The Functional and Imaging Implications of Left Bundle Branch Pacing in Ischemic Cardiomyopathy
Abstract
1. Introduction
2. Pathophysiology of Dyssynchrony in Left Bundle Branch Block
3. Imaging Modalities for Assessing Dyssynchrony
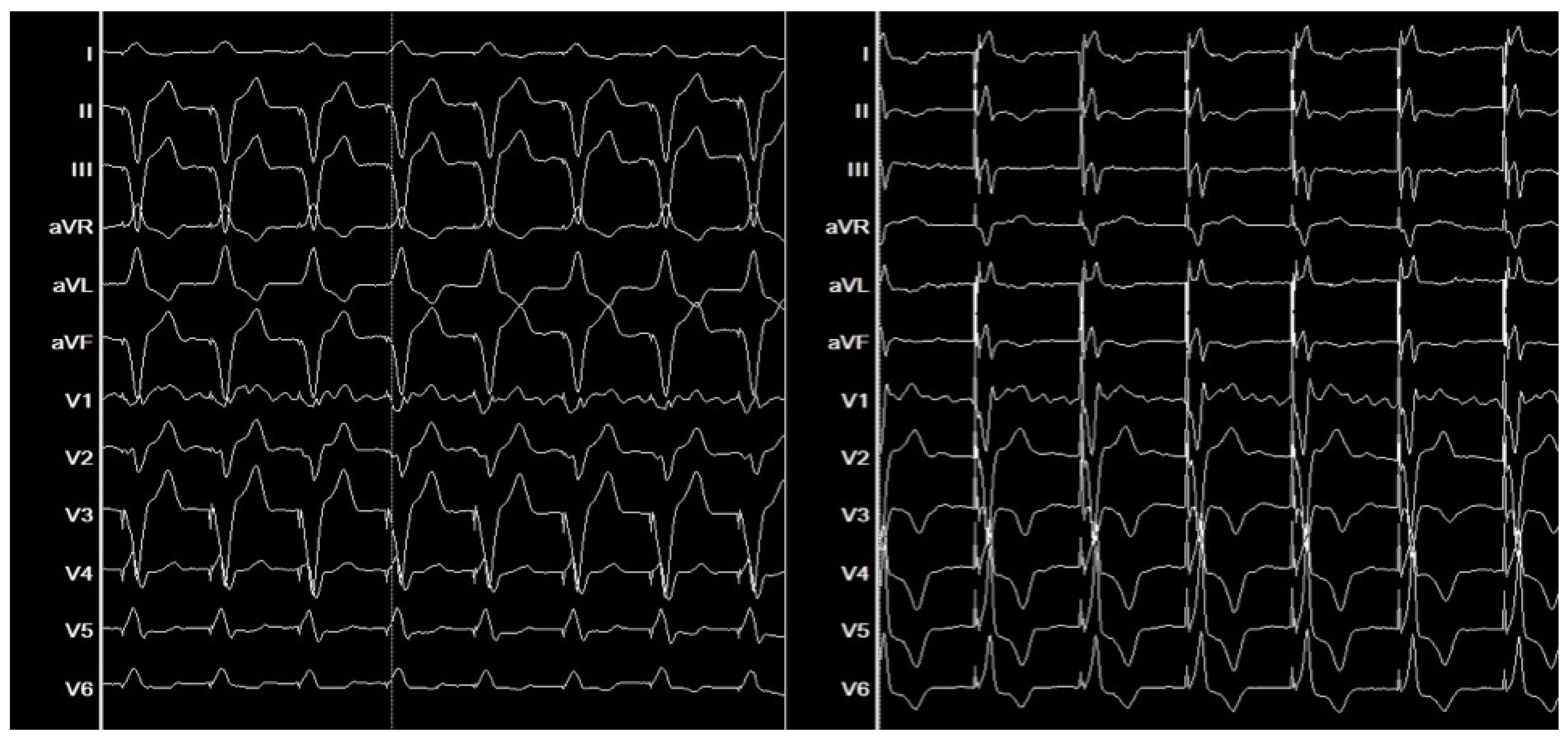
4. Clinical Outcomes of Bundle Branch Pacing
5. Echocardiographic Selection Criteria and Evidence of Reverse Remodeling Following Bundle Branch Pacing
6. Biomolecular Effects of Bundle Branch Stimulation
7. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBP | Bundle Branch Pacing |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| TDI | Tissue Doppler Imaging |
| STE | Speckle-Tracking Imaging |
| GLS | Global Longitudinal Strain |
| CRT | Cardiac Resynchronization Therapy |
| LBB | Left Bundle Branch Block |
References
- Vicent, L.; Álvarez-García, J.; Vazquez-Garcia, R.; González-Juanatey, J.R.; Rivera, M.; Segovia, J.; Pascual-Figal, D.; Bover, R.; Worner, F.; Fernández-Avilés, F.; et al. Coronary Artery Disease and Prognosis of Heart Failure with Reduced Ejection Fraction. J. Clin. Med. 2023, 12, 3028. [Google Scholar] [CrossRef]
- Friedman, D.J.; Emerek, K.; Kisslo, J.; Søgaard, P.; Atwater, B.D. Left bundle-branch block is associated with asimilar dyssynchronous phenotype in heart failure patients with normal and reduced ejection fractions. Am. Heart J. 2021, 231, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pujol-López, M.; Tolosana, J.M.; Upadhyay, G.A.; Mont, L.; Tung, R. Left Bundle Branch Block: Characterization, Definitions, and Recent Insights into Conduction System Physiology. Cardiol. Clin. 2023, 41, 379–391. [Google Scholar] [CrossRef]
- Sami, A.; Iftekhar, M.F.; Khan, I.; Jan, R. Intraventricular Dyssynchrony among patients with left bundle branch block. Pak. J. Med. Sci. 2018, 34, 390–392. [Google Scholar] [CrossRef]
- Leclercq, C.; Burri, H.; Delnoy, P.P.; Rinaldi, C.A.; Sperzel, J.; Calò, L.; Concha, J.F.; Fusco, A.; Al Samadi, F.; Lee, K.; et al. Cardiac resynchronization therapy non-responder to responder conversion rate in the MORE-CRT MPP trial. Europace 2023, 25, euad294. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Q.; Sun, H.; Qin, X.; Zheng, Q. Left Bundle Branch Pacing: Current Knowledge and Future Prospects. Front. Cardiovasc. Med. 2021, 8, 630399. [Google Scholar] [CrossRef]
- Siranart, N.; Chokesuwattanaskul, R.; Prasitlumkum, N.; Huntrakul, A.; Phanthong, T.; Sowalertrat, W.; Navaravong, L.; Cheungpasitporn, W.; Jongnarangsin, K. Reverse of left ventricular remodeling in heart failure patients with left bundle branch area pacing: Systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2023, 46, 459–466. [Google Scholar] [CrossRef] [PubMed]
- AlJaroudi, W. Left ventricular mechanical dyssynchrony in patient with CAD: The Saga continues. J. Nucl. Cardiol. 2021, 28, 3021–3024. [Google Scholar] [CrossRef]
