The C1473G Mutation in the Mouse Tph2 Gene: From Molecular Mechanism to Biological Consequences †
Abstract
1. Introduction
2. Materials and Methods
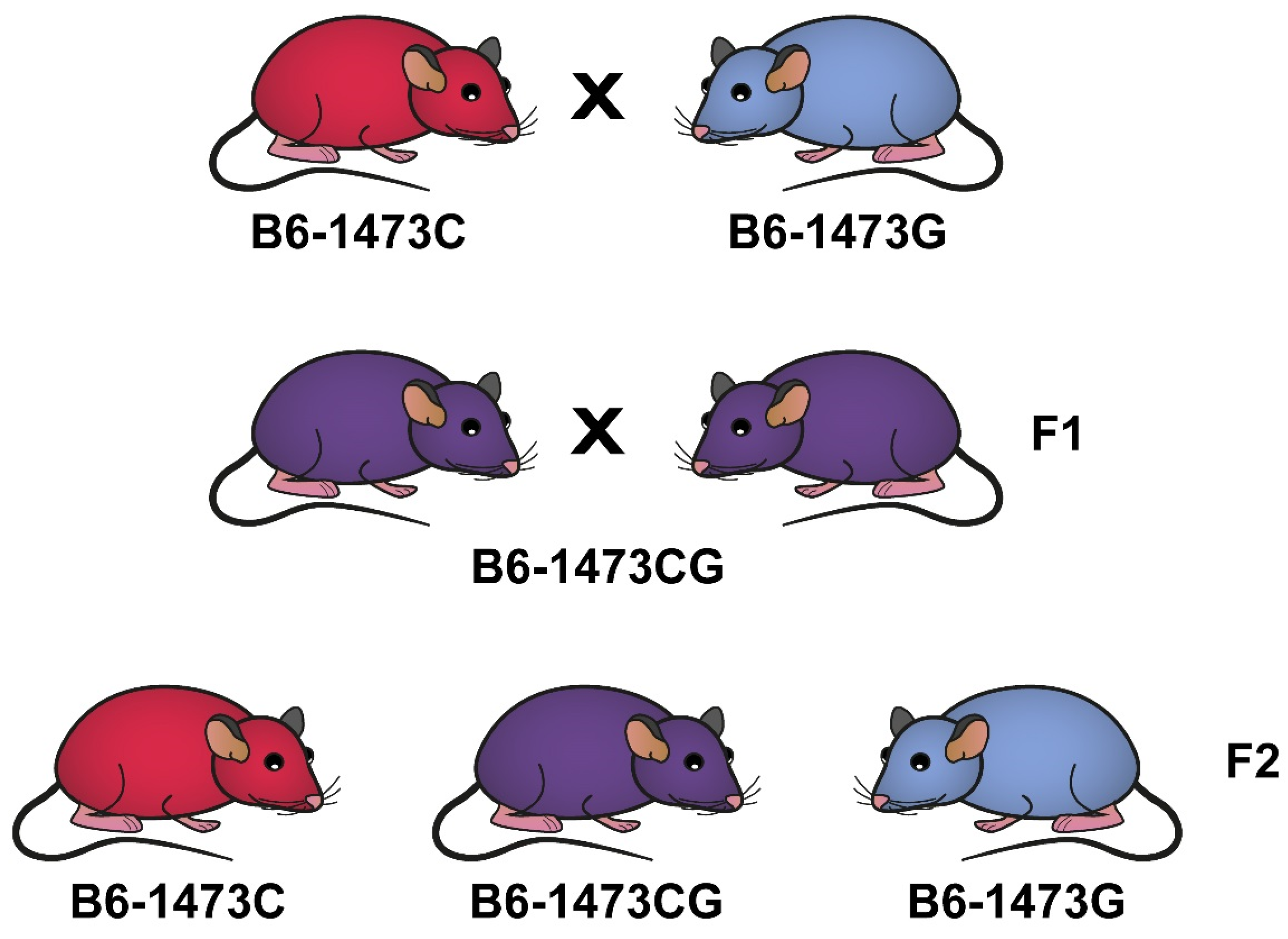
2.1. Animals and Experimental Design
2.2. Behavioral Tests
2.2.1. Tube Dominance Test
2.2.2. Olfactory Preference Test
2.2.3. Comparison of B6-1473C and B6-1473G Adult Males in Mixed Pairs for Their Ability to Mate Females and Pass 1473C and 1473G Alleles to Their Offspring
2.2.4. Tail Suspension Test
2.2.5. Home Cage Activity
2.2.6. Open Field Test
2.3. Embryos
2.4. Genotyping for C1473G Polymorphism
2.5. Biochemical Studies
2.5.1. Tissue Preparation
2.5.2. Assay of 5-HT and 5-HIAA Levels
2.5.3. Assay of the TPH Activity
2.5.4. Assay of TPH2 KM and Vmax Values
2.5.5. Assay of TPH2 Thermal Stability
2.5.6. TPH2 Protein Quantification with Western Blot Analysis
2.5.7. mRNA Level Assay by qPCR
2.6. Statistics
2.6.1. Calculation of KM and Vmax Values
2.6.2. Analysis of the Thermal Denaturation Curves
2.6.3. Statistical Tests
3. Results
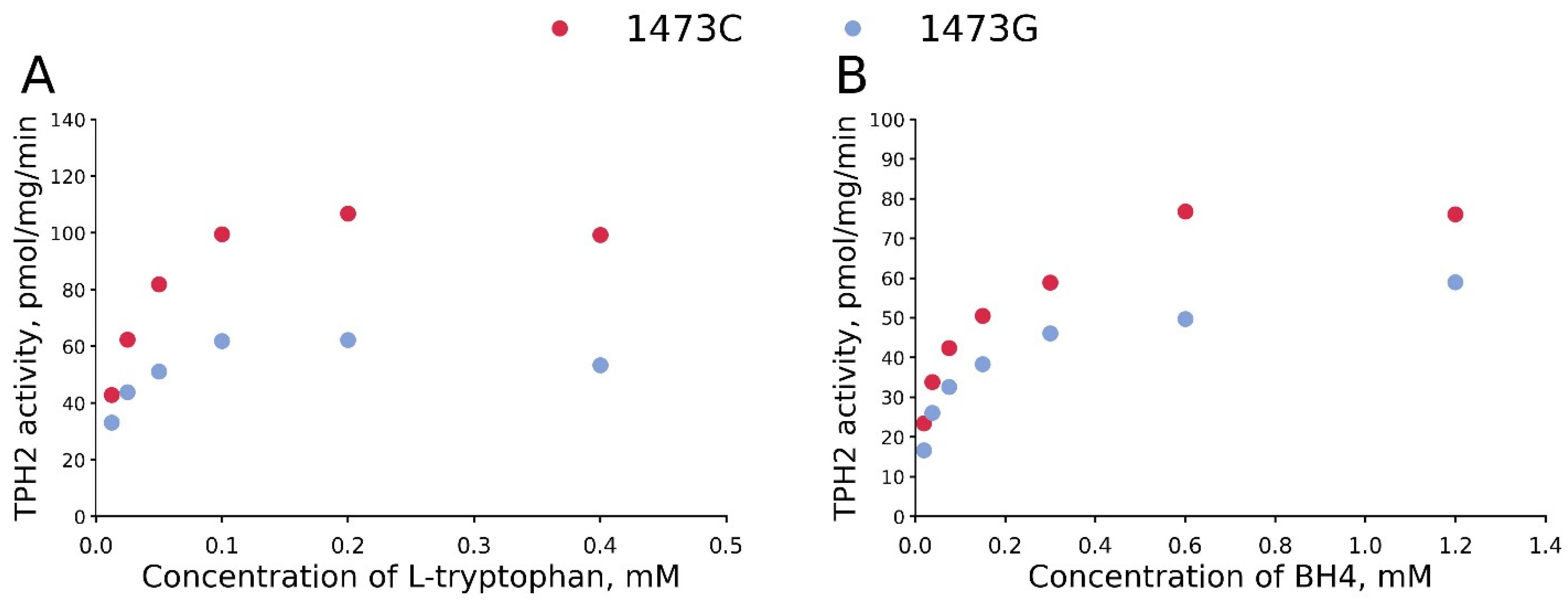
3.1. The KM and Vmax Values for BH4 and L-Tryptophan for TPH2 from the Midbrains of B6-1473C and B6-1473G Males
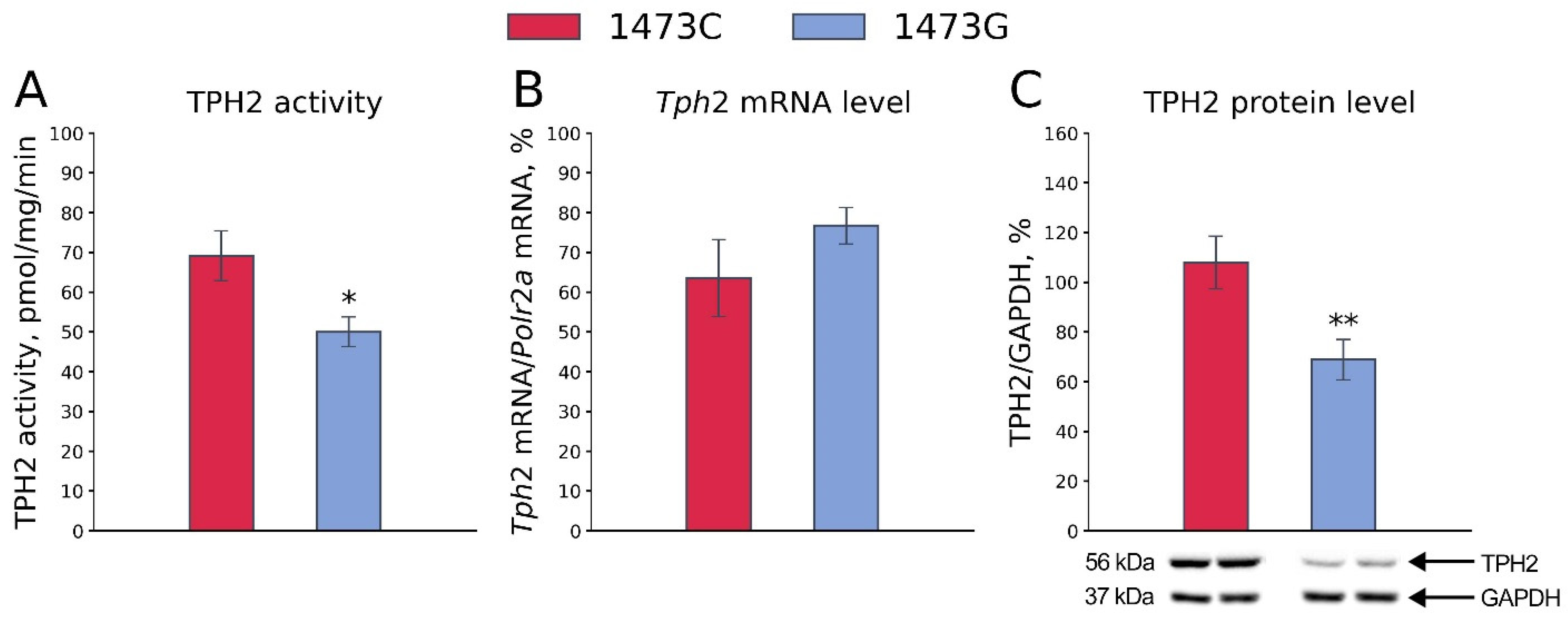
3.2. Tph2 Gene Expression and TPH2 Protein Density and Activity in the Midbrains of B6-1473C and B6-1473G Adult Males
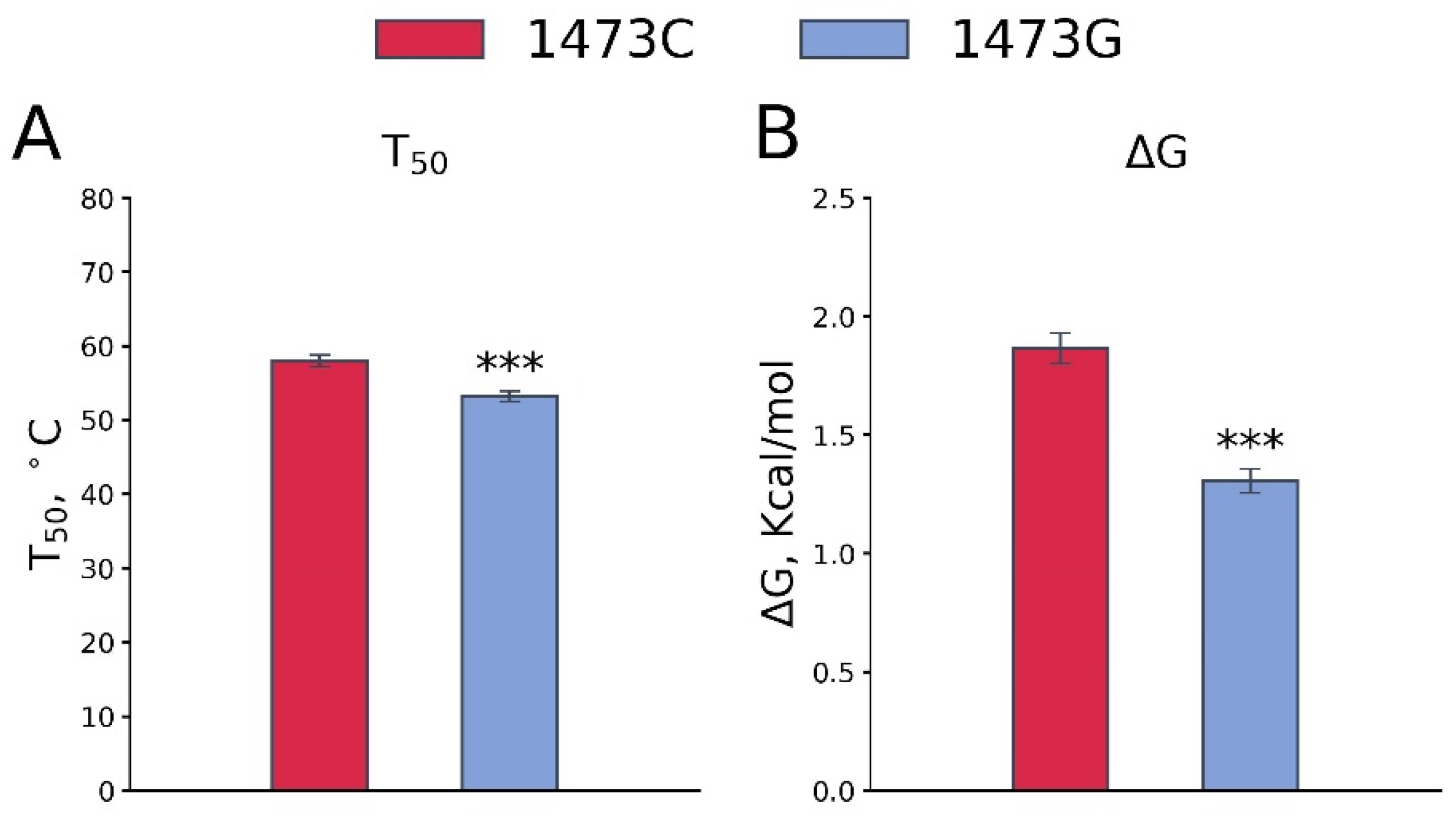
3.3. Effect of the C1473G Mutation on the TPH2 Molecule Thermal Stability In Vitro
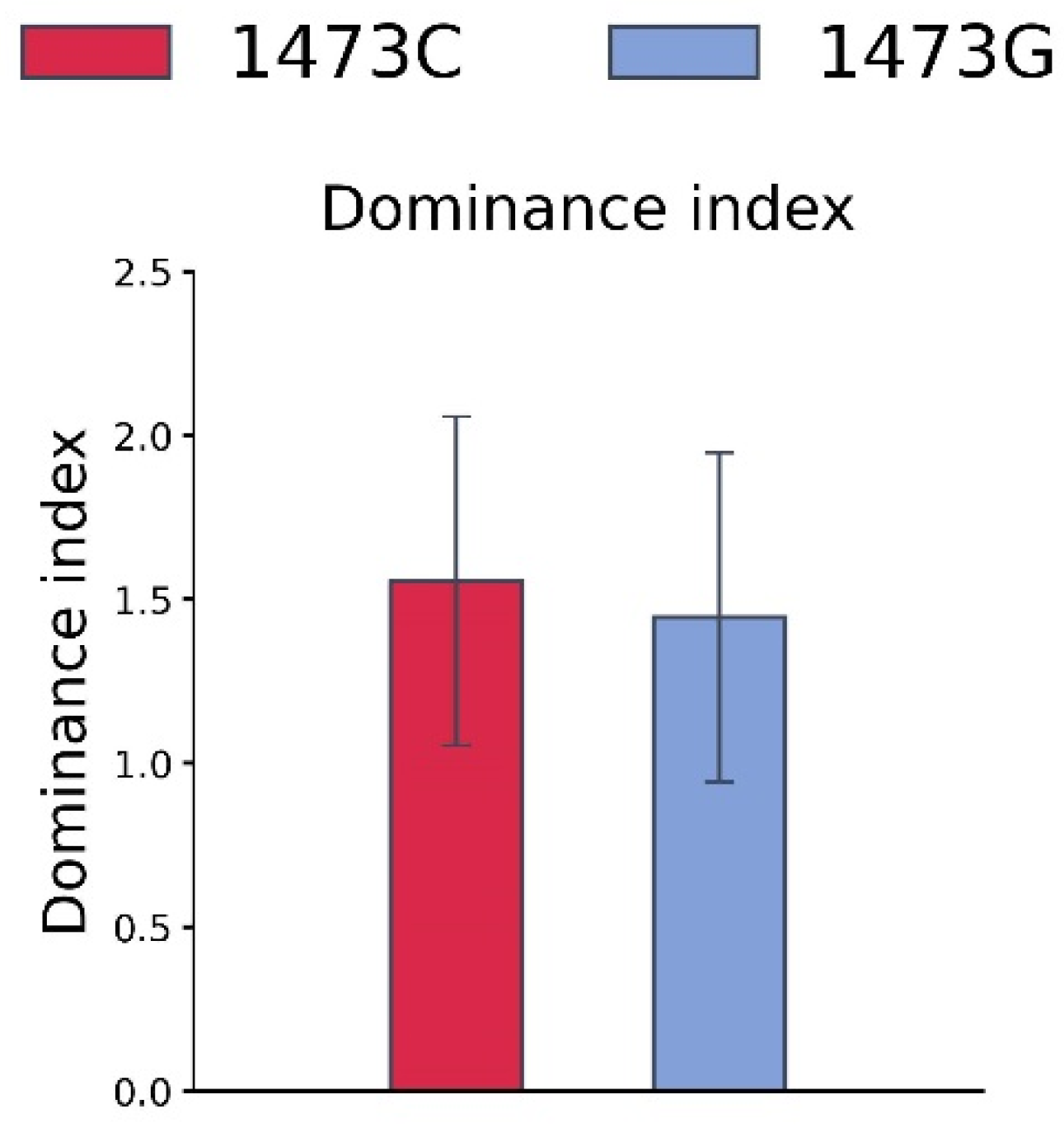
3.4. Tube Competition of B6-1473C and B6-1473G Adult Males
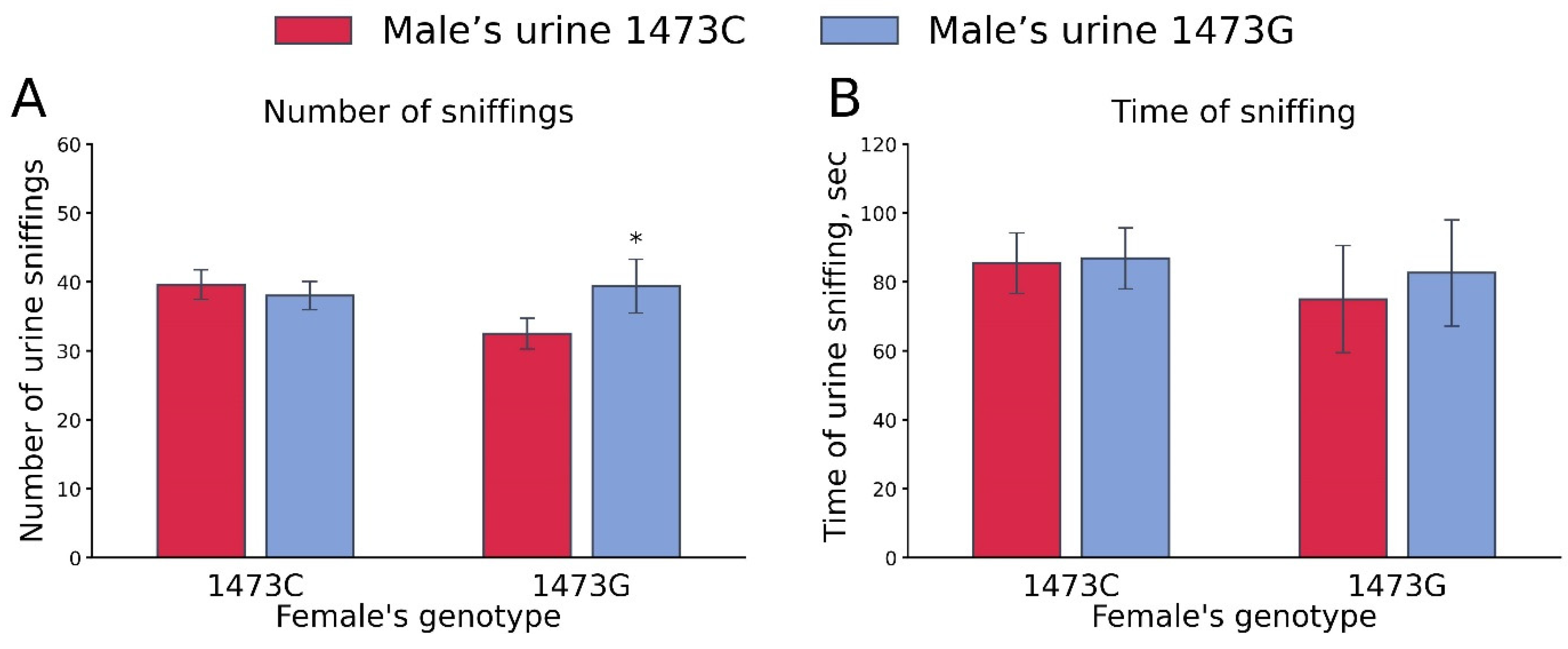
3.5. Olfactory Preference for B6-1473C and B6-1473G Adult Male Urine by Receptive B6-1473C and B6-1473G Adult Females
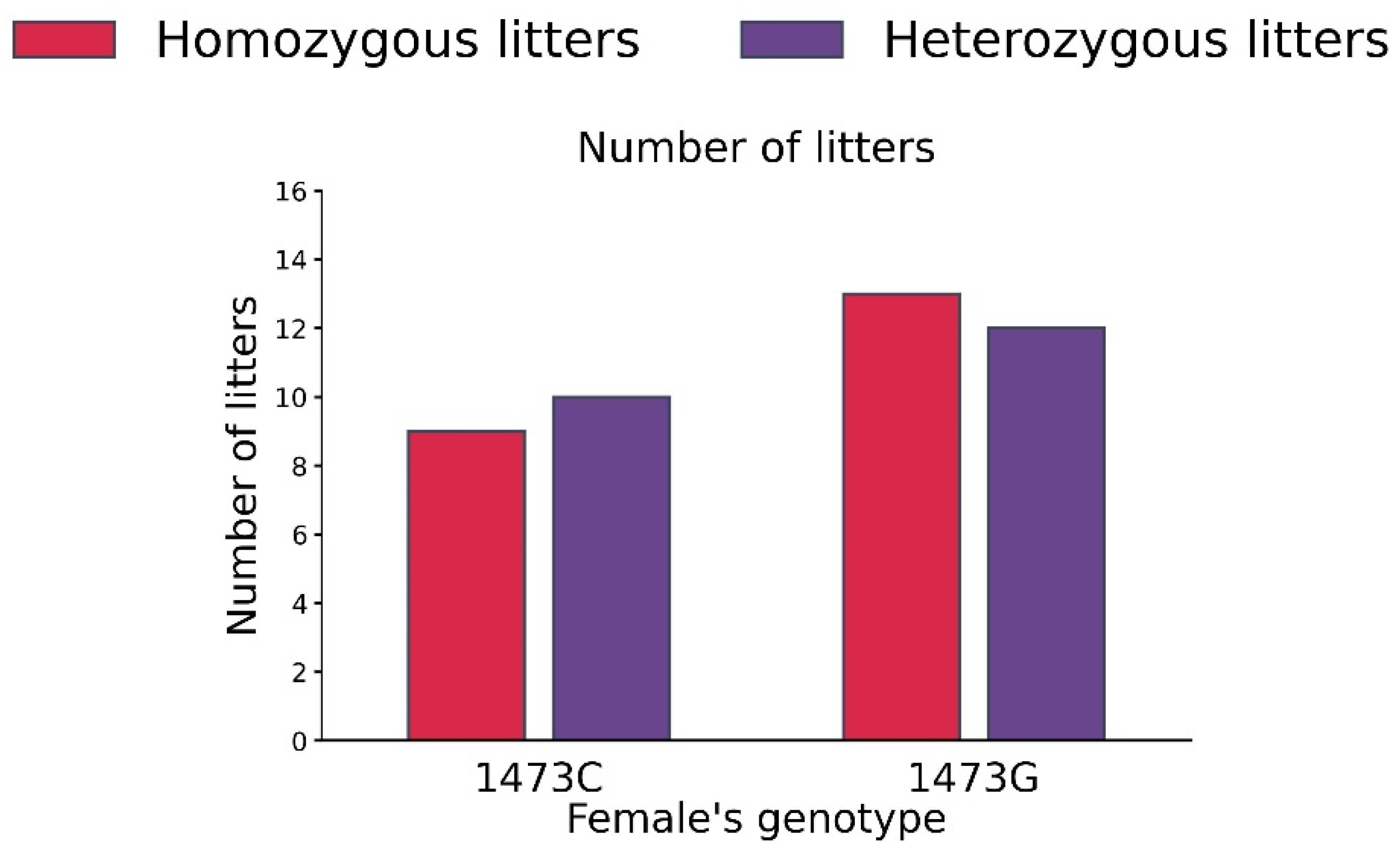
3.6. Distribution of Homo- and Heterozygote Litters in B6-1473C and B6-1473G Adult Females Put into Cage with a Pair of B6-1473C and B6-1473G Adult Males
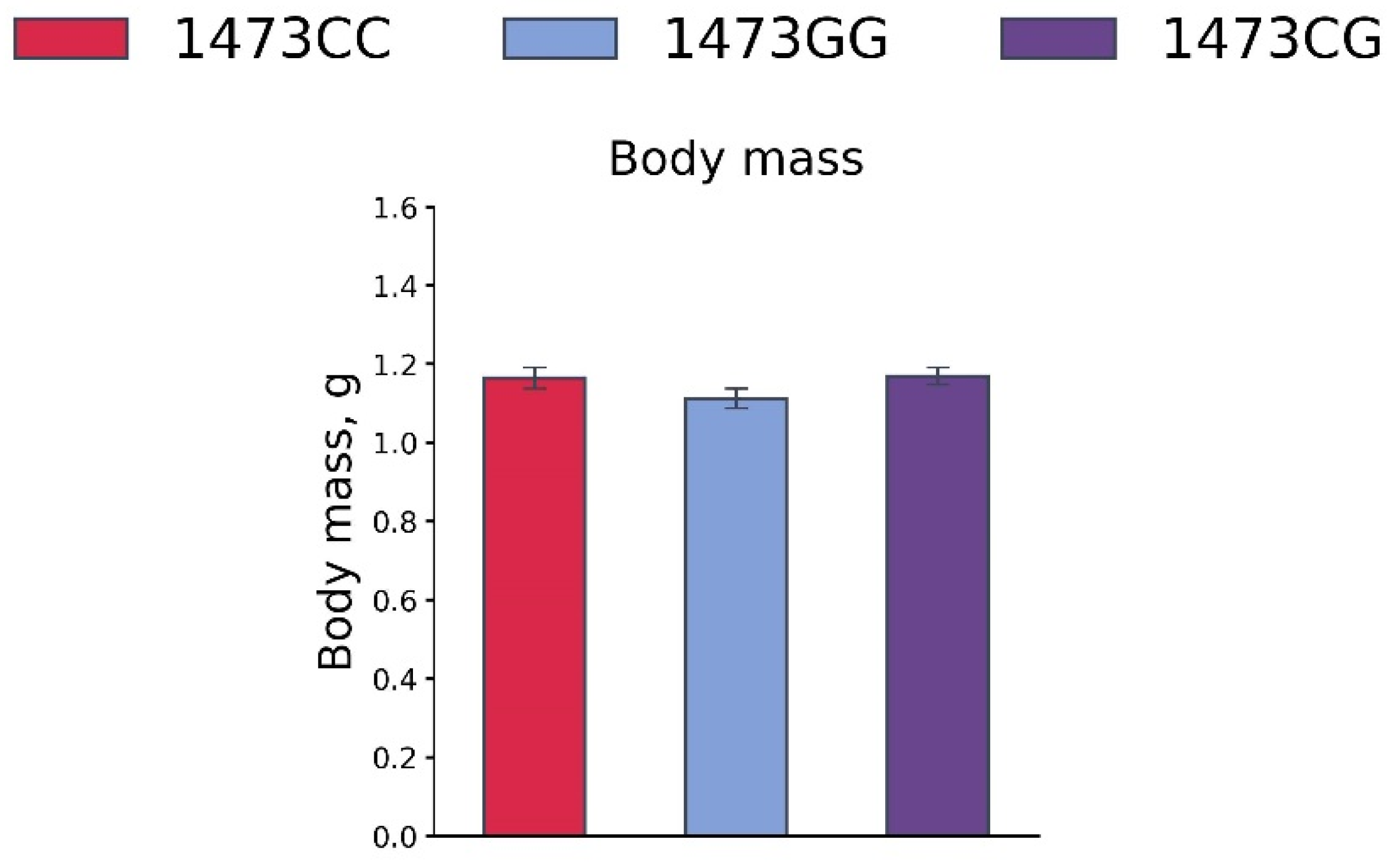
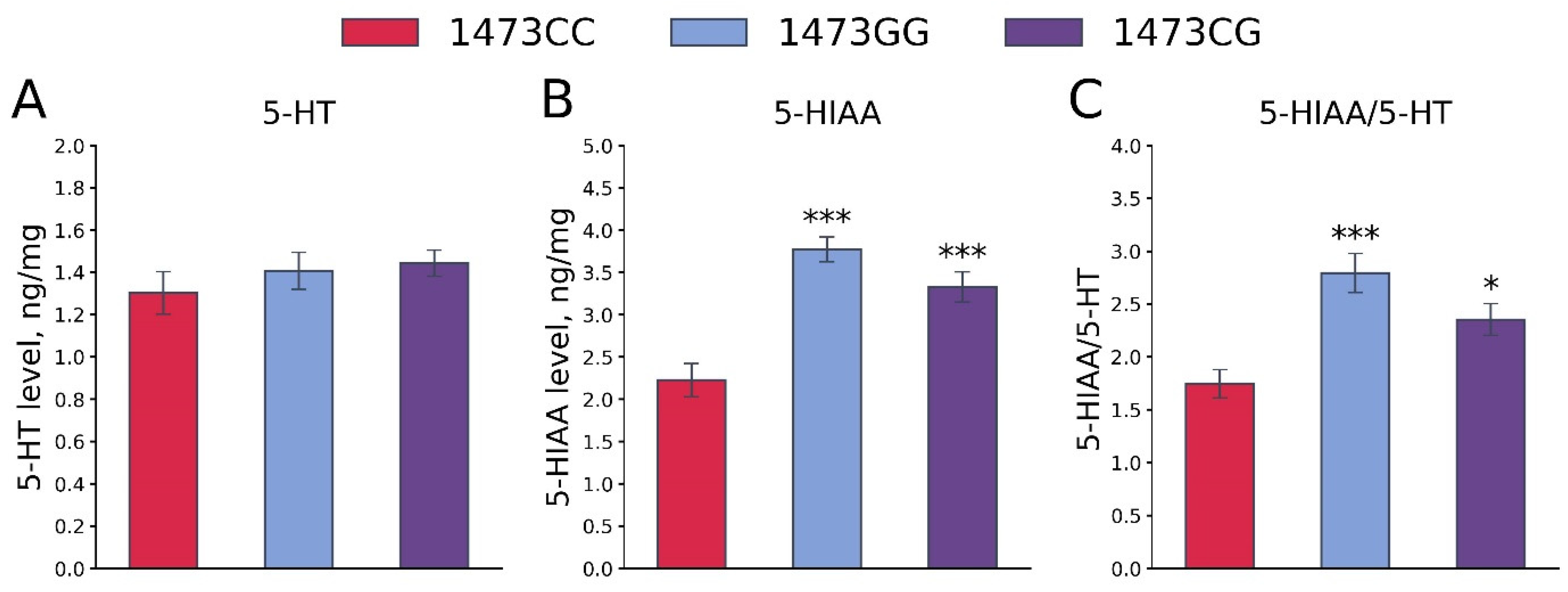
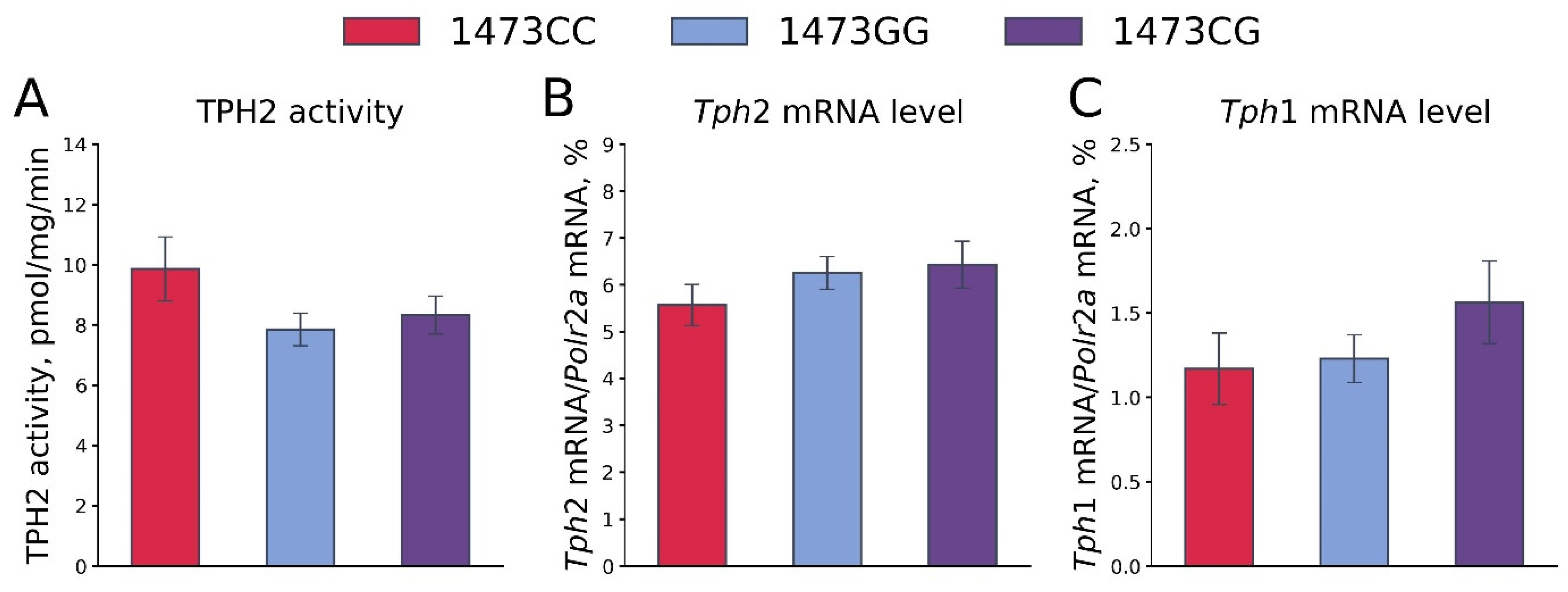
