Antitumor Assessment of Liposomal Beta-Carotene with Tamoxifen Against Breast Carcinoma Cell Line: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Liposomes
2.3. Measurement of Encapsulation Efficiency
2.4. Entrapment Efficiency Percentage
2.5. Dynamic Light Scattering and Zeta Potential
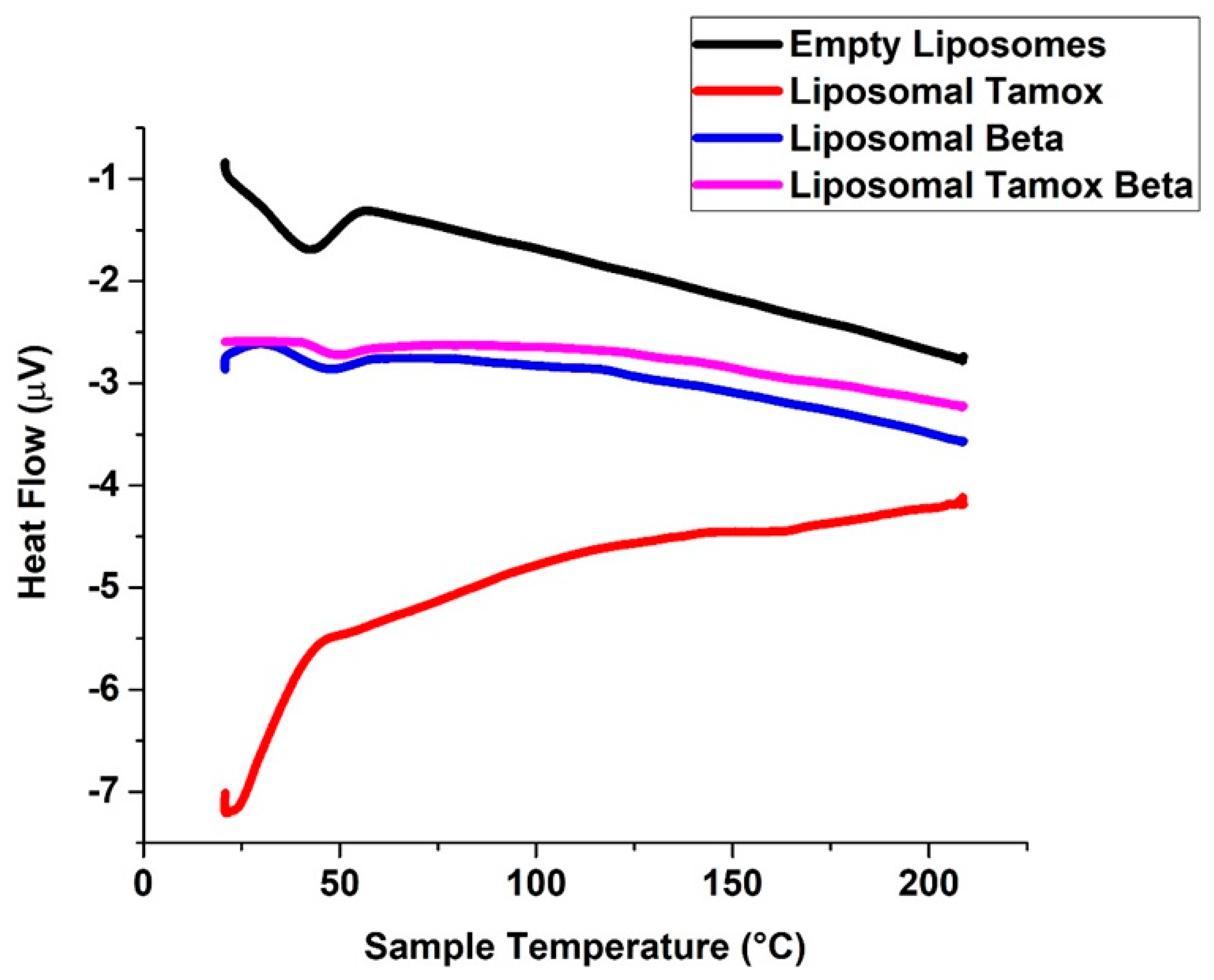
2.6. DSC Measurements
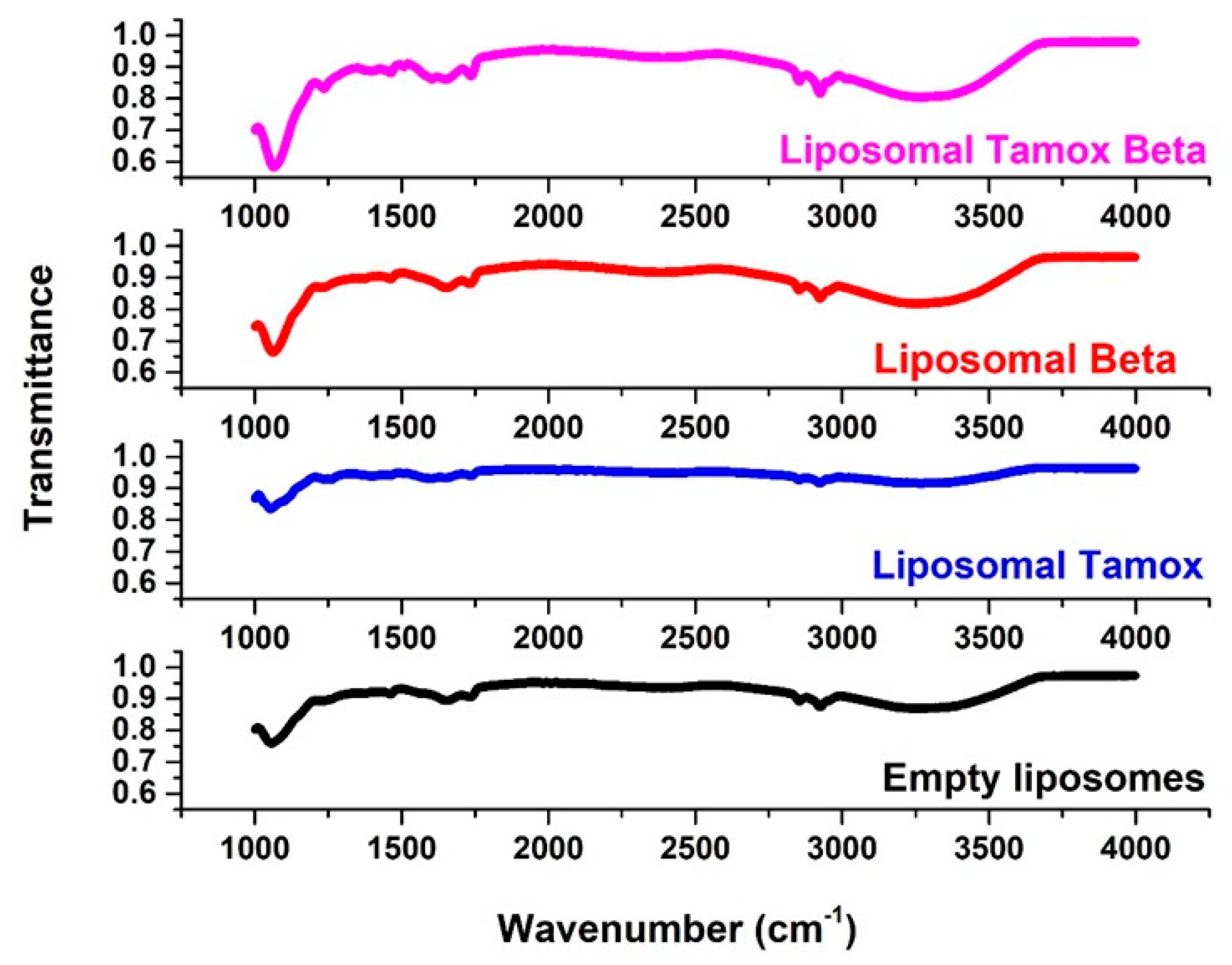
2.7. FTIR Spectroscopy
2.8. Cytotoxicity In Vitro Using the MTT Assay
2.9. Single-Cell Gel Electrophoresis (Comet Assay)
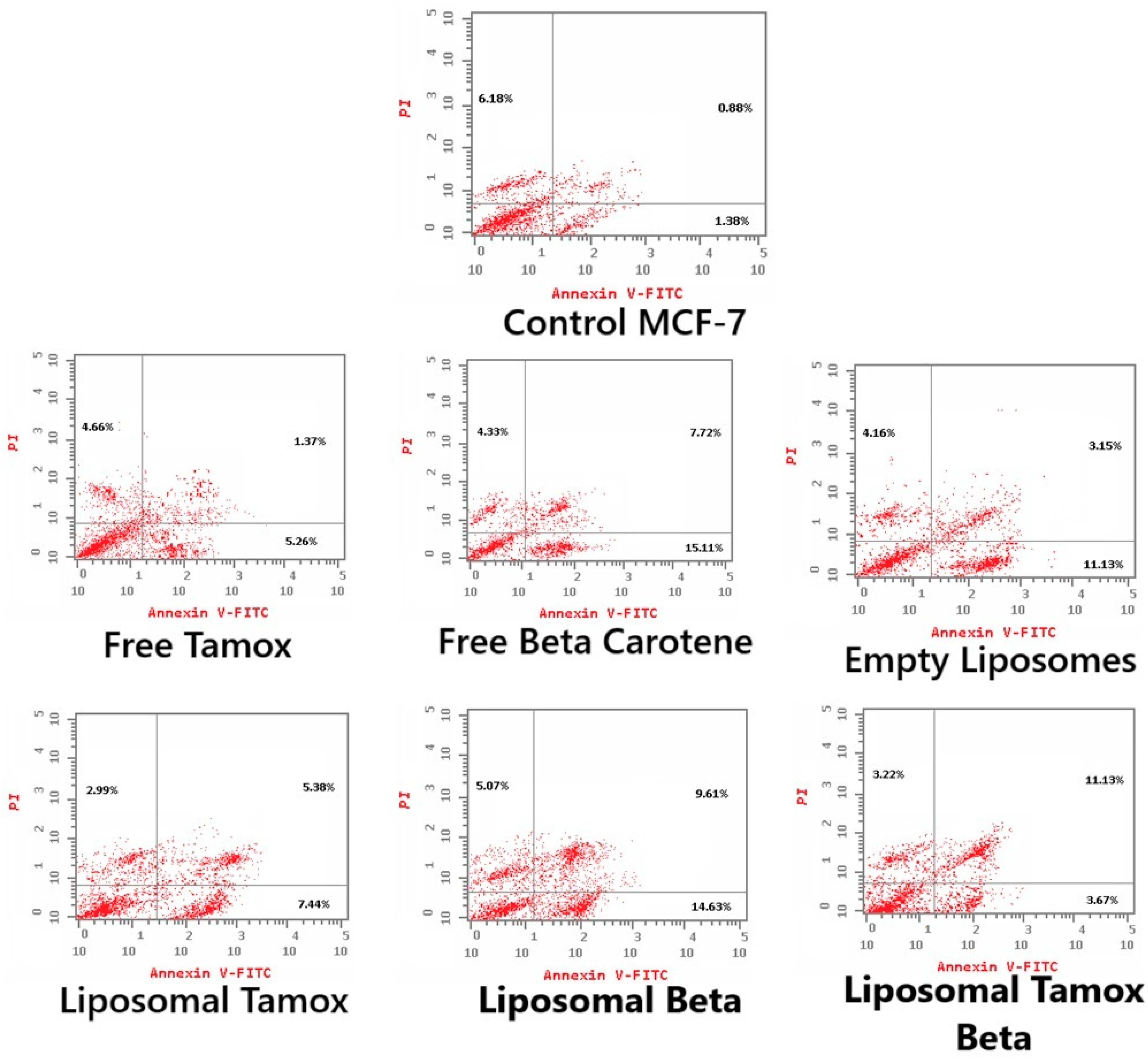
2.10. Apoptosis Detection Using Flow Cytometry
2.11. Analysis of Statistics
3. Results and Discussion
3.1. Characterization of Tamoxifen Combined with β-Carotene into Liposomes
3.2. Cytotoxicity Effects of Tamoxifen Combined with β-Carotene into Liposomes Toward Noncancerous and Cancerous Cells
3.3. Tamoxifen Combined with β-Carotene into Liposomes Induced DNA Damage
3.4. Tamoxifen Combined with β-Carotene into Liposomes Stimulates Apoptosis in MCF-7 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Singh, R.; Dutta, D.; Chandel, S.; Bhattacharya, A.; Ravichandiran, V.; Sukla, S. In vitro anticancer activity of methanolic extract of justicia adhatoda leaves with special emphasis on human breast cancer cell line. Molecules 2022, 27, 8222. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar]
- Talluri, S.V.; Kuppusamy, G.; Karri, V.V.S.R.; Tummala, S.; Madhunapantula, S.V. Lipid-based nanocarriers for breast cancer treatment—Comprehensive review. Drug Deliv. 2016, 23, 1291–1305. [Google Scholar] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [PubMed]
- Leucuta, S.E. Nanotechnology for delivery of drugs and biomedical applications. Curr. Clin. Pharmacol. 2010, 5, 257–280. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for tumor targeted therapy: A review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Delivery of green tea catechin and epigallocatechin gallate in liposomes incorporated into low-fat hard cheese. Food Chem. 2014, 156, 176–183. [Google Scholar]