- Bacharova, L.; de Luna, B. The Primary Alteration of Ventricular Myocardium Conduction: The Significant Determinant of Left Bundle Branch Block Pattern. Cardiol. Res. Pract. 2022, 2022, 3438603. [Google Scholar] [CrossRef]
- Sillanmäki, S.; Lipponen, J.A.; Tarvainen, M.P.; Laitinen, T.; Hedman, M.; Hedman, A.; Kivelä, A.; Hämäläinen, H.; Laitinen, T. Relationships between electrical and mechanical dyssynchrony in patients with left bundle branch block and healthy controls. J. Nucl. Cardiol. 2019, 26, 1228–1239. [Google Scholar] [CrossRef]
- Hayama, Y.; Miyazaki, A.; Ohuchi, H.; Miike, H.; Negishi, J.; Sakaguchi, H.; Kurosaki, K.; Shimizu, S.; Kawada, T.; Sugimachi, M. Septal Flash-like Motion of the Earlier Activated Ventricular Wall Represents the Pathophysiology of Mechanical Dyssynchrony in Single-Ventricle Anatomy. J. Am. Soc. Echocardiogr. 2020, 33, 612–621.e2. [Google Scholar] [CrossRef] [PubMed]
- Calle, S.; Kamoen, V.; De Buyzere, M.; De Pooter, J.; Timmermans, F. A Strain-Based Staging Classification of Left Bundle Branch Block-Induced Cardiac Remodeling. JACC Cardiovasc. Imaging 2021, 14, 1691–1702. [Google Scholar] [CrossRef] [PubMed]
- Sze, E.; Daubert, J.P. Left bundle branch block-induced left ventricular remodeling and its potential for reverse remodeling. J. Interv. Card. Electrophysiol. 2018, 52, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Degtiarova, G.; Claus, P.; Duchenne, J.; Schramm, G.; Nuyts, J.; Verberne, H.J.; Voigt, J.U.; Gheysens, O. Impact of left bundle branch block on myocardial perfusion and metabolism: A positron emission tomography study. J. Nucl. Cardiol. 2021, 28, 1730–1739. [Google Scholar] [CrossRef]
- Brenyo, A.; Pietrasik, G.; Barsheshet, A.; Huang, D.T.; Polonsky, B.; McNitt, S.; Moss, A.J.; Zareba, W. QRS fragmentation and the risk of sudden cardiac death in MADIT II. J. Cardiovasc. Electrophysiol. 2012, 23, 1343–1348. [Google Scholar] [CrossRef]
- Vezi, B.; Akinrimisi, O.P. Permanent Bi-Bundle Pacing in a Patient With Heart Failure and Left Bundle Branch Block. JACC Case Rep. 2022, 4, 101688. [Google Scholar] [CrossRef]
- Agricola, E.; Ancona, F. Is There Any Room Left for Echocardiographic-Dyssynchrony Parameters in the Field of CRT? JACC Cardiovasc. Imaging 2023, 16, 885–888. [Google Scholar] [CrossRef]
- Bazoukis, G.; Thomopoulos, C.; Tse, G.; Tsioufis, K.; Nihoyannopoulos, P. Global longitudinal strain predicts responders after cardiac resynchronization therapy-a systematic review and meta-analysis. Heart Fail Rev. 2022, 27, 827–836. [Google Scholar] [CrossRef]
