The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Pancreatic Cancer Cohort Characterization
2.3. Cell Culture
2.4. Cell Transfection
2.5. RNA Extraction
2.6. cDNA Synthesis
2.7. Quantitative Polymerase Chain Reaction (qPCR)
2.8. Statistical Analysis
2.9. TNMplot Database Analysis
3. Results
3.1. Association of Tumor and Age Range or Gender with the EMT Process
3.2. Association of Tumor Aggressiveness and Invasiveness with the EMT Process
3.3. Association of Tumor and Age Range or Gender with the MAPK/ERK Signaling Pathway
3.4. Association of Tumor Aggressiveness and Invasiveness with the MAPK/ERK Signaling Pathway
3.5. Pearson Correlation Coefficients Between AQPs, EMT, and MAPK/ERK Signaling Pathways
3.6. Experimental Validation of the Positive Associations Between AQPs and Markers of EMT and MAPK/ERK Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Li, Q.J.; Hao, F.B.; Wu, Y.Q.; Liu, S.; Zhong, G.C. Adherence to the 2018 World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and pancreatic cancer incidence and mortality: A prospective cohort study. Cancer Med. 2020, 9, 6843–6853. [Google Scholar] [CrossRef] [PubMed]
- Midha, S.; Chawla, S.; Garg, P.K. Modifiable and non-modifiable risk factors for pancreatic cancer: A review. Cancer Lett. 2016, 381, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Cwik, G.; Wallner, G.; Skoczylas, T.; Ciechanski, A.; Zinkiewicz, K. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch. Surg. 2006, 141, 968–973, discussion 974. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. Serum CA 19-9 as a Biomarker for Pancreatic Cancer—A Comprehensive Review. Indian J. Surg. Oncol. 2011, 2, 88–100. [Google Scholar] [CrossRef]
- Melisi, D.; Calvetti, L.; Frizziero, M.; Tortora, G. Pancreatic cancer: Systemic combination therapies for a heterogeneous disease. Curr. Pharm. Des. 2014, 20, 6660–6669. [Google Scholar] [CrossRef]
- Gaianigo, N.; Melisi, D.; Carbone, C. EMT and Treatment Resistance in Pancreatic Cancer. Cancers 2017, 9, 122. [Google Scholar] [CrossRef]
- Stickler, S.; Rath, B.; Hamilton, G. Targeting KRAS in pancreatic cancer. Oncol. Res. 2024, 32, 799–805. [Google Scholar] [CrossRef]
- Linehan, A.; O’Reilly, M.; McDermott, R.; O’Kane, G.M. Targeting KRAS mutations in pancreatic cancer: Opportunities for future strategies. Front. Med. 2024, 11, 1369136. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins: Translating bench research to human disease. J. Exp. Biol. 2009, 212, 1707–1715. [Google Scholar] [CrossRef]
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed]
- da Silva, I.V.; Mlinaric, M.; Lourenco, A.R.; Perez-Garcia, O.; Cipak Gasparovic, A.; Soveral, G. Peroxiporins and Oxidative Stress: Promising Targets to Tackle Inflammation and Cancer. Int. J. Mol. Sci. 2024, 25, 8381. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, L.; Zhu, Z.; Zheng, M.; Wang, D.; Chen, Z.; Sun, H. Aquaporins as diagnostic and therapeutic targets in cancer: How far we are? J. Transl. Med. 2015, 13, 96. [Google Scholar] [CrossRef]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Aquaporins—New players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef]
- Prata, C.; Hrelia, S.; Fiorentini, D. Peroxiporins in Cancer. Int. J. Mol. Sci. 2019, 20, 1371. [Google Scholar] [CrossRef]
- Moon, C.S.; Moon, D.; Kang, S.K. Aquaporins in Cancer Biology. Front. Oncol. 2022, 12, 782829. [Google Scholar] [CrossRef]
- Marlar, S.; Jensen, H.H.; Login, F.H.; Nejsum, L.N. Aquaporin-3 in Cancer. Int. J. Mol. Sci. 2017, 18, 2106. [Google Scholar] [CrossRef]
- Huang, X.W.; Huang, L.; Shao, M.H. Aquaporin 3 facilitates tumor growth in pancreatic cancer by modulating mTOR signaling. Biochem. Biophys. Res. Commun. 2017, 486, 1097–1102. [Google Scholar] [CrossRef]
- Direito, I.; Paulino, J.; Vigia, E.; Brito, M.A.; Soveral, G. Differential expression of aquaporin-3 and aquaporin-5 in pancreatic ductal adenocarcinoma. J. Surg. Oncol. 2017, 115, 980–996. [Google Scholar] [CrossRef]
- Chen, G.; Song, H.; Yang, Z.; Du, T.; Zheng, Y.; Lu, Z.; Zhang, K.; Wei, D. AQP5 Is a Novel Prognostic Biomarker in Pancreatic Adenocarcinoma. Front. Oncol. 2022, 12, 890193. [Google Scholar] [CrossRef]
