Metabolomics Insights into Salivary Profile in Dogs with Babesia canis Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Untargeted Metabolomics Analysis
2.2.1. Sample Preparation and LC-MS/MS Analysis
2.2.2. Data Processing for Untargeted Metabolomics
2.3. Targeted Metabolomics Analysis
2.3.1. Sample Preparation and Mass Spectroscopy Analysis (FIA-MS/MS and LC-MS/MS)
2.3.2. Data Processing for Targeted Metabolomics
2.4. Statistical and Bioinformatic Analysis
3. Results
3.1. Untargeted Metabolomics
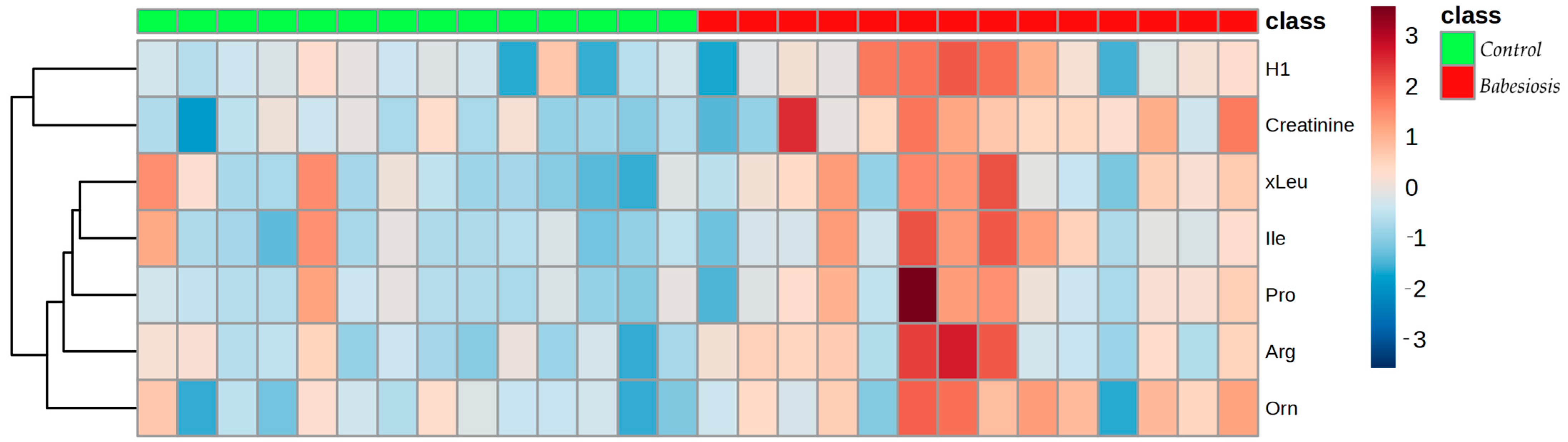
3.2. Targeted Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFadden, G.I.; Reith, M.E.; Munholland, J.; Lang-Unnasch, N. Plastid in human parasites. Nature 1996, 381, 482. [Google Scholar] [CrossRef]
- Bilić, P.; Kuleš, J.; Barić Rafaj, R.; Mrljak, V. Canine Babesiosis: Where Do We Stand? Acta Vet. Brno. 2018, 68, 127. [Google Scholar] [CrossRef]
- Beri, D.; Singh, M.; Rodriguez, M.; Goyal, N.; Rasquinha, G.; Liu, Y.; An, X.; Yazdanbakhsh, K.; Lobo, C.A. Global Metabolomic Profiling of Host Red Blood Cells Infected with Babesia divergens Reveals Novel Antiparasitic Target Pathways. Microbiol. Spectr. 2023, 11, e0468822. [Google Scholar] [CrossRef] [PubMed]
- Matijatko, V.; Torti, M.; Schetters, T.P. Canine babesiosis in Europe: How many diseases? Trends Parasitol. 2012, 28, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Koster, L.S.; Lobetti, R.G.; Kelly, P. Canine babesiosis: A perspective on clinical complications, biomarkers, and treatment. Vet. Med. Res. Rep. 2015, 6, 119–128. [Google Scholar]
- Kafsack, B.F.C.; Llinás, M. Eating at the Table of Another: Metabolomics of Host–parasite Interactions. Cell Host Microbe 2010, 7, 90–99. [Google Scholar] [CrossRef]
- Wang, Y.; Holmes, E.; Nicholson, J.K.; Cloarec, O.; Chollet, J.; Tanner, M.; Singer, B.H.; Utzinger, J. Metabonomic investigations in mice infected with Schistosoma mansoni: An approach for biomarker identification. Proc. Natl. Acad. Sci. USA 2004, 101, 12676–12681. [Google Scholar] [CrossRef]
- Martin, F.P.; Verdu, E.F.; Wang, Y.; Dumas, M.E.; Yap, I.K.; Cloarec, O.; Bergonzelli, G.E.; Corthesy-Theulaz, I.; Kochhar, S.; Holmes, E.; et al. Transgenomic metabolic interactions in a mouse disease model: Interactions of Trichinella spiralis infection with dietary Lactobacillus paracasei supplementation. J. Proteome Res. 2006, 5, 2185–2193. [Google Scholar] [CrossRef]