- Gurgu, A.; Luca, C.T.; Vacarescu, C.; Petrescu, L.; Goanta, E.V.; Lazar, M.A.; Arnăutu, D.A.; Cozma, D. Considering Diastolic Dyssynchrony as a Predictor of Favorable Response in LV-Only Fusion Pacing Cardiac Resynchronization Therapy. Diagnostics 2023, 13, 1186. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Cameli, M.; Pastore, M.C.; Benfari, G.; Malagoli, A.; D’Andrea, A.; Sperlongano, S.; Bandera, F.; Esposito, R.; Santoro, C.; et al. Speckle tracking echocardiography in early disease stages: A therapy modifier? J. Cardiovasc. Med. 2023, 24 (Suppl. S1), e55–e66. [Google Scholar] [CrossRef]
- Laufer-Perl, M.; Arnold, J.H.; Moshkovits, Y.; Havakuk, O.; Shmilovich, H.; Chausovsky, G.; Sivan, A.; Szekely, Y.; Arbel, Y.; Banai, S.; et al. Evaluating the role of left ventricle global longitudinal strain in myocardial perfusion defect assessment. Int. J. Cardiovasc. Imaging 2022, 38, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Pujol-López, M.; Jiménez-Arjona, R.; Garcia-Ribas, C.; Borràs, R.; Guasch, E.; Regany-Closa, M.; Graterol, F.R.; Niebla, M.; Carro, E.; Roca-Luque, I.; et al. Longitudinal comparison of dyssynchrony correction and ‘strain’ improvement by conduction system pacing: LEVEL-AT trial secondary findings. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Bragança, B.; Trêpa, M.; Santos, R.; Silveira, I.; Fontes-Oliveira, M.; Sousa, M.J.; Reis, H.; Torres, S.; Santos, M. Echocardiographic Assessment of Right Ventriculo-arterial Coupling: Clinical Correlates and Prognostic Impact in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. J. Cardiovasc. Imaging 2020, 28, 109–120. [Google Scholar] [CrossRef]
- Berlot, B.; Bucciarelli-Ducci, C.; Palazzuoli, A.; Marino, P. Myocardial phenotypes and dysfunction in HFpEF and HFrEF assessed by echocardiography and cardiac magnetic resonance. Heart Fail. Rev. 2020, 25, 75–84. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, X.; Gao, Y.; Wu, S.; Xu, N.; Chen, F.; Song, Y.; Li, C.; Lu, M.; Dai, Y.; et al. Cardiac magnetic resonance-derived myocardial scar is associated with echocardiographic response and clinical prognosis of left bundle branch area pacing for cardiac resynchronization therapy. Europace 2023, 25, euad326. [Google Scholar] [CrossRef]
- Bissell, M.M.; Raimondi, F.; Ali, L.A.; Allen, B.D.; Barker, A.J.; Bolger, A.; Burris, N.; Carhäll, C.J.; Collins, J.D.; Ebbers, T.; et al. 4D Flow cardiovascular magnetic resonance consensus statement: 2023 update. J. Cardiovasc. Magn. Reson. 2023, 25, 40. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Clarke, C.; Ramachenderam, L.; Matthews, G.; Broncano, J.; Garg, P. How can Four-Dimensional Magnetic Resonance Imaging Improve the Diagnosis of Heart Disease? Br. J. Hosp. Med. 2024, 85, 1–18. [Google Scholar] [CrossRef]
- Voigt, J.U.; Cvijic, M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc. Imaging 2019, 12, 1849–1863. [Google Scholar] [CrossRef]
