Single-nucleotide Polymorphism rs17860041 A/C in the Promoter of the PPIA Gene is Associated with Susceptibility to Kawasaki Disease in Chinese Children. Immunol. Investig. 2021, 50, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Vinitha, A.; Kutty, V.R.; Vivekanand, A.; Reshmi, G.; Divya, G.; Sumi, S.; Santosh, K.R.; Pratapachandran, N.S.; Ajit, M.S.; Kartha, C.C.; et al. PPIA rs6850: A > G single-nucleotide polymorphism is associated with raised plasma cyclophilin A levels in patients with coronary artery disease. Mol. Cell Biochem. 2016, 412, 259–268. [Google Scholar] [CrossRef]
- Rath, D.; von Ungern-Sternberg, S.; Heinzmann, D.; Sigle, M.; Monzien, M.; Horstmann, K.; Schaeffeler, E.; Winter, S.; Müller, K.; Groga-Bada, P.; et al. Platelet surface expression of cyclophilin A is associated with increased mortality in patients with symptomatic coronary artery disease. J. Thromb. Haemost. 2020, 18, 234–242. [Google Scholar] [CrossRef]
- Seizer, P.; Fuchs, C.; Ungern-Sternberg, S.N.; Heinzmann, D.; Langer, H.; Gawaz, M.; May, A.E.; Geisler, T. Platelet-bound cyclophilin A in patients with stable coronary artery disease and acute myocardial infarction. Platelets 2016, 27, 155–158. [Google Scholar] [CrossRef]