- Abdella, E.M. Short-term comparative study of the cyclophosphamide genotoxicity administered free and liposome-encapsulated in mice. Iran. J. Cancer Prev. 2012, 5, 51–60. [Google Scholar]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar]
- Dong, M.; Luo, L.; Ying, X.; Lu, X.; Shen, J.; Jiang, Z.; Wang, L. Comparable efficacy and less toxicity of pegylated liposomal doxorubicin versus epirubicin for neoadjuvant chemotherapy of breast cancer: A case-control study. Onco Targets Ther. 2018, 11, 4247–4252. [Google Scholar]
- Moosavian, S.A.; Bianconi, V.; Pirro, M.; Sahebkar, A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin. Cancer Biol. 2021, 69, 337–348. [Google Scholar]
- Franco, M.S.; Roque, M.C.; de Barros, A.L.B.; de Oliveira Silva, J.; Cassali, G.D.; Oliveira, M.C. Investigation of the antitumor activity and toxicity of long-circulating and fusogenic liposomes co-encapsulating paclitaxel and doxorubicin in a murine breast cancer animal model. Biomed. Pharmacother. 2019, 109, 1728–1739. [Google Scholar] [PubMed]
- Liu, S.; Song, X.L.; Wang, Y.H.; Wang, X.M.; Xiao, Y.; Wang, X.; Cheng, L.; Li, X.T. The efficacy of wga modified daunorubicin anti-resistant liposomes in treatment of drug-resistant mcf-7 breast cancer. J. Drug Target. 2017, 25, 541–553. [Google Scholar] [PubMed]
- Elzamly, S.; Badri, N.; Padilla, O.; Dwivedi, A.K.; Alvarado, L.A.; Hamilton, M.; Diab, N.; Rock, C.; Elfar, A.; Teleb, M.; et al. Epithelial-mesenchymal transition markers in breast cancer and pathological responseafter neoadjuvant chemotherapy. Breast Cancer 2018, 12, 1178223418788074. [Google Scholar] [PubMed]
