Neutrophils in Type 1 Diabetes: Untangling the Intricate Web of Pathways and Hypothesis
Abstract
1. Introduction: Neutrophils and Type 1 Diabetes
2. HYPOTHESIS-1: Neutrophils Engage Other Immune Cells Which in Turn Cause Dysfunction and Destruction of β-Cells
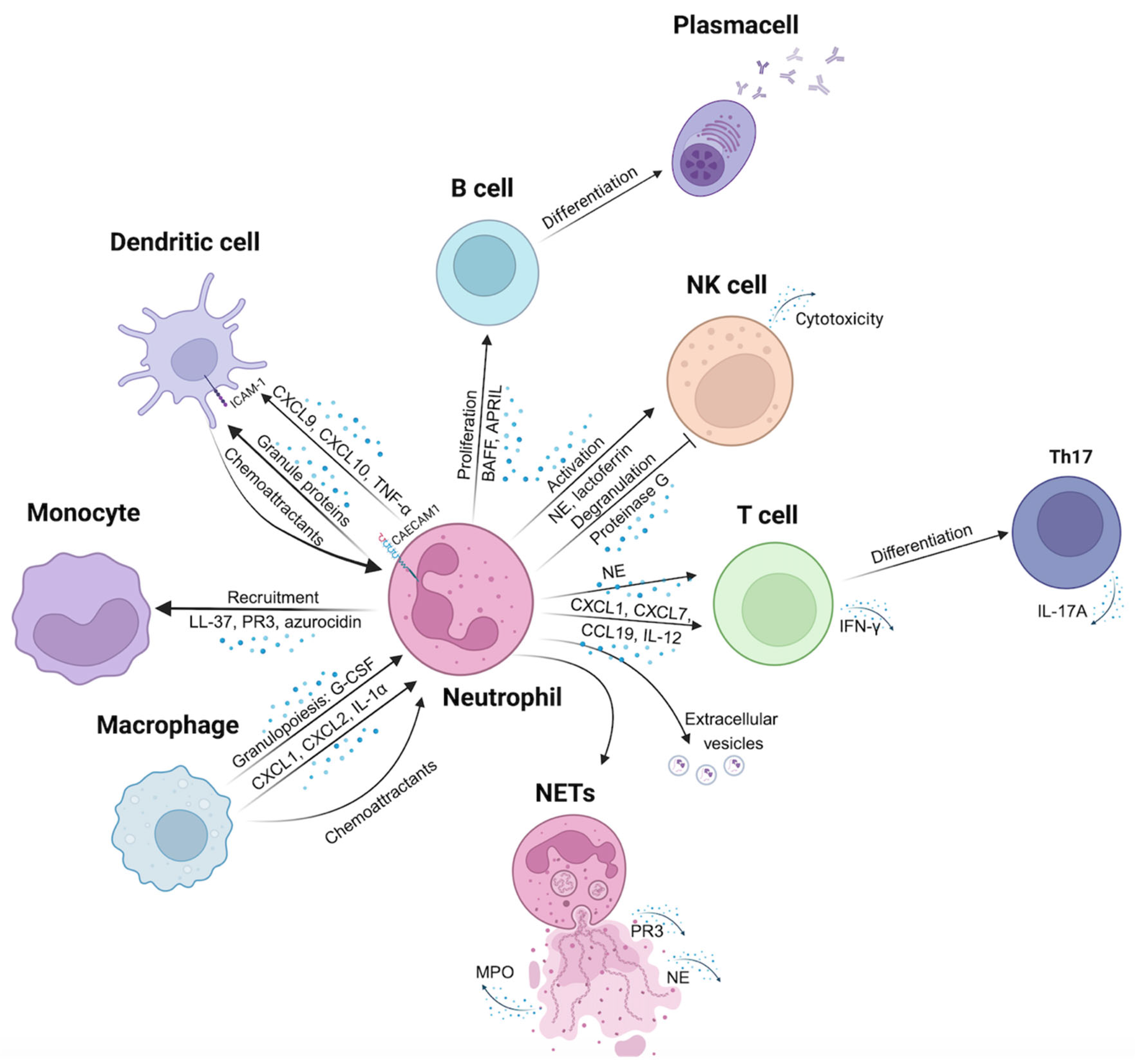
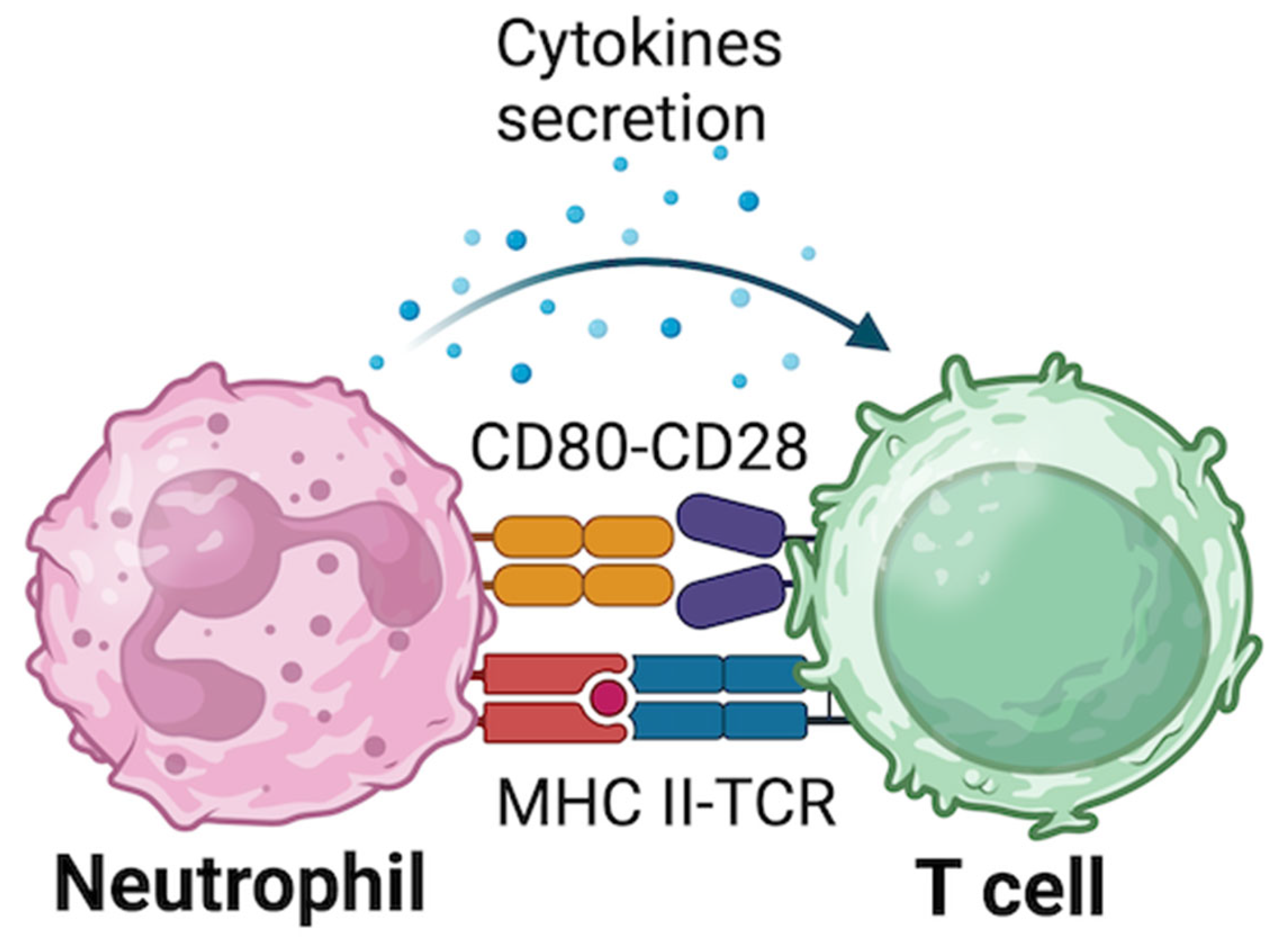
2.1. Direct Neutrophils-Immune Cells Interaction
2.2. Long-Range Signaling Among Neutrophils and Immune Cells via Cytokines, Chemokines, and/or Granule Proteins
2.2.1. Neutrophils-Dendritic Cells Interaction
2.2.2. Neutrophils-Monocytes/Macrophages Interaction
2.2.3. Neutrophils-B Cells Interaction
2.2.4. Neutrophils-T Cells Interaction
2.2.5. Neutrophils- NK Cells Interaction
2.3. Long-Range Communication Between Neutrophils and Immune Cells via Extracellular Vesicles (EVs), Neutrophils Extracellular Traps (NETs), and microRNAs
2.3.1. The Role of EVs in Neutrophils-Immune Cells Communication
2.3.2. The Role of NETs in Neutrophils-Immune Cells Interactions
2.3.3. The Role of Secreted miRNAs in Neutrophils-Immune Cells Interactions
3. HYPOTHESIS-2: Neutrophils Can Act as Antigen Presenting Cells Triggering Islet Autoimmunity
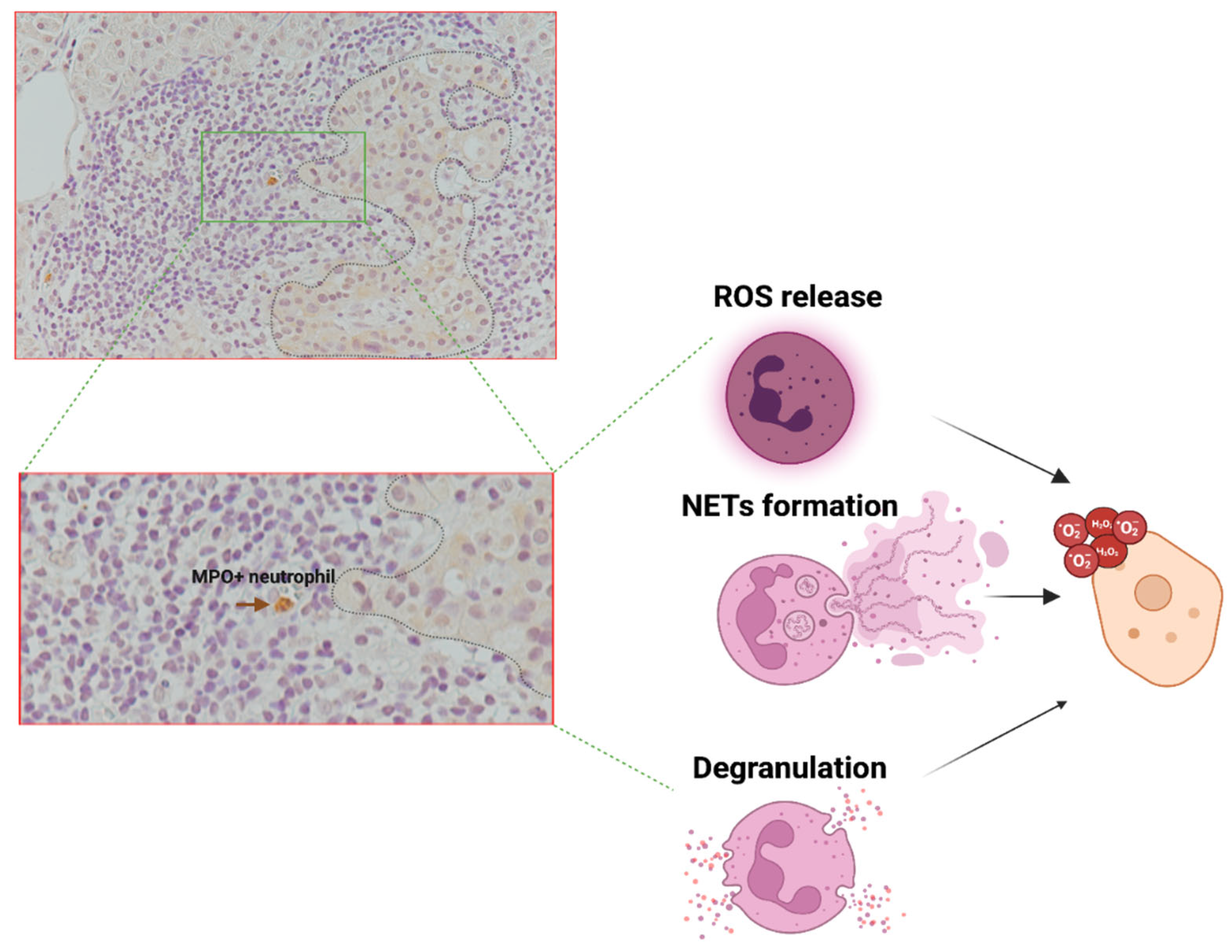
4. HYPOTHESIS-3: Neutrophils Can Release Factors That Are Involved in the Direct Damage of the β-Cells
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Citro, A.; Campo, F.; Dugnani, E.; Piemonti, L. Innate immunity mediated inflammation and beta cell function: Neighbors or enemies? Front. Endocrinol. 2020, 11, 606332. [Google Scholar] [CrossRef]
- Tsioumpekou, M.; Krijgsman, D.; Leusen, J.H.W.; Olofsen, P.A. The role of cytokines in neutrophil development, tissue homing, function and plasticity in health and disease. Cells 2023, 12, 1981. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-Y.; Hsieh, S.-C.; Liu, C.-W.; Lu, C.-S.; Wu, C.-H.; Liao, H.-T.; Chen, M.-H.; Li, K.-J.; Shen, C.-Y.; Kuo, Y.-M.; et al. Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils. Int. J. Mol. Sci. 2021, 22, 3119. [Google Scholar] [CrossRef]
- Battaglia, M.; Petrelli, A.; Vecchio, F. Neutrophils and type 1 diabetes: Current knowledge and suggested future directions. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 201–206. [Google Scholar] [CrossRef]
- Bissenova, S.; Ellis, D.; Mathieu, C.; Gysemans, C. Neutrophils in autoimmunity: When the hero becomes the villain. Clin. Exp. Immunol. 2022, 210, 128–140. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal. 2019, 17, 147. [Google Scholar] [CrossRef]
- Walrand, S.; Guillet, C.; Boirie, Y.; Vasson, M.-P. Insulin differentially regulates monocyte and polymorphonuclear neutrophil functions in healthy young and elderly humans. J. Clin. Endocrinol. Metab. 2006, 91, 2738–2748. [Google Scholar] [CrossRef]
- Cruz-Pineda, W.D.; Parra-Rojas, I.; Rodríguez-Ruíz, H.A.; Illades-Aguiar, B.; Matia-García, I.; Garibay-Cerdenares, O.L. The regulatory role of insulin in energy metabolism and leukocyte functions. J. Leukoc. Biol. 2022, 111, 197–208. [Google Scholar] [CrossRef]
- Walrand, S.; Guillet, C.; Gachon, P.; Giraudet, C.; Rousset, P.; Vasson, M.-P.; Boirie, Y. Insulin regulates protein synthesis rate in leukocytes from young and elderly healthy humans. Clin. Nutr. 2005, 24, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Anfossi, G.; Russo, I.; Mularoni, E.; Massucco, P.; Burzacca, S.; Mattiello, L.; Trovati, M. Insulin stimulates the polymorphonuclear leukocyte chemokinesis. Horm. Metab. Res. 1993, 25, 321–322. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Anfossi, G.; Russo, I.; Mularoni, E.; Massucco, P.; Burzacca, S.; Mattiello, L.; Trovati, M. Insulin, at physiological concentrations, enhances the polymorphonuclear leukocyte chemotactic properties. Horm. Metab. Res. 1992, 24, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Walrand, S.; Guillet, C.; Boirie, Y.; Vasson, M.P. In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions. J. Leukoc. Biol. 2004, 76, 1104–1110. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Q. The role of neutrophils in autoimmune diseases. Clin. Immunol. 2024, 266, 110334. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef]
- Nigi, L.; Maccora, C.; Dotta, F.; Sebastiani, G. From immunohistological to anatomical alterations of human pancreas in type 1 diabetes: New concepts on the stage. Diabetes Metab. Res. Rev. 2020, 36, e3264. [Google Scholar] [CrossRef]