- Palacín, M.; Rodríguez, I.; García-Castro, M.; Ortega, F.; Reguero, J.R.; López-Larrea, C.; Morís, C.; Alvarez, V.; Coto, E. A search for cyclophilin-A gene (PPIA) variation and its contribution to the risk of atherosclerosis and myocardial infarction. Int. J. Immunogenet. 2008, 35, 159–164. [Google Scholar] [CrossRef]
- Ramachandran, S.; Venugopal, A.; Kutty, V.R.; Vinitha, A.; Divya, G.; Chitrasree, V.; Mullassari, A.; Pratapchandran, N.S.; Santosh, K.R.; Pillai, M.R.; et al. Plasma level of cyclophilin A is increased in patients with type 2 diabetes mellitus and suggests presence of vascular disease. Cardiovasc. Diabetol. 2014, 13, 38. [Google Scholar] [CrossRef]
- Elvers, M.; Herrmann, A.; Seizer, P.; Münzer, P.; Beck, S.; Schönberger, T.; Borst, O.; Martin-Romero, F.J.; Lang, F.; May, A.E.; et al. Intracellular cyclophilin A is an important Ca2+ regulator in platelets and critically involved in arterial thrombus formation. Blood 2012, 120, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, U.; Amann, E.; Zettlmeissl, G.; Küpper, H.A. Characterization of cDNA coding for human factor XIIIa. Proc. Natl. Acad. Sci. USA 1986, 83, 8024–8028. [Google Scholar] [CrossRef] [PubMed]
- Aeschlimann, D.; Thomazy, V. Protein crosslinking in assembly and remodelling of extracellular matrices: The role of transglutaminases. Connect. Tissue Res. 2000, 41, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Zeri, G.; Orioli, E.; Mari, R.; Moratelli, S.; Vigliano, M.; Marchesini, J.; Grossi, M.E.; Pecoraro, A.; Cuneo, A.; et al. Factor XIII-A dynamics in acute myocardial infarction: A novel prognostic biomarker? Thromb. Haemost. 