- Yang, B.; Song, B.P.; Shankar, S.; Guller, A.; Deng, W. Recent advances in liposome formulations for breast cancer therapeutics. Cell. Mol. Life Sci. 2021, 78, 5225–5243. [Google Scholar]
- Yan, B.; Lu, M.S.; Wang, L.; Mo, X.F.; Luo, W.P.; Du, Y.F.; Zhang, C.X. Specific serum carotenoids are inversely associated with breast cancer risk among chinese women: A case-control study. Br. J. Nutr. 2016, 115, 129–137. [Google Scholar]
- Tufail, T.; Bader Ul Ain, H.; Noreen, S.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Nutritional benefits of lycopene and beta-carotene: A comprehensive overview. Food Sci. Nutr. 2024, 12, 8715–8741. [Google Scholar]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential role of natural antioxidant products in oncological diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef]
- Othman, M.S.; Obeidat, S.T.; Al-Bagawi, A.H.; Fareid, M.A.; Fehaid, A.; Moneim, A.E.A. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J. Integr. Med. 2022, 20, 65–72. [Google Scholar]
- Wiseman, H. Tamoxifen: New membrane-mediated mechanisms of action and therapeutic advances. Trends Pharmacol. Sci. 1994, 15, 83–89. [Google Scholar] [CrossRef]
- Bangham, A.D.; Hill, M.W.; Miller, N.G.A. Preparation and use of liposomes as models of biological membranes. In Methods in Membrane Biology: Volume 1; Korn, E.D., Ed.; Springer: Boston, MA, USA, 1974; pp. 1–68. [Google Scholar]
- Tan, C.; Xue, J.; Lou, X.; Abbas, S.; Guan, Y.; Feng, B.; Zhang, X.; Xia, S. Liposomes as delivery systems for carotenoids: Comparative studies of loading ability, storage stability and in vitro release. Food Funct. 2014, 5, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Perea-Flores, M.D.; Aguilar-Morán, H.F.; Calderón-Domínguez, G.; García-Hernández, A.B.; Díaz-Ramírez, M.; Romero-Campos, H.E.; Cortés-Sánchez, A.D.; Salgado-Cruz, M.D. Entrapment efficiency (ee) and release mechanism of rhodamine b encapsulated in a mixture of chia seed mucilage and sodium alginate. Appl. Sci. 2023, 13, 1213. [Google Scholar] [CrossRef]
