Cannabinoid Regulation of Murine Vaginal Secretion
Abstract
1. Introduction
2. Methods
2.1. Study Animals
2.2. Experimental Design
2.2.1. Sex and Disposition of Animals
2.2.2. Number of Mice Used for Each Experiment
- (1)
- THC, IP 4 mg/kg, active phase, n = 16;
- (2)
- THC, IP 10 mg/kg, active phase, n = 8;
- (3)
- THC, IP 4 mg/kg, rest phase, n = 8;
- (4)
- THC, Intravaginal (IVag) 1 mM (10 μL), n = 9;
- (5)
- CP55940, IP 0.5 mg/kg, n = 10;
- (6)
- CP55940, IntraVag 150 μM (10 μL), n = 13;
- (7)
- SR141716, IP 4 mg/kg, n = 12.
- (1)
- CP55940, IP 0.5 mg/kg, n = 12;
- (2)
- Vehicle, IP, n = 13;
- (3)
- CP55940, IVag 150 μM (10 μL), n = 30.
- (1)
- CB1 KO vs. WT (CD1 strain) baselines, n = 30, 32;
- (2)
- CB1 KO bedding responses, n = 16;
- (3)
- WT, SR141716, IP 4 mg/kg, n = 24;
- (4)
- WT, SR141716, IVag 1 mM (10 μL), n = 15.
- (1)
- THC, IP 4 mg/kg, daily, n = 10;
- (2)
- THC, IP 4 mg/kg, chronic (weekly), n = 16.
- (1)
- CP55940, IP 0.5 mg/kg (daily), CD1 strain, n = 13;
- (2)
- CP55940, IP 0.5 mg/kg (weekly), CD1 strain, n = 7;
- (3)
- CP55940, IP 0.5 mg/kg (weekly), CB1 knockout on CD1 strain, n = 8.