- Arsenijevic, T.; Perret, J.; Van Laethem, J.L.; Delporte, C. Aquaporins Involvement in Pancreas Physiology and in Pancreatic Diseases. Int. J. Mol. Sci. 2019, 20, 5052. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; da Silva, I.V.; Sarmento, M.J.; Silva, I.C.; Carvalho, F.A.; Soveral, G.; Santos, N.C. Aquaporin-3 and Aquaporin-5 Facilitate Migration and Cell-Cell Adhesion in Pancreatic Cancer by Modulating Cell Biomechanical Properties. Cells 2022, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Edamana, S.; Login, F.H.; Yamada, S.; Kwon, T.H.; Nejsum, L.N. Aquaporin water channels as regulators of cell-cell adhesion proteins. Am. J. Physiol. Cell Physiol. 2021, 320, C771–C777. [Google Scholar] [CrossRef]
- Rodrigues, C.; Pimpao, C.; Mosca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflugers Arch. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Direito, I.; Madeira, A.; Brito, M.A.; Soveral, G. Aquaporin-5: From structure to function and dysfunction in cancer. Cell. Mol. Life Sci. CMLS 2016, 73, 1623–1640. [Google Scholar] [CrossRef]
- Ryu, H.M.; Oh, E.J.; Park, S.H.; Kim, C.D.; Choi, J.Y.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Chung, H.Y.; Yoo, M.; et al. Aquaporin 3 expression is up-regulated by TGF-beta1 in rat peritoneal mesothelial cells and plays a role in wound healing. Am. J. Pathol. 2012, 181, 2047–2057. [Google Scholar] [CrossRef]
- Jung, H.J.; Jang, H.J.; Kwon, T.H. Aquaporins implicated in the cell proliferation and the signaling pathways of cell stemness. Biochimie 2021, 188, 52–60. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.; Zhou, H.; Du, Z.; Zhang, G. Silencing of aquaporin 5 inhibits the growth of A549 lung cancer cells in vitro and in vivo. Int. J. Oncol. 2018, 52, 1643–1650. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; He, Y.; Zhang, N.; Chang, W.; Niu, Y. Aquaporin-1 facilitates proliferation and invasion of gastric cancer cells via GRB7-mediated ERK and Ras activation. Anim. Cells Syst. 2020, 24, 253–259. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Zheng, B.; Wang, J.; Song, X.; Zheng, W.; Wang, L.; Yang, D.; Wang, J. Effect of AQP9 Expression in Androgen-Independent Prostate Cancer Cell PC3. Int. J. Mol. Sci. 2016, 17, 738. [Google Scholar] [CrossRef] [PubMed]
- Mlinaric, M.; Lucic, I.; Milkovic, L.; da Silva, I.V.; Tartaro Bujak, I.; Musani, V.; Soveral, G.; Cipak Gasparovic, A. AQP3-Dependent PI3K/Akt Modulation in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 8133. [Google Scholar] [CrossRef] [PubMed]
- Shabanian, K.; Shabanian, T.; Karsai, G.; Pontiggia, L.; Paneni, F.; Ruschitzka, F.; Beer, J.H.; Saeedi Saravi, S.S. AQP1 differentially orchestrates endothelial cell senescence. Redox Biol. 2024, 76, 103317. [Google Scholar] [CrossRef]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 2005, 434, 786–792. [Google Scholar] [CrossRef]
- Pimpao, C.; da Silva, I.V.; Mosca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef]
- da Silva, I.V.; Pimpao, C.; Paccetti-Alves, I.; Thomas, S.R.; Barateiro, A.; Casini, A.; Soveral, G. Blockage of aquaporin-3 peroxiporin activity by organogold compounds affects melanoma cell adhesion, proliferation and migration. J. Physiol. 2024, 602, 3111–3129. [Google Scholar] [CrossRef]
- Zhang, W.G.; Li, C.F.; Liu, M.; Chen, X.F.; Shuai, K.; Kong, X.; Lv, L.; Mei, Z.C. Aquaporin 9 is down-regulated in hepatocellular carcinoma and its over-expression suppresses hepatoma cell invasion through inhibiting epithelial-to-mesenchymal transition. Cancer Lett. 2016, 378, 111–119. [Google Scholar] [CrossRef]
- Shi, X.; Wu, S.; Yang, Y.; Tang, L.; Wang, Y.; Dong, J.; Lu, B.; Jiang, G.; Zhao, W. AQP5 silencing suppresses p38 MAPK signaling and improves drug resistance in colon cancer cells. Tumor Biol. 2014, 35, 7035–7045. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.N.; Chen, W.L.; Wang, G.S.; Mao, Q.; Li, S.Q.; Xiong, W.H.; Lin, Y.Y.; Ge, J.W.; Li, X.X.; et al. Effects of AQP5 gene silencing on proliferation, migration and apoptosis of human glioma cells through regulating EGFR/ERK/p38 MAPK signaling pathway. Oncotarget 2017, 8, 38444–38455. [Google Scholar] [CrossRef]
- Pimpão, C.; da Silva, I.V.; Soveral, G. The Expanding Role of Aquaporin-1, Aquaporin-3 and Aquaporin-5 as Transceptors: Involvement in Cancer Development and Potential Druggability. Int. J. Mol. Sci. 2025, 26, 1330. [Google Scholar] [CrossRef]
- Lopes, P.A.; Fonseca, E.; da Silva, I.V.; Vigia, E.; Paulino, J.; Soveral, G. Aquaporins Transcripts with Potential Prognostic Value in Pancreatic Cancer. Genes 2023, 14, 1694. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- SAS/STAT. User’s Guide. 2004, Version 9.1. Available online: https://support.sas.com/documentation/onlinedoc/91pdf/sasdoc_91/stat_ug_7313.pdf (accessed on 14 January 2025).