2015, 114, 123–132. [Google Scholar] [CrossRef]
- Gupta, S.; Biswas, A.; Akhter, M.S.; Krettler, C.; Reinhart, C.; Dodt, J.; Reuter, A.; Philippou, H.; Ivaskevicius, V.; Oldenburg, J. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 2016, 6, 30105. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Hu, K.; Frantz, S.; Jaffer, F.A.; Tung, C.H.; Hiller, K.H.; Voll, S.; Nordbeck, P.; Sosnovik, D.; Gattenlöhner, S.; et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 2006, 113, 1196–1202. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Weissleder, R.; Ertl, G. Does FXIII deficiency impair wound healing after myocardial infarction? PLoS ONE 2006, 1, e48. [Google Scholar] [CrossRef]
- Priyathilaka, T.T.; Bathige, S.D.N.K.; Herath, H.M.L.P.B.; Lee, S.; Lee, J. Molecular identification of disk abalone (Haliotis discus discus) tetraspanin 33 and CD63: Insights into potent players in the disk abalone host defense system. Fish Shellfish Immunol. 2017, 69, 173–184. [Google Scholar] [CrossRef]
- Heikens, M.J.; Cao, T.M.; Morita, C.; Dehart, S.L.; Tsai, S. Penumbra encodes a novel tetraspanin that is highly expressed in erythroid progenitors and promotes effective erythropoiesis. Blood 2007, 109, 3244–3252. [Google Scholar] [CrossRef]