2.2.3. Measurement of Vaginal Moisture
2.3. Bedding
2.3.1. General Method of Exposure to Odorant (Bedding)
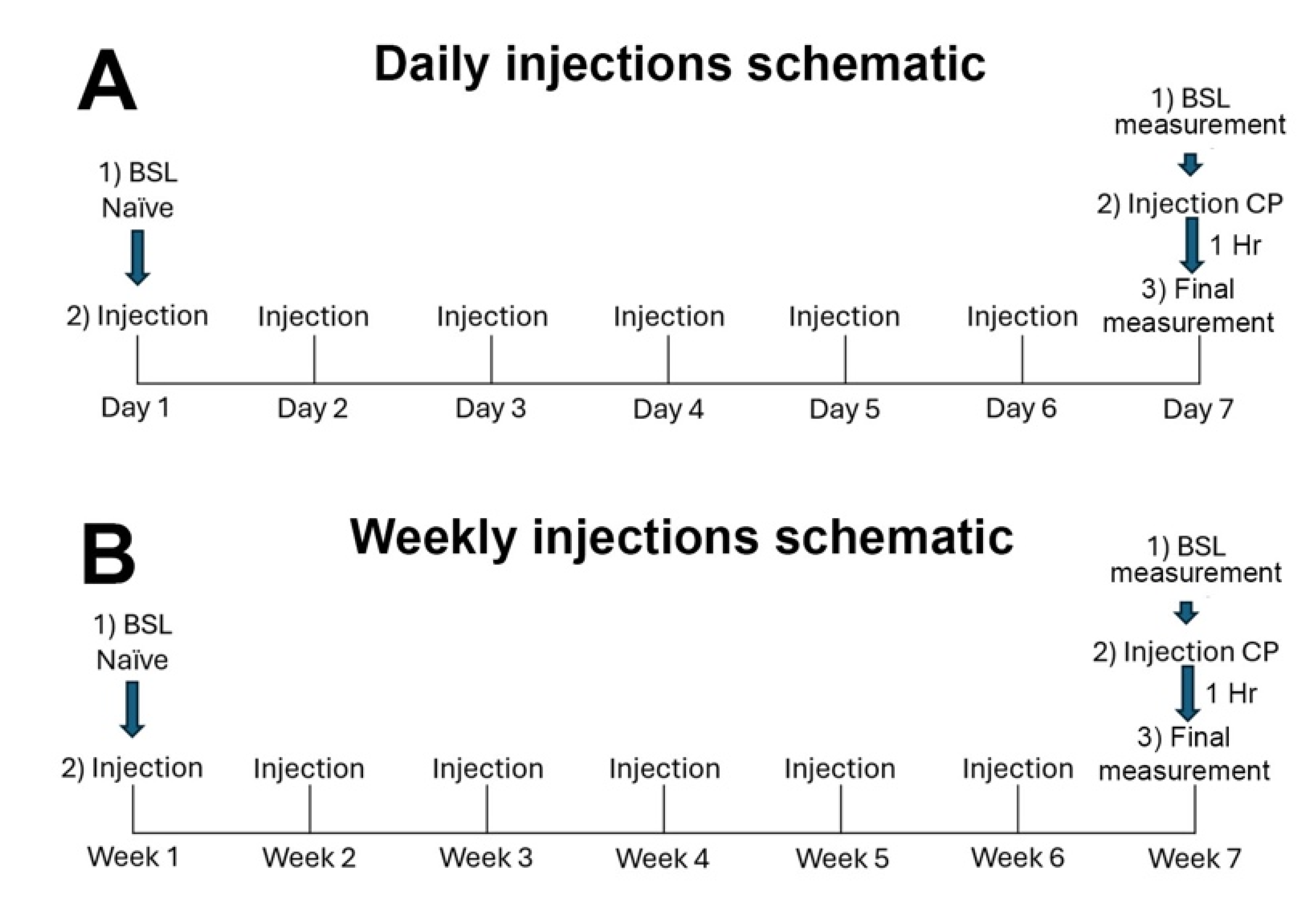
2.3.2. Chronic Treatments
2.4. Statistics
2.5. Drugs
3. Results
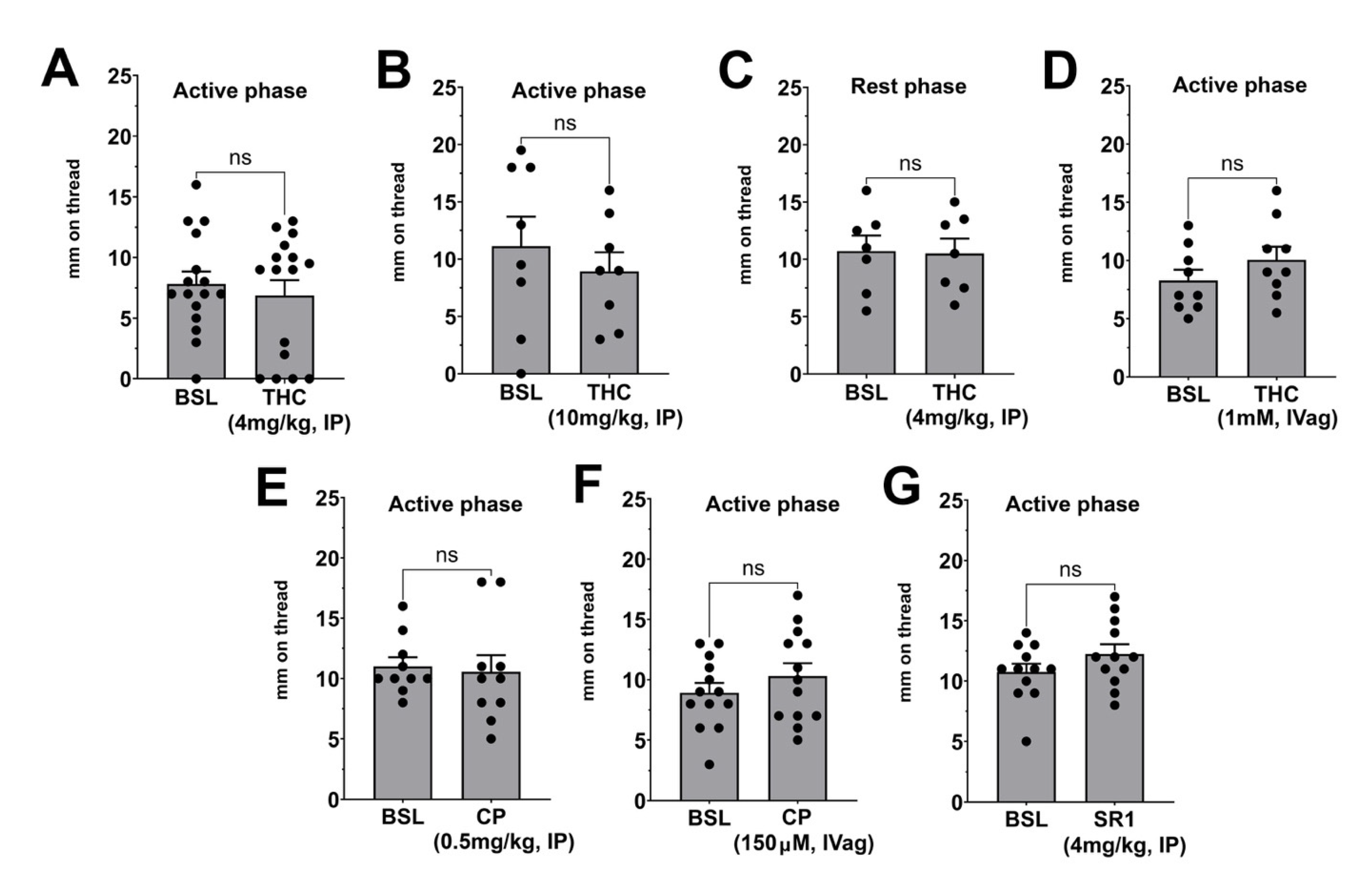
3.1. Effects of Acute Treatment with THC, CP55940, and SR141716 on Basal Vaginal Moisture
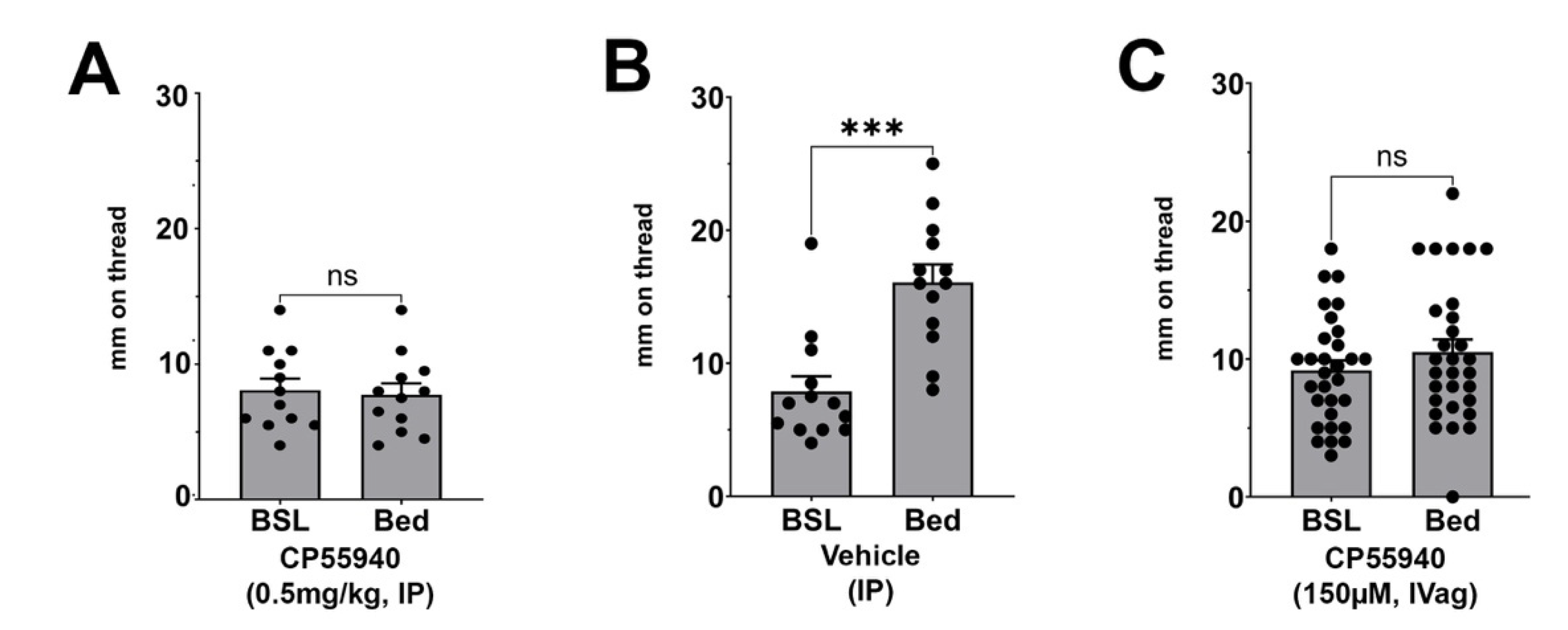
3.2. Effect of CP55940 on Stimulated Vaginal Secretion
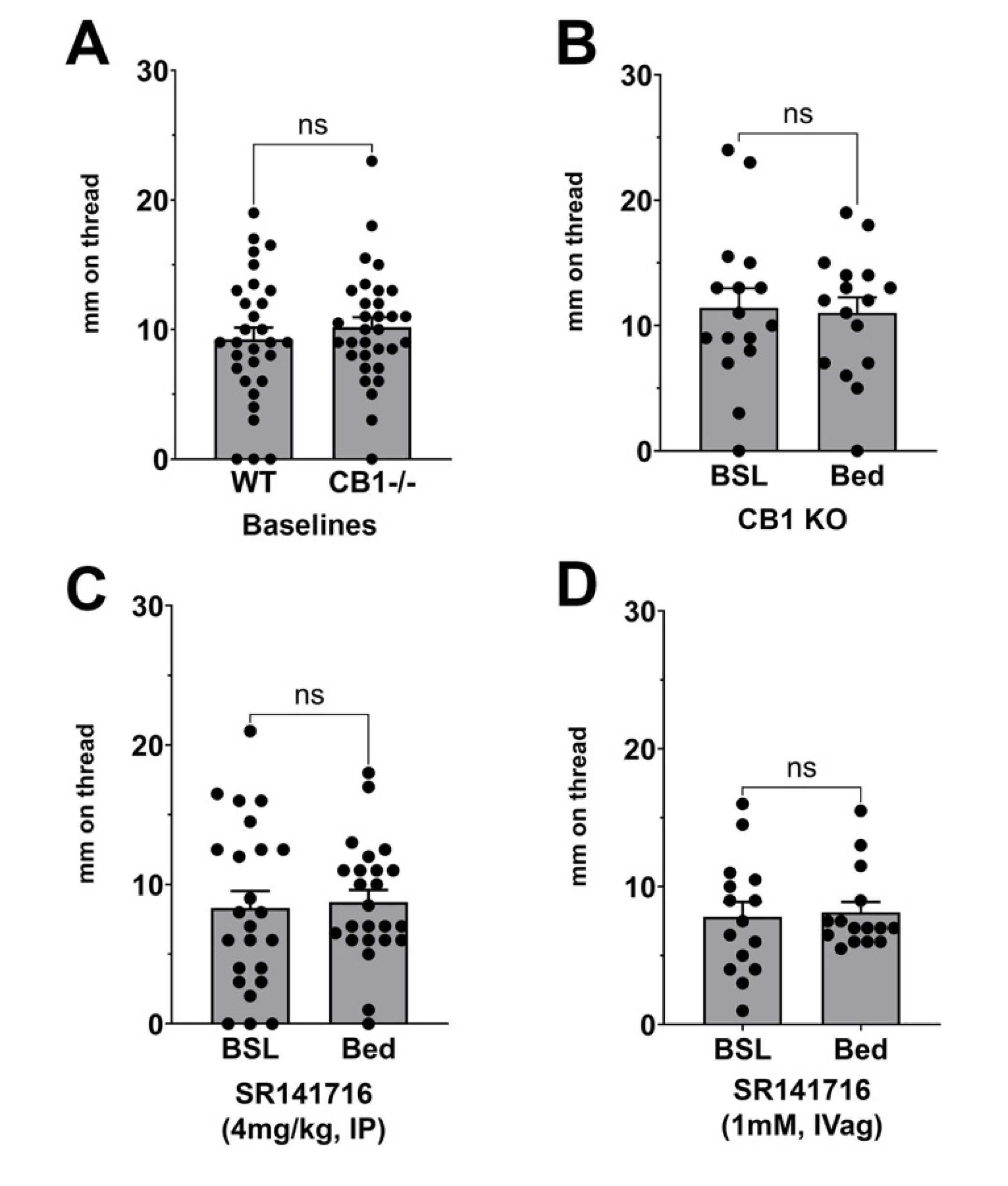
3.3. Effect of CB1 Receptor Block or Deletion on Stimulated Vaginal Secretion
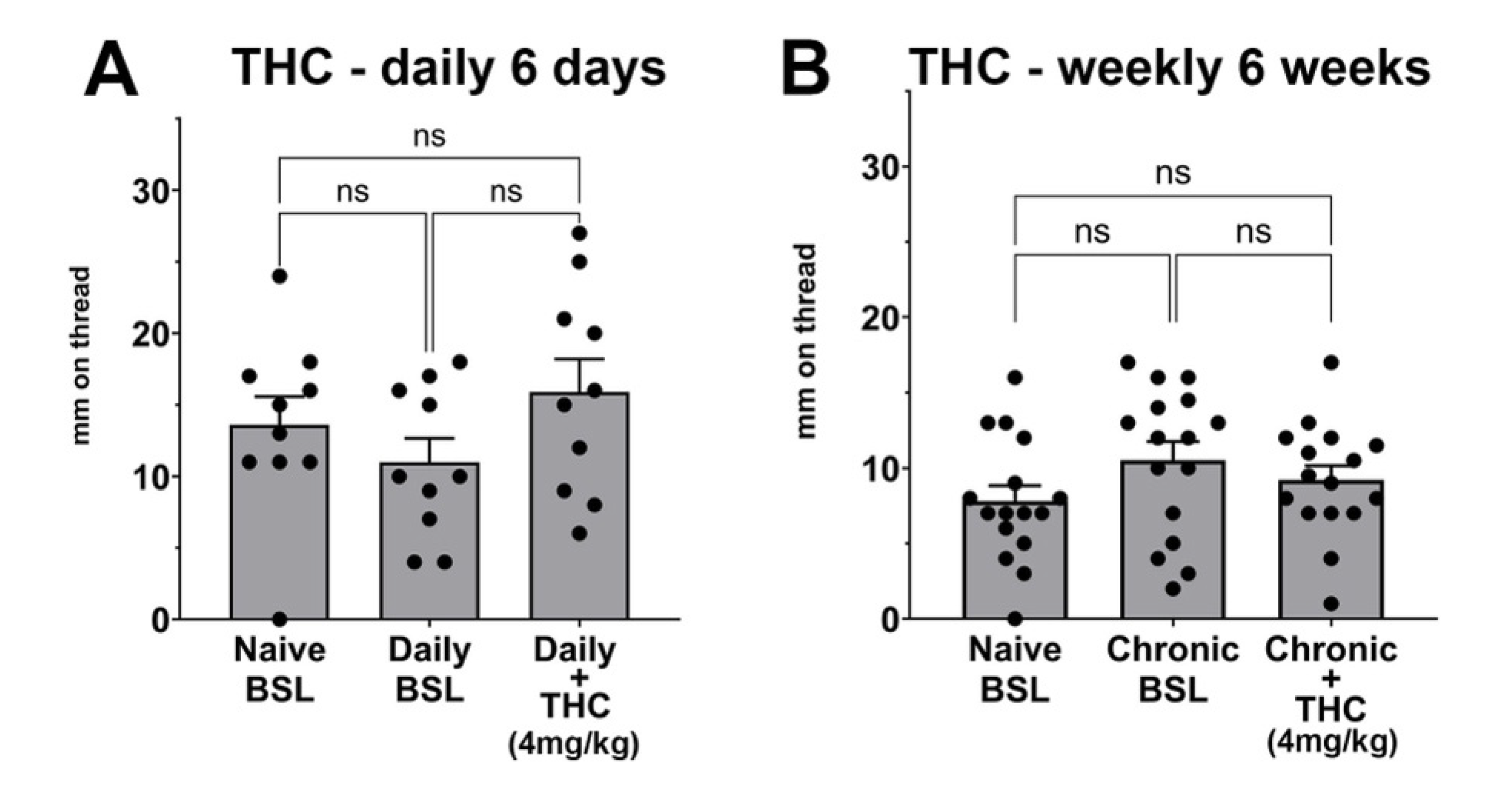
3.4. Chronic THC Does Not Alter Vaginal Moisture Levels
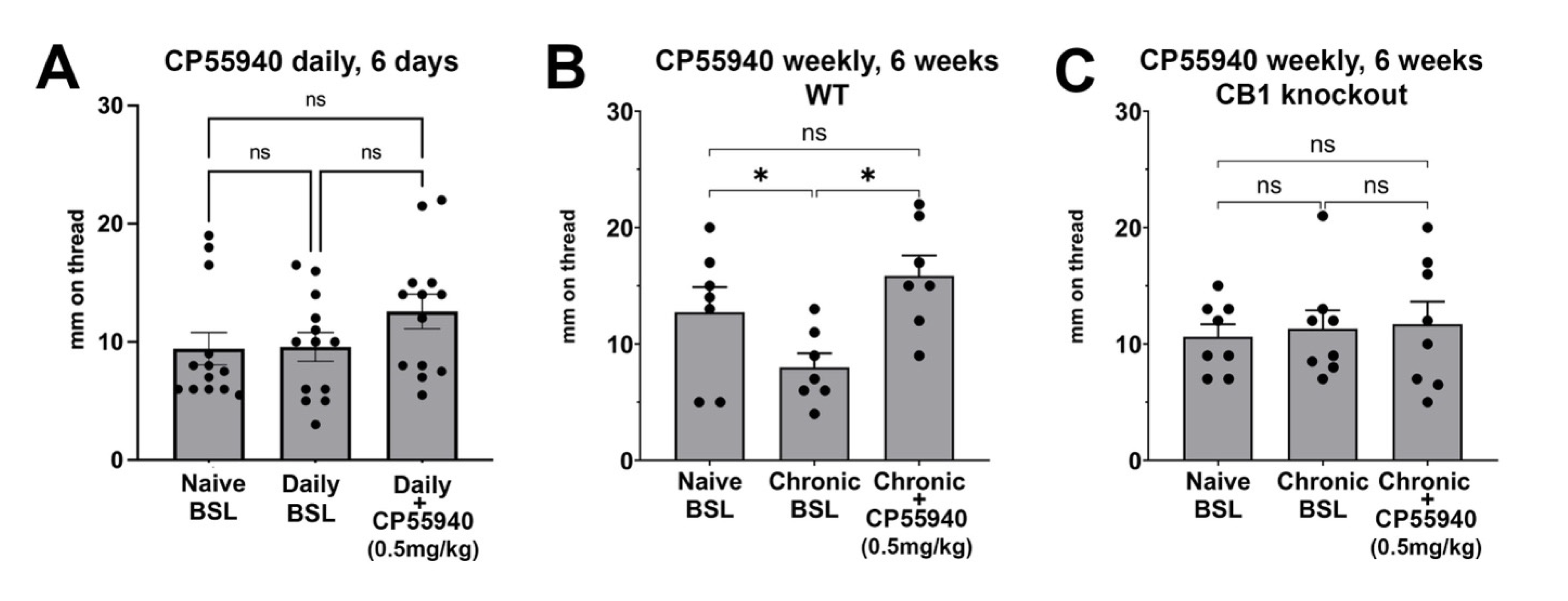
3.5. Chronic Intermittent CP55940 Reduces Basal Vaginal Moisture and Unveils a Stimulatory CB1-Mediated Effect
4. Discussion
4.1. An Effect on Stimulated but Not Basal Vaginal Moisture
4.2. The Mouse as a Model
4.3. Potential Mechanisms
4.4. THC, CP55940, and the Potential Effects of Cannabis