- Moriña-Vázquez, P.; Moraleda-Salas, M.T.; López-Masjuan-Ríos, Á.; Esteve-Ruiz, I.; Arce-León, Á.; Lluch-Requerey, C.; Rodríguez-Albarrán, A.; Venegas-Gamero, J.; Gómez-Menchero, A.E. Improvement in electrocardiographic parameters of repolarization related to sudden death in patients with ventricular dysfunction and left bundle branch block after cardiac resynchronization through His bundle pacing. J. Interv. Card Electrophysiol. 2023, 66, 2003–2010. [Google Scholar] [CrossRef]
- Sharma, P.S.; Patel, N.R.; Ravi, V.; Zalavadia, D.V.; Dommaraju, S.; Garg, V.; Larsen, T.R.; Naperkowski, A.M.; Wasserlauf, J.; Krishnan, K.; et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022, 19, 3–11, Erratum in Heart Rhythm. 2023, 20, 1100. https://doi.org/10.1016/j.hrthm.2023.05.001. [Google Scholar] [CrossRef]
- Zhuo, J.; Chen, C.; Lin, J.; Wang, J.; Fu, F. A long-term clinical comparative study of left bundle branch pacing versus biventricular pacing in patients with heart failure and complete left bundle branch block. Heart Vessel 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Felix, I.F.; Collini, M.; Fonseca, R.; Guida, C.; Armaganijan, L.; Healey, J.S.; Carvalho, G. Conduction system pacing versus biventricular pacing in heart failure with reduced ejection fraction: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2024, 21, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Kiełbasa, G.; Cano, O.; Curila, K.; Heckman, L.; De Pooter, J.; Chovanec, M.; Rademakers, L.; Huybrechts, W.; Grieco, D.; et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022, 43, 4161–4173. [Google Scholar] [CrossRef]
- Fletcher-Hall, S. Pacemaker-induced cardiomyopathy. JAAPA. 2023, 36, 1–4. [Google Scholar] [CrossRef]
- Shan, Q.J.; Xu, H.; Zhou, X.J.; Chang, Q.; Ji, L.; Chen, C.; Jiang, Z.X. Effects of permanent left bundle branch area pacing on QRS duration and short-term cardiac function in pacing-indicated patients with left bundle branch block. Chin. Med. J. 2021, 134, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraman, P.; Pokharel, P.; Subzposh, F.A.; Oren, J.W.; Storm, R.H.; Batul, S.A.; Beer, D.A.; Hughes, G.; Leri, G.; Manganiello, M.; et al. His-Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy vs Biventricular Pacing: HOT-CRT Clinical Trial. JACC Clin. Electrophysiol. 2023, 9, 2628–2638. [Google Scholar] [CrossRef]
- Madias, J.E. The impact of changing oedematous states on the QRS duration: Implications for cardiac resynchronization therapy and implantable cardioverter/defibrillator implantation. Europace 2005, 7, 158–164. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Li, Y.; Qi, J.; Liu, J. Left bundle branch pacing in heart failure patients with left bundle branch block: A systematic review and meta-analysis. Pacing Clin. Electrophysiol. 2022, 45, 212–218. [Google Scholar] [CrossRef]