- Arvelo, F.; Sojo, F.; Cotte, C. Cancer and the metastatic substrate. Ecancermedicalscience 2016, 10, 701. [Google Scholar] [CrossRef]
- Silva, I.V.D.; Coelho, D.; Prates, J.A.M.; Soveral, G.; Lopes, P.A. Tissue-Specific Variation in Aquaporins and Cytokines Transcriptional Profiles in Piglets being LPS Challenged. Front. Biosci. 2023, 28, 126. [Google Scholar] [CrossRef]
- Jang, S.J.; Moon, C. Aquaporin 5 (AQP5) expression in breast cancer and its clinicopathological characteristics. PLoS ONE 2023, 18, e0270752. [Google Scholar] [CrossRef]
- Charlestin, V.; Fulkerson, D.; Arias Matus, C.E.; Walker, Z.T.; Carthy, K.; Littlepage, L.E. Aquaporins: New players in breast cancer progression and treatment response. Front. Oncol. 2022, 12, 988119. [Google Scholar] [CrossRef]
- Shen, Q.; Lin, W.; Luo, H.; Zhao, C.; Cheng, H.; Jiang, W.; Zhu, X. Differential Expression of Aquaporins in Cervical Precursor Lesions and Invasive Cervical Cancer. Reprod. Sci. 2016, 23, 1551–1558. [Google Scholar] [CrossRef]
- Li, W.; Song, Y.; Pan, C.; Yu, J.; Zhang, J.; Zhu, X. Aquaporin-8 is a novel marker for progression of human cervical cancer cells. Cancer Biomark. 2021, 32, 391–400. [Google Scholar] [CrossRef]
- Zhang, G.; Hao, Y.; Chen, L.; Li, Z.; Gao, L.; Tian, J.; Qiao, Q.; Zhang, J. Expression of aquaporin 1, 3 and 5 in colorectal carcinoma: Correlation with clinicopathological characteristics and prognosis. Pathol. Oncol. Res. 2023, 29, 1611179. [Google Scholar] [CrossRef]
- Yamamoto, A.; Shimizu, H.; Takiguchi, K.; Shoda, K.; Shiraishi, K.; Furuya, S.; Akaike, H.; Kawaguchi, Y.; Amemiya, H.; Kawaida, H.; et al. The Expression and Role of Aquaporin 4 in Colon Cancer. Anticancer Res. 2024, 44, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, L.; Romaszko-Wojtowicz, A.; Doboszynska, A.; Skowronska, A. The Role of Aquaporin 5 (AQP5) in Lung Adenocarcinoma: A Review Article. Cells 2023, 12, 468. [Google Scholar] [CrossRef] [PubMed]
- Jaskiewicz, L.; Hejne, K.; Szostak, B.; Osowiecka, K.; Skowronski, M.T.; Lepiarczyk, E.; Doboszynska, A.; Majewska, M.; Kordowitzki, P.; Skowronska, A. Expression Profiles of AQP3 and AQP4 in Lung Adenocarcinoma Samples Generated via Bronchoscopic Biopsies. J. Clin. Med. 2022, 11, 5954. [Google Scholar] [CrossRef]