- Bellamakondi, P.K.; Godavarthi, A.; Ibrahim, M.K.; Kulkarni, S.; Naik, R.M.; Maradam, S. In vitro cytotoxicity of caralluma species by mtt and trypan blue dye exclusion. Asian J. Pharm. Clin. Res. 2014, 7, 17–19. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J.; Trzeciak, A.; Kowalik, J. Curcumin damages DNA in human gastric mucosa cells and lymphocytes. J. Environ. Pathol. Toxicol. Oncol. 1999, 18, 271–276. [Google Scholar]
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-beta-carotene) but not beta-carotene rigidifies lipid membranes: A 1h-nmr study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta 1996, 1285, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Babick, F.; Gropp, S.; Kätzel, U.; Vorbau, M. Dynamic light scattering of dispersed fumed silica aggregates. Powder Technol. 2012, 217, 39–45. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Amărandi, R.-M.; Neamṭu, A.; Ştiufiuc, R.-I.; Marin, L.; Drăgoi, B. Impact of lipid composition on vesicle protein adsorption: A bsa case study. ACS Omega 2024, 9, 17903–17918. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Wlodarczyk, M.; Witkowski, S.; Polewski, K. Phase transitions and structural changes in dppc liposomes induced by a 1-carba-alpha-tocopherol analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef] [PubMed]
- Melcrová, A.; Pokorna, S.; Pullanchery, S.; Kohagen, M.; Jurkiewicz, P.; Hof, M.; Jungwirth, P.; Cremer, P.S.; Cwiklik, L. The complex nature of calcium cation interactions with phospholipid bilayers. Sci. Rep. 2016, 6, 38035. [Google Scholar]
- Kolman, I.; Pippa, N.; Meristoudi, A.; Pispas, S.; Demetzos, C. A dual-stimuli-responsive polymer into phospholipid membranes. J. Therm. Anal. Calorim. 2016, 123, 2257–2271. [Google Scholar]