- Sun, L.; Xi, S.; He, G.; Li, Z.; Gang, X.; Sun, C.; Guo, W.; Wang, G. Two to Tango: Dialogue between Adaptive and Innate Immunity in Type 1 Diabetes. J. Diabetes Res. 2020, 2020, 4106518. [Google Scholar] [CrossRef]
- Grieco, F.A.; Vendrame, F.; Spagnuolo, I.; Dotta, F. Innate immunity and the pathogenesis of type 1 diabetes. Semin. Immunopathol. 2011, 33, 57–66. [Google Scholar] [CrossRef]
- Nigi, L.; Laiho, J.E.; Hyöty, H.; Dotta, F. Editorial: The contribution of viruses and innate immune system in the pathogenesis of type 1 diabetes. Front. Endocrinol. 2023, 14, 1335716. [Google Scholar] [CrossRef] [PubMed]
- Diana, J.; Simoni, Y.; Furio, L.; Beaudoin, L.; Agerberth, B.; Barrat, F.; Lehuen, A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 2013, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Citro, A.; Valle, A.; Cantarelli, E.; Mercalli, A.; Pellegrini, S.; Liberati, D.; Daffonchio, L.; Kastsiuchenka, O.; Ruffini, P.A.; Battaglia, M.; et al. CXCR1/2 inhibition blocks and reverses type 1 diabetes in mice. Diabetes 2015, 64, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Giamporcaro, G.M.; Scavini, M.; Stabilini, A.; Grogan, P.; Bianconi, E.; Sebastiani, G.; Masini, M.; Maugeri, N.; Porretti, L.; et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes 2013, 62, 2072–2077. [Google Scholar] [CrossRef]
- Vecchio, F.; Lo Buono, N.; Stabilini, A.; Nigi, L.; Dufort, M.J.; Geyer, S.; Rancoita, P.M.; Cugnata, F.; Mandelli, A.; Valle, A.; et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 2018, 3, e122146. [Google Scholar] [CrossRef]
- Petrelli, A.; Popp, S.K.; Fukuda, R.; Parish, C.R.; Bosi, E.; Simeonovic, C.J. The contribution of neutrophils and nets to the development of type 1 diabetes. Front. Immunol. 2022, 13, 930553. [Google Scholar] [CrossRef]
- Lübbers, J.; Brink, M.; van de Stadt, L.A.; Vosslamber, S.; Wesseling, J.G.; van Schaardenburg, D.; Rantapää-Dahlqvist, S.; Verweij, C.L. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 776–780. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef]
- Fresneda Alarcon, M.; McLaren, Z.; Wright, H.L. Neutrophils in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus: Same foe different M.O. Front. Immunol. 2021, 12, 649693. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Y.; Zheng, P.; Zhou, W.; Wang, Y.; Huang, G.; Xu, A.; Zhou, Z. Distinct neutrophil counts and functions in newly diagnosed type 1 diabetes, latent autoimmune diabetes in adults, and type 2 diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3064. [Google Scholar] [CrossRef]
- Marhoffer, W.; Stein, M.; Schleinkofer, L.; Federlin, K. Evidence of ex vivo and in vitro impaired neutrophil oxidative burst and phagocytic capacity in type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 1993, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.S.; Oliver, S.R.; Flores, R.L.; Ngo, J.; Milne, G.L.; Zaldivar, F.P.; Galassetti, P.R. Altered inflammatory, oxidative, and metabolic responses to exercise in pediatric obesity and type 1 diabetes. Pediatr. Diabetes 2011, 12, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, S.R.; Zhou, Z.; Lam, K.S.L.; Xu, A. Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Shu, P.; Wang, Y.; Ji, N.; Zhang, D. Interactions between neutrophils and T-helper 17 cells. Front. Immunol. 2023, 14, 1279837. [Google Scholar] [CrossRef]
- Parackova, Z.; Zentsova, I.; Vrabcova, P.; Klocperk, A.; Sumnik, Z.; Pruhova, S.; Petruzelkova, L.; Hasler, R.; Sediva, A. Neutrophil extracellular trap induced dendritic cell activation leads to th1 polarization in type 1 diabetes. Front. Immunol. 2020, 11, 661. [Google Scholar] [CrossRef]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Giovenzana, A.; Carnovale, D.; Phillips, B.; Petrelli, A.; Giannoukakis, N. Neutrophils and their role in the aetiopathogenesis of type 1 and type 2 diabetes. Diabetes Metab. Res. Rev. 2022, 38, e3483. [Google Scholar] [CrossRef]
- Battaglia, M. Neutrophils and type 1 autoimmune diabetes. Curr. Opin. Hematol. 2014, 21, 8–15. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Y.; Xu, A.; Zhou, Z. Neutrophils in type 1 diabetes. J. Diabetes Investig. 2016, 7, 652–663. [Google Scholar] [CrossRef]