- Chetry, M.; Li, S.; Liu, H.; Hu, X.; Zhu, X. Prognostic values of aquaporins mRNA expression in human ovarian cancer. Biosci. Rep. 2018, 38, BSR20180108. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Xu, D.; Liu, Y.; Gao, Y. Aquaporin 3 promotes prostate cancer cell motility and invasion via extracellular signal-regulated kinase 1/2-mediated matrix metalloproteinase-3 secretion. Mol. Med. Rep. 2015, 11, 2882–2888. [Google Scholar] [CrossRef]
- Brundl, J.; Wallinger, S.; Breyer, J.; Weber, F.; Evert, M.; Georgopoulos, N.T.; Rosenhammer, B.; Burger, M.; Otto, W.; Rubenwolf, P. Expression, localisation and potential significance of aquaporins in benign and malignant human prostate tissue. BMC Urol. 2018, 18, 75. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Dimas, K.; Sakellaridis, N.; Svokos, K.A.; Svokos, A.A.; Zacharoulis, D. Transcriptomic analysis of the Aquaporin (AQP) gene family interactome identifies a molecular panel of four prognostic markers in patients with pancreatic ductal adenocarcinoma. Pancreatology 2019, 19, 436–442. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Jana, A.; Bhattacharjee, S.; Mitra, S.; De, S.; Alghamdi, B.S.; Alam, M.Z.; Mahmoud, A.B.; Al Shareef, Z.; Abdel-Rahman, W.M.; et al. The role of Aquaporins in tumorigenesis: Implications for therapeutic development. Cell Commun. Signal 2024, 22, 106. [Google Scholar] [CrossRef]
- Cavallaro, U.; Christofori, G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer 2004, 4, 118–132. [Google Scholar] [CrossRef]
- Maier, H.J.; Wirth, T.; Beug, H. Epithelial-mesenchymal transition in pancreatic carcinoma. Cancers 2010, 2, 2058–2083. [Google Scholar] [CrossRef]
- Chae, Y.K.; Woo, J.; Kim, M.J.; Kang, S.K.; Kim, M.S.; Lee, J.; Lee, S.K.; Gong, G.; Kim, Y.H.; Soria, J.C.; et al. Expression of aquaporin 5 (AQP5) promotes tumor invasion in human non small cell lung cancer. PLoS ONE 2008, 3, e2162. [Google Scholar] [CrossRef]
- Soto, E.; Yanagisawa, M.; Marlow, L.A.; Copland, J.A.; Perez, E.A.; Anastasiadis, P.Z. p120 catenin induces opposing effects on tumor cell growth depending on E-cad herin expression. J. Cell Biol. 2008, 183, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Gottardi, C.J.; Wong, E.; Gumbiner, B.M. E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J. Cell Biol. 2001, 153, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, T.; Zhou, Y.C.; Gao, F.; Zhang, Z.H.; Xu, H.; Wang, S.L.; Shen, L.Z. Aquaporin 3 promotes epithelial-mesenchymal transition in gastric cancer. J. Exp. Clin. Cancer Res. 2014, 33, 38. [Google Scholar] [CrossRef]