- Riske, K.A.; Barroso, R.P.; Vequi-Suplicy, C.C.; Germano, R.; Henriques, V.B.; Lamy, M.T. Lipid bilayer pre-transition as the beginning of the melting process. Biochim. Biophys. Acta 2009, 1788, 954–963. [Google Scholar]
- Owusu-Ware, S.K.; Chowdhry, B.Z.; Leharne, S.A.; Antonijević, M.D. Phase behaviour of dehydrated phosphatidylcholines. J. Therm. Anal. Calorim. 2017, 127, 415–421. [Google Scholar]
- Shafaa, M.W.; Elshazly, A.H.; Dakrory, A.Z.; Elsyed, M.R. Interaction of coenzyme q10 with liposomes and its impact on suppression of selenite—Induced experimental cataract. Adv. Pharm. Bull. 2018, 8, 1–9. [Google Scholar]
- Pedersen, T.B.; Kaasgaard, T.; Jensen, M.Ø.; Frokjaer, S.; Mouritsen, O.G.; Jørgensen, K. Phase behavior and nanoscale structure of phospholipid membranes incorporated with acylated c14-peptides. Biophys. J. 2005, 89, 2494–2503. [Google Scholar] [PubMed]
- Popova, A.V.; Hincha, D.K. Effects of cholesterol on dry bilayers: Interactions between phosphatidylcholine unsaturation and glycolipid or free sugar. Biophys. J. 2007, 93, 1204–1214. [Google Scholar] [CrossRef]
- Fa, N.; Ronkart, S.; Schanck, A.; Deleu, M.; Gaigneaux, A.; Goormaghtigh, E.; Mingeot-Leclercq, M.P. Effect of the antibiotic azithromycin on thermotropic behavior of dopc or dppc bilayers. Chem. Phys. Lipids 2006, 144, 108–116. [Google Scholar]
- Bafna, S.S.; Sun, T.; Baird, D.G. The role of partial miscibility on the properties of blends of a polyetherimide and two liquid crystalline polymers. Polymer 1993, 34, 708–715. [Google Scholar] [CrossRef]
- Yarce, C.J.; Alhajj, M.J.; Sanchez, J.D.; Onate-Garzon, J.; Salamanca, C.H. Development of antioxidant-loaded nanoliposomes employing lecithins with different purity grades. Molecules 2020, 25, 5344. [Google Scholar] [CrossRef]