- van Gisbergen, K.P.J.M.; Sanchez-Hernandez, M.; Geijtenbeek, T.B.H.; van Kooyk, Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005, 201, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- van Gisbergen, K.P.J.M.; Ludwig, I.S.; Geijtenbeek, T.B.H.; van Kooyk, Y. Interactions of DC-SIGN with Mac-1 and CEACAM1 regulate contact between dendritic cells and neutrophils. FEBS Lett. 2005, 579, 6159–6168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Müller, I.; Munder, M.; Kropf, P.; Hänsch, G.M. Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol. 2009, 30, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef]
- Melbouci, D.; Haidar Ahmad, A.; Decker, P. Neutrophil extracellular traps (NET): Not only antimicrobial but also modulators of innate and adaptive immunities in inflammatory autoimmune diseases. RMD Open 2023, 9, e003104. [Google Scholar] [CrossRef]
- Riaz, B.; Sohn, S. Neutrophils in inflammatory diseases: Unraveling the impact of their derived molecules and heterogeneity. Cells 2023, 12, 2621. [Google Scholar] [CrossRef]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Fu, X.; Liu, H.; Huang, G.; Dai, S.-S. The emerging role of neutrophils in autoimmune-associated disorders: Effector, predictor, and therapeutic targets. MedComm 2021, 2, 402–413. [Google Scholar] [CrossRef]
- Kupor, D.; Felder, M.L.; Kodikalla, S.; Chu, X.; Eniola-Adefeso, O. Nanoparticle-neutrophils interactions for autoimmune regulation. Adv. Drug Deliv. Rev. 2024, 209, 115316. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Yang, X.; Nan, F.; Zhang, T.; Ji, S.; Rao, D.; Feng, H.; Gao, K.; Gu, X.; et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell 2024, 187, 1422–1439.e24. [Google Scholar] [CrossRef]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Padgett, L.E.; Broniowska, K.A.; Hansen, P.A.; Corbett, J.A.; Tse, H.M. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Ann. N. Y. Acad. Sci. 2013, 1281, 16–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Kasahara, S.; Jhingran, A.; Tosini, N.L.; Zhai, B.; Aufiero, M.A.; Mills, K.A.M.; Gjonbalaj, M.; Espinosa, V.; Rivera, A.; et al. During Aspergillus Infection, Monocyte-Derived DCs, Neutrophils, and Plasmacytoid DCs Enhance Innate Immune Defense through CXCR3-Dependent Crosstalk. Cell Host Microbe 2020, 28, 104–116.e4. [Google Scholar] [CrossRef] [PubMed]
- Nigi, L.; Brusco, N.; Grieco, G.E.; Licata, G.; Krogvold, L.; Marselli, L.; Gysemans, C.; Overbergh, L.; Marchetti, P.; Mathieu, C.; et al. Pancreatic Alpha-Cells Contribute Together With Beta-Cells to CXCL10 Expression in Type 1 Diabetes. Front. Endocrinol. 2020, 11, 630. [Google Scholar] [CrossRef]
- Schuster, S.; Hurrell, B.; Tacchini-Cottier, F. Crosstalk between neutrophils and dendritic cells: A context-dependent process. J. Leukoc. Biol. 2013, 94, 671–675. [Google Scholar] [CrossRef]
- Vaschetto, R.; Grinstein, J.; Del Sorbo, L.; Khine, A.A.; Voglis, S.; Tullis, E.; Slutsky, A.S.; Zhang, H. Role of human neutrophil peptides in the initial interaction between lung epithelial cells and CD4+ lymphocytes. J. Leukoc. Biol. 2007, 81, 1022–1031. [Google Scholar] [CrossRef]
- Wang, F.; Qiao, L.; Lv, X.; Trivett, A.; Yang, R.; Oppenheim, J.J.; Yang, D.; Zhang, N. Alarmin human α defensin HNP1 activates plasmacytoid dendritic cells by triggering NF-κB and IRF1 signaling pathways. Cytokine 2016, 83, 53–60. [Google Scholar] [CrossRef]
- Maier-Begandt, D.; Alonso-Gonzalez, N.; Klotz, L.; Erpenbeck, L.; Jablonska, J.; Immler, R.; Hasenberg, A.; Mueller, T.T.; Herrero-Cervera, A.; Aranda-Pardos, I.; et al. Neutrophils-biology and diversity. Nephrol. Dial. Transplant. 2024, 39, 1551–1564. [Google Scholar] [CrossRef]
- Megyeri, P.; Sadowska, J.; Issekutz, T.B.; Issekutz, A.C. Endotoxin-stimulated human macrophages produce a factor that induces polymorphonuclear leucocyte infiltration and is distinct from interleukin-1, tumour necrosis factor alpha and chemotactic factors. Immunology 1990, 69, 155–161. [Google Scholar]
- Schulz, C.; Petzold, T.; Ishikawa-Ankerhold, H. Macrophage regulation of granulopoiesis and neutrophil functions. Antioxid. Redox Signal. 2021, 35, 182–191. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Demkow, U. Molecular mechanisms of neutrophil extracellular trap (nets) degradation. Int. J. Mol. Sci. 2023, 24, 4896. [Google Scholar] [CrossRef] [PubMed]
- Diana, J.; Lehuen, A. Macrophages and β-cells are responsible for CXCR2-mediated neutrophil infiltration of the pancreas during autoimmune diabetes. EMBO Mol. Med. 2014, 6, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Chertov, O.; Yang, D.; Howard, O.M.; Oppenheim, J.J. Leukocyte granule proteins mobilize innate host defenses and adaptive immune responses. Immunol. Rev. 2000, 177, 68–78. [Google Scholar] [CrossRef]