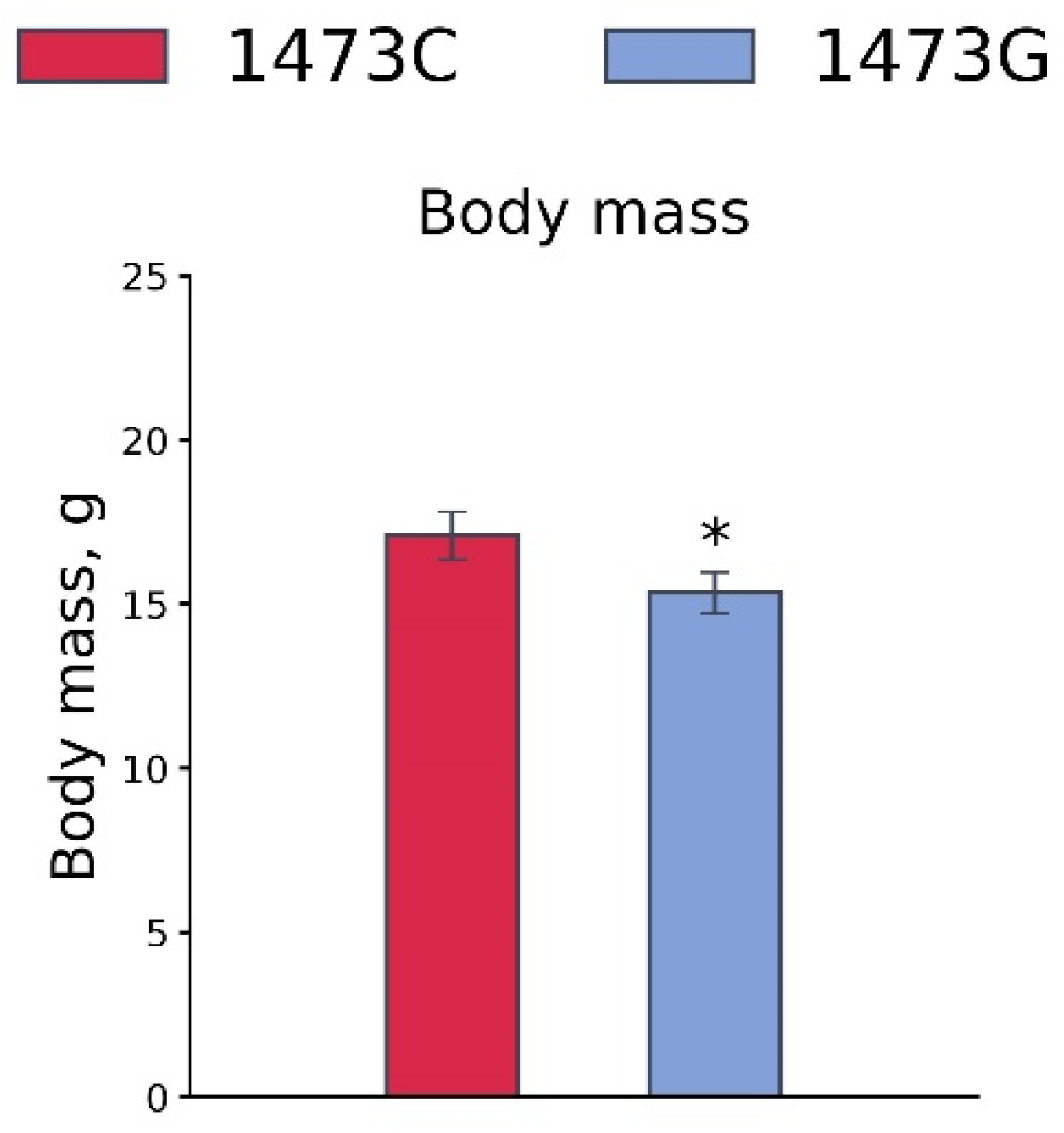
3.7. Body Masses, 5-HT and 5-HIAA Levels, TPH2 Activity, and Tph1 and Tph2 Gene mRNA Levels in the Brains of 19-Day-Old Embryos of the B6-1473CC, B6-1473CG and B6-1473GG Genotypes
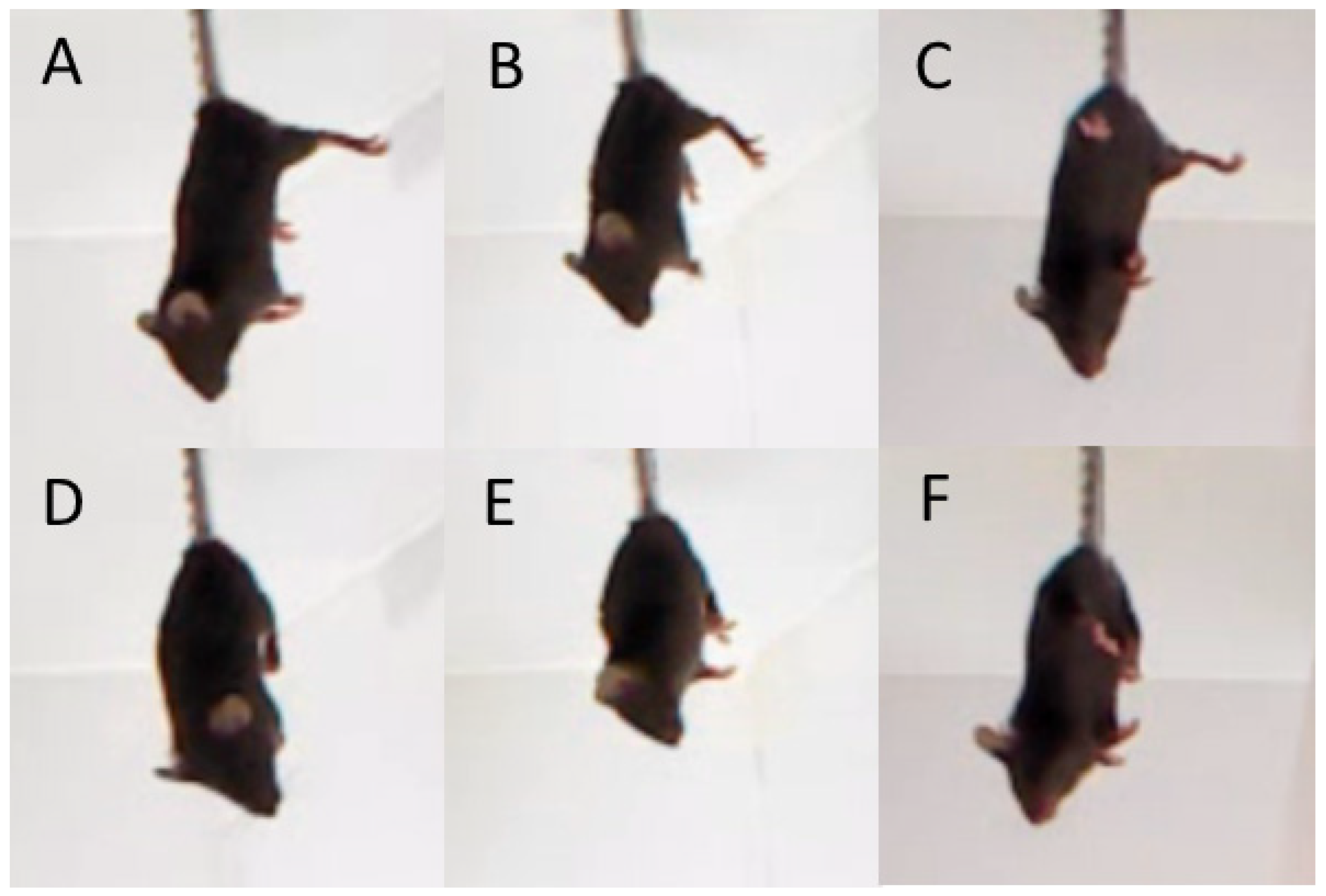
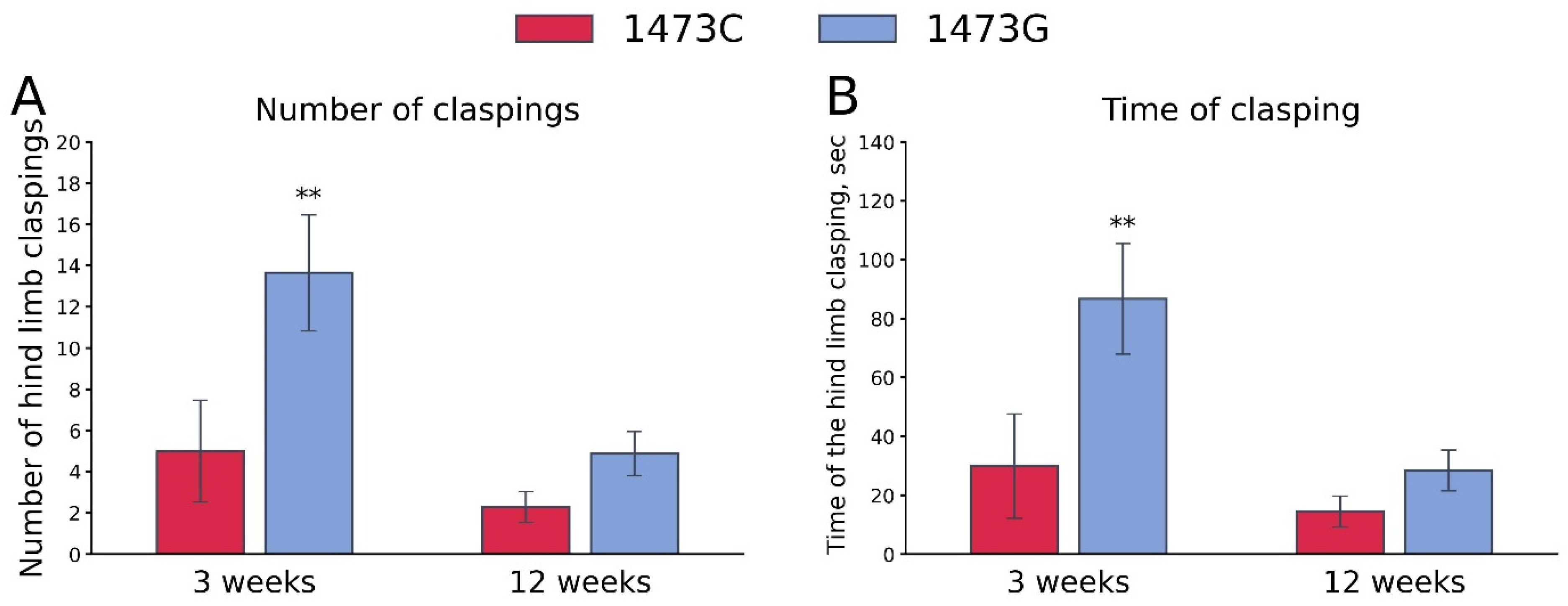
3.8. Hind Legs Clasping in Juvenile and Adult B6-1473CC and B6-1473GG Males
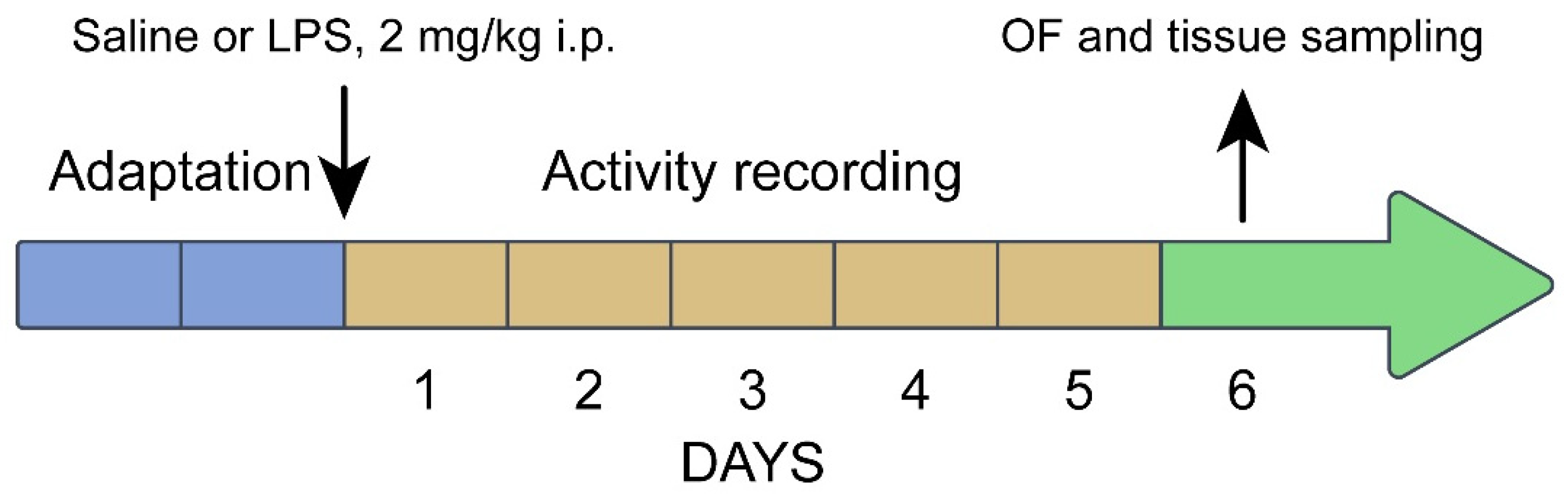
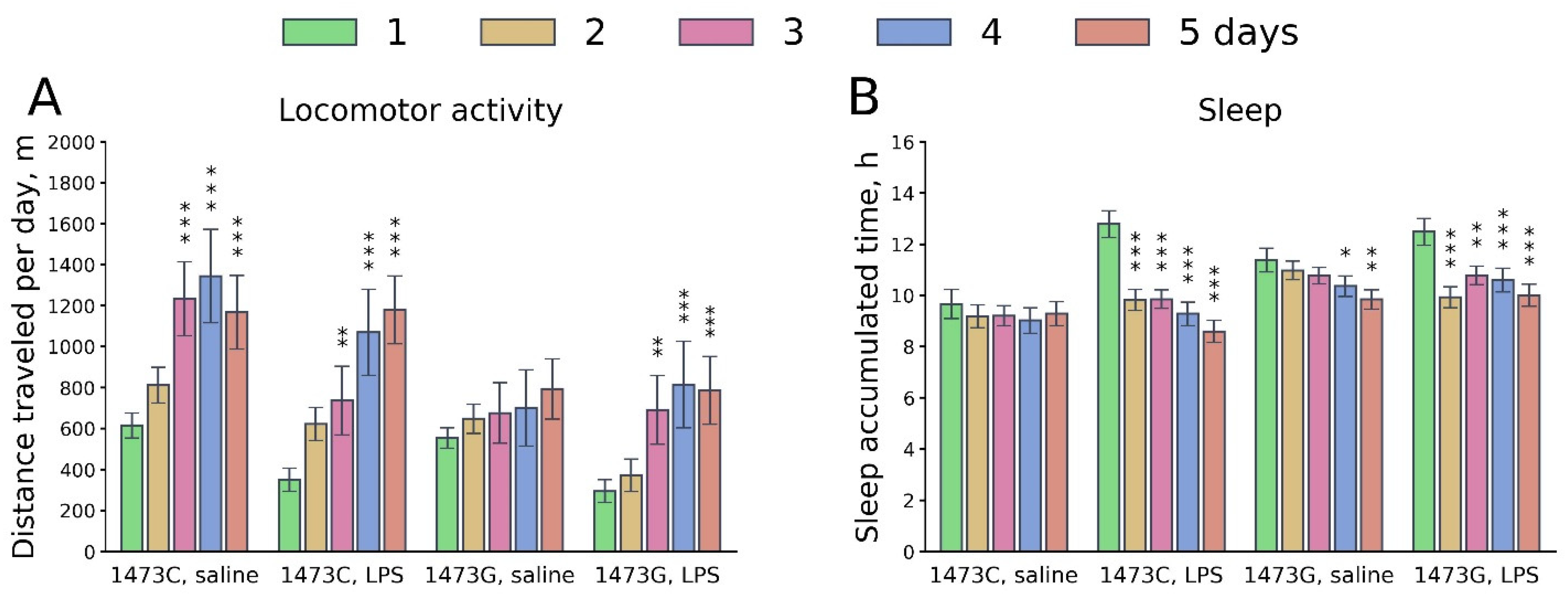
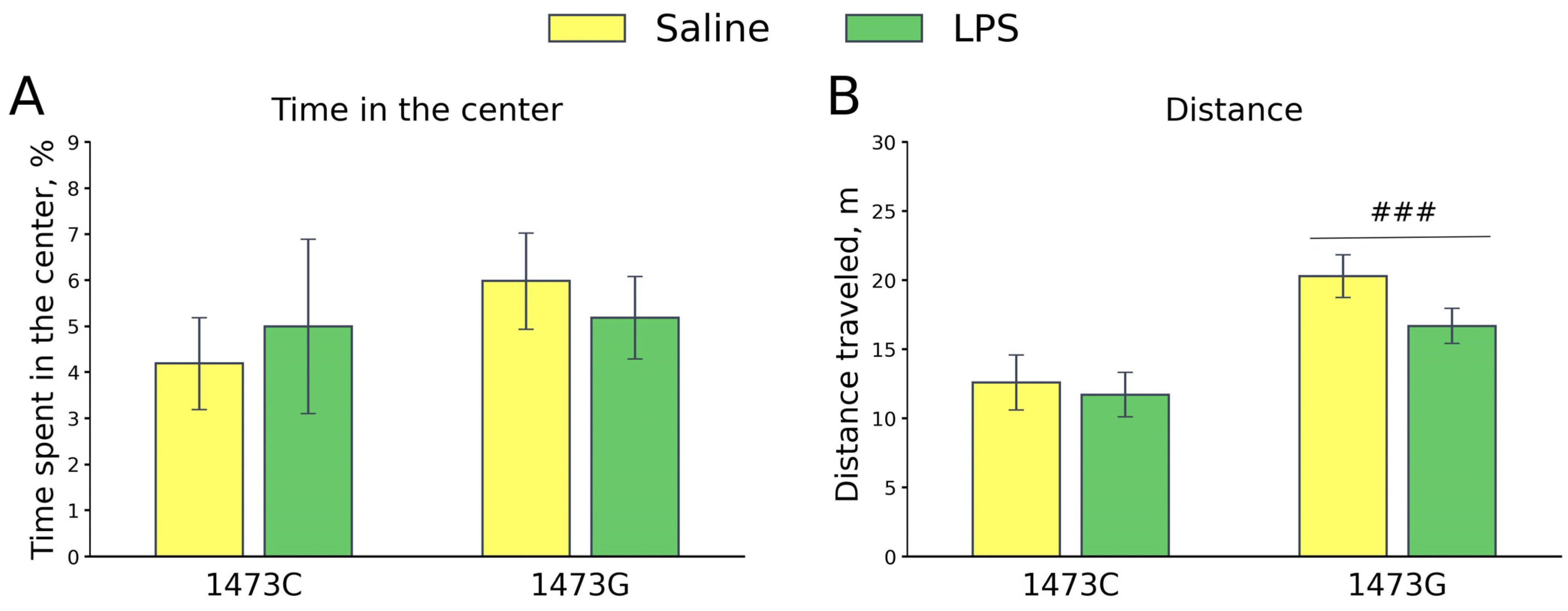
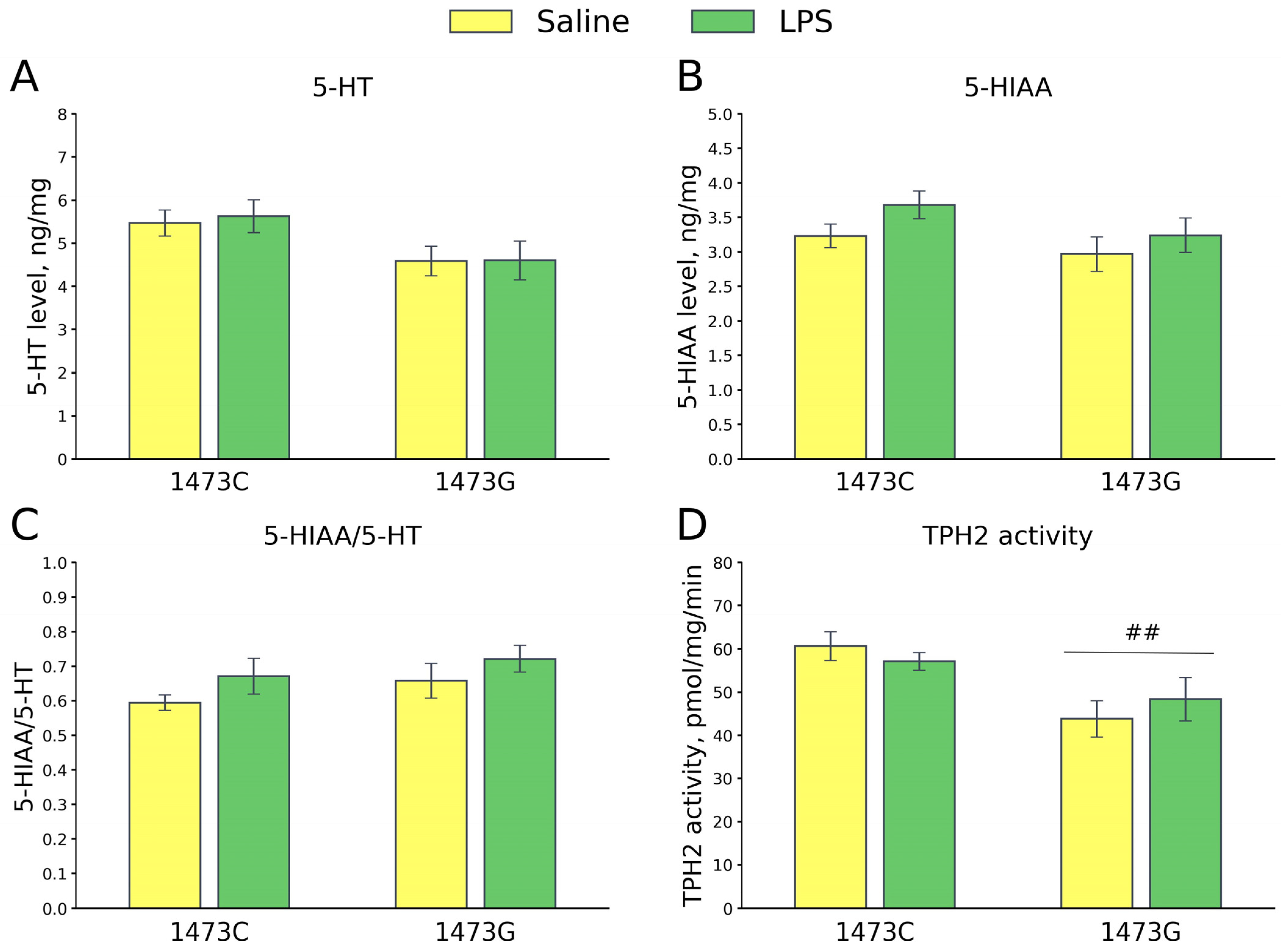
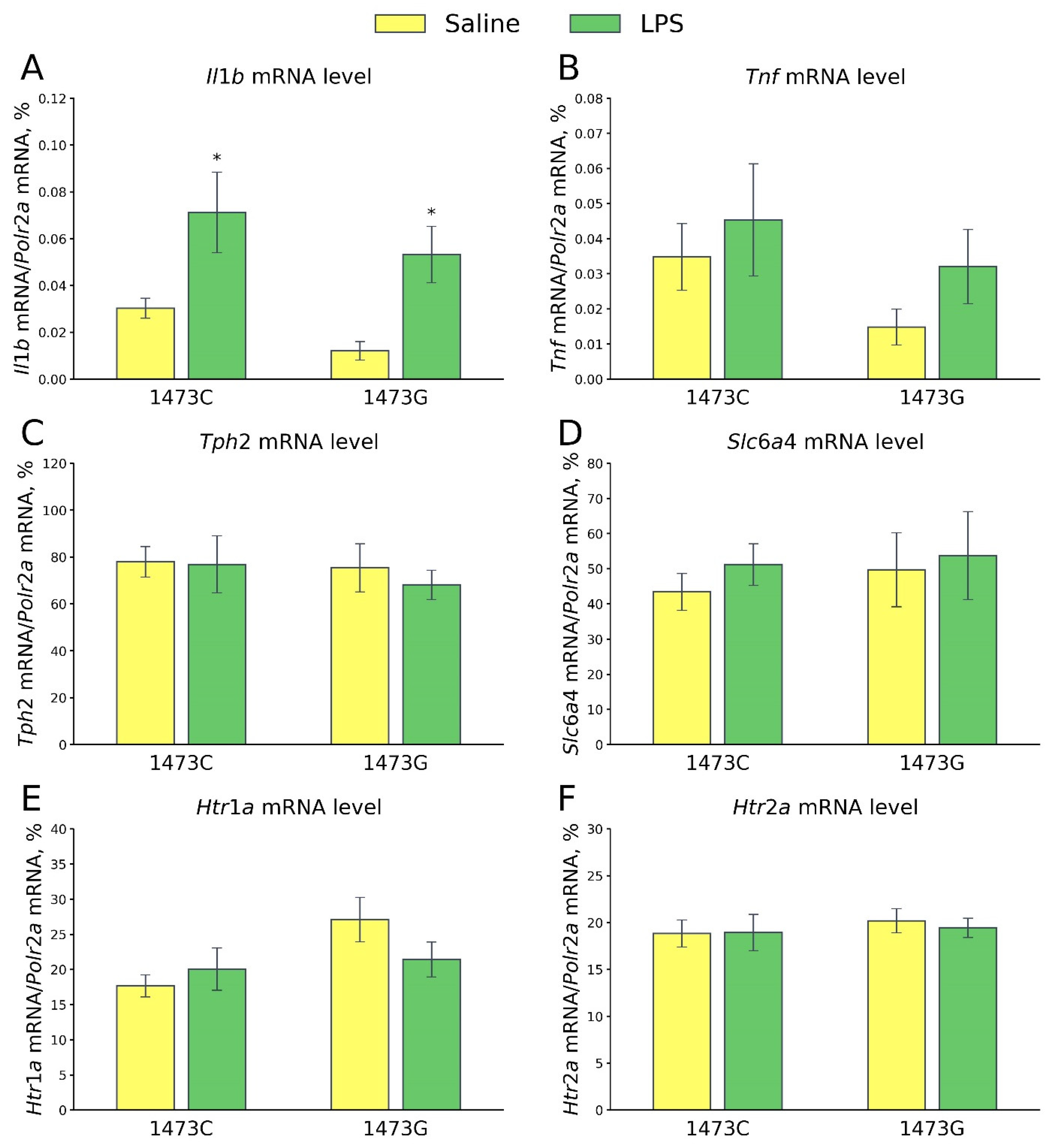
3.9. Prolonged Effects of LPS and Saline Administration on Survival, Home Cage Activity, Open Field Behavior and 5-HT System Characteristics in the Midbrains of Juvenile B6-1473C and B6-1473G Males
4. Discussion
4.1. Molecular Mechanism Decreasing the Mutant TPH2 Activity
4.2. Physiological and Behavioral Traits Altered by the C1473G Mutation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzpatrick, P.F. Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999, 68, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, P.F. The aromatic amino acid hydroxylases: Structures, catalysis, and regulation of phenylalanine hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase. Arch. Biochem. Biophys. 2023, 735, 109518. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.J.; Peter, J.U.; Bashammakh, S.; Hörtnagl, H.; Voits, M.; Fink, H.; Bader, M. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 2003, 299, 76. [Google Scholar] [CrossRef]
- Walther, D.J.; Bader, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, L.; Waider, J.; Kraft, S.; Kriegebaum, C.; Holtmann, B.; Reif, A.; Schmitt, A.; Lesch, K.P. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. J. Neural. Transm. 2008, 115, 1127–1132. [Google Scholar] [CrossRef]
- Savelieva, K.V.; Zhao, S.; Pogorelov, V.M.; Rajan, I.; Yang, Q.; Cullinan, E.; Lanthorn, T.H. Genetic disruption of both tryp-tophan hydroxylase genes dramatically reduces serotonin and affects behavior in models sensitive to antidepressants. PLoS ONE 2008, 3, e3301. [Google Scholar] [CrossRef]