- Severcan, F.; Sahin, I.; Kazancı, N. Melatonin strongly interacts with zwitterionic model membranes—Evidence from fourier transform infrared spectroscopy and differential scanning calorimetry. Biochim. Biophys. Acta (BBA)—Biomembr. 2005, 1668, 215–222. [Google Scholar]
- Saxena, J. Detection of carotenoids in psychrotrophic bacteria by spectroscopic approach. J. BioSci. Biotechnol. 2014, 3, 253–260. [Google Scholar]
- Llansola-Portoles, M.J.; Pascal, A.A.; Robert, B. Electronic and vibrational properties of carotenoids: From in vitro to in vivo. J. R. Soc. Interface 2017, 14, 20170504. [Google Scholar] [CrossRef] [PubMed]
- Hannon, J.P.; Trammell, G.T.; Blume, M.; Gibbs, D. X-ray resonance exchange scattering. Phys. Rev. Lett. 1988, 61, 1245–1248. [Google Scholar] [PubMed]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar]
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Di Nicuolo, F.; Ranelletti, F.O.; Calviello, G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin a and bcl-2 family proteins. Carcinogenesis 2002, 23, 11–18. [Google Scholar]
- Williams, A.W.; Boileau, T.W.; Zhou, J.R.; Clinton, S.K.; Erdman, J.W., Jr. Beta-carotene modulates human prostate cancer cell growth and may undergo intracellular metabolism to retinol. J. Nutr. 2000, 130, 728–732. [Google Scholar]
- Hazuka, M.B.; Edwards-Prasad, J.; Newman, F.; Kinzie, J.J.; Prasad, K.N. Beta-carotene induces morphological differentiation and decreases adenylate cyclase activity in melanoma cells in culture. J. Am. Coll. Nutr. 1990, 9, 143–149. [Google Scholar] [CrossRef]
- Dulinska-Litewka, J.; Dykas, K.; Boznanski, S.; Halubiec, P.; Kaczor-Kaminska, M.; Zagajewski, J.; Bohn, T.; Wator, G. The influence of beta-carotene and its liposomal form on the expression of emt markers and androgen-dependent pathways in different prostate cell lines. Antioxidants 2024, 13, 902. [Google Scholar] [CrossRef]
