The Impact of Two Elicitors and Harvest Ripening Stage on the Quality of Monastrell Grapes and Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Field Treatments, and Meteorological Information
2.2. Vinifications
2.3. Physicochemical Parameters in Grapes and Wines
2.4. Extractability Parameters in Grapes
2.5. Spectrophotometric Parameters in Wines
2.6. Analysis of Phenolic Compounds in Grapes and Wines
2.6.1. Anthocyanins and Flavonols
2.6.2. Proanthocyanidins
2.7. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters in Grapes and Wines
3.2. Extractability Parameters in Grapes
3.3. Spectrophotometric Parameters in Wines
3.4. Phenolic Composition in Grapes and Wines
3.4.1. Anthocyanins
3.4.2. Flavonols
3.4.3. Proanthocyanidins
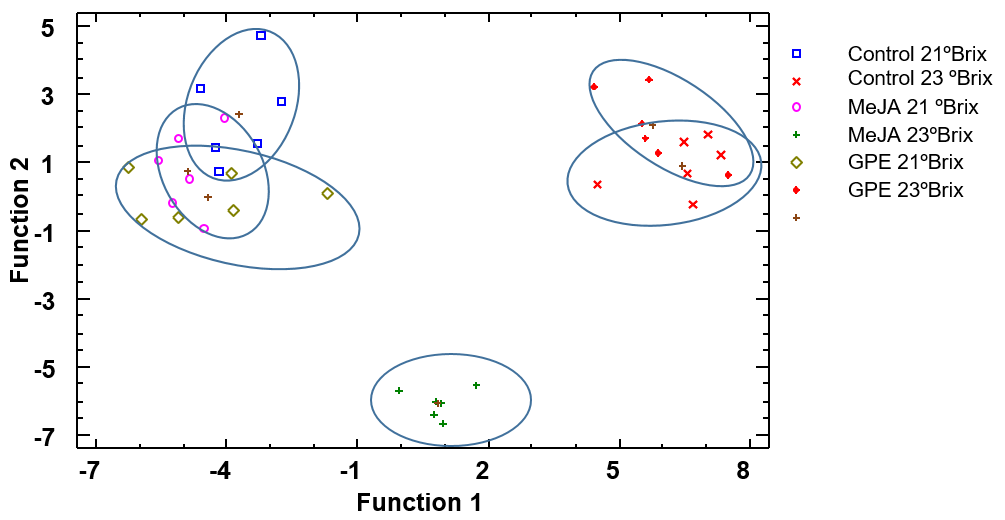
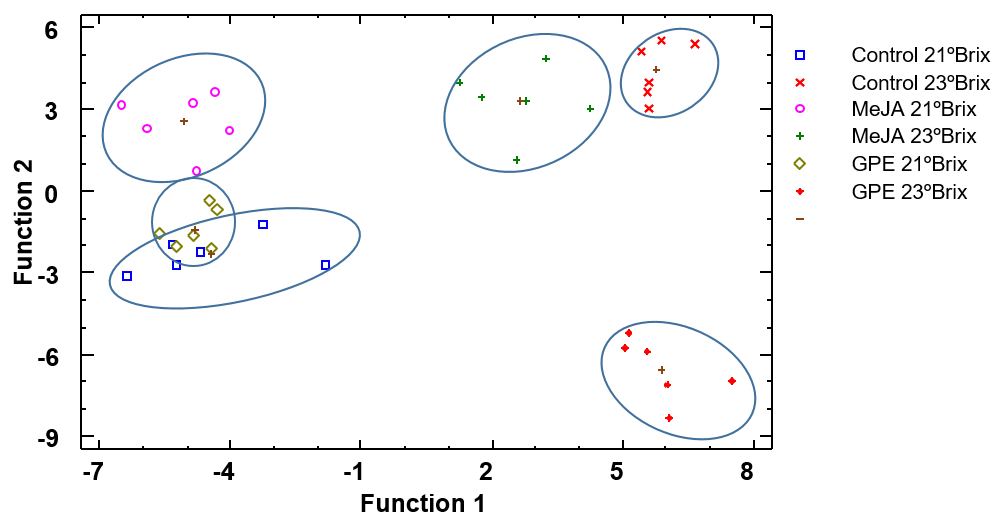
3.5. Multivariable Discriminant Analysis
3.5.1. Grape
3.5.2. Wine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MeJA | methyl jasmonate |
| JA | jasmonic acid |
| JAs | jasmonates |
| GPE | grape pomace extract |
| SIAM | Agricultural Information System of the Region of Murcia |
| TP | total phenol content |
| IMC | index of cellular maturity |
| SMI | seed maturity index |
| WA | total wine anthocyanins |
| CI | color intensity |
| mDP | mean degree polymerization |
| LSD | least significant difference test |
| Df-3g | delphinidin-3-O-glucoside |
| Cy-3g | cyanidin-3-O-glucoside |
| Pet-3g | petunidin-3-O-glucoside |
| Pn-3g | peonidin-3-O-glucoside |
| Mv-3g | malvidin-3-O-glucoside |
| ac3g | acetyl-3-O-glucosides |
| cum | coumaryl-3-O-glucosides |
| pyr | pyruvate |
| Vit | vitisin |
| epi | epicatechin |
| BTH | Benzothiadiazole |
| Myr | myricetin |
| Quer | quercetin |
| Lar | laricitrin |
| Kam | kampherol |
| Iso | isorhamnetin |
| Syr | syrigetin |
| gU | O-glucuronide |
| gal | O-galactoside |
| g | O-glucoside |
| PAs | proanthocyanidins |
| % Gal | % galloylation |
| EGC | epigallocatechin |
| Cat | catechin |
| ECG | epicatechin gallate |
| ext | extension units |
| T | terminal units |
References
- Varela, A.C.; Wagner, M.; Masseroni, M.L.; Sartor, P.D.; Zaldarriaga Heredia, J.; Jofré, F.C.; Fanzone, M.L. Assessing chemical composition variations on vintage, vineyard location and grape ripeness: A regional study of red wines produced in the colorado river middle basin, La Pampa (Argentina). J. Food Compost. Anal. 2024, 134, 106496. [Google Scholar] [CrossRef]
- Dupuis, J.; Knoepfel, P. Les barrières à la mise en auvre des politiques d’adaptation au changement climatique: Le cas de la Suisse. SPSR 2011, 17, 188–219. [Google Scholar] [CrossRef]
- Zoecklein, B.W.; Gump, B.H. Practical methods of evaluating grape quality and quality potential. In Managing Wine Quality: Volume One: Viticulture and Wine Quality; Elsevier: Amsterdam, The Netherlands, 2021; pp. 135–185. [Google Scholar] [CrossRef]
- Kurtural, S.K.; Gambetta, G.A. Global warming and wine quality: Are we close to the tipping point? Oeno One 2021, 55, 353–361. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Torres, R.E. Effect of controlled day and night temperatures on grape coloration. Am. J. Enol. Vitic. 1972, 23, 71–77. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and Cabernet Franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of elevated temperature in grapevine. II juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. The concept of terroir in viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Goode, J. Viticulture. Fruity with a hint of drought. Nature 2012, 492, 351–353. [Google Scholar] [CrossRef][Green Version]