4.5. Ruling out the Role of Olfactory Cannabinoid Receptors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russo, E.B.; Cuttler, C.; Cooper, Z.D.; Stueber, A.; Whiteley, V.L.; Sexton, M. Survey of Patients Employing Cannabigerol-Predominant Cannabis Preparations: Perceived Medical Effects, Adverse Events, and Withdrawal Symptoms. Cannabis Cannabinoid Res. 2021, 7, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Woodard, T.L.; Diamond, M.P. Physiologic measures of sexual function in women: A review. Fertil. Steril. 2009, 92, 19–34. [Google Scholar] [CrossRef]
- Oyewolo, T. Anatomy, Physiology and Neurology. In Mosby’s Guide to Women’s Health; Mosby: Maryland Heights, MO, USA, 2007; p. 306. [Google Scholar]
- Lee, M.Y.; Dalpiaz, A.; Schwamb, R.; Miao, Y.; Waltzer, W.; Khan, A. Clinical Pathology of Bartholin’s Glands: A Review of the Literature. Curr. Urol. 2015, 8, 22–25. [Google Scholar] [CrossRef]
- Skene, A.J.C. Diseases of the Glands of the Female Urethra. Chic. Med. J. Exam. 1881, 42, 177–187. [Google Scholar] [PubMed]
- Rodriguez, F.D.; Camacho, A.; Bordes, S.J.; Gardner, B.; Levin, R.J.; Tubbs, R.S. Female ejaculation: An update on anatomy, history, and controversies. Clin. Anat. 2021, 34, 103–107. [Google Scholar] [CrossRef]