- Li, J.V.; Wang, Y.; Saric, J.; Nicholson, J.K.; Dirnhofer, S.; Singer, B.H.; Tanner, M.; Wittlin, S.; Holmes, E.; Utzinger, J. Global metabolic responses of NMRI mice to an experimental Plasmodium berghei infection. J. Proteome Res. 2008, 7, 3948–3956. [Google Scholar] [CrossRef]
- Brayton, K.A.; Lau, A.O.T.; Herndon, D.R.; Hannick, L.; Kappmeyer, L.S.; Berens, S.J.; Bidwell, S.L.; Brown, W.C.; Crabtree, J.; Fadrosh, D.; et al. Genome Sequence of Babesia bovis and Comparative Analysis of Apicomplexan Hemoprotozoa. PLoS Pathog. 2007, 3, e148. [Google Scholar] [CrossRef]
- Shen, L.; Wang, C.; Wang, R.; Hu, X.; Liao, S.; Liu, W.; Du, A.; Ji, S.; Galon, E.M.; Li, H.; et al. Serum metabolomic profiles in BALB/c mice induced by Babesia microti infection. Front. Cell. Infect. Microbiol. 2023, 13, 1179967. [Google Scholar] [CrossRef] [PubMed]
- Rubić, I.; Burchmore, R.; Weidt, S.; Regnault, C.; Kuleš, J.; Barić Rafaj, R.; Mašek, T.; Horvatić, A.; Crnogaj, M.; Eckersall, P.D.; et al. Multi Platforms Strategies and Metabolomics Approaches for the Investigation of Comprehensive Metabolite Profile in Dogs with Babesia canis Infection. Int. J. Mol. Sci. 2022, 23, 1575. [Google Scholar] [CrossRef] [PubMed]
- Kuleš, J.; Rubić, I.; Beer Ljubić, B.; Bilić, P.; Barić Rafaj, R.; Brkljačić, M.; Burchmore, R.; Eckersall, D.; Mrljak, V. Combined Untargeted and Targeted Metabolomics Approaches Reveal Urinary Changes of Amino Acids and Energy Metabolism in Canine Babesiosis With Different Levels of Kidney Function. Front. Microbiol. 2021, 12, 715701. [Google Scholar] [CrossRef]
- Yoshizawa Janice, M.; Schafer Christopher, A.; Schafer Jason, J.; Farrell James, J.; Paster Bruce, J.; Wong David, T.W. Salivary Biomarkers: Toward Future Clinical and Diagnostic Utilities. Clin. Microbiol. Rev. 2013, 26, 781–791. [Google Scholar] [CrossRef]
- Drobitch, R.K.; Svensson, C.K. Therapeutic Drug Monitoring in Saliva. Clin. Pharmacokinet. 1992, 23, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Haeckel, R.; Hänecke, P. The application of saliva, sweat and tear fluid for diagnostic purposes. Ann. Biol. Clin. 1993, 51, 903–910. [Google Scholar]
- Jusko, W.J.; Milsap, R.L. Pharmacokinetic Principles of Drug Distribution in Saliva. Ann. N. Y. Acad. Sci. 1993, 694, 36–47. [Google Scholar] [CrossRef]
- González-Arostegui, L.G.; Rubio, C.P.; Rubić, I.; Rafaj, R.B.; Gotić, J.; Cerón, J.J.; Tvarijonaviciute, A.; Mrljak, V.; Muñoz-Prieto, A. Changes in the salivary metabolome in canine hypothyroidism: A pilot study. Res. Vet. Sci. 2022, 151, 189–195. [Google Scholar] [CrossRef]