- D’Onofrio, C.; Matarese, F.; Cuzzola, A. Effect of methyl jasmonate on the aroma of Sangiovese grapes and wines. Food Chem. 2018, 242, 352–361. [Google Scholar] [CrossRef]
- Casassa, L.F.; Fanzone, M.L.; Sari, S.E. Comparative phenolic, chromatic, and sensory composition of five monovarietal wines processed with microwave technology. Heliyon 2022, 8, 1–10. [Google Scholar] [CrossRef]
- Fanzone, M.; Zamora, F.; Jofré, V.; Assof, M.; Gómez-Cordovés, C.; Peña-Neira, A. Phenolic characterisation of red wines from different grape varieties cultivated in Mendoza province (Argentina). J. Sci. Food Agric. 2012, 92, 704–718. [Google Scholar] [CrossRef]
- Keller, M. The Sciences of Grapevines: Anatomy and Physiology, 2nd ed.; Elsevier, Inc.: Oxford, UK, 2015. [Google Scholar]
- Espitia-Lopez, J.; Escalona-Buendía, H.B.; Luna, H.; Verde-Calvo, J.R. Multivariate study of the evolution of phenolic composition and sensory profile on mouth of Mexican red Merlot wine aged in barrels vs wood chips. CYTA—J. Food 2015, 13, 26–31. [Google Scholar] [CrossRef]
- Creelman, R.A.; Mullet, A.; John, E.T. Biosynthesis and action of jasmonates in plant. Annu. Rev. Plant Biol. 1997, 48, 355–381. [Google Scholar] [CrossRef]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates counter plant stress: A review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.H. Jasmonic acid signaling pathway in response to abiotic stresses in plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Portu, J.; González-Arenzana, L.; Hermosín-Gutiérrez, I.; Santamaría, P.; Garde- Cerdán, T. Phenylalanine and urea foliar applications to grapevine: Effect on wine phenolic content. Food Chem. 2015, 180, 55–63. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Ewald, P.; Santamaría, P.; Winterhalter, P.; Garde-Cerdán, T. Evaluation of Grenache, Graciano and Tempranillo grape stilbene content after field applications of elicitors and nitrogen compounds. J. Sci. Food Agric. 2018, 98, 1856–1862. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Effect of elicitors on the evolution of grape phenolic compounds during the ripening period. J. Sci. Food Agric. 2017, 97, 977–983. [Google Scholar] [CrossRef]
- Goupil, P.; Benouaret, R.; Charrier, O.; ter Halle, A.; Richard, C.; Eyheraguibel, B.; Thiery, D.; Ledoigt, G. Grape pomace extract acts as elicitor of plant defence responses. Ecotoxicology 2012, 21, 1541–1549. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Cebrıán-Pérez, A.; Fernández-Fernández, J.I. Elicitors: A strategy to improve phenolic composition in wines. Acta Hortic. 2020, 1274, 9–16. [Google Scholar] [CrossRef]
- Saint Cricq, N.; Vivas, N.; Glories, Y. Maturité pjhénolique: Définition et contrôle. Rev. Fr. Oenol. 1998, 173, 22–25. [Google Scholar]
- Glories, Y. La couleur des vins rouges. lre partie: Les équilibres des anthocyanes et des tanins. Oeno One 1984, 18, 195–217. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Pontallier, P.; Glories, Y. Some interpretations of colour changes in young red wines during their conservation. J. Sci. Food Agric. 1983, 34, 505–516. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Paladines-Quezada, D.F.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. Effect of methyl jasmonate doped nanoparticles on nitrogen composition of Monastrell grapes and wines. Biomolecules 2021, 11, 1631. [Google Scholar] [CrossRef]