- Buonocore, S.; Surquin, M.; Le Moine, A.; Abramowicz, D.; Flamand, V.; Goldman, M. Amplification of T-cell responses by neutrophils: Relevance to allograft immunity. Immunol. Lett. 2004, 94, 163–166. [Google Scholar] [CrossRef]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L.; Weber, C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood 2009, 114, 4613–4623. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef]
- Påhlman, L.I.; Mörgelin, M.; Eckert, J.; Johansson, L.; Russell, W.; Riesbeck, K.; Soehnlein, O.; Lindbom, L.; Norrby-Teglund, A.; Schumann, R.R.; et al. Streptococcal M protein: A multipotent and powerful inducer of inflammation. J. Immunol. 2006, 177, 1221–1228. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Swirski, F.K. Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis. Science 2015, 349, 237–238. [Google Scholar] [CrossRef]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2011, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Scapini, P.; Bazzoni, F.; Cassatella, M.A. Regulation of B-cell-activating factor (BAFF)/B lymphocyte stimulator (BLyS) expression in human neutrophils. Immunol. Lett. 2008, 116, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, U.; Vermeren, S. Crosstalk between B cells and neutrophils in rheumatoid arthritis. Immunology 2021, 164, 689–700. [Google Scholar] [CrossRef]
- Kristyanto, H.; Blomberg, N.J.; Slot, L.M.; van der Voort, E.I.H.; Kerkman, P.F.; Bakker, A.; Burgers, L.E.; Ten Brinck, R.M.; van der Helm-van Mil, A.H.M.; Spits, H.; et al. Persistently activated, proliferative memory autoreactive B cells promote inflammation in rheumatoid arthritis. Sci. Transl. Med. 2020, 12, eaaz5327. [Google Scholar] [CrossRef]
- Souwer, Y.; Groot Kormelink, T.; Taanman-Kueter, E.W.; Muller, F.J.; van Capel, T.M.M.; Varga, D.V.; Bar-Ephraim, Y.E.; Teunissen, M.B.M.; van Ham, S.M.; Kuijpers, T.W.; et al. Human TH17 cell development requires processing of dendritic cell-derived CXCL8 by neutrophil elastase. J. Allergy Clin. Immunol. 2018, 141, 2286–2289.e5. [Google Scholar] [CrossRef]
- Agerberth, B.; Charo, J.; Werr, J.; Olsson, B.; Idali, F.; Lindbom, L.; Kiessling, R.; Jörnvall, H.; Wigzell, H.; Gudmundsson, G.H. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood 2000, 96, 3086–3093. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Alessandrini, V.; Hardisty, G.; Melrose, L.; Jackson-Jones, L.; MacDonald, A.S.; Davidson, D.J.; Gwyer Findlay, E. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef]
- Ellis, T.N.; Beaman, B.L. Interferon-gamma activation of polymorphonuclear neutrophil function. Immunology 2004, 112, 2–12. [Google Scholar] [CrossRef]
- Pelletier, M.; Maggi, L.; Micheletti, A.; Lazzeri, E.; Tamassia, N.; Costantini, C.; Cosmi, L.; Lunardi, C.; Annunziato, F.; Romagnani, S.; et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010, 115, 335–343. [Google Scholar] [CrossRef]
- Tamassia, N.; Arruda-Silva, F.; Wright, H.L.; Moots, R.J.; Gardiman, E.; Bianchetto-Aguilera, F.; Gasperini, S.; Capone, M.; Maggi, L.; Annunziato, F.; et al. Human neutrophils activated via TLR8 promote Th17 polarization through IL-23. J. Leukoc. Biol. 2019, 105, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Abi Abdallah, D.S.; Egan, C.E.; Butcher, B.A.; Denkers, E.Y. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 2011, 23, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Fousert, E.; Toes, R.; Desai, J. Neutrophil extracellular traps (nets) take the central stage in driving autoimmune responses. Cells 2020, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Sagar, D.; Hanna, R.N.; Lightfoot, Y.L.; Mistry, P.; Smith, C.K.; Manna, Z.; Hasni, S.; Siegel, R.M.; Sanjuan, M.A.; et al. Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 957–966. [Google Scholar] [CrossRef]
- Grieco, F.A.; Moore, F.; Vigneron, F.; Santin, I.; Villate, O.; Marselli, L.; Rondas, D.; Korf, H.; Overbergh, L.; Dotta, F.; et al. IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia 2014, 57, 502–511. [Google Scholar] [CrossRef]
- Maffia, P.C.; Zittermann, S.E.; Scimone, M.L.; Tateosian, N.; Amiano, N.; Guerrieri, D.; Lutzky, V.; Rosso, D.; Romeo, H.E.; Garcia, V.E.; et al. Neutrophil elastase converts human immature dendritic cells into transforming growth factor-beta1-secreting cells and reduces allostimulatory ability. Am. J. Pathol. 2007, 171, 928–937. [Google Scholar] [CrossRef]