- Alenina, N.; Kikic, D.; Todiras, M.; Mosienko, V.; Qadri, F.; Plehm, R.; Boyé, P.; Vilianovitch, L.; Sohr, R.; Tenner, K.; et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proc. Natl. Acad. Sci. USA 2009, 106, 10332–10337. [Google Scholar] [CrossRef]
- Cervo, L.; Canetta, A.; Calcagno, E.; Burbassi, S.; Sacchetti, G.; Caccia, S.; Fracasso, C.; Albani, D.; Forloni, G.; Invernizzi, R.W. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J. Neurosci. 2005, 25, 8165–8172. [Google Scholar] [CrossRef]
- Mehta, H.; Saravanan, K.S.; Mohanakumar, K.P. Serotonin synthesis inhibition in olivo-cerebellar system attenuates harmaline-induced tremor in Swiss albino mice. Behav. Brain Res. 2003, 145, 31–36. [Google Scholar] [CrossRef]
- Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.M.; Sotnikova, T.D.; Burch, L.H.; Williams, R.B.; Schwartz, D.A.; Krishnan, K.R.; Caron, M.G. Loss-of-function mutation in tryptophan hydroxylase-2 identified in unipolar major depression. Neuron 2005, 45, 11–16. [Google Scholar] [CrossRef]
- Popova, N.K.; Kulikov, A.V. Targeting tryptophan hydroxylase 2 in affective disorder. Expert Opin. Ther. Targets 2010, 14, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.B.; Miranda, D.M.; Souza, B.R.; Pereira, P.A.; Neves, F.S.; Tramontina, J.; Kapczinski, F.; Romano-Silva, M.A.; Correa, H. Association study of tryptophan hydroxylase 2 gene polymorphisms in bipolar disorder patients with panic disorder comorbidity. Psychiatr. Genet. 2011, 21, 106–111. [Google Scholar] [CrossRef]
- Ottenhof, K.W.; Sild, M.; Lévesque, M.L.; Ruhé, H.G.; Booij, L. TPH2 polymorphisms across the spectrum of psychiatric morbidity: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 29–42. [Google Scholar] [CrossRef]
- Zhang, X.; Beaulieu, J.M.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science 2004, 305, 217. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Popova, N.K. Genetic control of tryptophan hydroxylase activity in the mouse brain. Genetika. 1983, 19, 784–788. [Google Scholar]