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roca, J.M.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B. Effect of different enological practices on skin and seed proanthocyanidins in three varietal wines. J. Agric. Food Chem. 2010, 58, 11333–11339. [Google Scholar] [CrossRef]
- Del Rio, J.L.P.; Kennedy, J.A. Development of proanthocyanidins in Vitis vinifera L. cv. Pinot noir grapes and extraction into wine. Am. J. Enol. Vitic. 2006, 57, 125–132. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.; Fernández-Fernández, J.; Moreno-Olivares, J.; Bleda-Sánchez, J.; Gómez-Martínez, J.; Martínez-Jiménez, J.; Gil-Muñoz, R. Application of elicitors in two ripening periods of Vitis vinifera L. cv Monastrell: Influence on anthocyanin concentration of grapes and wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Improving phenolic and chromatic characteristics of Monastrell, Merlot and Syrah wines by using methyl jasmonate and benzothiadiazole . J. Int. Sci. Vigne Vin 2017, 51, 17–27. [Google Scholar]
- Garde-Cerdán, T.; Sáenz de Urturi, I.; Rubio-Bretón, P.; Marín-San Román, S.; Baroja, E.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M.; Pérez-Álvarez, E.P. Foliar application of methyl jasmonate and methyl jasmonate supported on nanoparticles: Incidence on grape phenolic composition over two seasons. Food Chem. 2023, 402, 134244. [Google Scholar] [CrossRef] [PubMed]
- Gil-Muñoz, R.; Paladines-Quezada, D.F.; Giménez-Bañón, M.J.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.M. The Effect of methyl jasmonate-doped nano-particles and methyl jasmonate on the phenolics and quality in Monastrell grapes during the ripening period. Appl. Sci. 2023, 13, 1906. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Romero-Cascales, I.; Gil-Muñoz, R.; Fernández-Fernández, J.I.; López-Roca, J.M.; Gómez-Plaza, E. Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J. Agric. Food Chem. 2012, 60, 1283–1290. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Methyl jasmonate treatment to increase grape and wine phenolic content inTempranillo and Graciano varieties during two growing seasons. Sci. Hortic. 2018, 240, 378–386. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gil-Muñoz, R. Influence of methyl jasmonate and benzothiadiazole on the composition of grape skin cell walls and wines. Food Chem. 2019, 277, 691–697. [Google Scholar] [CrossRef]
- Huglin, P.; Schneider, C. Biologie et Écologie de la Vigne; Éditions Tec & Doc Lavoisier: Cachan, France, 1998. [Google Scholar]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Principles and Practices of Winemaking; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Traité d’Oenologie II; Dunod: Paris, France, 1998. [Google Scholar]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M. The composition of cell walls from grape pomaces is affected by grape origin and enological technique. Food Chem. 2015, 167, 370–377. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Elicitation with methyl jasmonate supported by precursor feeding with phenylalanine: Effect on Garnacha grape phenolic content. Food Chem. 2017, 237, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Ribereau-Gayon, R.; Dubordieu, D.; Donèche, B.; Lonvauda, A. Handbook of Enology Vol. 1. The Microbiology of WineVinification; John Wiley & Sons: Chichester, UK, 2000. [Google Scholar]
- Ortega-Regules, A.E. Antocianos, Taninos y Composición de la Pared Celular en Distintas Variedades de uva. Evolución durante la Maduración e Implicaciones Tecnológicas. Doctoral Thesis, Food Technology, Murcia University, Murcia, Spain, 2006. [Google Scholar]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Downey, M.O.; Harvey, J.S.; Robinson, S.P. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 2003, 9, 110–121. [Google Scholar] [CrossRef]