- Vijayaraman, P.; Sharma, P.S.; Cano, Ó.; Ponnusamy, S.S.; Herweg, B.; Zanon, F.; Jastrzebski, M.; Zou, J.; Chelu, M.G.; Vernooy, K.; et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023, 82, 228–241. [Google Scholar] [CrossRef]
- Auricchio, A.; Prinzen, F.W. Enhancing Response in the Cardiac Resynchronization Therapy Patient: The 3B Perspective-Bench, Bits, and Bedside. JACC Clin. Electrophysiol. 2017, 3, 1203–1219. [Google Scholar] [CrossRef]
- Ali, N.; Arnold, A.D.; Miyazawa, A.A.; Keene, D.; Peters, N.S.; Kanagaratnam, P.; Qureshi, N.; Ng, F.S.; Linton, N.W.F.; Lefroy, D.C.; et al. Septal scar as a barrier to left bundle branch area pacing. Pacing Clin. Electrophysiol. 2023, 46, 1077–1084. [Google Scholar] [CrossRef]
- Lisi, M.; Cameli, M.; Mandoli, G.E.; Pastore, M.C.; Righini, F.M.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; Mondillo, S.; Henein, M.Y. Detection of myocardial fibrosis by speckle-tracking echocardiography: From prediction to clinical applications. Heart Fail. Rev. 2022, 27, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Kayani, W.T. Cardiac Resynchronization Therapy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Yang, Z.; Liang, J.; Chen, R.; Pang, N.; Zhang, N.; Guo, M.; Gao, J.; Wang, R. Clinical outcomes of left bundle branch area pacing: Prognosis and specific applications. Pacing Clin. Electrophysiol. 2024, 47, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Fan, X.; Wang, Q.; Wang, Z.; Li, H.; Tao, J.; Wang, H.; Liu, Z.; Yao, Y. Tricuspid regurgitation outcomes in left bundle branch area pacing and comparison with right ventricular septal pacing. Heart Rhythm. 2022, 19, 1202–1203. [Google Scholar] [CrossRef] [PubMed]
- Cacciapuoti, F.; Marfella, R.; Paolisso, G.; Cacciapuoti, F. Is the aging heart similar to the diabetic heart? Evaluation of LV function of the aging heart with Tissue Doppler Imaging. Aging Clin. Exp. Res. 2009, 21, 22–26. [Google Scholar] [CrossRef]
- Schmitt, J.; Althoff, T.; Busch, S.; Chun, K.R.J.; Dahme, T.; Ebert, M.; Estner, H.; Gunawardene, M.; Heeger, C.; Iden, L.; et al. Left bundle branch (area) pacing“: Sondenpositionierung und Erfolgskriterien—Schritt für Schritt [Left bundle branch (area) pacing: Lead positioning and implant criteria-step for step]. Herzschrittmacherther Elektrophysiol. 2024, 36, 82–90. [Google Scholar] [CrossRef]
- Guo, J.; Li, L.; Xiao, G.; Huang, X.; Li, Q.; Wang, Y.; Cai, B. Feasibility and stability of left bundle branch pacing in patients after prosthetic valve implantation. Clin. Cardiol. 2020, 43, 1110–1118. [Google Scholar] [CrossRef]