- Kulikov, A.V.; Osipova, D.V.; Naumenko, V.S.; Popova, N.K. Association between Tph2 gene polymorphism, brain tryptophan hydroxylase activity and aggressiveness in mouse strains. Genes Brain Behav. 2005, 4, 482–485. [Google Scholar] [CrossRef]
- Osipova, D.V.; Kulikov, A.V.; Popova, N.K. C1473G polymorphism in mouse tph2 gene is linked to tryptophan hydroxylase-2 activity in the brain, intermale aggression, and depressive-like behavior in the forced swim test. J. Neurosci. Res. 2009, 87, 1168–1174. [Google Scholar] [CrossRef]
- Siesser, W.B.; Zhang, X.; Jacobsen, J.P.; Sotnikova, T.D.; Gainetdinov, R.R.; Caron, M.G. Tryptophan hydroxylase 2 genotype determines brain serotonin synthesis but not tissue content in C57Bl/6J and BALB/cJ congenic mice. Neurosci. Lett. 2010, 481, 6–11. [Google Scholar] [CrossRef]
- Koshimizu, H.; Hirata, N.; Takao, K.; Toyama, K.; Ichinose, T.; Furuya, S.; Miyakawa, T. Comprehensive behavioral analysis and quantification of brain free amino acids of C57BL/6J congenic mice carrying the 1473G allele in tryptophan hydroxylase-2. Neuropsychopharmacol. Rep. 2019, 39, 56–60. [Google Scholar] [CrossRef]
- Osipova, D.V.; Kulikov, A.V.; Mekada, K.; Yoshiki, A.; Moshkin, M.P.; Kotenkova, E.V.; Popova, N.K. Distribution of the C1473G polymorphism in tryptophan hydroxylase 2 gene in laboratory and wild mice. Genes Brain Behav. 2010, 9, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Khotskin, N.V.; Komleva, P.D.; Arefieva, A.B.; Bazovkina, D.V.; Kulikov, A.V. The C1473G mutation in the mouse Tph2 gene: From molecular mechanism to biological consequences. In Proceedings of the 14th International Conference on Bioinformatics of Genome Regulation and Structure/Systems Biology (BGRS/SB-2024), Novosibirsk, Russia, 5–10 August 2024; pp. 1240–1243. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, H.; Zhou, T.; Wang, S.; Wu, Y.; Hu, H. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 2019, 14, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sumi-Ichinose, C.; Kaji, R.; Ikemoto, K.; Nomura, T.; Nagatsu, I.; Ichinose, H.; Ito, M.; Sako, W.; Nagahiro, S.; et al. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. Proc. Natl. Acad. Sci. USA 2008, 105, 12551–12556. [Google Scholar] [CrossRef] [PubMed]