- Farenhorst, C.A.; de Wolff, L.; Arends, S.; van Nimwegen, J.F.; Kroese, F.G.M.; Verstappen, G.M.; Bootsma, H. Clinical determinants of vaginal dryness in patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 133), 73–79. [Google Scholar] [CrossRef]
- Lynn, B.K.; Lopez, J.D.; Miller, C.; Thompson, J.; Campian, E.C. The Relationship between Marijuana Use Prior to Sex and Sexual Function in Women. Sex. Med. 2019, 7, 192–197. [Google Scholar] [CrossRef]
- Thayer, A.; Murataeva, N.; Delcroix, V.; Wager-Miller, J.; Makarenkova, H.P.; Straiker, A. THC Regulates Tearing via Cannabinoid CB1 Receptors. Invest. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef]
- Andreis, K.; Billingsley, J.; Naimi Shirazi, K.; Wager-Miller, J.; Johnson, C.; Bradshaw, H.; Straiker, A. Cannabinoid CB1 receptors regulate salivation. Sci. Rep. 2022, 12, 14182. [Google Scholar] [CrossRef]
- Murataeva, N.; Mattox, S.; Lemieux, J.; Griffis, J.; Yust, K.; Du, W.; Heinbockel, T.; Straiker, A. Cannabinoid regulation of sex-dependent murine odorant-stimulated salivation. Sci. Rep. 2024, 14, 26720. [Google Scholar] [CrossRef]