- Mei, Y.; Han, R.; Cheng, L.; Li, R.; He, Y.; Xie, J.; Wang, Z.; Wu, Y. Assessment of Cardiac Function and Ventricular Mechanical Synchronization in Left Bundle Branch Area Pacing by Speckle Tracking and Three-Dimensional Echocardiography. Am. J. Cardiol. 2023, 187, 1–9. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, C.; Du, A.; Wang, Q.; He, C.; Zou, F.; Li, X.; Tao, J.; Wang, C.; Liu, Z.; et al. Comparisons of long-term clinical outcomes with left bundle branch pacing, left ventricular septal pacing, and biventricular pacing for cardiac resynchronization therapy. Heart Rhythm. 2024, 21, 1342–1353. [Google Scholar] [CrossRef]
- Ponnusamy, S.S.; Arora, V.; Namboodiri, N.; Kumar, V.; Kapoor, A.; Vijayaraman, P. Left bundle branch pacing: A comprehensive review. J. Cardiovasc. Electrophysiol. 2020, 31, 2462–2473. [Google Scholar] [CrossRef]
- Cheng, S.; Li, H.; Hu, Y.; Jin, H.; Weng, S.; He, P.; Huang, H.; Liu, X.; Gu, M.; Niu, H.; et al. Left Bundle Branch Area Pacing With or Without Conduction System Capture in Heart Failure Models. JACC Clin. Electrophysiol. 2024, 10, 2234–2246. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Wu, Y.; Qin, C.; Xue, S.; Hu, G.; Zou, J.; Shan, Q.; Zhou, X.; Hou, X.; et al. The association between paced left ventricular activation time and cardiac reverse remodeling in heart failure patients with left bundle branch block. J. Cardiovasc. Electrophysiol. 2024, 35, 1636–1644. [Google Scholar] [CrossRef]
- Peigh, G.; Steinberg, B.A. Mechanisms for structural remodeling with left bundle branch area pacing: More than meets the eye. J. Interv. Card Electrophysiol. 2024, 67, 221–223. [Google Scholar] [CrossRef]
- Chen, X.; Huang, M.; Chen, Y.; Xu, H.; Wu, M. Mineralocorticoid receptor antagonists and heart failure with preserved ejection fraction: Current understanding and future prospects. Heart Fail. Rev. 2025, 30, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, Y.; Zhou, C.; Xie, J.; Zhang, Y.; Yang, X.; Xiao, J.; Wang, D.W.; Shan, C.; Zhou, X.; et al. Hyperactivation of ATF4/TGF-β1 signaling contributes to the progressive cardiac fibrosis in Arrhythmogenic cardiomyopathy caused by DSG2 Variant. BMC Med. 2024, 22, 361. [Google Scholar] [CrossRef]
- Wang, J.; Gong, X.; Chen, H.; Qin, S.; Zhou, N.; Su, Y.; Ge, J. Effect of Cardiac Resynchronization Therapy on Myocardial Fibrosis and Relevant Cytokines in a Canine Model With Experimental Heart Failure. J. Cardiovasc. Electrophysiol. 2017, 28, 438–445. [Google Scholar] [CrossRef]
- Loh, Y.H.; Lv, J.; Goh, Y.; Sun, X.; Zhu, X.; Muheyati, M.; Luan, Y. Remodelling of T-Tubules and Associated Calcium Handling Dysfunction in Heart Failure: Mechanisms and Therapeutic Insights. Can. J. Cardiol. 2024, 40, 2569–2588. [Google Scholar] [CrossRef] [PubMed]
- Uchinoumi, H.; Nakamura, Y.; Suetomi, T.; Nawata, T.; Fujinaka, M.; Kobayashi, S.; Yamamoto, T.; Yano, M.; Sano, M. Structural Instability of Ryanodine Receptor 2 Causes Endoplasmic Reticulum (ER) Dysfunction as Well as Sarcoplasmic Reticulum (SR) Dysfunction. J. Cardiol. 2025, in press. [Google Scholar] [CrossRef]