- Yun, S.; Sun, P.L.; Jin, Y.; Kim, H.; Park, E.; Park, S.Y.; Lee, K.; Lee, K.; Chung, J.H. Aquaporin 1 Is an Independent Marker of Poor Prognosis in Lung Adenocarcinoma. J. Pathol. Transl. Med. 2016, 50, 251–257. [Google Scholar] [CrossRef]
- Liu, W.; Wang, K.; Gong, K.; Li, X.; Luo, K. Epidermal growth factor enhances MPC-83 pancreatic cancer cell migration through the upregulation of aquaporin 3. Mol. Med. Rep. 2012, 6, 607–610. [Google Scholar] [CrossRef]
- Li, A.; Lu, D.; Zhang, Y.; Li, J.; Fang, Y.; Li, F.; Sun, J. Critical role of aquaporin-3 in epidermal growth factor-induced migration of colorectal carcinoma cells and its clinical significance. Oncol. Rep. 2013, 29, 535–540. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Z.; Sun, M.; Wang, J.; Guo, R.; Shen, L.; Wu, W. Critical role of aquaporin-3 in the human epidermal growth factor-induced migration and proliferation in the human gastric adenocarcinoma cells. Cancer Biol. Ther. 2010, 9, 1000–1007. [Google Scholar] [CrossRef]
- Li, C.F.; Zhang, W.G.; Liu, M.; Qiu, L.W.; Chen, X.F.; Lv, L.; Mei, Z.C. Aquaporin 9 inhibits hepatocellular carcinoma through up-regulating FOXO1 expression. Oncotarget 2016, 7, 44161–44170. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Monje, P.; Hernandez-Losa, J.; Lyons, R.J.; Castellone, M.D.; Gutkind, J.S. Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J. Biol. Chem. 2005, 280, 35081–35084. [Google Scholar] [CrossRef] [PubMed]
- Virtakoivu, R.; Mai, A.; Mattila, E.; De Franceschi, N.; Imanishi, S.Y.; Corthals, G.; Kaukonen, R.; Saari, M.; Cheng, F.; Torvaldson, E.; et al. Vimentin-ERK Signaling Uncouples Slug Gene Regulatory Function. Cancer Res. 2015, 75, 2349–2362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Song, Y.; Zhang, P.; Hu, J.; Bai, C. Expression of aquaporin 5 increases proliferation and metastasis potential of lung cancer. J. Pathol. 2010, 221, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.C.; Zhang, W.R.; Cao, W.F.; Liu, B.W.; Zhang, F.; Zhao, H.M.; Meng, R.; Zhang, L.; Niu, R.F.; Hao, X.S.; et al. Aquaporin3 is required for FGF-2-induced migration of human breast cancers. PLoS ONE 2013, 8, e56735. [Google Scholar] [CrossRef]
- Liao, S.; Chen, H.; Liu, M.; Gan, L.; Li, C.; Zhang, W.; Lv, L.; Mei, Z. Aquaporin 9 inhibits growth and metastasis of hepatocellular carcinoma cells via Wnt/beta-catenin pathway. Aging 2020, 12, 1527–1544. [Google Scholar] [CrossRef]