- James, A.K.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skins and seeds of Cabernet Sauvignon, Syrah, and Pinot noir berries during ripening. Am. J. Enol. Vitic. 2002, 53, 54–59. [Google Scholar] [CrossRef]
- González-Lázaro, M.; Sáenz de Urturi, I.; Marín-San Román, S.; Murillo-Peña, R.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Effects of foliar applications of methyl jasmonate alone or with urea on anthocyanins content during grape ripening. Sci Hortic. 2024, 338, 113782. [Google Scholar] [CrossRef]
- Ruiz-García, Y.; Gil-Muñoz, R.; López-Roca, J.M.; Martínez-Cutillas, A.; Romero-Cascales, I.; Gómez-Plaza, E. Increasing the phenolic compound content of grapes by preharvest application of abcisic acid and a combination of methyl jasmonate and benzothiadiazole. J. Agric. Food Chem. 2013, 61, 3978–3983. [Google Scholar] [CrossRef]
- Giménez-Bañón, M.J.; Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Fernández-Fernández, J.I.; Parra-Torrejón, B.; Ramírez-Rodríguez, G.B.; Delgado-López, J.; Gil-Muñoz, R. Methyl jasmonate and nanoparticles doped with methyl jasmonate affect the cell wall composition of monastrell grape skins. Molecules 2023, 28, 1478. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Fernández-Fernández, J.I.; Portu, J.; Garde-Cerdán, T. Methyl jasmonate: Effect on proanthocyanidin content in Monastrell and Tempranillo grapes and wines. Eur. Food Res. Technol. 2018, 244, 611–621. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage. Am. J. Clin. Nutr. 2005, 81, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, M.D.; Dambergs, R.G.; Cozzolino, D.; Herderich, M.J.; Smith, P.A. Relationship between red wine grades and phenolics.1. Tannin and total phenolics concentrations. J. Agric. Food Chem. 2010, 58, 12313–12319. [Google Scholar] [CrossRef] [PubMed]


| °Brix | Total Acidity * | pH | Tartaric Acid (g/L) | Malic Acid (g/L) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Treatment | ||||||||||
| Control | 21.6 b,α | 23.32 d,β | 3.76 b,α | 3.28 a,β | 3.90 b,α | 4.08 d,β | 4.30 b,α | 5.13 c,β | 2.58 c,α | 2.14 b,β |
| MeJA | 20.23 a,α | 22.43 c,β | 4.00 b,α | 3.41 a,β | 3.83 a,α | 4.07 d,β | 4.16 ab,α | 4.84 c,β | 2.58 c,α | 2.19 b,β |
| GPE | 20.38 a,α | 22.82 cd,β | 3.77 b,α | 3.26 a,β | 3.88 b,α | 3.98 c,β | 3.82 a,α | 4.94, c,β | 2.73 c,α | 1.85 a,β |
| Year | ||||||||||
| 2022 | 21.70 b,α | 22.82 c,β | 3.77 b,α | 3.79 b,α | 3.89 a,α | 3.98 b,β | 4.87 b,α | 5.00 b,α | 2.45 b,α | 2.48 b,α |
| 2023 | 20.62 a,α | 22.52 bc,β | 3.54 ab,α | 3.29 a,β | 3.87 a,α | 4.05 c,β | 3.79 a,α | 4.86 b,β | 2.57 b,α | 2.0 a,β |
| Alcohol (%v/v) | Volatil Acidity (g/L) | Reductor Sugars (g/L) | Total Acidity (g/L) | pH | Tartaric Acid (g/L) | Malic Acid (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Treament | ||||||||||||||
| Control | 12.09 b,α | 13.99 d,β | 0.32 a,α | 0.41 c,β | 0.97 a,α | 1.15 b,β | 6.45 ab,α | 7.20 d,β | 3.57 b,α | 3.51 a,β | 2.82 a,α | 2.57 a,α | 2.36 c,α | 1.90 abc,α |
| MeJA | 11.33 a,α | 13.19 c,β | 0.37 abc,α | 0.41 c,α | 0.94 a,α | 1.12 b,β | 6.45 ab,α | 6.65 c,β | 3.56 b,α | 3.57 b,α | 2.85 a,α | 2.51 a,α | 1.80 ab,α | 2.05 abc,α |
| GPE | 11.78 b,α | 13.80 d,β | 0.34 ab,α | 0.40 bc,α | 0.94 a,α | 1.10 b,β | 6.31 a,α | 6.64 bc,β | 3.60 b,α | 3.48 a,β | 2.88 a,α | 2.78 a,α | 2.22 bc,α | 1.66 a,β |
| Year | ||||||||||||||
| 2022 | 11.77 a,α | 13.80 c,β | 0.31 a,α | 0.37 b,β | 0.94 a,α | 1.15 c,β | 6.48 b,α | 6.84 c,β | 3.51 a,β | 3.51 a,β | 3.14 d,α | 2.95 c,β | 1.86 ab,α | 1.75 a,α |