- Jacobsen, L.C.; Theilgaard-Mönch, K.; Christensen, E.I.; Borregaard, N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood 2007, 109, 3084–3087. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Deng, Y.; Tai, Y.; Zeng, K.; Zhang, Y.; Liu, W.; Zhang, Q.; Yang, Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 422. [Google Scholar] [CrossRef]
- Shen, G.; Krienke, S.; Schiller, P.; Nießen, A.; Neu, S.; Eckstein, V.; Schiller, M.; Lorenz, H.-M.; Tykocinski, L.-O. Microvesicles released by apoptotic human neutrophils suppress proliferation and IL-2/IL-2 receptor expression of resting T helper cells. Eur. J. Immunol. 2017, 47, 900–910. [Google Scholar] [CrossRef]
- Minns, D.; Smith, K.J.; Hardisty, G.; Rossi, A.G.; Gwyer Findlay, E. The Outcome of Neutrophil-T Cell Contact Differs Depending on Activation Status of Both Cell Types. Front. Immunol. 2021, 12, 633486. [Google Scholar] [CrossRef]
- Costantini, C.; Cassatella, M.A. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J. Leukoc. Biol. 2011, 89, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Riise, R.E.; Bernson, E.; Aurelius, J.; Martner, A.; Pesce, S.; Della Chiesa, M.; Marcenaro, E.; Bylund, J.; Hellstrand, K.; Moretta, L.; et al. TLR-Stimulated Neutrophils Instruct NK Cells To Trigger Dendritic Cell Maturation and Promote Adaptive T Cell Responses. J. Immunol. 2015, 195, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, B.N.; Donadieu, J.; Cognet, C.; Bernat, C.; Ordoñez-Rueda, D.; Barlogis, V.; Mahlaoui, N.; Fenis, A.; Narni-Mancinelli, E.; Beaupain, B.; et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 2012, 209, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Valayer, A.; Brea, D.; Lajoie, L.; Avezard, L.; Combes-Soia, L.; Labas, V.; Korkmaz, B.; Thibault, G.; Baranek, T.; Si-Tahar, M. Neutrophils can disarm NK cell response through cleavage of NKp46. J. Leukoc. Biol. 2017, 101, 253–259. [Google Scholar] [CrossRef]
- Dotta, F.; Censini, S.; van Halteren, A.G.S.; Marselli, L.; Masini, M.; Dionisi, S.; Mosca, F.; Boggi, U.; Muda, A.O.; Del Prato, S.; et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 2007, 104, 5115–5120. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Majumdar, R.; Tavakoli Tameh, A.; Arya, S.B.; Parent, C.A. Exosomes mediate LTB4 release during neutrophil chemotaxis. PLoS Biol. 2021, 19, e3001271. [Google Scholar] [CrossRef]
- Glémain, A.; Néel, M.; Néel, A.; André-Grégoire, G.; Gavard, J.; Martinet, B.; Le Bloas, R.; Riquin, K.; Hamidou, M.; Fakhouri, F.; et al. Neutrophil-derived extracellular vesicles induce endothelial inflammation and damage through the transfer of miRNAs. J. Autoimmun. 2022, 129, 102826. [Google Scholar] [CrossRef]
- Bonaventura, A.; Vecchié, A.; Abbate, A.; Montecucco, F. Neutrophil extracellular traps and cardiovascular diseases: An update. Cells 2020, 9, 231. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Jiang, J.-J.; Zhao, Y.-C.; Zheng, X.-R.; Xi, N.; Zhao, G.-R.; Huang, X.-W.; Wang, S.-L. Neutrophil extracellular traps in central nervous system (CNS) diseases. PeerJ 2024, 12, e16465. [Google Scholar] [CrossRef] [PubMed]
- Stutz, A.; Kolbe, C.-C.; Stahl, R.; Horvath, G.L.; Franklin, B.S.; van Ray, O.; Brinkschulte, R.; Geyer, M.; Meissner, F.; Latz, E. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 2017, 214, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Fitzgerald, K.A. Inflammasome complexes: Emerging mechanisms and effector functions. Cell 2016, 165, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef]
- Monteith, A.J.; Miller, J.M.; Maxwell, C.N.; Chazin, W.J.; Skaar, E.P. Neutrophil extracellular traps enhance macrophage killing of bacterial pathogens. Sci. Adv. 2021, 7, eabj2101. [Google Scholar] [CrossRef]
- Soehnlein, O.; Kai-Larsen, Y.; Frithiof, R.; Sorensen, O.E.; Kenne, E.; Scharffetter-Kochanek, K.; Eriksson, E.E.; Herwald, H.; Agerberth, B.; Lindbom, L. Neutrophil primary granule proteins HBP and HNP1-3 boost bacterial phagocytosis by human and murine macrophages. J. Clin. Investig. 2008, 118, 3491–3502. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Carlucci, P.M.; Moore, E.; Lingampalli, N.; Uchtenhagen, H.; James, E.; Liu, Y.; Bicker, K.L.; Wahamaa, H.; Hoffmann, V.; et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017, 2, eaag3358. [Google Scholar] [CrossRef]
- Tillack, K.; Breiden, P.; Martin, R.; Sospedra, M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J. Immunol. 2012, 188, 3150–3159. [Google Scholar] [CrossRef]