- Ploypetch, S.; Luo, X.; Zhao, S.; Roytrakul, S.; Li, L.; Suriyaphol, G. Salivary metabolomic identification of biomarker candidates for oral melanoma and oral squamous cell carcinoma in dogs. J. Vet. Intern. Med. 2024, 38, 2293–2304. [Google Scholar] [CrossRef]
- Muñoz-Prieto, A.; Rubić, I.; Horvatić, A.; Rafaj, R.B.; Cerón, J.J.; Tvarijonaviciute, A.; Mrljak, V. Evaluation of Changes in Metabolites of Saliva in Canine Obesity Using a Targeted Metabolomic Approach. Animals 2021, 11, 2501. [Google Scholar] [CrossRef]
- Turunen, S.; Puurunen, J.; Auriola, S.; Kullaa, A.; Kärkkäinen, O.; Lohi, H.; Hanhineva, K. Metabolome of canine and human saliva: A non-targeted metabolomics study. Metabolomics 2020, 16, 90. [Google Scholar] [CrossRef] [PubMed]
- Carlos, G.; dos Santos, F.P.; Fröehlich, P.E. Canine metabolomics advances. Metabolomics 2020, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.; Vojta, L.; Mrljak, V.; Marinculić, A.; Beck, A.; Živičnjak, T.; Cacciò, S.M. Diversity of Babesia and Theileria species in symptomatic and asymptomatic dogs in Croatia. Int. J. Parasitol. 2009, 39, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, Y.; Morton, F.; Daly, R.; Gurden, R.; Rogers, S.; Wandy, J.; Wilson, D.; Barrett, M.; Burgess, K.; Wren, J. PiMP my metabolome: An integrated, web-based tool for LC-MS metabolomics data. Bioinformatics 2017, 33, 4007–4009. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; E MacDonald, P.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Babbitt, S.E.; Altenhofen, L.; Cobbold, S.A.; Istvan, E.S.; Fennell, C.; Doerig, C.; Llinás, M.; Goldberg, D.E. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc. Natl. Acad. Sci. USA 2012, 109, E3278–E3287. [Google Scholar] [CrossRef]
- Goldberg, D.E. Complex nature of malaria parasite hemoglobin degradation. Proc. Natl. Acad. Sci. USA 2013, 110, 5283–5284. [Google Scholar] [CrossRef]
- Krishnan, A.; Soldati-Favre, D. Amino Acid Metabolism in Apicomplexan Parasites. Metabolites 2021, 11, 61. [Google Scholar] [CrossRef]
- Gafan, C.; Wilson, J.; Berger, L.C.; Berger, B.J. Characterization of the ornithine aminotransferase from Plasmodium falciparum. Mol. Biochem. Parasitol. 2001, 118, 1–10. [Google Scholar] [CrossRef]
- Jortzik, E.; Fritz-Wolf, K.; Sturm, N.; Hipp, M.; Rahlfs, S.; Becker, K. Redox Regulation of Plasmodium falciparum Ornithine δ-Aminotransferase. J. Mol. Biol. 2010, 402, 445–459. [Google Scholar] [CrossRef]