- Bassingthwaighte, J.B. Linking cellular energetics to local flow regulation in the heart. Ann. N. Y. Acad. Sci. 2008, 1123, 126–133. [Google Scholar] [CrossRef][Green Version]
- Sahu, P.; Acharya, S.; Totade, M. Evolution of Pacemakers and Implantable Cardioverter Defibrillators (ICDs) in Cardiology. Cureus 2023, 15, e46389. [Google Scholar] [CrossRef]
- Nantsupawat, T.; Gumrai, P.; Apaijai, N.; Phrommintikul, A.; Prasertwitayakij, N.; Chattipakorn, S.C.; Chattipakorn, N.; Wongcharoen, W. Atrial pacing improves mitochondrial function in peripheral blood mononuclear cells in patients with cardiac implantable electronic devices. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H1146–H1152. [Google Scholar] [CrossRef] [PubMed]
- Furquim, S.R.; Bocchi, E.A.; Lira, M.T.S.S.; Wanderley, M.R.B., Jr.; de Marchi, D.C.; Maciel, P.C.; Zimerman, A.; Ramires, F.J.A.; Nastari, L.; Biselli, B.; et al. Predictors of sustained reverse remodelling in patients with heart failure with reduced ejection fraction. ESC Heart Fail. 2025. early view. [Google Scholar] [CrossRef] [PubMed]
- Moraleda-Salas, M.T.; Amigo-Otero, E.; Esteve-Ruiz, I.; Arce-León, Á.; Carreño-Lineros, J.M.; Torralba, E.I.; Roldan, F.N.; Moriña-Vázquez, P. Early Improvement in Cardiac Function and Dyssynchrony After Physiological Upgrading in Pacing-Induced Cardiomyopathy. Pacing Clin. Electrophysiol. 2024, 48, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Verheule, S.; Kaese, S. Connexin diversity in the heart: Insights from transgenic mouse models. Front. Pharmacol. 2013, 4, 81. [Google Scholar] [CrossRef]
- Ladenvall, P.; Andersson, B.; Dellborg, M.; Hansson, P.O.; Eriksson, H.; Thelle, D.; Eriksson, P. Genetic variation at the human connexin 43 locus but not at the connexin 40 locus is associated with left bundle branch block. Open Heart 2015, 2, e000187. [Google Scholar] [CrossRef]
- Aiba, T.; Tomaselli, G.F. Electrical remodeling in the failing heart. Curr. Opin. Cardiol. 2010, 25, 29–36. [Google Scholar] [CrossRef]
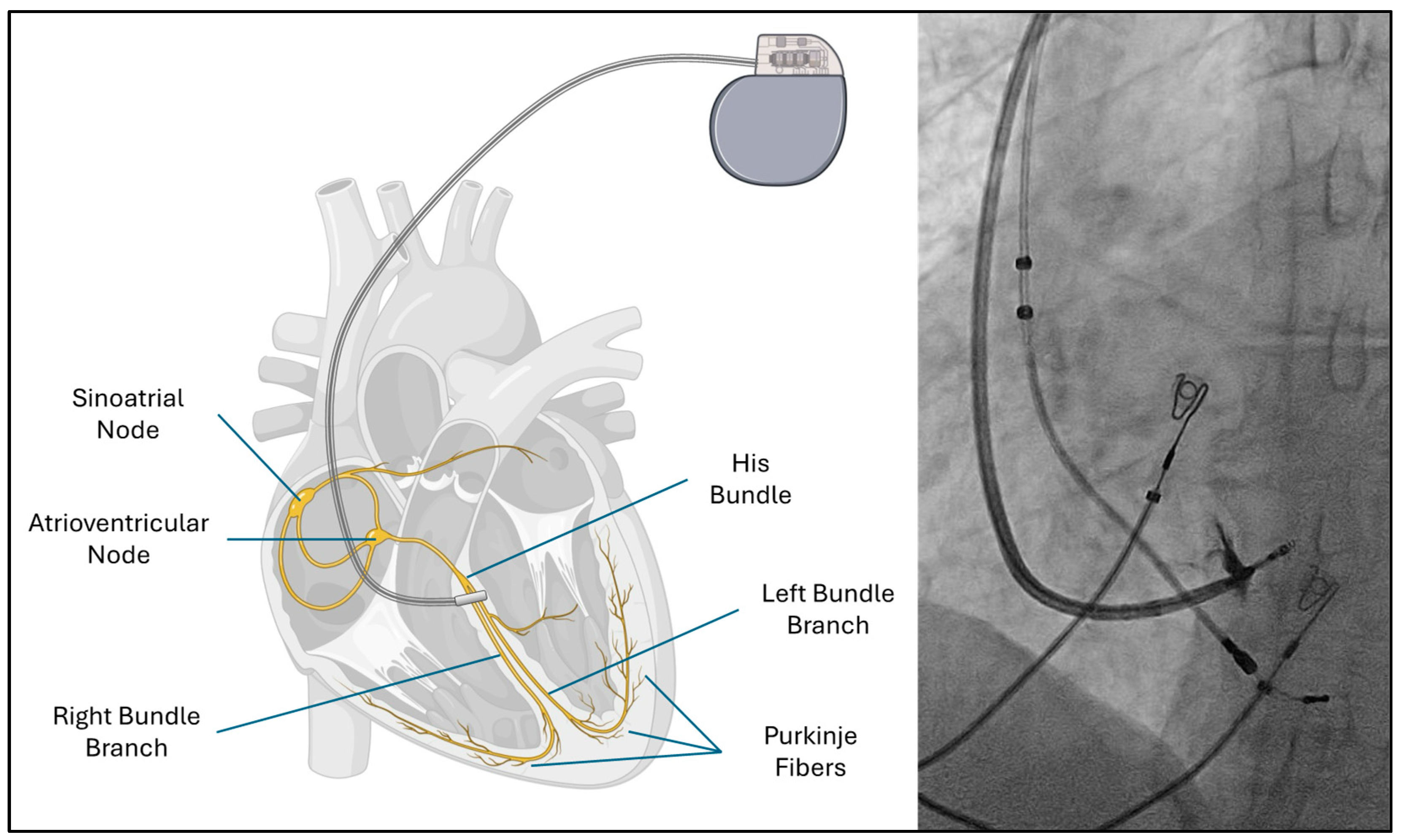


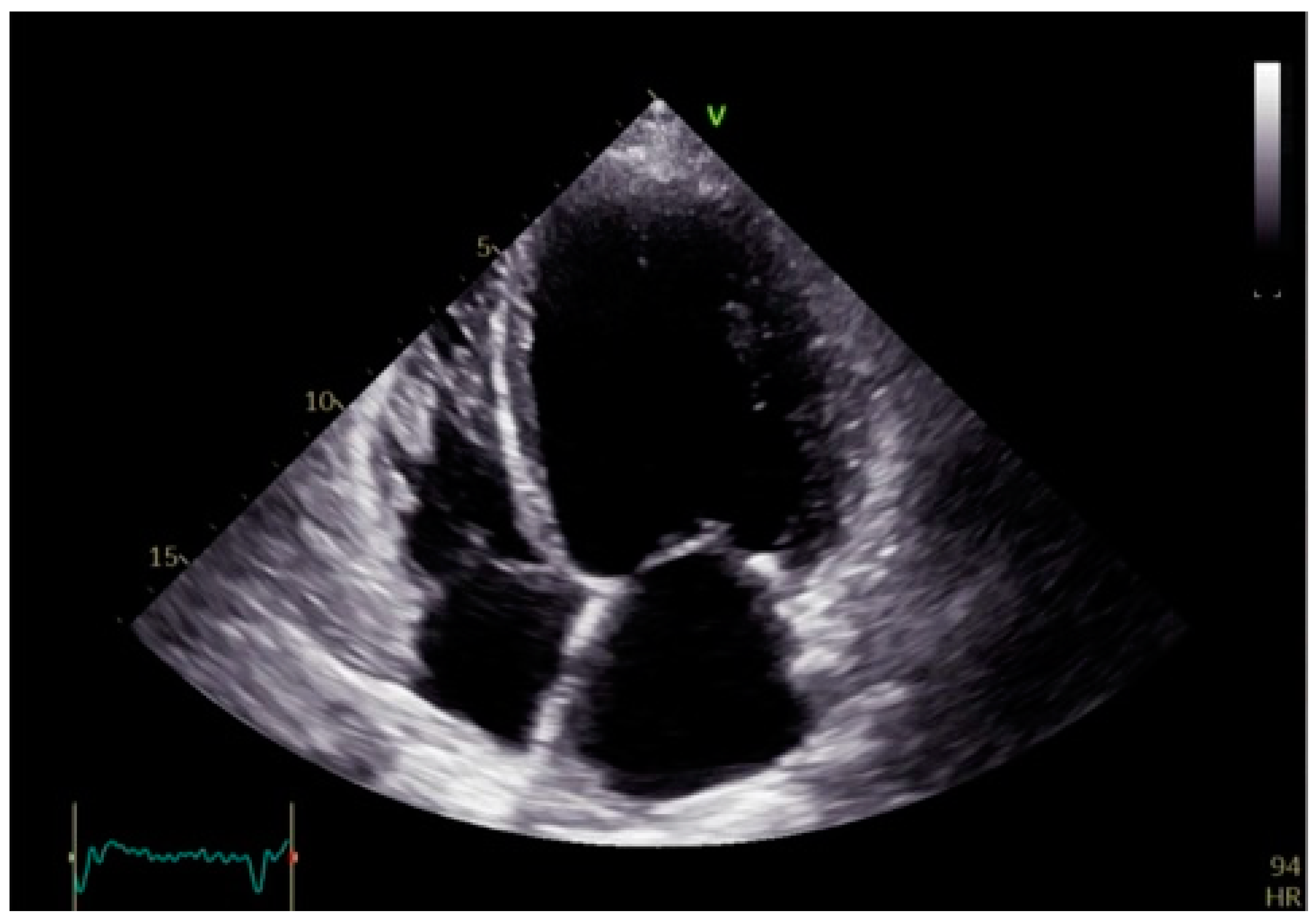


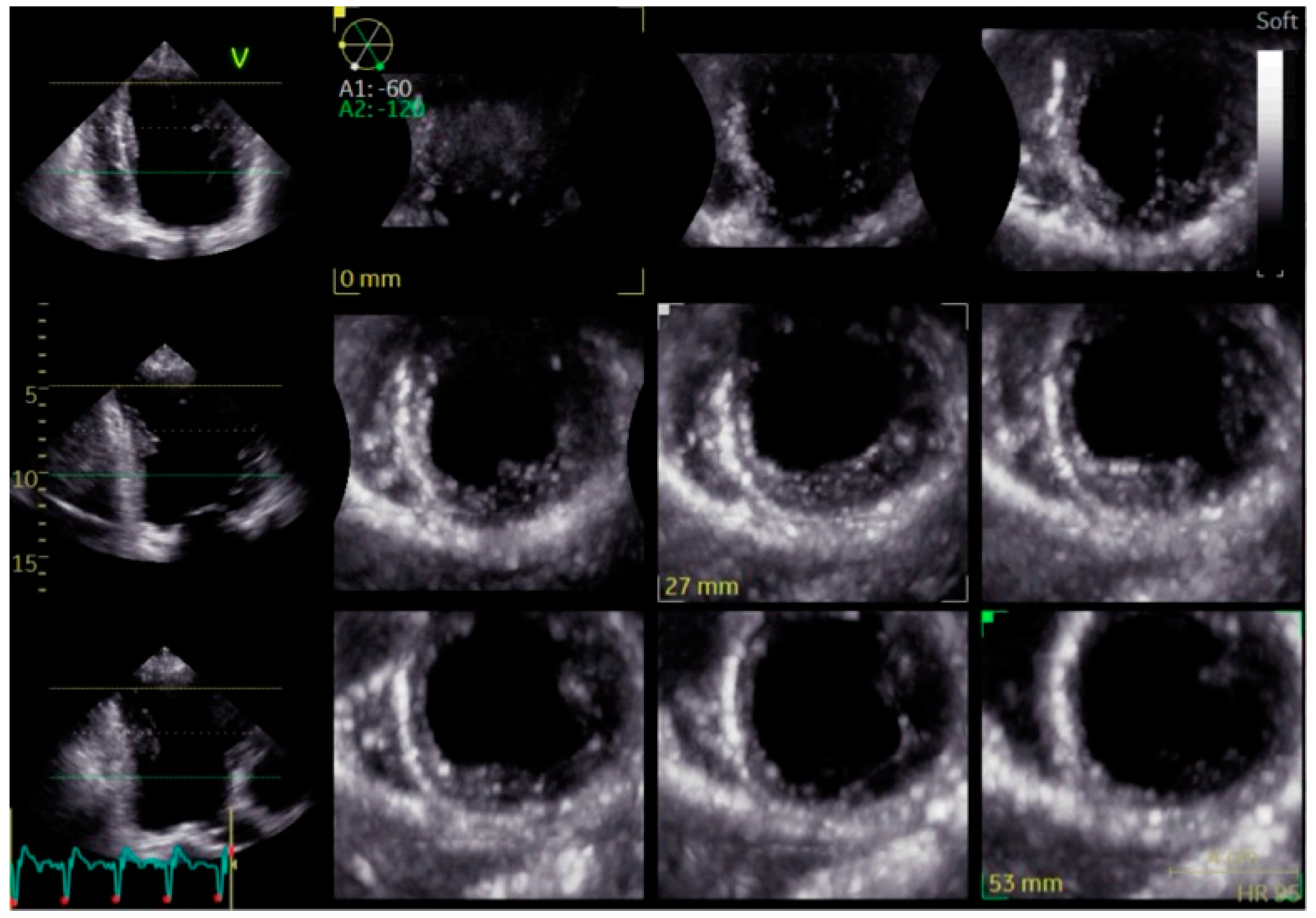
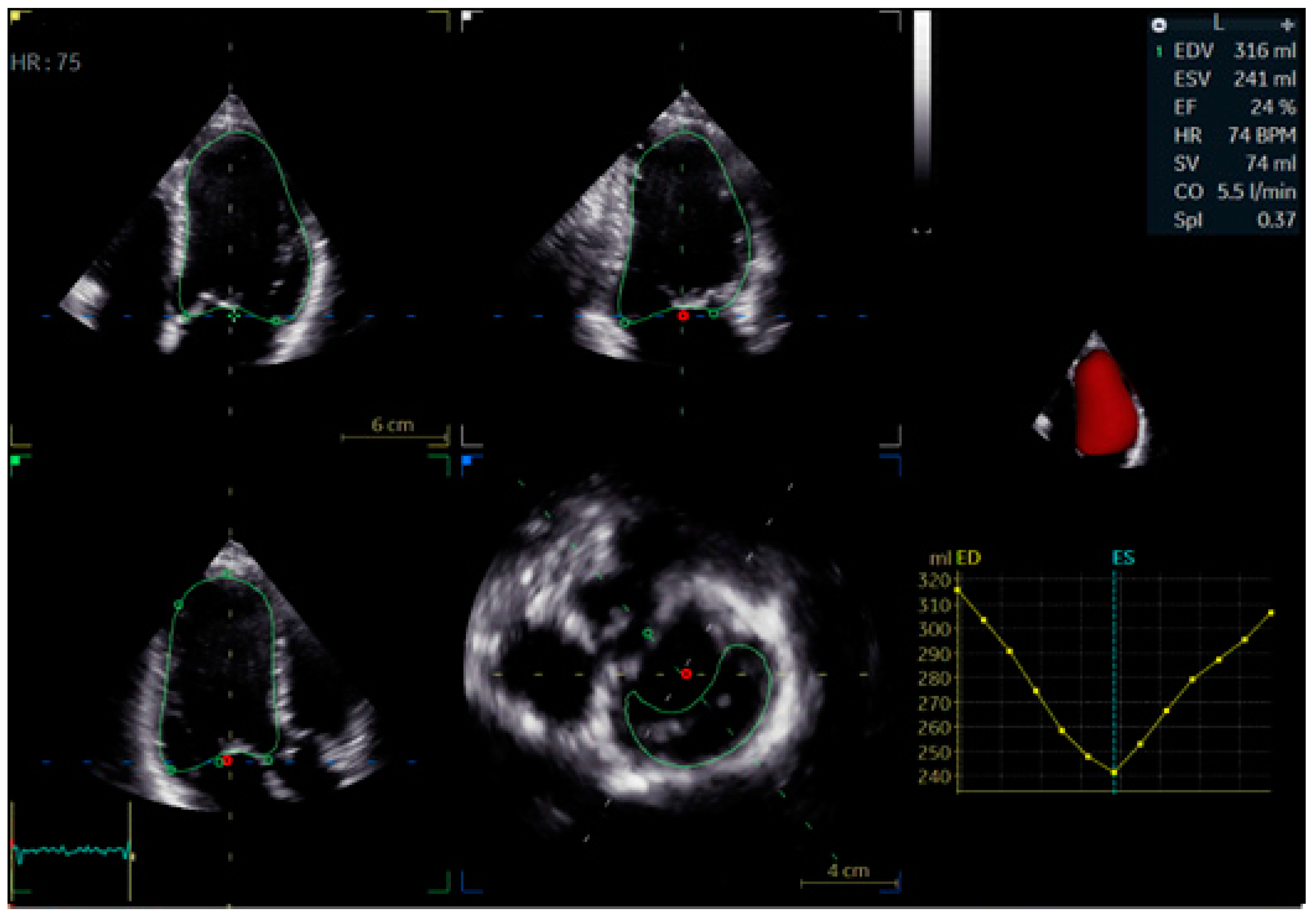
| Electrophysiological Effect | Bundle Branch Pacing (BBP) | Cardiac Resynchronization Therapy (CRT) |
|---|---|---|
| Conduction System Engagement | Utilizes the His-Purkinje system, preserving conduction integrity | Direct myocardial stimulation, bypassing conduction system |
| Ventricular Activation Pattern | More physiological, mimicking natural ventricular depolarization | Non-physiological but enhances synchrony in dyssynchronous ventricles |
| QRS Duration | Narrower QRS compared to traditional pacing | QRS narrowing possible but may remain wider than BBP |
| Ventricular Activation Time | Reduced due to activation through native pathways | Can reduce activation time but depends on lead placement |