- Komleva, P.D.; Alhalabi, G.; Izyurov, A.E.; Khotskin, N.V.; Kulikov, A.V. Effects of the Combination of the C1473G Mutation in the Tph2 Gene and Lethal Yellow Mutations in the Raly-Agouti Locus on Behavior, Brain 5-HT and Melanocortin Systems in Mice. Biomolecules 2023, 13, 963. [Google Scholar] [CrossRef]
- Pack, A.I.; Galante, R.J.; Maislin, G.; Cater, J.; Metaxas, D.; Lu, S.; Zhang, L.; Von Smith, R.; Kay, T.; Lian, J.; et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol. Genomics 2007, 28, 232–238. [Google Scholar] [CrossRef]
- Fisher, S.P.; Godinho, S.I.; Pothecary, C.A.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Rapid assessment of sleep-wake behavior in mice. J. Biol. Rhythms 2012, 27, 48–58. [Google Scholar] [CrossRef]
- Bains, R.S.; Wells, S.; Sillito, R.R.; Armstrong, J.D.; Cater, H.L.; Banks, G.; Nolan, P.M. Assessing mouse behaviour throughout the light/dark cycle using automated incage analysis tools. J. Neurosci. Methods 2017, 300, 37–47. [Google Scholar] [CrossRef]
- Arefieva, A.B.; Komleva, P.D.; Naumenko, V.S.; Khotskin, N.V.; Kulikov, A.V. In Vitro and In Vivo Chaperone Effect of (R)-2-amino-6-(1R,2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin-4(3H)-one on the C1473G Mutant Tryptophan Hydroxylase 2. Biomolecules 2023, 13, 1458. [Google Scholar] [CrossRef]
- Moskaliuk, V.S.; Kozhemyakina, R.V.; Khomenko, T.M.; Volcho, K.P.; Salakhutdinov, N.F.; Kulikov, A.V.; Naumenko, V.S.; Kulikova, E.A. Key Enzymes of the Serotonergic System Tryptophan Hydroxylase 2 and Monoamine Oxidase A—In the Brain of Rats Selectively Bred for a Reaction toward Humans: Effects of Benzopentathiepin TC-2153. Biochemistry 2024, 89, 1109–1121. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. Principles of Enzyme Kinetics; Butterworth & Co.: London, UK, 1976; 281p. [Google Scholar]