- Murataeva, N.; Yust, K.; Mattox, S.; Du, W.; Straiker, A. A Sex-Dependent Cannabinoid CB1 Receptor Role in Circadian Tearing of the Mouse. Invest. Ophthalmol. Vis. Sci. 2024, 65, 10. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar]
- Herkenham, M.; Lynn, A.B.; Johnson, M.R.; Melvin, L.S.; de Costa, B.R.; Rice, K.C. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J. Neurosci. 1991, 11, 563–583. [Google Scholar] [PubMed]
- Cabral, G.A.; Raborn, E.S.; Griffin, L.; Dennis, J.; Marciano-Cabral, F. CB2 receptors in the brain: Role in central immune function. Br. J. Pharmacol. 2008, 153, 240–251. [Google Scholar]
- Piomelli, D. The molecular logic of endocannabinoid signalling. Nat. Rev. Neurosci. 2003, 4, 873–884. [Google Scholar] [PubMed]
- Bilbao, A.; Spanagel, R. Medical cannabinoids: A pharmacology-based systematic review and meta-analysis for all relevant medical indications. BMC Med. 2022, 20, 259. [Google Scholar] [CrossRef]
- Murataeva, N.; Mattox, S.; Yust, K.; Du, W.; Straiker, A. Murine vaginal secretory responses to a male volatile chemical messenger. Sci. Rep. 2024, 14, 27707. [Google Scholar] [CrossRef]