- Navarro-Hernandez, I.C.; López-Ortega, O.; Acevedo-Ochoa, E.; Cervantes-Díaz, R.; Romero-Ramírez, S.; Sosa-Hernández, V.A.; Meza-Sánchez, D.E.; Juárez-Vega, G.; Pérez-Martínez, C.A.; Chávez-Munguía, B.; et al. Tetraspanin 33 (TSPAN33) regulates endocytosis and migration of human B lymphocytes by affecting the tension of the plasma membrane. FEBS J. 2020, 287, 3449–3471. [Google Scholar] [CrossRef]
- Luu, V.P.; Hevezi, P.; Vences-Catalan, F.; Maravillas-Montero, J.L.; White, C.A.; Casali, P.; Llorente, L.; Jakez-Ocampo, J.; Lima, G.; Vilches-Cisneros, N.; et al. TSPAN33 is a novel marker of activated and malignant B cells. Clin. Immunol. 2013, 149, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Fadista, J.; Vikman, P.; Laakso, E.O.; Mollet, I.G.; Esguerra, J.L.; Taneera, J.; Storm, P.; Osmark, P.; Ladenvall, C.; Prasad, R.B.; et al. Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc. Natl. Acad. Sci. USA 2014, 111, 13924–13929. [Google Scholar] [CrossRef] [PubMed]


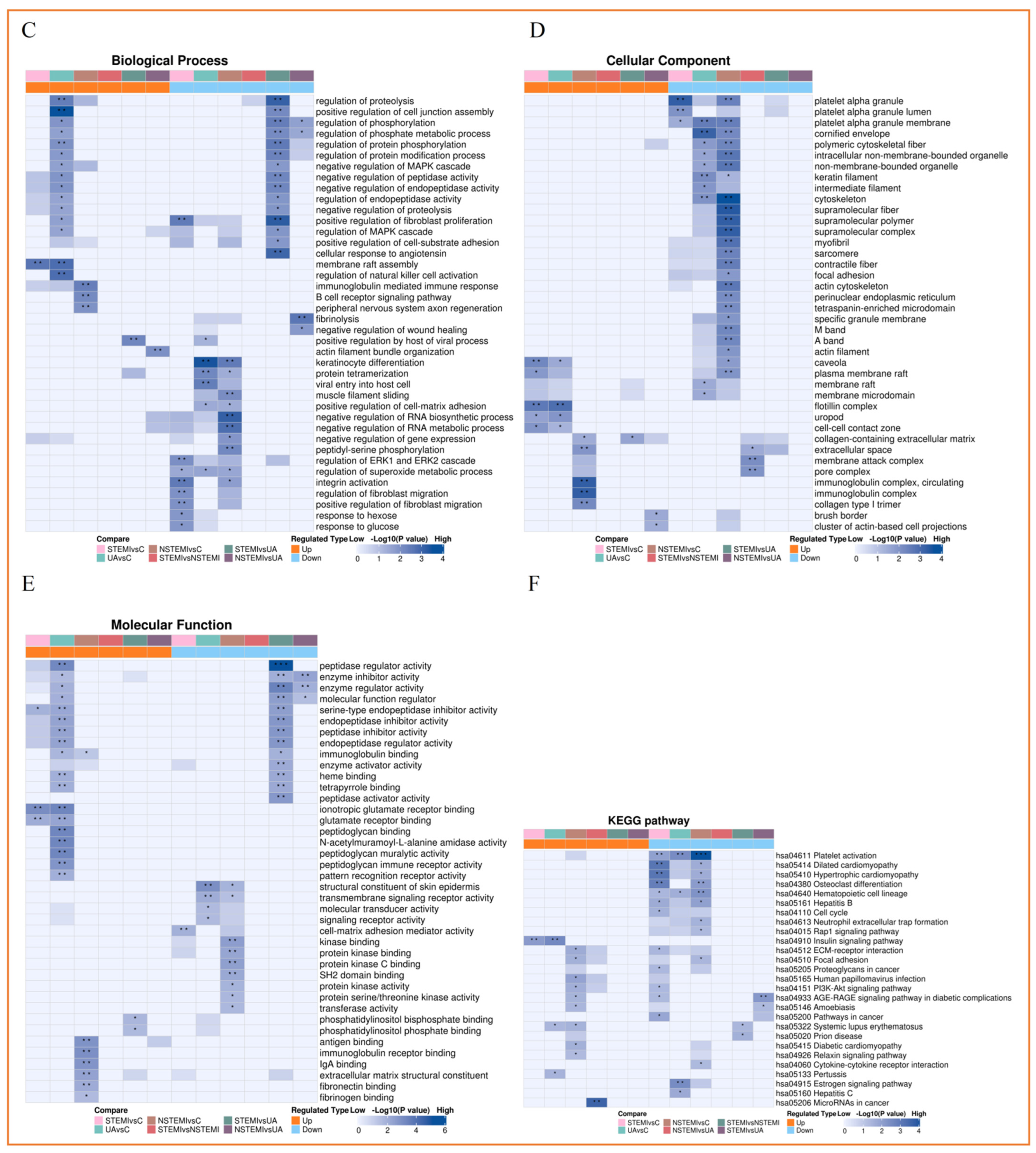
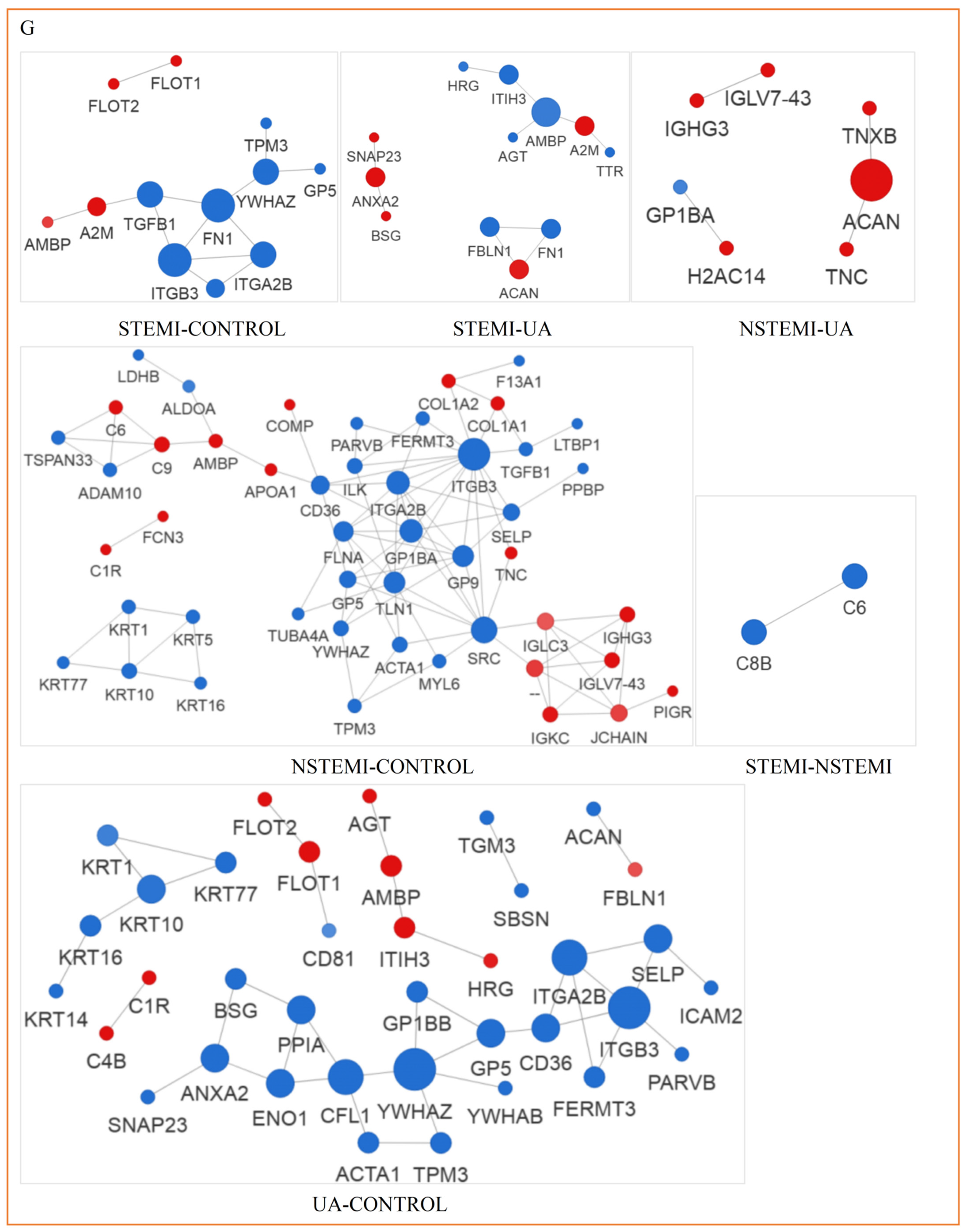
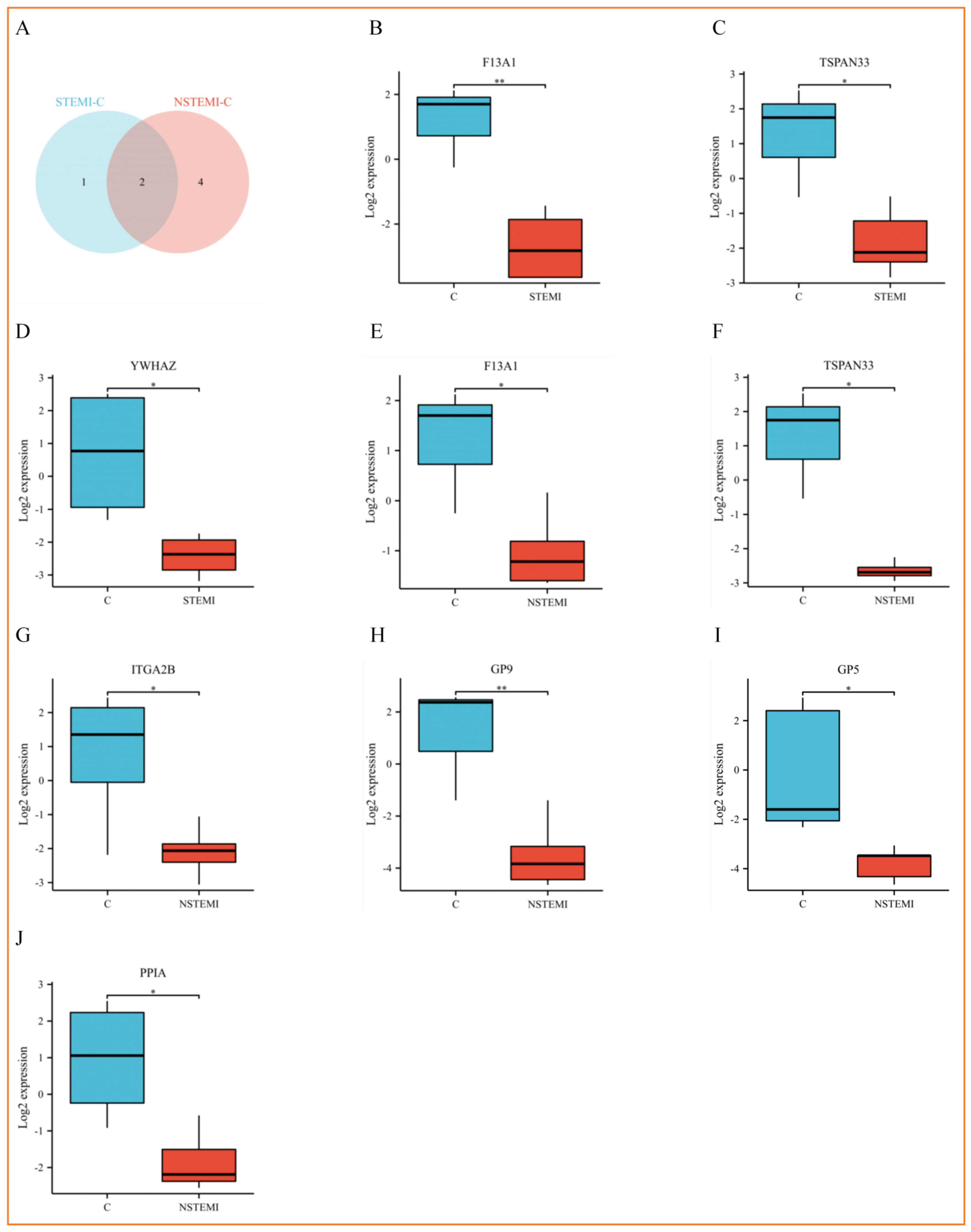
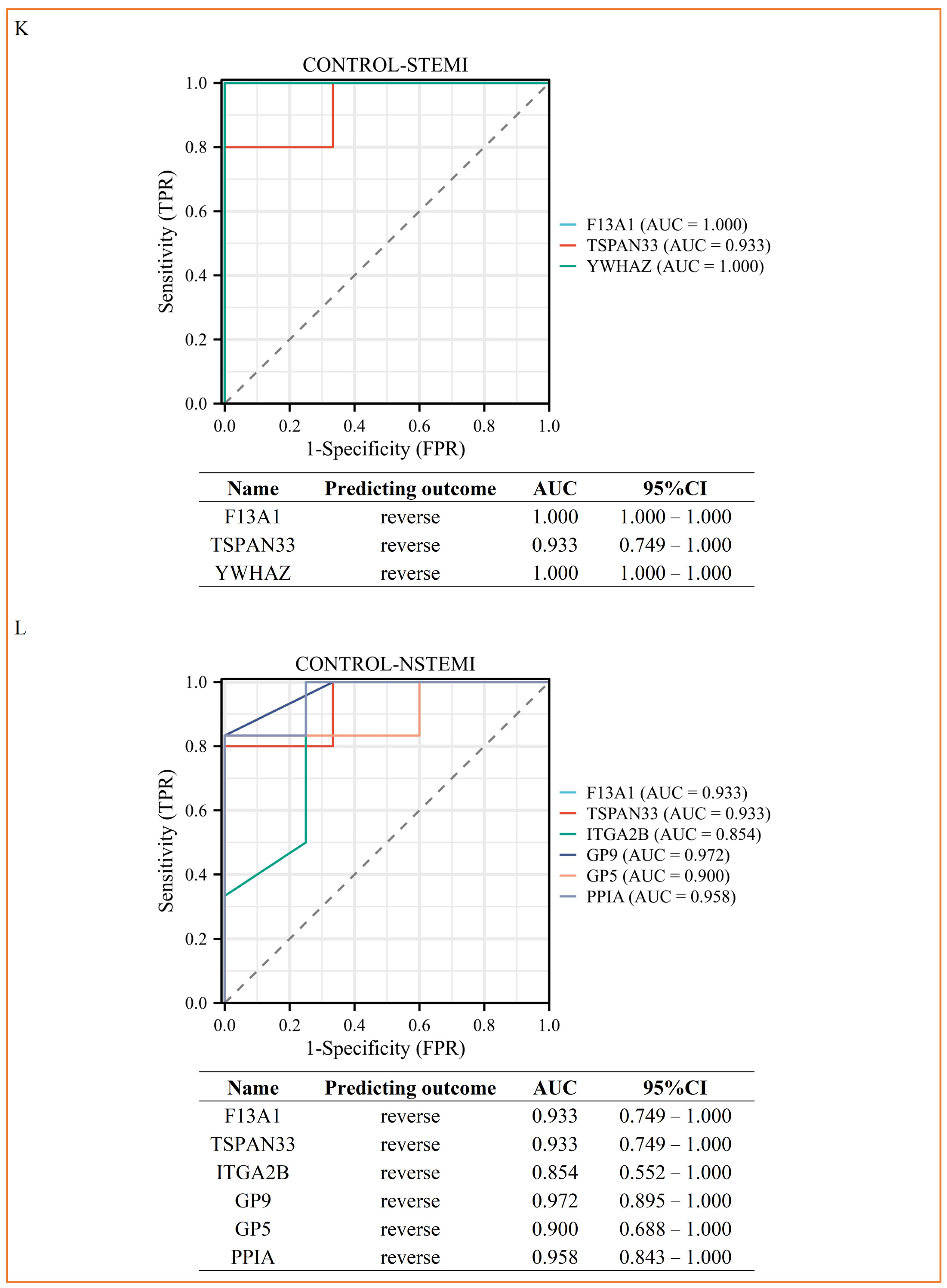
| CONTROL | STEMI | NSTEMI | UA | p | |
|---|---|---|---|---|---|
| (n = 11) | (n = 11) | (n = 14) | (n = 5) | ||