- Kuleš, J.; Bilić, P.; Beer Ljubić, B.; Gotić, J.; Crnogaj, M.; Brkljačić, M.; Mrljak, V. Glomerular and tubular kidney damage markers in canine babesiosis caused by Babesia canis. Ticks Tick Borne Dis. 2018, 9, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Torti, M.; Kuleš, J.; Matijatko, V.; Brkljačić, M.; Kiš, I.; Gotić, J.; Mrljak, V.; Šmit, I. Acid-base status in canine babesiosis caused by Babesia canis. Vet. Arh. 2020, 90, 603–610. [Google Scholar]
- Kropf, P.; Fuentes, J.M.; Fähnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Müller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005, 19, 1000–1002. [Google Scholar] [CrossRef]
- Sherman, I.W.; Eda, S.; Winograd, E. Cytoadherence and sequestration in Plasmodium falciparum: Defining the ties that bind. Microbes Infect. 2003, 5, 897–909. [Google Scholar] [CrossRef]
- Olszewski, K.L.; Morrisey, J.M.; Wilinski, D.; Burns, J.M.; Vaidya, A.B.; Rabinowitz, J.D.; Llinás, M. Host–parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe 2009, 5, 191–199. [Google Scholar] [CrossRef]
- Barić Rafaj, R.; Kuleš, J.; Selanec, J.; Vrkić, N.; Zovko, V.; Zupančič, M.; Trampuš Bakija, A.; Matijatko, V.; Crnogaj, M.; Mrljak, V. Markers of coagulation activation, endothelial stimulation, and inflammation in dogs with babesiosis. J. Vet. Intern. Med. 2013, 27, 1172–1178. [Google Scholar] [CrossRef]
- Martin, R.E.; Kirk, K. Transport of the essential nutrient isoleucine in human erythrocytes infected with the malaria parasite Plasmodium falciparum. Blood 2006, 109, 2217–2224. [Google Scholar] [CrossRef]
- McLean, K.J.; Jacobs-Lorena, M. The response of Plasmodium falciparum to isoleucine withdrawal is dependent on the stage of progression through the intraerythrocytic cell cycle. Malar. J. 2020, 19, 147. [Google Scholar] [CrossRef]
- Prior, K.F.; Middleton, B.; Owolabi, A.T.Y.; Westwood, M.L.; Holland, J.; O’Donnell, A.J.; Blackman, M.; Skene, D.J.; Reece, S.E. Synchrony between daily rhythms of malaria parasites and hosts is driven by an essential amino acid. bioRxiv 2021. bioRxiv:12020.2008.2024.264689. [Google Scholar] [CrossRef]
- Zhenyukh, O.; González-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Zygner, W.; Gójska-Zygner, O.; Bąska, P.; Długosz, E. Increased concentration of serum TNF alpha and its correlations with arterial blood pressure and indices of renal damage in dogs infected with Babesia canis. Parasitol. Res. 2014, 113, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Goddard, A.; Leisewitz, A.L.; Kjelgaard-Hansen, M.; Kristensen, A.T.; Schoeman, J.P. Excessive Pro-Inflammatory Serum Cytokine Concentrations in Virulent Canine Babesiosis. PLoS ONE 2016, 11, e0150113. [Google Scholar] [CrossRef]