- Sheng, B.; Li, L.; Zhang, X.; Jiao, W.; Zhao, D.; Wang, X.; Wan, L.; Li, B.; Rong, H. Physicochemical properties and chemical stability of beta-carotene bilayer emulsion coated with bovine serum albumin and arabic gum compared to monolayer emulsions. Molecules 2018, 23, 495. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Song, M.H.; Oh, J.W.; Keum, Y.S.; Saini, R.K. Pro-oxidant actions of carotenoids in triggering apoptosis of cancer cells: A review of emerging evidence. Antioxidants 2020, 9, 532. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Misran, M.; Kalantari, K.; Webster, T.J.; Kia, P.; Basrowi, N.A.; Rasouli, E.; Shameli, K. Advancements in liposomal nanomedicines: Innovative formulations, therapeutic applications, and future directions in precision medicine. Int. J. Nanomed. 2025, 20, 1213–1262. [Google Scholar]
- Dutta, R.S.; Elhassan, G.O.; Devi, T.B.; Bhattacharjee, B.; Singh, M.; Jana, B.K.; Sahu, S.; Mazumder, B.; Sahu, R.K.; Khan, J. Enhanced efficacy of β-carotene loaded solid lipid nanoparticles optimized and developed via central composite design on breast cancer cell lines. Heliyon 2024, 10, e28457. [Google Scholar] [CrossRef] [PubMed]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and beta-carotene induce cell-cycle arrest and apoptosis in human breast cancer cell lines. Anticancer. Res. 2014, 34, 1377. [Google Scholar]
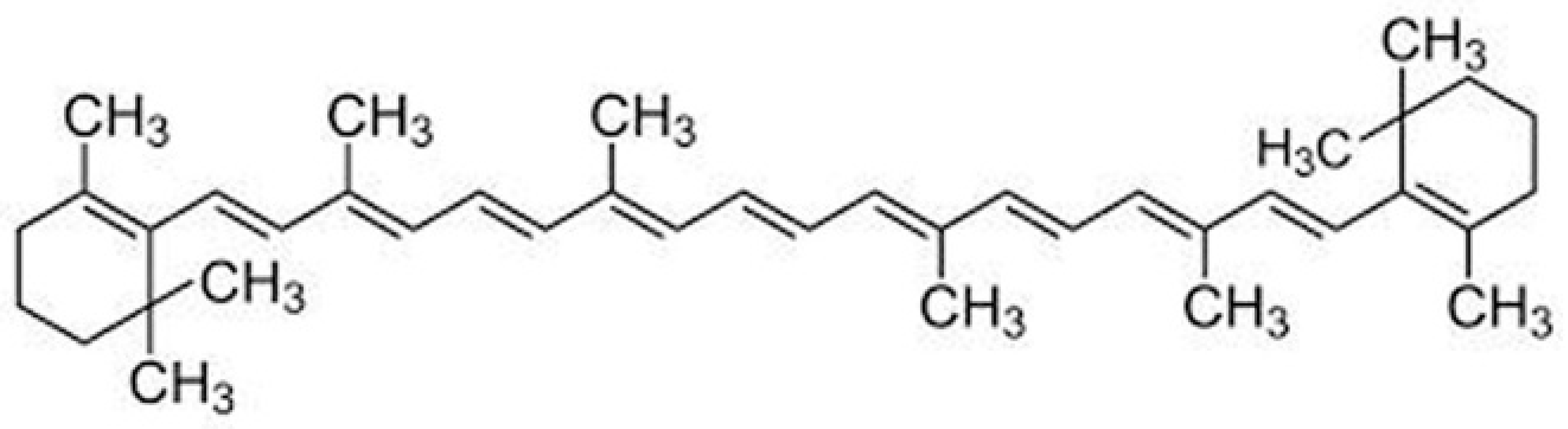

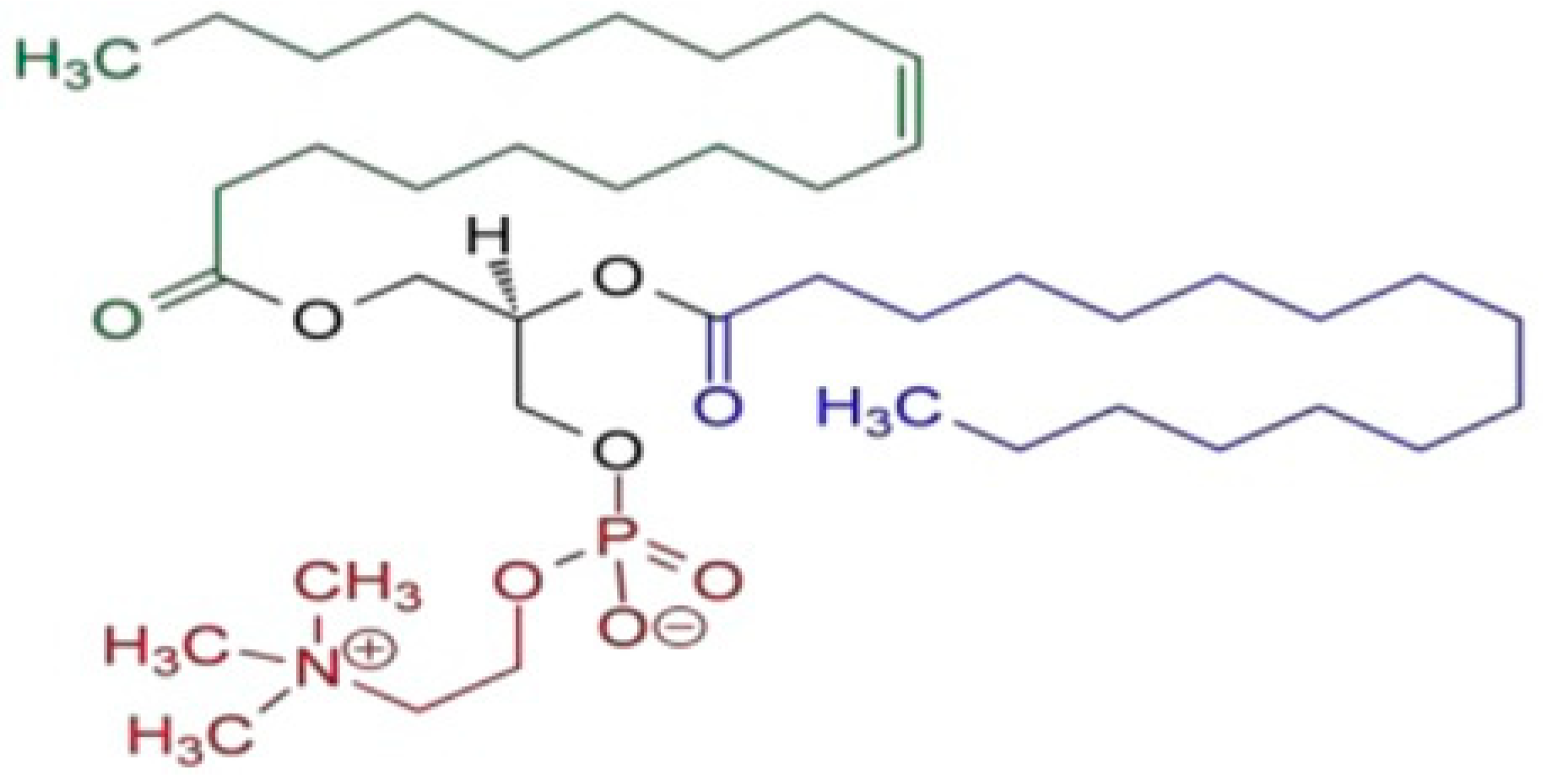

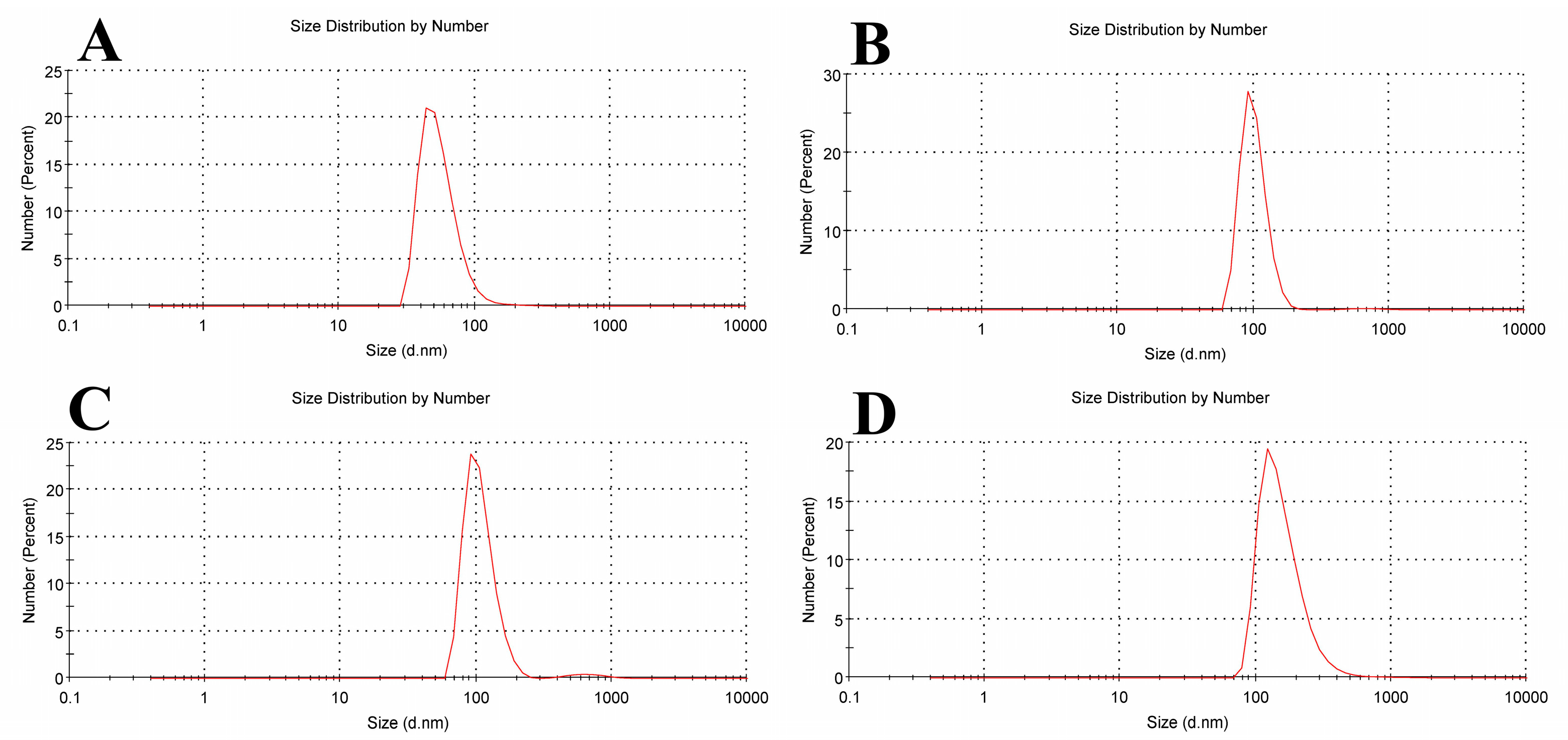





| Sample Name | Mean Size Diameter (nm) ± SD (nm) | PDI Average | Mean Zeta Potential ± SD (mV) |
|---|---|---|---|
| Empty Liposomes | 43.82 ± 21.86 | 0.477 | −23.8 ± 12.4 |
| Liposomal Tamox | 91.28 ± 22.13 | 0.546 | −17.6 ± 11.7 |
| Liposomal β-carotene | 91.28 ± 27.26 | 0.674 | −23.5 ± 14.9 |