| Age, median (IQR) | 62.00 (60.00–62.50) | 62.00 (58.00–64.50) | 64.50 (59.75–70.00) | 62.00 (59.00–63.00) | 0.147 |
| Male, n (%) | 6 (54.5%) | 6 (54.5%) | 7 (50%) | 3 (60%) | 0.983 |
| BMI, kg/m2, median (IQR) | 25.22 (23.22–28.17) | 23.88 (23.56–27.25) | 24.36 (23.27–25.33) | 28.32 (27.73–28.41) | 0.079 |
| Risk factors, n (%) | |||||
| Hypertension, n (%) | 7 (63.6%) | 8 (72.7%) | 9 (64.3%) | 4 (80.0%) | 0.889 |
| Diabetes, n (%) | 6 (54.5%) | 3 (27.3%) | 6 (42.9%) | 4 (80.0%) | 0.235 |
| Dyslipidemia, n (%) | 4 (36.4%) | 2 (18.2%) | 1 (7.1%) | 1 (20.0%) | 0.339 |
| Current smoker, n (%) | 4 (36.4%) | 4 (36.4%) | 3 (21.4%) | 0 (0%) | 0.382 |
| Cardiac Biomarkers, median (IQR) | |||||
| Myo (ng/mL) | NA | 423.00 (261.00–500.00) | 267.00 (201.75–500.00) | 113.00 (74.20–137.00) | 0.055 |
| CKMB (ng/mL) | NA | 15.90 (2.65–59.35) | 4.55 (3.03–9.60) | 1.70 (1.10–2.10) | 0.067 |
| hs-cTnI (ng/mL) | NA | 0.330 (0.115–4.330) | 0.150 (0.050–1.435) | 0.050 (0.050–0.050) | 0.057 |
| BNP (pg/mL) | NA | 33.00 (21.50–55.80) | 342.00 (96.55–811.00) | 120.00 (10.30–123.00) | 0.013 |
| D-Dimer(ng/mL) | NA | 240.0 (127.5–576.0) | 463.0 (100.0–1389.2) | 355.0 (207.0–480.0) | 0.884 |
| Vessel lesion, n (%) | |||||
| Single-vessel disease | 0 (0%) | 2 (18.2%) | 0 (0%) * | 1 (25.0%) * | 0.301 |
| Multi-vessel disease | 0 (0%) | 9 (81.8%) | 9 (90.0%) * | 3 (75.0%) * | 0.760 |
| Laboratory data | |||||