- Jackson, M.B. Molecular and Cellular Biophysics; Cambridge University Press: Cambridge, UK, 2006; 552p. [Google Scholar]
- Sakowski, S.A.; Geddes, T.J.; Kuhn, D.M. Mouse tryptophan hydroxylase isoform 2 and the role of proline 447 in enzyme function. J Neurochem. 2006, 96, 758–765. [Google Scholar] [CrossRef]
- Lowry, C.A.; Hale, M.W.; Evans, A.K.; Heerkens, J.; Staub, D.R.; Gasser, P.J.; Shekhar, A. Serotonergic systems, anxiety, and affective disorder: Focus on the dorsomedial part of the dorsal raphe nucleus. Ann. N. Y. Acad. Sci. 2008, 1148, 86–94. [Google Scholar] [CrossRef]
- Alenina, N.; Bashammakh, S.; Bader, M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006, 2, 5–10. [Google Scholar] [CrossRef]
- Bazhenova, E.Y.; Sinyakova, N.A.; Kulikova, E.A.; Kazarinova, I.A.; Bazovkina, D.V.; Gainetdinov, R.R.; Kulikov, A.V. No effect of C1473G polymorphism in the tryptophan hydroxylase 2 gene on the response of the brain serotonin system to chronic fluoxetine treatment in mice. Neurosci. Lett. 2017, 653, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Bazhenova, E.Y.; Fursenko, D.V.; Kulikova, E.A.; Khotskin, N.V.; Sinyakova, N.A.; Kulikov, A.A. Effect of photoperiodic alterations on depression-like behavior and the brain serotonin system in mice genetically different in tryptophan hydroxylase 2 activity. Neurosci. Lett. 2019, 699, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pey, A.L.; Ying, M.; Cremades, N.; Velazquez-Campoy, A.; Scherer, T.; Thöny, B.; Sancho, J.; Martinez, A. Identification of pharmacological chaperones as potential therapeutic agents to treat phenylketonuria. J. Clin. Investig. 2008, 118, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.C.; Scherer, T.; Pey, A.L.; Ying, M.; Winge, I.; McKinney, J.; Haavik, J.; Thöny, B.; Martinez, A. Effect of pharmaco-logical chaperones on brain tyrosine hydroxylase and tryptophan hydroxylase. J. Neurochem. 2010, 114, 853–863. [Google Scholar] [CrossRef]