- Ernstsen, C.V.; Riishede, A.; Iversen, A.K.S.; Bay, L.; Bjarnsholt, T.; Nejsum, L.N. E-cadherin and aquaporin-3 are downregulated in wound edges of human chronic wounds. APMIS 2023, 131, 403–409. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
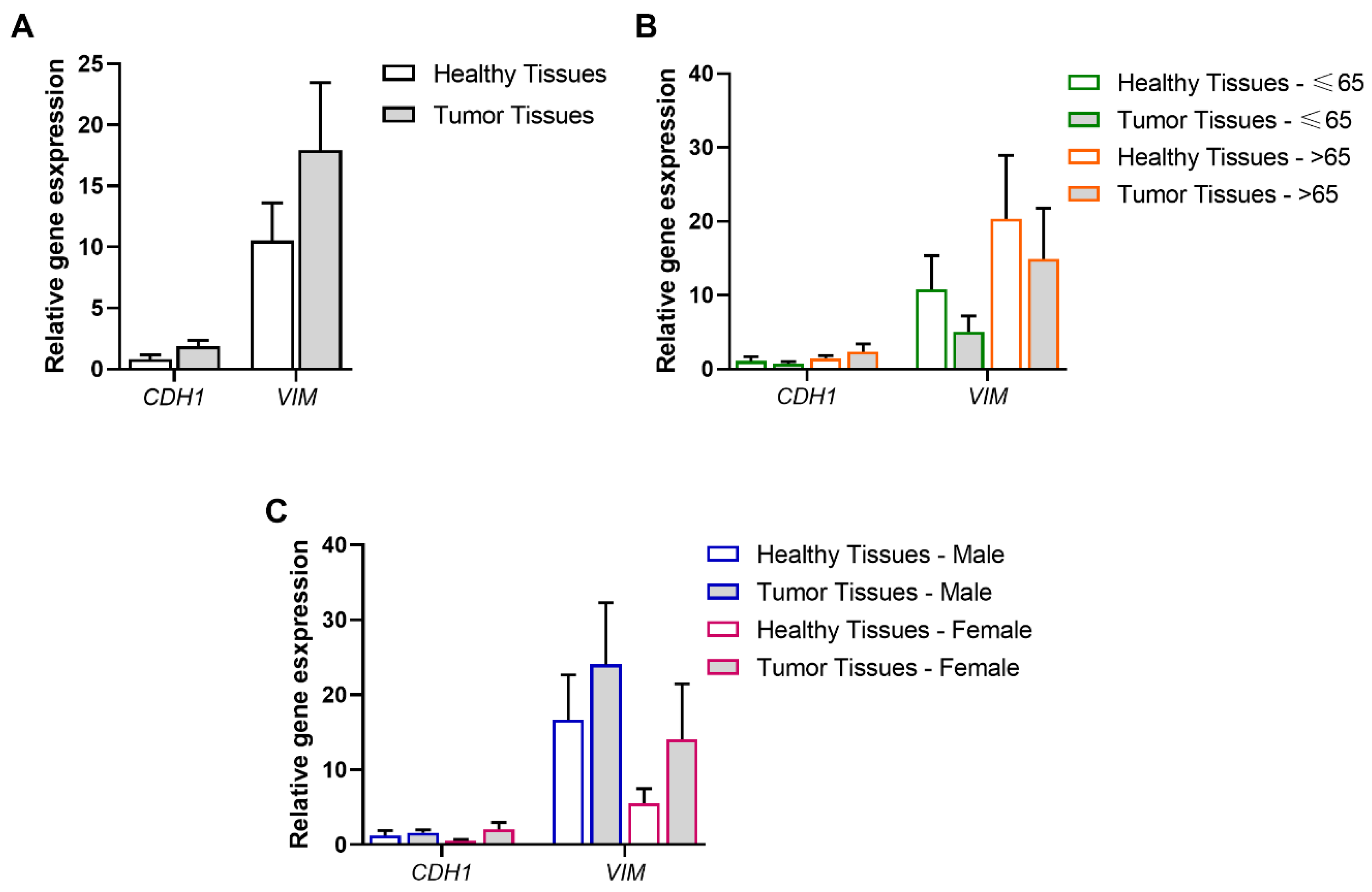
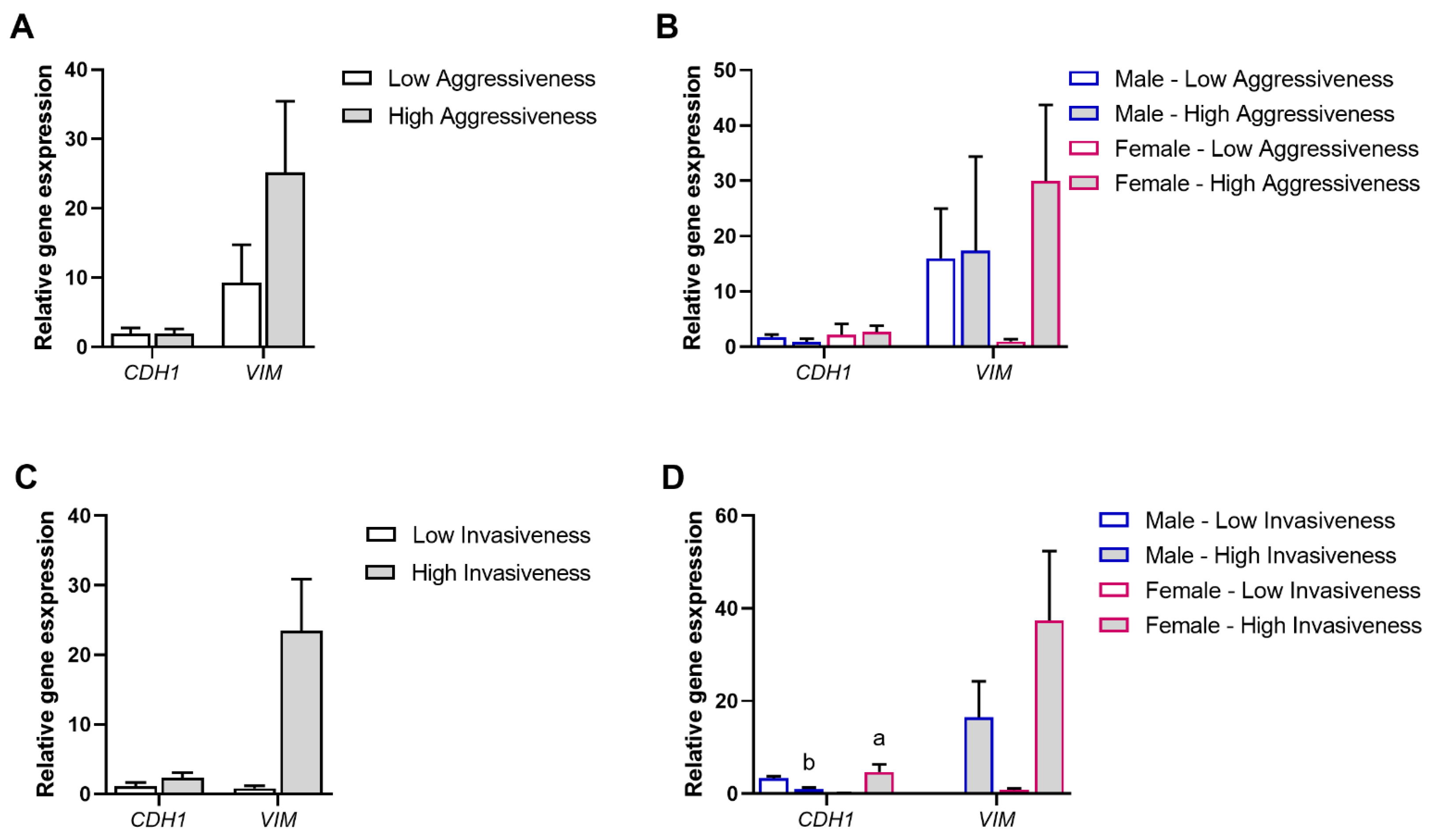
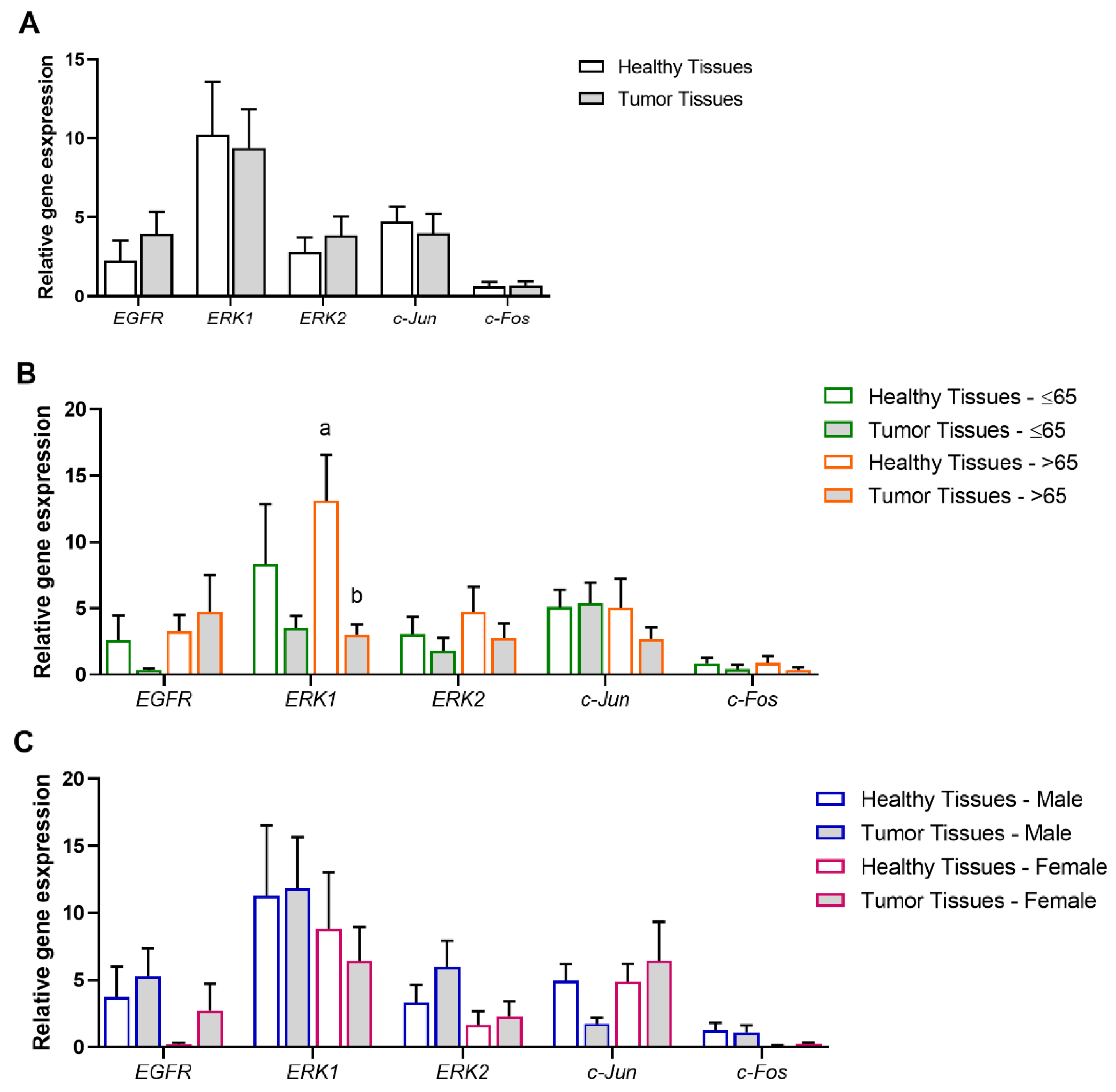


| Patient | Pancreatic Cancer Type | Age | Gender | Aggressiveness Grade | Invasiveness Grade |
|---|---|---|---|---|---|
| 1 | Distal cholangiocarcinoma | 50 | M | Low | High |
| 2 | Invasive intraductal papillary mucinous neoplasm | 52 | M | Low | High |
| 3 | Kidney metastasis | 55 | M | Low | Low |
| 4 | Cystic neuroendocrine tumor | 56 | M | Low | Low |
| 5 | Ampulla adenocarcinoma | 56 | M | Low | High |
| 6 | Distal cholangiocarcinoma | 58 | M | Low | High |
| 7 | Ductal adenocarcinoma | 59 | M | High | High |
| 8 | Ampulla adenocarcinoma | 63 | M | Low | High |
| 9 | Ductal adenocarcinoma | 64 | M | High | High |
| 10 | Ductal adenocarcinoma | 67 | M | High | High |
| 11 | Ampulla adenocarcinoma | 69 | M | Low | High |
| 12 | Distal cholangiocarcinoma | 69 | M | High | High |
| 13 | Ductal adenocarcinoma | 80 | M | Low | High |
| 14 | Cystic neuroendocrine tumor | 51 | F | Low | Low |
| 15 | Ductal adenocarcinoma | 55 | F | High | High |
| 16 | Ductal adenocarcinoma | 60 | F | High | High |
| 17 | Ductal adenocarcinoma | 65 | F | High | High |
| 18 | Non-invasive intraductal papillary mucinous neoplasm | 65 | F | Low | Low |
| 19 | Endocrine neoplasia | 69 | F | High | Low |
| 20 | Non-invasive intraductal papillary mucinous neoplasm | 69 | F | Low | Low |
| 21 | Ampulla adenocarcinoma | 72 | F | Low | High |
| 22 | Ductal adenocarcinoma | 78 | F | High | High |
| 23 | Invasive intraductal papillary mucinous neoplasm | 80 | F | Low | Low |
| 24 | Ductal adenocarcinoma | 82 | F | High | High |
| Gene | Gene Symbol | Primer Sequence |
|---|---|---|
| E-cadherin | CDH1 | FWD: 5′ TCGACACCCGATTCAAAGTG 3′ REV: 5′ GTCCCAGGCGTAGACCAAGA 3′ |
| Vimentin | VIM | FWD: 5′ TGCCCTTAAAGGAACCAATGAG 3′ REV: 5′ AGGCGGCCAATAGTGTCTTG 3′ |
| Epidermal growth factor receptor | EGFR | FWD: 5′ GAAATCCTCGATGAAGCCTACGTG 3′ REV: 5′ GTCTTTGTGTTCCCGGACATAGTC 3′ |
| Extracellular signal-regulated kinase 1 | ERK1 | FWD: 5′ AAGATCAGCCCCTTCGAACATC 3′ REV: 5′ CTTGTACAGGTCAGTCTCCATCAG 3′ |