| Blood glucose (mmol/L) | 6.05 (5.15–8.53) | 8.60 (6.20–9.90) | 9.85 (7.15–12.15) | 7.40 (6.80–10.70) | 0.113 |
| ALT (U/L) | 24.00 (19.25–28.50) | 32.00 (19.50–59.00) | 24.00 (14.50–39.50) | 23.00 (23.00–35.00) | 0.608 |
| AST (U/L) | 22.00 (19.25–23.00) | 108.00 (43.00–197.00) | 48.50 (24.25–86.25) | 23.00 (17.00–37.00) | <0.001 |
| AST/ALT | 0.90 (0.80–1.00) | 2.80 (2.50–3.80) | 1.85 (1.23–3.18) | 1.00 (1.00–1.10) | <0.001 |
| GGT (U/L) | 21.00 (16.00–26.75) | 31.00 (17.00–40.00) | 22.00 (13.75–34.50) | 22.00 (18.00–27.00) | 0.809 |
| TP (g/L) | 74.50 (68.50–75.75) | 65.00 (63.50–68.50) | 64.00 (59.50–66.50) | 63.00 (62.00–69.00) | 0.006 |
| TBIL (μmol/L) | 11.40 (8.45–12.93) | 9.30 (6.40–12.95) | 8.95 (7.83–13.73) | 8.30 (5.00–13.90) | 0.899 |
| ALP (U/L) | 73.00 (63.75–86.50) | 87.00 (72.50–102.00) | 74.50 (61.25–89.00) | 74.00 (74.00–92.00) | 0.567 |
| Urea (mmol/L) | 5.40 (4.60–5.95) | 6.10 (5.65–7.45) | 7.00 (5.40–10.38) | 7.90 (6.10–9.50) | 0.0498 |
| Uric acid (μmol/L) | 335.00 (247.50–351.5) | 349.00 (311.00–376.00) | 385.50 (312.25–454.5) | 383.00 (349.00–437.00) | 0.142 |
| CREA (μmol/L) | 60.00 (50.50–64.50) | 60.00 (56.50–67.00) | 83.50 (64.25–106.00) | 65.00 (60.00–93.00) | 0.075 |
| Total cholesterol (mmol/L) | 5.50 (3.98–6.38) | 4.50 (3.85–4.90) | 3.75 (3.20–4.68) | 4.30 (4.10–5.60) | 0.081 |