- Galán, A.; Mayer, I.; Rafaj, R.B.; Bendelja, K.; Sušić, V.; Cerón, J.J.; Mrljak, V. MCP-1, KC-like and IL-8 as critical mediators of pathogenesis caused by Babesia canis. PLoS ONE 2018, 13, e0190474. [Google Scholar] [CrossRef]
- Wagner, I.; Musso, H. New Naturally Occurring Amino Acids. Angew. Chem. Int. Ed. Engl. 1983, 22, 816–828. [Google Scholar] [CrossRef]
- Xuan, M.; Gu, X.; Li, J.; Huang, D.; Xue, C.; He, Y. Polyamines: Their significance for maintaining health and contributing to diseases. Cell Commun. Signal. 2023, 21, 348. [Google Scholar] [CrossRef]
- Lobetti, R.G.; Reyers, F.; Nesbit, J.W. The comparative role of haemoglobinaemia and hypoxia in the development of canine babesial nephropathy. J. S. Afr. Vet. Assoc. 1996, 67, 188–198. [Google Scholar]
- Jacobson, L.S.; Lobetti, R.G.; Vaughan-Scott, T. Blood pressure changes in dogs with babesiosis. J. S. Afr. Vet. Assoc. 2000, 71, 7. [Google Scholar] [CrossRef]
- Zygner, W.; Gójska-Zygner, O. Association between decreased blood pressure and azotaemia in canine babesiosis. Pol. J. Vet. Sci. 2014, 17, 3. [Google Scholar]
- Temilola, D.O.; Bezuidenhout, K.; Erasmus, R.T.; Stephen, L.; Davids, M.R.; Holmes, H. Salivary creatinine as a diagnostic tool for evaluating patients with chronic kidney disease. BMC Nephrol. 2019, 20, 387. [Google Scholar] [CrossRef]
- Lasisi, T.J.; Raji, Y.R.; Salako, B.L. Salivary creatinine and urea analysis in patients with chronic kidney disease: A case control study. BMC Nephrol. 2016, 17, 10. [Google Scholar] [CrossRef]
- Tewari, S.G.; Swift, R.P.; Reifman, J.; Prigge, S.T.; Wallqvist, A. Metabolic alterations in the erythrocyte during blood-stage development of the malaria parasite. Malar. J. 2020, 19, 94. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, K.; Tewari, S.G.; Wallqvist, A.; Prigge, S.T. Metabolic changes accompanying the loss of fumarate hydratase and malate–quinone oxidoreductase in the asexual blood stage of Plasmodium falciparum. J. Biol. Chem. 2022, 298, 101897. [Google Scholar] [CrossRef]
- Olson, W.J.; Martorelli Di Genova, B.; Gallego-Lopez, G.; Dawson, A.R.; Stevenson, D.; Amador-Noguez, D.; Knoll, L.J. Dual metabolomic profiling uncovers Toxoplasma manipulation of the host metabolome and the discovery of a novel parasite metabolic capability. PLoS Pathog. 2020, 16, e1008432. [Google Scholar] [CrossRef]