| 2023 | 11.67 a,α | 13.38 b,β | 0.40 b,α | 0.47 c,β | 0.97 a,α | 1.07 b,β | 6.24 a,α | 6.81 c,β | 3.54 b,β | 3.54 b,β | 2.27 b,α | 1.95 a,β | 2.65 c,α | 2.12 b,β |
| Extractable Polyphenols (mg/Kg) | Extractable Anthocyanins (mg/Kg) | Total Anthocyanins (mg/Kg) | IMC | SMI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Treatment | ||||||||||
| Control | 50.56 c,α | 47.49 bc,α | 335.81 a,α | 481.1 b,β | 577.09 a,α | 876.33 c,β | 40.78 ab,α | 44.78 abc,α | 73.29 c,α | 59.31 b,β |
| MeJA | 45.50 a,α | 46.32 abc,α | 319.65 a,α | 538.37 c,β | 667.44 ab,α | 1005.57 d,β | 51.91 c,α | 45.31 abc,α | 72.13 c,α | 58.23 a,β |
| GPE | 42.61 a,α | 44.20 ab,α | 305.42 a,α | 444.1 b,β | 568.35 a,α | 735.5 b,β | 46.35 bc,α | 38.00 a,β | 71.74 c,α | 58.94 b,β |
| Year | ||||||||||
| 2022 | 47.91 bc,α | 49.93 c,α | 359.22 b,α | 513.18 d,β | 702.22 b,α | 959.53 d,β | 48.32 b,α | 46.27 b,α | 69.91 b,α | 58.76 a,β |
| 2023 | 44.54 ab,α | 42.18 a,α | 281.36 a,α | 463.70 c,β | 506.37 a,α | 798.38 c,β | 44.38 ab,α | 39.03 a,α | 74.86 c,α | 55.13 a,β |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Anthocyanins ** | 353.08 ab,α | 352.75 ab,α | 340.25 ab,α | 401.58 b,α | 325.83 a,α | 308.00 a,α | 310.22 a,α | 365.0 ab | 369.22 b,α | 343.22 ab,α |
| IC | 6.856 ab,α | 9.456 c,β | 6.922 ab,α | 9.445 c,β | 6.181 a,α | 7.979 b,β | 6.499 a,α | 8.971 b,β | 6.813 a,α | 9.130 b,β |
| Tint | 0.495 a,α | 0.570 c,β | 0.522 ab,α | 0.552 bc,α | 0.503 a,α | 0.534 abc,α | 0.495 a,α | 0.531 b,β | 0.519 ab,α | 0.574 c,β |
| L* | 26.26 bc,α | 18.75 a,β | 24.75 b,α | 19.08 a,β | 28.97 c,α | 22.88 ab,β | 27.64 b,α | 21.17 a,β | 25.68 b,α | 19.30 a,β |
| a* | 58.47 cd,α | 51.14 a,β | 55.27 bc,α | 51.46 ab,α | 60.05 d,α | 55.55 c,β | 59.48 c,α | 54.21 ab,β | 56.39 bc,α | 51.23 a,β |
| b* | 34.21 ab,α | 31.35 a,α | 31.75 a,α | 31.27 a,α | 32.91 a,α | 36.17 b,β | 34.05 a,α | 34.06 a,α | 31.86 a,α | 31.80 a,α |
| C* | 67.77 bc,α | 59.99 a,β | 63.76 ab,α | 60.24 a,α | 68.55 c,α | 66.29 bc,α | 68.56 c,α | 64.03 ab,β | 64.83 bc,α | 60.32 a,β |
| H* | 30.35 ab,α | 31.48 bc,α | 29.85 ab,α | 31.17 bc,α | 28.75 a,α | 33.06 c,β | 29.79 a,α | 32.10 b,β | 29.51 a,α | 31.72 b,β |
| Tannins ** | 667.72 a,α | 968.47 b,β | 678.00 a,α | 1002.16 b,β | 646.20 a,α | 926.38 b,β | 483.26 a,α | 955.69 c,β | 844.68 b,α | 975.65 c,β |
| TP | 32.60 a,α | 41.45 b,β | 32.35 a,α | 42.08 b,β | 30.26 a,α | 38.20 b,β | 33.06 b,α | 45.47 d,β | 30.41a,α | 35.68 c,β |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Df-3g* | 62.37 ab,α | 68.15 b,α | 69.05 b,α | 97.72 c,β | 47.69 a,α | 60.13 ab,α | 68.56 ab,α | 80.86 b,α | 58.85 a,α | 69.81 b,β |
| Cy-3g | 50.21 a,α | 120.34 b,β | 72.25 a,α | 141.50 b,β | 39.73 a,α | 74.25 a,α | 74.82 b,α | 130.32 c,β | 33.30 a,α | 93.71 b,β |
| Pet-3g | 74.92 a,α | 79.56 a,α | 75.49 a,α | 106.81 b,β | 60.94 a,α | 73.38 a,α | 80.75 b,α | 93.69 b,α | 60.14 a,α | 79.47 b,β |
| Pn-3g | 49.17 a,α | 130.22 c,β | 53.92 ab,α | 140.91 c,β | 43.95 a,α | 87.25 b,β | 66.38 b,α | 125.37 c,β | 31.65 a,α | 116.88 c,β |
| Mv-3g | 208.81 a,α | 230.34 a,α | 190.24 a,α | 297.32 b,β | 193.36 a, α | 234.09 a,α | 230.12 b, α | 267.74 b,α | 164.82 a,α | 240.08 b,β |
| Df-ac3g | 6.15 a,α | 5.84 a,α | 6.41 a,α | 6.66 a,α | 5.26 a,α | 5.62 a,α | 4.18 a,α | 4.62 a,α | 7.69 b,α | 7.46 b,α |
| Pet-ac3g | 8.20 a,α | 7.50 a,α | 8.21 a,α | 8.64 a,α | 7.52 a,α | 7.83 a,α | 7.12 a,α | 7.26 a,α | 8.83 b,α | 8.71 b,α |
| Pn-ac3g | 5.67 a,α | 8.63 b,β | 5.92 a,α | 8.75 b,β | 5.51 a,α | 6.64 ab,α | 4.01 a,α | 7.01 b,β | 7.39 b,α | 9.01 c,β |