| TG (mmol/L) | 1.40 (1.02–1.78) | 1.60 (1.22–1.86) | 1.13 (0.76–1.88) | 1.65 (1.46–3.67) | 0.216 |
| HDL-C (mmol/L) | 1.16 (1.04–1.25) | 1.00 (0.84–1.16) | 0.92 (0.76–1.09) | 0.80 (0.77–0.96) | 0.125 |
| LDL-C (mmol/L) | 3.77 (2.31–4.49) | 2.95 (2.14–3.30) | 2.30 (1.89–2.80) | 2.66 (2.35–2.98) | 0.061 |
| PLT(×109/L) | 242.00 (212.50–291.50) | 242.00 (185.00–277.00) | 207.00 (158.00–229.25) | 247.00 (234.00–252.00) | 0.292 |
| Length of stay (days) | 1.00 (1.00–2.00) | 8.00 (6.50–9.00) | 6.00 (4.25–7.75) | 6.00 (6.00–13.00) | 0.0494 |
| Comparison | Protein Name | Gene Name | Discovery Phase | Validation Phase | ||
|---|---|---|---|---|---|---|
| FC | p Value | FC(PRM/PRO) | p Value | |||
| CONTROL-STEMI | Coagulation factor XIII A chain | F13A1 | 0.466204068 | 0.040562299 | 0.07/0.47 | 8.05 × 10−3 |
| Tetraspanin-33 | TSPAN33 | 0.001 | 0.001 | 0.1/0 | 1.25 × 10−2 | |
| 14-3-3 protein zeta/delta | YWHAZ | 0.277126634 | 0.017480742 | 0.07/0.28 | 4.93 × 10−2 | |
| CONTROL-NSTEMI | Coagulation factor XIII A chain | F13A1 | 0.416860933 | 0.011631169 | 0.2/0.42 | 1.86 × 10−2 |
| Tetraspanin-33 | TSPAN33 | 0.001 | 0.001 | 0.09/0 | 1.08 × 10−2 | |
| Integrin alpha-IIb | ITGA2B | 0.090198556 | 0.000373439 | 0.09/0.09 | 1.35 × 10−2 | |
| Platelet glycoprotein IX | GP9 | 0.001 | 0.001 | 0.03/0 | 3.94 × 10−3 | |
| Platelet glycoprotein V | GP5 | 0.001 | 0.001 | 0.05/0 | 2.22 × 10−2 | |