- Cornillot, E.; Hadj-Kaddour, K.; Dassouli, A.; Noel, B.; Ranwez, V.; Vacherie, B.; Augagneur, Y.; Brès, V.; Duclos, A.; Randazzo, S.; et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti†. Nucleic Acids Res. 2012, 40, 9102–9114. [Google Scholar] [CrossRef]
- Bryant, C.; Voller, A.; Smith, M.J. The incorporation of Radioactivity From (14C)Glucose into the Soluble Metabolic Intermediates of Malaria Parasites. Am. J. Trop. Med. Hyg. 1964, 13, 515–519. [Google Scholar] [CrossRef]
- Jacot, D.; Waller, R.F.; Soldati-Favre, D.; MacPherson, D.A.; MacRae, J.I. Apicomplexan Energy Metabolism: Carbon Source Promiscuity and the Quiescence Hyperbole. Trends Parasitol. 2016, 32, 56–70. [Google Scholar] [CrossRef]
- MacRae, J.I.; Dixon, M.W.; Dearnley, M.K.; Chua, H.H.; Chambers, J.M.; Kenny, S.; Bottova, I.; Tilley, L.; McConville, M.J. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013, 11, 67. [Google Scholar] [CrossRef]
- Slavic, K.; Straschil, U.; Reininger, L.; Doerig, C.; Morin, C.; Tewari, R.; Krishna, S. Life cycle studies of the hexose transporter of Plasmodium species and genetic validation of their essentiality. Mol. Microbiol. 2010, 75, 1402–1413. [Google Scholar] [CrossRef]
- Blume, M.; Hliscs, M.; Rodriguez-Contreras, D.; Sanchez, M.; Landfear, S.; Lucius, R.; Matuschewski, K.; Gupta, N. A constitutive pan-hexose permease for the Plasmodium life cycle and transgenic models for screening of antimalarial sugar analogs. FASEB J. 2011, 25, 1218–1229. [Google Scholar] [CrossRef]
- Salcedo-Sora, J.E.; Caamano-Gutierrez, E.; Ward, S.A.; Biagini, G.A. The proliferating cell hypothesis: A metabolic framework for Plasmodium growth and development. Trends Parasitol. 2014, 30, 170–175. [Google Scholar] [CrossRef]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Rep. 2020, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Crnogaj, M.; Cerón, J.J.; Šmit, I.; Kiš, I.; Gotić, J.; Brkljačić, M.; Matijatko, V.; Rubio, C.P.; Kučer, N.; Mrljak, V. Relation of antioxidant status at admission and disease severity and outcome in dogs naturally infected with Babesia canis canis. BMC Vet. Res. 2017, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Crnogaj, M.; Petlevski, R.; Mrljak, V.; Kis, I.; Torti, M.; Kucer, N.; Matijatko, V.; Sacer, I.; Stokovic, I. Malondialdehyde levels in serum of dogs infected with Babesia canis. Vet. Med. 2010, 55, 163–171. [Google Scholar] [CrossRef]
- Rossi, G.; Kuleš, J.; Rafaj, R.B.; Mrljak, V.; Lauzi, S.; Giordano, A.; Paltrinieri, S. Relationship between paraoxonase 1 activity and high density lipoprotein concentration during naturally occurring babesiosis in dogs. Res. Vet. Sci. 2014, 97, 318–324. [Google Scholar] [CrossRef]