| Liposomal Tamox + Beta | 122.4 ± 83.37 | 0.408 | −15.2 ± 14.5 |
| Peak Assignment | Wavenumber (cm−1) | Wavenumber (cm−1) | |||
|---|---|---|---|---|---|
| Control | Tamoxifen | Beta-Carotene | Tamox Mixed with Beta | ||
| Symmetric stretching vibration of CH2 in acyl chain | (2800–2855) | 2853 | 2852.61 | 2852 | 2858 |
| Antisymmetric stretching vibration of CH2 in acyl chain | (2916–2921) | 2923 | 2926. 61 | 2920 | 2920 |
| Carbonyl stretching vibration C=O | (1730–1740) | 1732 | 1735.58 | 1741 | 1735 |
| Antisymmetric PO2− stretching vibrations | (1215–1260) | 1241 | 1241 | 1228 | 1241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsayed, M.H.; Shafaa, M.W.; Abdalla, M.S.; El-Khadragy, M.F.; Moneim, A.E.A.; Ramadan, S.S. Antitumor Assessment of Liposomal Beta-Carotene with Tamoxifen Against Breast Carcinoma Cell Line: An In Vitro Study. Biomolecules 2025, 15, 486. https://doi.org/10.3390/biom15040486
Elsayed MH, Shafaa MW, Abdalla MS, El-Khadragy MF, Moneim AEA, Ramadan SS. Antitumor Assessment of Liposomal Beta-Carotene with Tamoxifen Against Breast Carcinoma Cell Line: An In Vitro Study. Biomolecules. 2025; 15(4):486. https://doi.org/10.3390/biom15040486
Chicago/Turabian StyleElsayed, Marim H., Medhat W. Shafaa, Mohga S. Abdalla, Manal F. El-Khadragy, Ahmed E. Abdel Moneim, and Shimaa S. Ramadan. 2025. "Antitumor Assessment of Liposomal Beta-Carotene with Tamoxifen Against Breast Carcinoma Cell Line: An In Vitro Study" Biomolecules 15, no. 4: 486. https://doi.org/10.3390/biom15040486
APA StyleElsayed, M. H., Shafaa, M. W., Abdalla, M. S., El-Khadragy, M. F., Moneim, A. E. A., & Ramadan, S. S. (2025). Antitumor Assessment of Liposomal Beta-Carotene with Tamoxifen Against Breast Carcinoma Cell Line: An In Vitro Study. Biomolecules, 15(4), 486. https://doi.org/10.3390/biom15040486