| Repolarization Effects | Minimal repolarization abnormalities | Potentially alters repolarization due to non-specific myocardial activation |
| Effect in Extensive Fibrosis | Limited effectiveness if conduction system is severely diseased | More effective in cases with significant fibrosis or conduction block |
| Clinical Outcome | Bundle Branch Pacing (BBP) | Cardiac Resynchronization Therapy (CRT) |
|---|---|---|
| Changes in QRS Duration | Significant QRS narrowing by engaging the conduction system | QRS narrowing depends on lead placement; may not be as physiological as BBP |
| LVEF Improvements | Moderate improvement, particularly in patients with conduction disease | Greater improvement, especially in patients with LBBB and wide QRS |
| NYHA Class Improvements | Mild to moderate improvement in symptoms | More substantial symptomatic improvement, particularly in NYHA class III-IV |
| Reduction in Heart Failure Hospitalizations | Reduces hospitalizations in select patients, but less studied than CRT | Well-documented reduction in heart failure hospitalizations |
| Reverse Remodeling | Modest reductions in LV volumes due to improved contraction efficiency | More pronounced reductions in LVEDV and LVESV due to improved synchrony |
| Arrhythmia Burden | Lower risk of ventricular arrhythmias; uncertain effect on atrial fibrillation | May reduce ventricular arrhythmias but can increase atrial fibrillation risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacciapuoti, F.; Mauro, C.; Caso, I.; Crispo, S.; Gottilla, R.; Capone, V.; Ambrosino, S.; Pirozzi, C.; Munciguerra, O.; Volpicelli, M. The Functional and Imaging Implications of Left Bundle Branch Pacing in Ischemic Cardiomyopathy. Biomolecules 2025, 15, 489. https://doi.org/10.3390/biom15040489
Cacciapuoti F, Mauro C, Caso I, Crispo S, Gottilla R, Capone V, Ambrosino S, Pirozzi C, Munciguerra O, Volpicelli M. The Functional and Imaging Implications of Left Bundle Branch Pacing in Ischemic Cardiomyopathy. Biomolecules. 2025; 15(4):489. https://doi.org/10.3390/biom15040489
Chicago/Turabian StyleCacciapuoti, Fulvio, Ciro Mauro, Ilaria Caso, Salvatore Crispo, Rossella Gottilla, Valentina Capone, Saverio Ambrosino, Ciro Pirozzi, Orlando Munciguerra, and Mario Volpicelli. 2025. "The Functional and Imaging Implications of Left Bundle Branch Pacing in Ischemic Cardiomyopathy" Biomolecules 15, no. 4: 489. https://doi.org/10.3390/biom15040489
APA StyleCacciapuoti, F., Mauro, C., Caso, I., Crispo, S., Gottilla, R., Capone, V., Ambrosino, S., Pirozzi, C., Munciguerra, O., & Volpicelli, M. (2025). The Functional and Imaging Implications of Left Bundle Branch Pacing in Ischemic Cardiomyopathy. Biomolecules, 15(4), 489. https://doi.org/10.3390/biom15040489