- Ledent, C.; Valverde, O.; Cossu, G.; Petitet, F.; Aubert, J.F.; Beslot, F.; Bohme, G.A.; Imperato, A.; Pedrazzini, T.; Roques, B.P.; et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 1999, 283, 401–404. [Google Scholar]
- Welch, S.P.; Huffman, J.W.; Lowe, J. Differential blockade of the antinociceptive effects of centrally administered cannabinoids by SR141716A. J. Pharmacol. Exp. Ther. 1998, 286, 1301–1308. [Google Scholar]
- Long, L.E.; Chesworth, R.; Huang, X.F.; McGregor, I.S.; Arnold, J.C.; Karl, T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int. J. Neuropsychopharmacol. 2010, 13, 861–876. [Google Scholar] [CrossRef]
- Sain, N.M.; Liang, A.; Kane, S.A.; Urban, M.O. Antinociceptive effects of the non-selective cannabinoid receptor agonist CP 55,940 are absent in CB1(-/-) and not CB2(-/-) mice in models of acute and persistent pain. Neuropharmacology 2009, 57, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, M.; Bhattacharyya, S. Cannabis use and the development of tolerance: A systematic review of human evidence. Neurosci. Biobehav. Rev. 2018, 93, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.J.; Suschinsky, K.D.; Lalumiere, M.L. Habituation of sexual responses in men and women: A test of the preparation hypothesis of women’s genital responses. J. Sex. Med. 2013, 10, 990–1000. [Google Scholar] [CrossRef]
- Biancardi, M.F.; Santos, F.C.; Madi-Ravazzi, L.; Goes, R.M.; Vilamaior, P.S.; Felisbino, S.L.; Taboga, S.R. Testosterone promotes an anabolic increase in the rat female prostate (Skene’s paraurethral gland) which acquires a male ventral prostate phenotype. Anat. Rec. 2010, 293, 2163–2175. [Google Scholar] [CrossRef]
- Kannan, S.; Archunan, G. Chemistry of clitoral gland secretions of the laboratory rat: Assessment of behavioural response to identified compounds. J. Biosci. 2001, 26, 247–252. [Google Scholar] [CrossRef]
- Gomez-Baena, G.; Pounder, K.C.; Halstead, J.O.; Roberts, S.A.; Davidson, A.J.; Prescott, M.; Beynon, R.J.; Hurst, J.L. Unraveling female communication through scent marks in the Norway rat. Proc. Natl. Acad. Sci. USA 2023, 120, e2300794120. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB and CB. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar]