- Jurecka, A. Inborn errors of purine and pyrimidine metabolism. J. Inherit. Metab. Dis. 2009, 32, 247–263. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Irvin, A.D.; Young, E.R.; Purnell, R.E. The in vitro uptake of tritiated nucleic acid precursors by Babesia spp. of cattle and mice. Int. J. Parasitol. 1978, 8, 19–24. [Google Scholar] [CrossRef]
- Wannemacher, R.W., Jr.; Klainer, A.S.; Dinterman, R.E.; Beisel, W.R. The significance and mechanism of an increased serum phenylalanine-tyrosine ratio during infection. Am. J. Clin. Nutr. 1976, 29, 997–1006. [Google Scholar] [CrossRef]
- Scholl-Bürgi, S.; Schroecksnadel, S.; Jenny, M.; Karall, D.; Fuchs, D. Chronic Immune Stimulation May Cause Moderate Impairment of Phenylalanine 4-hydroxylase. Pteridines 2011, 22, 120–125. [Google Scholar] [CrossRef]
- Birungi, G.; Achar, J.B.; Byamugisha, D. Characterization of urinary metabolites associated with malaria infection using infra-red spectroscopy and liquid chromatography–mass spectrometry in South Western Uganda. Discov. Appl. Sci. 2024, 6, 356. [Google Scholar] [CrossRef]
- Basant, A.; Rege, M.; Sharma, S.; Sonawat, H.M. Alterations in urine, serum and brain metabolomic profiles exhibit sexual dimorphism during malaria disease progression. Malar. J. 2010, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.N.; Brown, S.H.J.; Rug, M.; Ridgway, M.C.; Mitchell, T.W.; Maier, A.G. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Florin-Christensen, J.; Suarez, C.E.; Florin-Christensen, M.; Hines, S.A.; McElwain, T.F.; Palmer, G.H. Phosphatidylcholine formation is the predominant lipid biosynthetic event in the hemoparasite Babesia bovis. Mol. Biochem. Parasitol. 2000, 106, 147–156. [Google Scholar] [CrossRef] [PubMed]

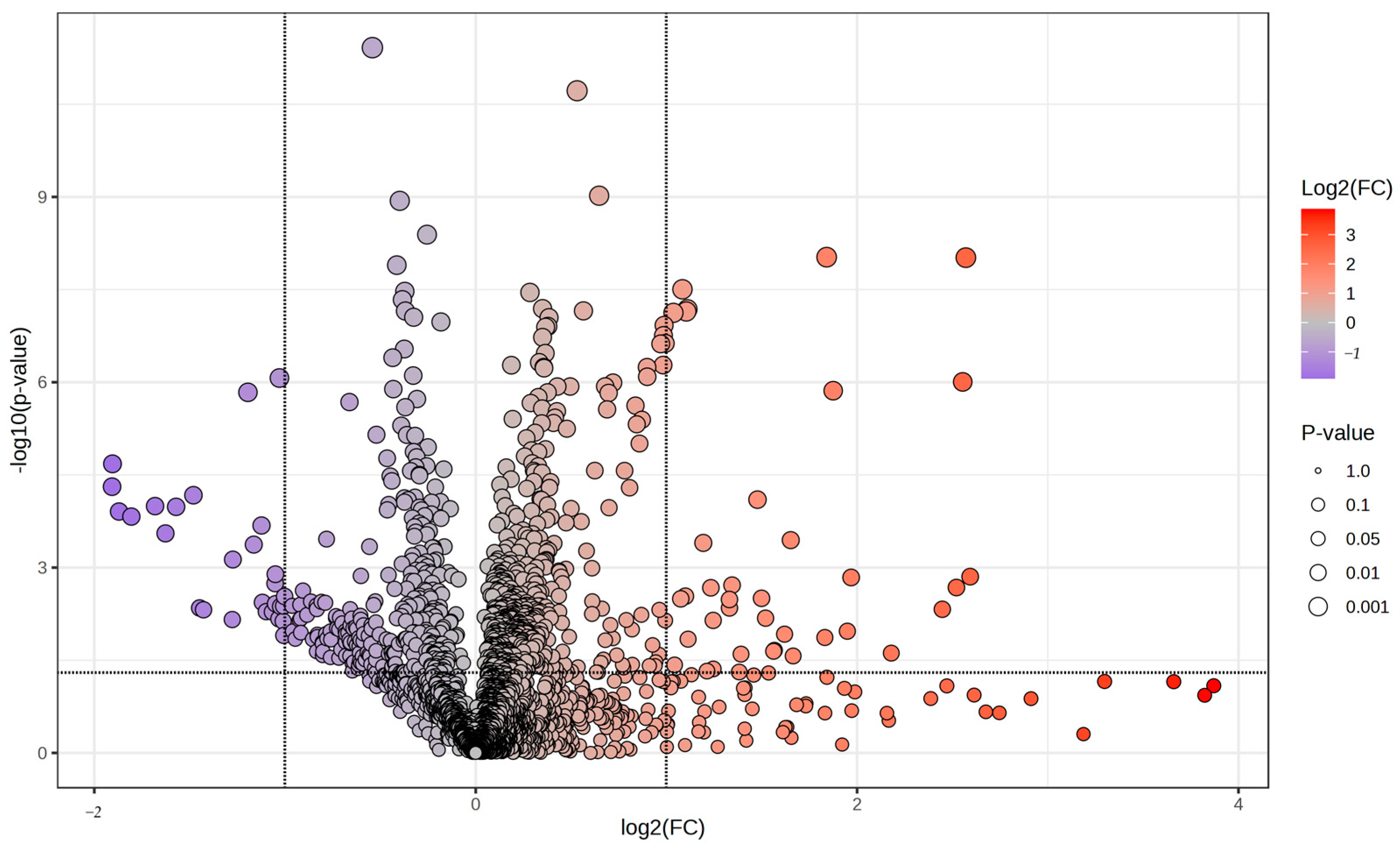


| Peak ID | FrAnK Annotation | Mass | RT | Polarity | log2(FC) | p Value |
|---|---|---|---|---|---|---|
| 25 | Adenine | 136.0619 | 502.68 | positive | −0.79 | 0.012 |
| 64 | Hypoxanthine | 137.0459 | 556.11 | positive | −1.63 | 0.000 |
| 66 | Inosine | 269.0881 | 556.45 | positive | −1.68 | 0.000 |
| 686 | Cytidine | 244.0928 | 599.26 | positive | −1.05 | 0.002 |
| 1493 | Inosine | 267.0738 | 555.21 | negative | −1.57 | 0.000 |
| 1998 | Cysteic acid | 167.9975 | 736.79 | negative | −1.16 | 0.000 |
| 6 | Betaine | 118.0862 | 559 | positive | −0.42 | 0.048 |