| Peptidyl-prolyl cis-trans isomerase A | PPIA | 0.33860234 | 0.022349546 | 0.11/0.34 | 3.68 × 10−2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Hou, H.-T.; Chen, H.-X.; Song, Y.; Zhou, X.-L.; Zhang, L.-L.; Xue, H.-M.; Yang, Q.; He, G.-W. Plasma Exosomal Proteomics Identifies Differentially Expressed Proteins as Biomarkers for Acute Myocardial Infarction. Biomolecules 2025, 15, 583. https://doi.org/10.3390/biom15040583
Zhou J, Hou H-T, Chen H-X, Song Y, Zhou X-L, Zhang L-L, Xue H-M, Yang Q, He G-W. Plasma Exosomal Proteomics Identifies Differentially Expressed Proteins as Biomarkers for Acute Myocardial Infarction. Biomolecules. 2025; 15(4):583. https://doi.org/10.3390/biom15040583
Chicago/Turabian StyleZhou, Jie, Hai-Tao Hou, Huan-Xin Chen, Yu Song, Xiao-Lin Zhou, Li-Li Zhang, Hong-Mei Xue, Qin Yang, and Guo-Wei He. 2025. "Plasma Exosomal Proteomics Identifies Differentially Expressed Proteins as Biomarkers for Acute Myocardial Infarction" Biomolecules 15, no. 4: 583. https://doi.org/10.3390/biom15040583
APA StyleZhou, J., Hou, H.-T., Chen, H.-X., Song, Y., Zhou, X.-L., Zhang, L.-L., Xue, H.-M., Yang, Q., & He, G.-W. (2025). Plasma Exosomal Proteomics Identifies Differentially Expressed Proteins as Biomarkers for Acute Myocardial Infarction. Biomolecules, 15(4), 583. https://doi.org/10.3390/biom15040583