| Mv-ac3g | 26.31 ab,α | 27.44 ab,α | 24.08 a,α | 31.38 c,β | 26.67 ab,α | 29.67 bc,α | 26.86 ab,α | 29.68 b,α | 24.51 a,α | 29.32 b,β |
| Pn-cum3g | 14.24 ab,α | 22.32 b,α | 11.74 a,α | 15.80 ab,α | 13.50 ab,α | 17.84 a,α | 14.72 b,α | 28.51 c,β | 11.60 b,α | 7.18 a,β |
| Mv-cum3g | 52.06 a,α | 43.43 a,α | 46.33 a,α | 39.27 a,α | 51.89 a,α | 45.95 a,α | 56.79 c,α | 67.06 d,β | 43.39 b,α | 28.71 a,β |
| Total * | 558.12 a,α | 743.80 bc,β | 563.65 a, α | 899.98 c,β | 496.06 a,α | 640.24 ab,α | 634.35 b,α | 842.15 c,β | 444.20 a,α | 680.40 b,β |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Df-3g* | 22.49 b,α | 20.12 ab,α | 20.24 ab,α | 21.58 ab,α | 19.79 ab,α | 18.51 a,α | 21.09 a,α | 19.28 a,α | 20.59 a,α | 20.86 a,α |
| Cy-3g | 11.00 a,α | 13.47 a,α | 11.77 a,α | 13.83 a,α | 10.93 a,α | 12.29 a,α | 9.14 a,α | 11.13 b,β | 13.33 c,α | 15.26 d,β |
| Pet-3g | 34.22 a,α | 32.35 a,α | 29.60 a,α | 32.16 a,α | 31.12 a,α | 28.85 a,α | 34.56 b,α | 32.50 ab,α | 28.74 a,α | 29.74 a,α |
| Cy-3g VitA | 6.76 a,α | 6.97 a,α | 6.77 a,α | 6.86 a,α | 4.87 a,α | 6.71 a,α | 2.06 a,α | 2.11 a,α | 10.21 b,α | 11.58 b,α |
| Pn-3g | 17.97 a,α | 30.90 c,β | 17.60 a,α | 27.42 c,β | 19.47 a,α | 23.61 b,β | 18.99 a,α | 26.23 b,β | 17.71 a,α | 28.38 b,β |
| Mv-3g | 128.52 a,α | 130.30 a,α | 113.78 a,α | 128.24 a,α | 122.52 a,α | 112.93 a,α | 134.09 c,α | 131.14 bc,α | 109.13 a,α | 116.51 ab,α |
| Pn-3g pyruvate | 0.00 a,α | 0.98 b,β | 0.00 a,α | 0.95 b,β | 0.00 a,α | 0.00 a,α | 0.00 a,α | 1.29 b,β | 0.00 a,α | 0.00 a,α |
| Df-ac3g | 7.75 a,α | 7.21 a,α | 7.31 a,α | 7.05 a,α | 7.18 a,α | 7.01 a,α | 2.72 a,α | 2.58 a,α | 12.31 c,α | 11.59 b,β |
| Mv-3g pyr(Vit A) | 7.51 a,α | 8.20 a,α | 7.32 a,α | 7.91 a,α | 7.35 a,α | 7.72 a,α | 2.65 a,α | 3.64 b,β | 12.14 c,α | 12.25 c,α |
| Mv-3g acet (Vit B) | 7.25 a,α | 7.43 a,α | 7.42 a,α | 7.80 a,α | 7.38 a,α | 7.48 a,α | 2.75 a,α | 3.14 b,β | 11.95 c,α | 12.00 c,α |
| Acetil VitA | 0.00 a,α | 0.98 b,β | 0.00 a,α | 0.00 a,α | 0.00 a,α | 0.00 a,α | 0.00 a,α | 0.65 b,β | 0.00 a,α | 0.00 a,α |
| Acetil VitB | 5.71 b,α | 0.00 a,β | 3.82 b,α | 0.94 a,β | 0.00 a,α | 0.96 a,α | 0.00 a,α | 1.27 a,α | 6.35 b,α | 0.00 a,α |
| Pet-ac-3g | 8.63 a,α | 7.75 a,α | 7.87 a,α | 7.73 a,α | 7.83 a,α | 7.71 a,α | 3.62 a,α | 3.50 a,α | 12.60 c,α | 11.96 b,β |
| Mv-3Og-ethyl epi2 | 0.99 a,α | 0.98 a,α | 0.98 a,α | 0.96 a,α | 0.95 a,α | 0.99 a,α | 1.95 b,α | 1.92 b,α | 0.00 a,α | 0.00 a,α |
| Mv-3Og-ethyl epi3 | 2.92 a,α | 4.93 a,α | 4.79 a,α | 4.87 a,α | 4.80 a,α | 6.76 a,α | 1.99 a,α | 2.14 a,α | 6.35 b,α | 8.89 b,α |
| Pn-ac3g | 7.24 a,α | 7.63 a,α | 7.16 a,α | 7.49 a,α | 7.21 a,α | 7.39 a,α | 2.60 a,α | 3.03 b,β | 11.81 c,α | 11.98 c,α |
| Mv-ac3g | 20.10 b,α | 16.50 ab,α | 16.78 ab,α | 15.46 a,α | 17.00 ab,α | 16.11 a,α | 15.23 ab,α | 14.52 a,α | 20.70 c,α | 17.53 b,β |
| Mv6cum-3g pyruvate | 0.00 a,α | 2.91 ab,α | 5.71 b,α | 0.00 a,β | 3.81 ab,α | 6.067 b,α | 0.00 a,α | 1.30 ab,α | 6.35 c,α | 5.08 bc,α |
| Mv6cum-3g acetaldehyde | 7.28 a,α | 8.34 a,α | 6.77 a,α | 7.49 a,α | 7.16 a,α | 7.70 a,α | 2.51 a,α | 3.59 b,β | 11.74 c,α | 12.09 c,α |
| Mv-3Og-ethyl epi4 | 7.26 a,α | 7.20 a,α | 6.93 a,α | 6.94 a,α | 7.15 a,α | 7.10 a,α | 2.54 a,α | 2.56 a,α | 11.69 b,α | 11.60 b,α |
| Pn-cum3g | 8.31 a,α | 9.97 a,α | 7.54 a,α | 8.95 a,α | 8.26 a,α | 9.08 a,α | 3.84 a,α | 5.17 b,β | 12.23 c,α | 13.49 d,β |
| Mv-cum3g | 23.85 c,α | 24.12 c,α | 17.47 a,α | 20.01 ab,α | 22.19 bc,α | 22.30 bc,α | 20.95 a,α | 22.41 a,α | 21.39 a,α | 21.88 a,α |