- Waløen, K.; Kleppe, R.; Martinez, A.; Haavik, J. Tyrosine and tryptophan hydroxylases as therapeutic targets in human disease. Expert Opin. Ther. Targets 2017, 21, 167–180. [Google Scholar] [CrossRef]
- Hole, M.; Jorge-Finnigan, A.; Underhaug, J.; Teigen, K.; Martinez, A. Pharmacological Chaperones that Protect Tetrahydrobiopterin Dependent Aromatic Amino Acid Hydroxylases Through Different Mechanisms. Curr. Drug Targets 2016, 17, 1515–1526. [Google Scholar] [CrossRef]
- Santos-Sierra, S.; Kirchmair, J.; Perna, A.M.; Reiss, D.; Kemter, K.; Röschinger, W.; Glossmann, H.; Gersting, S.W.; Muntau, A.C.; Wolber, G.; et al. Novel pharmacological chaperones that correct phenylketonuria in mice. Hum. Mol. Genet. 2012, 21, 1877–1887. [Google Scholar] [CrossRef]
- Sakowski, S.A.; Geddes, T.J.; Thomas, D.M.; Levi, E.; Hatfield, J.S.; Kuhn, D.M. Differential tissue distribution of tryptophan hydroxylase isoforms 1 and 2 as revealed with monospecific antibodies. Brain Res. 2006, 1085, 11–18. [Google Scholar] [CrossRef]
- Patel, P.D.; Pontrello, C.; Burke, S. Robust and tissue-specific expression of TPH2 versus TPH1 in rat raphe and pineal gland. Biol. Psychiatry 2004, 55, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Bonthuis, P.J.; Huang, W.C.; Stacher Hörndli, C.N.; Ferris, E.; Cheng, T.; Gregg, C. Noncanonical Genomic Imprinting Effects in Offspring. Cell Rep. 2015, 12, 979–991. [Google Scholar] [CrossRef]
- Hart, B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988, 12, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine-induced sickness behavior: Mechanisms and implications. Ann. N. Y. Acad. Sci. 2001, 933, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J. Effects of cytokines and infections on brain neurochemistry. Clin. Neurosci. Res. 2006, 6, 52–68. [Google Scholar] [CrossRef]
- Komleva, P.D.; Bazhenova, E.Y.; Khotskin, N.V.; Kulikova, E.A.; Kulikov, A.V. C1473G Polymorphism in Tph2 Gene Affects Activation of Serotonin Metabolism in Mouse Brain 24 h after LPS Administration. Bull. Exp. Biol. Med. 2022, 174, 199–204. [Google Scholar] [CrossRef]