| Extracellular signal-regulated kinase 1 | ERK2 | FWD: 5′ TACACCAACCTCTCGTAACATC 3′ REV: 5′ CATGTCTGAAGCGCAGTAAGATT 3′ |
| Jun AP-1 Transcription Factor Subunit | c-Jun | FWD: 5′ GTATCCTGCCCAGTGTTGTTTG 3′ REV: 5′ GCAGAAAAGAGGTTAGGGGAGTAC 3′ |
| Fos AP-1 Transcription Factor Subunit | c-Fos | FWD: 5′ CCGGGGATAGCCTCTCTTACT 3′ REV: 5′ CCAGGTCCGTGCAGAAGTC 3′ |
| Hypoxanthine-guanine phosphoribosyltransferase 1 | HPRT1 | FWD: 5′ ACTGAACGTCTTGCTCGAGATG 3′ REV: 5′ AGCAGGTCAGCAAAGAATTTATAGC 3′ |
| CDH1 | VIM | EGFR | ERK1 | ERK2 | c-Jun | c-Fos | |||||
| AQP1 | −0.254 0.310 | −0.116 0.648 | −0.255 0.307 | −0.289 0.244 | −0.142 0.588 | −0.257 0.273 | −0.361 0.141 | ||||
| AQP3 | −0.195 0.469 | 0.038 0.890 | −0.196 0.468 | −0.094 0.730 | −0.008 0.663 | −0.108 0.671 | −0.213 0.413 | ||||
| AQP5 | −0.325 0.477 | −0.294 0.480 | −0.293 0.444 | −0.332 0.422 | −0.203 0.601 | 0.169 0.664 | −0.423 0.344 | ||||
| AQP1 | AQP3 | AQP5 | AQP9 | −0.312 0.452 | −0.036 0.932 | −0.237 0.511 | −0.143 0.694 | −0.258 0.473 | −0.184 0.611 | −0.266 0.489 | |
| CDH1 | −0.254 0.310 | −0.195 0.469 | −0.325 0.477 | −0.312 0.452 | 0.487 0.041 | 0.397 0.115 | 0.211 0.401 | 0.400 0.111 | −0.144 0.557 | 0.892 <0.001 | |
| VIM | −0.116 0.648 | 0.038 0.890 | −0.294 0.480 | −0.036 0.932 | 0.487 0.041 | 0.466 0.059 | 0.505 0.033 | 0.707 0.002 | −0.177 0.470 | 0.742 <0.001 | |
| EGFR | −0.255 0.307 | −0.196 0.468 | −0.293 0.444 | −0.237 0.511 | 0.397 0.115 | 0.466 0.059 | 0.135 0.592 | 0.058 0.826 | 0.397 0.103 | 0.617 0.008 | |
| ERK1 | −0.289 0.244 | −0.094 0.730 | −0.332 0.422 | −0.143 0.694 | 0.211 0.401 | 0.505 0.033 | 0.135 0.592 | 0.842 <0.001 | 0.002 0.994 | 0.607 0.008 | |
| ERK2 | −0.142 0.588 | −0.008 0.663 | −0.203 0.601 | −0.258 0.473 | 0.400 0.111 | 0.707 0.002 | 0.058 0.826 | 0.842 <0.001 | −0.137 0.587 | 0.567 0.022 | |
| c-Jun | −0.257 0.273 | −0.108 0.671 | 0.169 0.664 | −0.184 0.611 | −0.144 0.557 | −0.177 0.470 | 0.397 0.103 | 0.002 0.994 | −0.137 0.587 | 0.020 0.937 | |
| c-Fos | −0.361 0.141 | −0.213 0.413 | −0.423 0.344 | −0.266 0.489 | 0.892 <0.001 | 0.742 <0.001 | 0.617 0.008 | 0.607 0.008 | 0.567 0.022 | 0.020 0.937 | |
| CDH1 | VIM | EGFR | ERK1 | ERK2 | c-Jun | c-Fos | |||||
| AQP1 | −0.235 0.319 | −0.051 0.850 | −0.145 0.520 | −0.238 0.357 | −0.255 0.266 | 0.282 0.228 | −0.239 0.311 | ||||
| AQP3 | −0.210 0.361 | −0.287 0.265 | −0.128 0.562 | −0.202 0.421 | −0.212 0.343 | 0.852 <0.001 | −0.083 0.721 | ||||