| Total * | 335.78 a,α | 349.24 a,α | 307.91 a,α | 334.70 a,α | 317.04 a,α | 317.94 a,α | 283.32 a,α | 295.19 a,α | 357.18 b,α | 372.74 b,α |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Myr-3-gU* | 1.20 a,α | 1.52 ab,α | 0.67 a,α | 2.49 b,β | 0.73 a,α | 1.51 ab,β | 0.81 a,α | 0.78 a,α | 0.93 a,α | 2.90 b,β |
| Myr-3gal | 1.21 ab,α | 2.41 c,β | 0.73 a,α | 2.06 c,β | 0.89 a,α | 1.94 bc,β | 1.03 ab,α | 1.45 b,α | 0.86 a,α | 2.83 c,β |
| Myr-3g | 9.72 bc,α | 15.94 d,β | 6.30 a,α | 12.54 cd,β | 7.94 ab,α | 12.14 c,β | 8.24 a,α | 14.01 b,β | 7.74 a,α | 13.07 b,β |
| Quer-3gal | 1.09 a,α | 3.67 a,α | 1.67 a,α | 2.86 a,α | 1.44 a,α | 1.74 a,α | 2.81 b,α | 5.43 c,β | 0 a,α | 0.08 a,α |
| Quer-3gU | 11.95 d,α | 9.36 bc,β | 10.84 cd,α | 8.79 ab,β | 9.12 bc,α | 7.06 a,β | 10.17 bc,α | 8.26 a,β | 11.10 c,α | 8.55 ab,β |
| Quer-3g | 22.64 a,α | 38.81 b,β | 15.12 a,α | 20.95 a,α | 17.48 a,α | 20.71 a,α | 21.21 a,α | 32.20 b,β | 15.6 a,α | 21.45 a,α |
| Lar-3g | 1.69 a,α | 2.98 b,β | 1.01 a,α | 2.57 b,β | 1.50 a,α | 2.47 b,β | 1.51 a,α | 2.48 b,β | 1.29 a,α | 2.87 b,β |
| Kam-3g | 1.65 a,α | 3.37 a,α | 1.70 a,α | 2.23 a,α | 2.18 a,α | 1.69 a,α | 3.25 b,α | 4.47 c,β | 0.44 a,α | 0.38 a,α |
| Iso-3g | 0.12 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α | 0.01 a,α |
| Syr-3-g | 0.64 a,α | 1.22 b,β | 0.48 a,α | 1.08 b,β | 0.64 a,α | 1.03 b,β | 0.65 a,α | 1.16 b,β | 0.52 a,α | 1.07 b,β |
| Total * | 51.94 ab,α | 79.29 c,β | 38.55 a,α | 55.61 b,β | 41.95 ab,α | 50.31 ab,α | 49.70 a,α | 70.28 b,β | 38.59 a,α | 53.21 a,α |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| Myr-3-gU* | 1.67 a,α | 2.38 ab,α | 0.99 a,α | 4.31 b,β | 0.96 a,α | 3.08 ab,α | 0.85 a,α | 0.95 a,α | 1.57 a,α | 5.56 b,β |
| Myr-3-gal | 1.11 a,α | 3.12 b,β | 0.90 a,α | 3.11 b,β | 0.91 a,α | 2.95 b,β | 0.48 a,α | 0.70 a,α | 1.47 b,α | 5.42 c,β |
| Myr-3-g | 11.87 ab,α | 16.29 ab,α | 8.19 a,α | 17.33 b,β | 9.06 ab,α | 16.27 ab,α | 6.22 a,α | 8.06 a,α | 13.19 b,α | 25.20 c,β |
| Quer-3-gal | 0.40 a,α | 0.90 a,α | 0.39 a,α | 0.66 a,α | 0.50 a,α | 0.63 a,α | 0.85 c,α | 1.30 d,β | 0.00 a,α | 0.16 b,β |
| Quer-3-gU | 14.06 a,α | 12.81 a,α | 12.31 a,α | 12.33 a,α | 9.93 a,α | 10.29 a,α | 5.30 a,α | 6.62 a,α | 18.91 b,α | 16.94 b,α |
| Quer-3-g | 24.28 a,α | 30.02 a,α | 15.84 a,α | 24.96 a,α | 13.31 a,α | 24.03 a,α | 9.33 a,α | 9.18 a,α | 26.28 b,α | 43.49 c,β |
| Lar-3-g | 1.99 abc,α | 3.70 cd,α | 1.37 a,α | 3.87 d,β | 1.80 ab,α | 3.55 bcd,α | 1.22 a,α | 1.90 b,β | 2.22 b,α | 5.51 c,β |
| Kam-3-g | 0.94 ab,α | 0.70 a,α | 1.09 b,α | 0.85 ab,α | 0.84 ab,α | 0.73 a,α | 1.16 b,α | 0.79 a,β | 0.75 a,α | 0.73 a,α |
| Iso-3-g | 0.99 a,α | 1.65 b,β | 0.78 a,α | 1.28 ab,α | 0.74 a,α | 1.35 ab,α | 0.59 a,α | 0.85 b,β | 1.09 c,α | 2.01 d,β |
| Syr-3-g | 0.84 a,α | 1.71 b,β | 0.66 a,α | 1.57 b,β | 0.84 a,α | 1.50 b,β | 0.66 a,α | 1.14 c,β | 0.90 b,α | 2.04 d,β |
| Total * | 58.87 a,α | 76.57 b,β | 43.03 a,α | 72.18 b,β | 39.57 a,α | 66.40 ab,α | 27.92 a,α | 34.62 a,α | 66.40 b,α | 104.82 c,β |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| mDP | 15.66 b,α | 13.29 ab,α | 14.24 ab,α | 15.78 b,α | 15.25 ab,α | 12.89 a,α | 17.21 c,α | 15.04 b,β | 12.89 a,α | 12.92 a,α |
| % Gal | 1.51 b,α | 1.75 c,β | 1.54 bc,α | 0.95 a,β | 1.42 b,α | 1.50 b,α | 1.50 ab,α | 1.56 b,α | 1.48 ab,α | 1.24 a,α |
| EGC-Ext* | 234.56 bc,α | 135.14 a,β | 237.35 c,α | 237.4 c,α | 220.34 bc,α | 160.55 ab,α | 272.91 b,α | 195.75 a,β | 188.59 a,α | 159.65 a,α |
| Cat-Ext | 12.54 a,α | 8.77 a,β | 13.63 b,α | 10.50 ab,α | 12.62 b,α | 11.03 ab,α | 32.60 a,α | 31.71 a,α | 48.70 b,α | 35.08 a,β |