- Gomez-Sotres, P.; Skupio, U.; Dalla Tor, T.; Julio-Kalajzic, F.; Cannich, A.; Gisquet, D.; Bonilla-Del Rio, I.; Drago, F.; Puente, N.; Grandes, P.; et al. Olfactory bulb astrocytes link social transmission of stress to cognitive adaptation in male mice. Nat. Commun. 2024, 15, 7103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murataeva, N.; Mattox, S.; Yust, K.; Straiker, A. Cannabinoid Regulation of Murine Vaginal Secretion. Biomolecules 2025, 15, 472. https://doi.org/10.3390/biom15040472
Murataeva N, Mattox S, Yust K, Straiker A. Cannabinoid Regulation of Murine Vaginal Secretion. Biomolecules. 2025; 15(4):472. https://doi.org/10.3390/biom15040472
Chicago/Turabian StyleMurataeva, Natalia, Sam Mattox, Kyle Yust, and Alex Straiker. 2025. "Cannabinoid Regulation of Murine Vaginal Secretion" Biomolecules 15, no. 4: 472. https://doi.org/10.3390/biom15040472
APA StyleMurataeva, N., Mattox, S., Yust, K., & Straiker, A. (2025). Cannabinoid Regulation of Murine Vaginal Secretion. Biomolecules, 15(4), 472. https://doi.org/10.3390/biom15040472