- Wilson, A.S.; Randall, K.L.; Pettitt, J.A.; Ellyard, J.I.; Blumenthal, A.; Enders, A.; Quah, B.J.; Bopp, T.; Parish, C.R.; Brüstle, A. Neutrophil extracellular traps and their histones promote Th17 cell differentiation directly via TLR2. Nat. Commun. 2022, 13, 528. [Google Scholar] [CrossRef] [PubMed]
- Auddino, S.; Aiello, E.; Grieco, G.E.; Dotta, F.; Sebastiani, G. A three-layer perspective on miRNA regulation in β cell inflammation. Trends Endocrinol. Metab. 2024. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, G.; Nigi, L.; Sebastiani, G.; Dotta, F. MicroRNAs: Novel Players in the Dialogue between Pancreatic Islets and Immune System in Autoimmune Diabetes. BioMed Res. Int. 2015, 2015, 749734. [Google Scholar] [CrossRef] [PubMed]
- Hussen, B.M.; Rasul, M.F.; Faraj, G.S.H.; Abdullah, S.R.; Sulaiman, S.H.; Pourmoshtagh, H.; Taheri, M. Role of microRNAs in neutrophil extracellular trap formation and prevention: Systematic narrative review. Mol. Cell. Probes 2024, 78, 101986. [Google Scholar] [CrossRef]
- Linhares-Lacerda, L.; Temerozo, J.R.; Ribeiro-Alves, M.; Azevedo, E.P.; Mojoli, A.; Nascimento, M.T.C.; Silva-Oliveira, G.; Savino, W.; Foguel, D.; Bou-Habib, D.C.; et al. Neutrophil extracellular trap-enriched supernatants carry microRNAs able to modulate TNF-α production by macrophages. Sci. Rep. 2020, 10, 2715. [Google Scholar] [CrossRef]
- Águila, S.; de Los Reyes-García, A.M.; Fernández-Pérez, M.P.; Reguilón-Gallego, L.; Zapata-Martínez, L.; Ruiz-Lorente, I.; Vicente, V.; González-Conejero, R.; Martínez, C. Micrornas as new regulators of neutrophil extracellular trap formation. Int. J. Mol. Sci. 2021, 22, 2116. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Song, Y.; Guo, X.-L.; Miao, R.-Y.; Fu, Y.-Q.; Miao, C.-F.; Zhang, C. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle 2019, 18, 2674–2684. [Google Scholar] [CrossRef]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef]
- Eiz-Vesper, B.; Schmetzer, H.M. Antigen-Presenting Cells: Potential of Proven und New Players in Immune Therapies. Transfus Med. Hemother. 2020, 47, 429–431. [Google Scholar] [CrossRef]
- Podojil, J.R.; Miller, S.D. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol. Rev. 2009, 229, 337–355. [Google Scholar] [CrossRef]
- Karavitis, J.; Kovacs, E.J. Macrophage phagocytosis: Effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J. Leukoc. Biol. 2011, 90, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Segovia, M.; Louvet, C.; Charnet, P.; Savina, A.; Tilly, G.; Gautreau, L.; Carretero-Iglesia, L.; Beriou, G.; Cebrian, I.; Cens, T.; et al. Autologous dendritic cells prolong allograft survival through Tmem176b-dependent antigen cross-presentation. Am. J. Transplant. 2014, 14, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Savina, A.; Amigorena, S. Phagocytosis and antigen presentation in dendritic cells. Immunol. Rev. 2007, 219, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef]
- Meinderts, S.M.; Baker, G.; van Wijk, S.; Beuger, B.M.; Geissler, J.; Jansen, M.H.; Saris, A.; Ten Brinke, A.; Kuijpers, T.W.; van den Berg, T.K.; et al. Neutrophils acquire antigen-presenting cell features after phagocytosis of IgG-opsonized erythrocytes. Blood Adv. 2019, 3, 1761–1773. [Google Scholar] [CrossRef]
- Moffat, A.; Gwyer Findlay, E. Evidence for antigen presentation by human neutrophils. Blood 2024, 143, 2455–2463. [Google Scholar] [CrossRef]
- Shafqat, A.; Khan, J.A.; Alkachem, A.Y.; Sabur, H.; Alkattan, K.; Yaqinuddin, A.; Sing, G.K. How neutrophils shape the immune response: Reassessing their multifaceted role in health and disease. Int. J. Mol. Sci. 2023, 24, 7583. [Google Scholar] [CrossRef]
- Sandilands, G.P.; Ahmed, Z.; Perry, N.; Davison, M.; Lupton, A.; Young, B. Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology 2005, 114, 354–368. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Nigrovic, P.A. Neutrophil Heterogeneity as Therapeutic Opportunity in Immune-Mediated Disease. Front. Immunol. 2019, 10, 346. [Google Scholar] [CrossRef]
- Takeda, Y.; Kato, T.; Sabrina, S.; Naito, S.; Ito, H.; Emi, N.; Kuboki, Y.; Takai, Y.; Fukuhara, H.; Ushijima, M.; et al. Intracellular Major Histocompatibility Complex Class II and C-X-C Motif Chemokine Ligand 10-Expressing Neutrophils Indicate the State of Anti-Tumor Activity Induced by Bacillus Calmette-Guérin. Biomedicines 2023, 11, 3062. [Google Scholar] [CrossRef]