| Epi-Ext | 604.94 c,α | 392.33 a,β | 596.49 c,α | 537.87 bc,α | 531.80 bc,α | 456.72 bc,α | 568.76 b,α | 504.31 ab,α | 586.74 b,α | 420.3 a,β |
| Cat-T | 39.57 ab,α | 29.63 a,α | 44.28 b,α | 32.06 a,β | 38.21 ab,α | 38.50 ab,α | 32.60 a,α | 31.71 a,α | 48.78 b,α | 35.08 a,β |
| Epi-T | 18.76 ab,α | 13.54 a,α | 19.85 ab,α | 22.39 b,α | 17.65 ab,α | 13.41 a,α | 19.97 a,α | 18.63 a,α | 17.53 a,α | 14.26 a,α |
| ECG-Ext | 20.28 cd,α | 15.53 abc,α | 20.56 d,α | 11.79 a,β | 17.48 bcd,α | 15.21 ab,α | 20.55 b,α | 17.61 b,α | 18.32 b.α | 10.74 a,β |
| ECG-T | 0.62 ab,α | 0.29 ab,α | 0.86 b,α | 0.00 a,β | 0.42 ab,α | 0.72 ab,α | 0.19 ab,α | 1.08 c,β | 0.63 bc,α | 0.04 a,β |
| Totalµg/g skin | 8427.21 b,α | 5407.84 a,β | 7531.04 b,α | 5565.62 a,β | 7395.41 b,α | 5731.76 a,β | 8284.88 b,α | 6049.96 b,β | 7285.02 b,α | 5086.85 a,β |
| Total mg/Kg | 606.61c,α | 358.54 a,β | 540.85 bc,α | 486.52 b,α | 496.70 bc,α | 368.60 a,β | 589.49 c,α | 480.22 b,β | 506.61 bc,α | 328.89 a,β |
| Treatment | Year | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | MeJA | GPE | 2022 | 2023 | ||||||
| 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | 21 °Brix | 23 °Brix | |
| GPm | 5.43 a,α | 4.90 a,α | 5.24 a,α | 5.48 a,α | 5.13 a,α | 4.99 a,α | 4.68 a,α | 4.88 a,α | 6.02 c,α | 5.24 b,β |
| % Gal | 1.28 a,α | 2.25 a,α | 1.50 a,α | 1.50 a,α | 1.48 a,α | 2.15 a,α | 2.17 c,α | 2.75 d,β | 0.66 a,α | 1.18 b,β |
| EGC-Ext* | 105.28 ab,α | 83.02 a,α | 122.41 b,α | 117.52 b,α | 86.47 a,α | 96.10 ab,α | 85.43 a,α | 109.46 bc,β | 124.0 c,α | 88.30 ab,β |
| Cat-Ext | 22.79 a,α | 21.05 a,α | 23.06 a,α | 22.57 a,α | 19.74 a,α | 23.08 a,α | 10.18 a,α | 12.97 b,β | 33.56 c,α | 31.50 c,α |
| Epi-Ext | 310.42 a,α | 261.31 a,α | 314.10 a,α | 283.50 a,α | 248.08 a,α | 282.79 a,α | 229.09 a,α | 296.68 b,β | 352.64 c,α | 225.07 ab,β |
| Cat-T | 72.17 a,α | 63.61 a,α | 76.22 a,α | 68.43 a,α | 66.07 a,α | 69.94 a,α | 64.61 b,α | 79.12 c,β | 78.37 c,α | 55.53 a,β |
| Epi-T | 23.43 a,α | 30.00 b,β | 23.09 a,α | 29.82 b,β | 18.57 a,α | 30.48 b,β | 23.02 a,α | 28.64 b,β | 20.26 a,α | 31.57 a,β |
| ECG-Ext | 7.56 a,α | 14.39 a,α | 8.82 a,α | 10.80 a,α | 7.72 a,α | 14.05 a,α | 12.98 b,α | 19.45 c,β | 3.87 a,α | 6.71 a,α |
| ECG-T | 2.21 ab,α | 2.34 ab,α | 2.08 ab,α | 1.31 a,α | 1.70 ab,α | 3.02 b,α | 1.85 ab,α | 2.98 b,α | 2.14 ab,α | 1.47 a,α |
| Total mg/L | 543.69 a,α | 475.74 a,α | 569.81 a,α | 533.98 a,α | 448.39 a,α | 519.49 a,α | 426.38 a,α | 549.33 bc,β | 614.87 c,α | 470.15 ab,β |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Muñoz, R.; Giménez-Bañón, M.J.; Bleda-Sánchez, J.A.; Moreno-Olivares, J.D. The Impact of Two Elicitors and Harvest Ripening Stage on the Quality of Monastrell Grapes and Wines. Biomolecules 2025, 15, 474. https://doi.org/10.3390/biom15040474
Gil-Muñoz R, Giménez-Bañón MJ, Bleda-Sánchez JA, Moreno-Olivares JD. The Impact of Two Elicitors and Harvest Ripening Stage on the Quality of Monastrell Grapes and Wines. Biomolecules. 2025; 15(4):474. https://doi.org/10.3390/biom15040474
Chicago/Turabian StyleGil-Muñoz, Rocío, Maria José Giménez-Bañón, Juan Antonio Bleda-Sánchez, and Juan Daniel Moreno-Olivares. 2025. "The Impact of Two Elicitors and Harvest Ripening Stage on the Quality of Monastrell Grapes and Wines" Biomolecules 15, no. 4: 474. https://doi.org/10.3390/biom15040474
APA StyleGil-Muñoz, R., Giménez-Bañón, M. J., Bleda-Sánchez, J. A., & Moreno-Olivares, J. D. (2025). The Impact of Two Elicitors and Harvest Ripening Stage on the Quality of Monastrell Grapes and Wines. Biomolecules, 15(4), 474. https://doi.org/10.3390/biom15040474