| 1378 | Malonate | 103.0039 | 727.34 | negative | 1.25 | 0.044 |
| Metabolite | Babesiosis Group | Control Group | ||
|---|---|---|---|---|
| (μmol dm−³) | Mean | SD | Mean | SD |
| AC (0:0) | 24.19 | 13.86 | NA | NA |
| AC (4:0) | NA | NA | 0.61 | 0.28 |
| alpha-AAA | 13.28 | 28.36 | NA | NA |
| Cer (34:1) | NA | NA | 0.07 | 0.06 |
| Cer (42:1) | 2.69 | 1.65 | NA | NA |
| LPC (17:0) | 2.78 | 1.76 | NA | NA |
| LPC (20:3) | 0.33 | 0.32 | NA | NA |
| Lys | 95.08 | 159.55 | NA | NA |
| PC (25:0) | NA | NA | 0.05 | 0.02 |
| PC (31:2) | 0.31 | 0.23 | NA | NA |
| PC (32:6) | NA | NA | 0.28 | 0.14 |
| PC (33:2) | 1.18 | 0.41 | NA | NA |
| PC (37:5) | NA | NA | 0.28 | 0.09 |
| PC (40:8) | 1.40 | 1.36 | NA | NA |
| PC-O (34:4) | 0.45 | 0.27 | NA | NA |
| Phe | 41.81 | 58.23 | NA | NA |
| SM (32:2) | NA | NA | 0.32 | 0.19 |
| SM (38:1) | 4.30 | 3.79 | NA | NA |
| t4-OH-Pro | NA | NA | 5.46 | 4.94 |
| TG (51:1) | 0.65 | 0.23 | NA | NA |
| TG (52:7) | 1.77 | 0.79 | NA | NA |
| Metabolite Name | p Value | log2(FC) |
|---|---|---|
| Creatinine | 0.002 | 1.63 |
| Ornithine | 0.005 | 1.38 |
| Arginine | 0.008 | 2.09 |
| Isoleucine | 0.025 | 0.99 |
| H1 (hexoses, including glucose) | 0.035 | 2.26 |
| Proline | 0.038 | 1.66 |
| xLeu (leucine + isoleucine) | 0.049 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuleš, J.; Rubić, I.; Rešetar Maslov, D.; Efendić, M.; Martinković, K.; Pongrac, E.; Šmit, I.; Potočnjak, D.; Barić Rafaj, R.; Mrljak, V. Metabolomics Insights into Salivary Profile in Dogs with Babesia canis Infection. Biomolecules 2025, 15, 520. https://doi.org/10.3390/biom15040520
Kuleš J, Rubić I, Rešetar Maslov D, Efendić M, Martinković K, Pongrac E, Šmit I, Potočnjak D, Barić Rafaj R, Mrljak V. Metabolomics Insights into Salivary Profile in Dogs with Babesia canis Infection. Biomolecules. 2025; 15(4):520. https://doi.org/10.3390/biom15040520
Chicago/Turabian StyleKuleš, Josipa, Ivana Rubić, Dina Rešetar Maslov, Maša Efendić, Krešimir Martinković, Elizabeta Pongrac, Iva Šmit, Dalibor Potočnjak, Renata Barić Rafaj, and Vladimir Mrljak. 2025. "Metabolomics Insights into Salivary Profile in Dogs with Babesia canis Infection" Biomolecules 15, no. 4: 520. https://doi.org/10.3390/biom15040520
APA StyleKuleš, J., Rubić, I., Rešetar Maslov, D., Efendić, M., Martinković, K., Pongrac, E., Šmit, I., Potočnjak, D., Barić Rafaj, R., & Mrljak, V. (2025). Metabolomics Insights into Salivary Profile in Dogs with Babesia canis Infection. Biomolecules, 15(4), 520. https://doi.org/10.3390/biom15040520