- Forrer, P.; Palianina, D.; Stühler, C.; Kreuzaler, M.; Roux, J.; Li, J.; Schmutz, C.; Burckhardt, D.; Franzeck, F.; Finke, D.; et al. Unveiling signaling pathways inducing MHC class II expression in neutrophils. Front. Immunol. 2024, 15, 1444558. [Google Scholar] [CrossRef]
- Sandilands, G.P.; McCrae, J.; Hill, K.; Perry, M.; Baxter, D. Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology 2006, 119, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, A.; Maniak, S.; Gebert, A.; Ferger, I.; Wurster, U.; Heidenreich, F. Human polymorphonuclear neutrophils express a B7-1-like molecule. J. Leukoc. Biol. 1999, 66, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.V.; Unanue, E.R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature 1990, 346, 574–576. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, R.A.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Bankey, P.E.; Banerjee, S.; Zucchiatti, A.; De, M.; Sleem, R.W.; Lin, C.-F.L.; Miller-Graziano, C.L.; De, A.K. Cytokine induced expression of programmed death ligands in human neutrophils. Immunol. Lett. 2010, 129, 100–107. [Google Scholar] [CrossRef]
- Radsak, M.; Iking-Konert, C.; Stegmaier, S.; Andrassy, K.; Hänsch, G.M. Polymorphonuclear neutrophils as accessory cells for T-cell activation: Major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology 2000, 101, 521–530. [Google Scholar] [CrossRef]
- Geng, S.; Matsushima, H.; Okamoto, T.; Yao, Y.; Lu, R.; Page, K.; Blumenthal, R.M.; Ward, N.L.; Miyazaki, T.; Takashima, A. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood 2013, 121, 1690–1700. [Google Scholar] [CrossRef][Green Version]
- Chan, L.; Morovati, S.; Karimi, N.; Alizadeh, K.; Vanderkamp, S.; Kakish, J.E.; Bridle, B.W.; Karimi, K. Neutrophil functional heterogeneity and implications for viral infections and treatments. Cells 2022, 11, 1322. [Google Scholar] [CrossRef]
- Abadie, V.; Badell, E.; Douillard, P.; Ensergueix, D.; Leenen, P.J.M.; Tanguy, M.; Fiette, L.; Saeland, S.; Gicquel, B.; Winter, N. Neutrophils rapidly migrate via lymphatics after Mycobacterium bovis BCG intradermal vaccination and shuttle live bacilli to the draining lymph nodes. Blood 2005, 106, 1843–1850. [Google Scholar] [CrossRef]
- Liang, F.; Lindgren, G.; Sandgren, K.J.; Thompson, E.A.; Francica, J.R.; Seubert, A.; De Gregorio, E.; Barnett, S.; O’Hagan, D.T.; Sullivan, N.J.; et al. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Sci. Transl. Med. 2017, 9, eaal2094. [Google Scholar] [CrossRef] [PubMed]
- Ostanin, D.V.; Kurmaeva, E.; Furr, K.; Bao, R.; Hoffman, J.; Berney, S.; Grisham, M.B. Acquisition of antigen-presenting functions by neutrophils isolated from mice with chronic colitis. J. Immunol. 2012, 188, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Morgan, M.P.; Liuzzi, A.R.; Tyler, C.J.; Khan, M.W.A.; Szakmany, T.; Hall, J.E.; Moser, B.; Eberl, M. Microbe-specific unconventional T cells induce human neutrophil differentiation into antigen cross-presenting cells. J. Immunol. 2014, 193, 3704–3716. [Google Scholar] [CrossRef] [PubMed]
- Iking-Konert, C.; Vogt, S.; Radsak, M.; Wagner, C.; Hänsch, G.M.; Andrassy, K. Polymorphonuclear neutrophils in Wegener’s granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001, 60, 2247–2262. [Google Scholar] [CrossRef]
- Cascão, R.; Rosário, H.S.; Souto-Carneiro, M.M.; Fonseca, J.E. Neutrophils in rheumatoid arthritis: More than simple final effectors. Autoimmun. Rev. 2010, 9, 531–535. [Google Scholar] [CrossRef]
- Gysemans, C.; Beya, M.; Pedace, E.; Mathieu, C. Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease. Biomedicines 2025, 13, 597. [Google Scholar] [CrossRef]
- Robertson, R.P. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Brorsson, C.; Johansen, C.H.; Banasik, K.; Mazzoni, G.; Moulder, R.; Hirvonen, K.; Suomi, T.; Rasool, O.; Bruggraber, S.F.A.; et al. Multi-omics analysis reveals drivers of loss of β-cell function after newly diagnosed autoimmune type 1 diabetes: An INNODIA multicenter study. Diabetes Metab. Res. Rev. 2024, 40, e3833. [Google Scholar] [CrossRef]
- Garciafigueroa, Y.; Phillips, B.E.; Engman, C.; Trucco, M.; Giannoukakis, N. Neutrophil-Associated Inflammatory Changes in the Pre-Diabetic Pancreas of Early-Age NOD Mice. Front. Endocrinol. 2021, 12, 565981. [Google Scholar] [CrossRef]
- Dinić, S.; Arambašić Jovanović, J.; Uskoković, A.; Mihailović, M.; Grdović, N.; Tolić, A.; Rajić, J.; Đorđević, M.; Vidaković, M. Oxidative stress-mediated beta cell death and dysfunction as a target for diabetes management. Front. Endocrinol. 2022, 13, 1006376. [Google Scholar] [CrossRef]