| AQP5 | 0.737 <0.001 | −0.064 0.827 | 0.457 0.043 | −0.334 0.223 | 0.148 0.546 | 0.158 0.532 | −0.121 0.631 | ||||
| AQP1 | AQP3 | AQP5 | AQP9 | −0.124 0.625 | −0.272 0.347 | −0.092 0.698 | −0.185 0.508 | −0.144 0.545 | 0.952 <0.001 | −0.049 0.848 | |
| CDH1 | −0.235 0.319 | −0.210 0.361 | 0.737 <0.001 | −0.124 0.625 | 0.441 0.100 | 0.744 <0.001 | 0.689 0.001 | 0.395 0.076 | −0.320 0.169 | 0.063 0.787 | |
| VIM | −0.051 0.850 | −0.287 0.265 | −0.064 0.827 | −0.272 0.347 | 0.441 0.100 | −0.073 0.781 | 0.719 0.006 | 0.361 0.170 | −0.237 0.359 | 0.066 0.808 | |
| EGFR | −0.145 0.520 | −0.128 0.562 | 0.457 0.043 | −0.092 0.698 | 0.744 <0.001 | −0.073 0.781 | 0.259 0.285 | 0.098 0.658 | −0.239 0.284 | 0.150 0.506 | |
| ERK1 | −0.238 0.357 | −0.202 0.421 | −0.334 0.223 | −0.185 0.508 | 0.689 0.001 | 0.719 0.006 | 0.259 0.285 | 0.796 <0.001 | −0.192 0.445 | 0.567 0.014 | |
| ERK2 | −0.255 0.266 | −0.212 0.343 | 0.148 0.546 | −0.144 0.545 | 0.395 0.076 | 0.361 0.170 | 0.098 0.658 | 0.796 <0.001 | −0.217 0.345 | 0.667 0.001 | |
| c-Jun | 0.282 0.228 | 0.852 <0.001 | 0.158 0.532 | 0.952 <0.001 | −0.320 0.169 | −0.237 0.359 | −0.239 0.284 | −0.192 0.445 | −0.217 0.345 | −0.153 0.508 | |
| c-Fos | −0.239 0.311 | −0.083 0.721 | −0.121 0.631 | −0.049 0.848 | 0.063 0.787 | 0.066 0.808 | 0.150 0.506 | 0.567 0.014 | 0.667 0.001 | −0.153 0.508 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, I.V.; Lopes, P.A.; Fonseca, E.; Vigia, E.; Paulino, J.; Soveral, G. The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach. Biomolecules 2025, 15, 488. https://doi.org/10.3390/biom15040488
da Silva IV, Lopes PA, Fonseca E, Vigia E, Paulino J, Soveral G. The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach. Biomolecules. 2025; 15(4):488. https://doi.org/10.3390/biom15040488
Chicago/Turabian Styleda Silva, Inês V., Paula A. Lopes, Elisabete Fonseca, Emanuel Vigia, Jorge Paulino, and Graça Soveral. 2025. "The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach" Biomolecules 15, no. 4: 488. https://doi.org/10.3390/biom15040488
APA Styleda Silva, I. V., Lopes, P. A., Fonseca, E., Vigia, E., Paulino, J., & Soveral, G. (2025). The Association of Aquaporins with MAPK Signaling Pathway Unveils Potential Prognostic Biomarkers for Pancreatic Cancer: A Transcriptomics Approach. Biomolecules, 15(4), 488. https://doi.org/10.3390/biom15040488