- Leenders, F.; Groen, N.; de Graaf, N.; Engelse, M.A.; Rabelink, T.J.; de Koning, E.J.P.; Carlotti, F. Oxidative Stress Leads to β-Cell Dysfunction Through Loss of β-Cell Identity. Front. Immunol. 2021, 12, 690379. [Google Scholar] [CrossRef]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Grankvist, K.; Marklund, S.L.; Täljedal, I.B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem. J. 1981, 199, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Tiedge, M.; Lortz, S.; Drinkgern, J.; Lenzen, S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997, 46, 1733–1742. [Google Scholar] [CrossRef]
- Tonooka, N.; Oseid, E.; Zhou, H.; Harmon, J.S.; Robertson, R.P. Glutathione peroxidase protein expression and activity in human islets isolated for transplantation. Clin. Transplant. 2007, 21, 767–772. [Google Scholar] [CrossRef]
- Roep, B.O.; Thomaidou, S.; van Tienhoven, R.; Zaldumbide, A. Type 1 diabetes mellitus as a disease of the β-cell (do not blame the immune system?). Nat. Rev. Endocrinol. 2021, 17, 150–161. [Google Scholar] [CrossRef]
- Stowe, A.M.; Adair-Kirk, T.L.; Gonzales, E.R.; Perez, R.S.; Shah, A.R.; Park, T.S.; Gidday, J.M. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol. Dis. 2009, 35, 82–90. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Carden, D.; Xiao, F.; Moak, C.; Willis, B.H.; Robinson-Jackson, S.; Alexander, S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am. J. Physiol. 1998, 275, H385–H392. [Google Scholar] [CrossRef]
- Hoenderdos, K.; Lodge, K.M.; Hirst, R.A.; Chen, C.; Palazzo, S.G.C.; Emerenciana, A.; Summers, C.; Angyal, A.; Porter, L.; Juss, J.K.; et al. Hypoxia upregulates neutrophil degranulation and potential for tissue injury. Thorax 2016, 71, 1030–1038. [Google Scholar] [CrossRef]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, R.R.; Zaldumbide, A.; Taylor, C.T. Role of hypoxia-inducible factor 1 in type 1 diabetes. Trends Pharmacol. Sci. 2024, 45, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Popp, S.K.; Vecchio, F.; Brown, D.J.; Fukuda, R.; Suzuki, Y.; Takeda, Y.; Wakamatsu, R.; Sarma, M.A.; Garrett, J.; Giovenzana, A.; et al. Circulating platelet-neutrophil aggregates characterize the development of type 1 diabetes in humans and NOD mice. JCI Insight 2022, 7, eaal2094. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Y.; Ji, Q.; Fu, S.; Gu, J.; Tai, N.; Wang, X. Interplay between Extracellular Matrix and Neutrophils in Diseases. J. Immunol. Res. 2021, 2021, 8243378. [Google Scholar] [CrossRef]
- van Tienhoven, R.; O’Meally, D.; Scott, T.A.; Morris, K.V.; Williams, J.C.; Kaddis, J.S.; Zaldumbide, A.; Roep, B.O. Genetic protection from type 1 diabetes resulting from accelerated insulin mRNA decay. Cell 2025. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Su, J.; Quan, F.; Zhou, H.; Li, Q.; Feng, Q.; Lin, C.; Wang, D.; Jiang, Z. Neutrophil diversity and function in health and disease. Signal Transduct. Target. Ther. 2024, 9, 343. [Google Scholar] [CrossRef]
- Salemme, R.; Peralta, L.N.; Meka, S.H.; Pushpanathan, N.; Alexander, J.J. The role of netosis in systemic lupus erythematosus. J. Cell. Immunol. 2019, 1, 33–42. [Google Scholar] [CrossRef]
- Benguigui, M.; Cooper, T.J.; Kalkar, P.; Schif-Zuck, S.; Halaban, R.; Bacchiocchi, A.; Kamer, I.; Deo, A.; Manobla, B.; Menachem, R.; et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 2024, 42, 253–265.e12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigi, L.; Pedace, E.; Dotta, F.; Sebastiani, G. Neutrophils in Type 1 Diabetes: Untangling the Intricate Web of Pathways and Hypothesis. Biomolecules 2025, 15, 505. https://doi.org/10.3390/biom15040505
Nigi L, Pedace E, Dotta F, Sebastiani G. Neutrophils in Type 1 Diabetes: Untangling the Intricate Web of Pathways and Hypothesis. Biomolecules. 2025; 15(4):505. https://doi.org/10.3390/biom15040505
Chicago/Turabian StyleNigi, Laura, Erika Pedace, Francesco Dotta, and Guido Sebastiani. 2025. "Neutrophils in Type 1 Diabetes: Untangling the Intricate Web of Pathways and Hypothesis" Biomolecules 15, no. 4: 505. https://doi.org/10.3390/biom15040505
APA StyleNigi, L., Pedace, E., Dotta, F., & Sebastiani, G. (2025). Neutrophils in Type 1 Diabetes: Untangling the Intricate Web of Pathways and Hypothesis. Biomolecules, 15(4), 505. https://doi.org/10.3390/biom15040505