| Gene | Sequence | Annealing Temperature, °C | Amplicon Size, bp |
|---|---|---|---|
| Positive control | 5′-TTTGACCCAAAGACGACCTGCTTGCA-3′ 5′-TGCATGCTTACTAGCCAACCATGAGACA-3′ | 62 | 523 |
| 1473C specific | 5′-CAGAATTTCAATGCTCTGCGTGTGGG-3′ | 62 | 307 |
| 1473G specific | 5′-CAGAATTTCAATGCTXTGCGTGTGGC-3′ | 62 | 307 |
| Polr2a | 5′-TGTGACAACTCCATACAATGC-3′ 5′-CTCTCTTACTGAATTTGCGTACT-3’ | 60 | 194 |
| Htr1a | 5′-GACTGCCACCCTCTGCCCTATATC-3′ 5′-TCAGCAAGGCAAACAATTCCAG-3′ | 62 | 200 |
| Htr2a | 5′-AGAAGCCACCTTGTGTGTGA-3′ 5′-TTGCTCATTGCTGATGGACT-3 | 61 | 169 |
| Tph2 | 5′-CATTCCTCGCACAATTCCAGTCG-3′ 5′-AGTCTACATCCATCCCAACTGCTG-3′ | 62 | 239 |
| Tph1 | 5′-ACAAACTCTACCCGACCCAC-3′ 5′- CAGTTGCGGGATGTTGTCTT-3′ | 63 | 197 |
| Slc6a4 | 5′-CGCTCTACTACCTCATCTCCTCC-3′ 5′-GTCCTGGGCGAAGTAGTTGG-3′ | 63 | 101 |
| Il1b | 5′-GATCCCAAGCAATACCCAAA-3′ 5′-TAGAAACAGTCCAGCCCATAC-3′ | 61 | 226 |
| Tnf | 5′-AGCCGATGGGTTGTACCTTG-3′ 5′-GGTTGACTTTCTCCTGGTATGAGA-3′ | 59 | 211 |
| Trait | 1473C | 1473G | p |
|---|---|---|---|
| L-tryptophan | |||
| Vmax | 113.9 ± 2.6 pmol/mg/min | 63.0 ± 2.2 pmol/mg/min | t10 = 21.1, p < 0.001 |
| KM | 20.3 ± 1.4 μM | 11.0 ± 1.6 μM | t10 = 6.4, p < 0.001 |
| BH4 | |||
| Vmax | 74.6 ± 3.0 pmol/mg/min | 54.7 ± 2.1 pmol/mg/min | t12 = 3.86, p = 0.002 |
| KM | 45.1 ± 4.6 μM | 43.5 ± 4.3 μM | t12 = 0.35, p = 0.73 |
| Trait | “Genotype” | “Age” | Interaction |
|---|---|---|---|
| Clasping frequency | F1,85 = 7.2, p = 0.009 | F1,85 = 6.9, p = 0.01 | F1,85 = 2.0, p = 0.16 |
| Clasping duration | F1,85 = 6.5, p = 0.013 | F1,85 = 5.9, p = 0.017 | F1,85 = 2.2, p = 0.14 |
| Distance Travelled | Sleep Duration | |
|---|---|---|
| “Genotype” | F1,25 = 6.03, p = 0.022 | F1,25 = 13.54, p = 0.001 |
| “Treatment” | F1,25 = 2.02, p = 0.17 | F1,25 = 2.47, p = 0.13 |
| “Genotype” × “Treatment” | F1,25 = 0.49, p = 0.49 | F1,25 = 1.52, p = 0.23 |
| “Day” | F4,100 = 20.4, p < 0.001 | F4,100 = 18.9, p < 0.001 |
| “Day” × “Genotype” | F4,100 = 2.2, p = 0.07 | F4,100 = 0.43, p = 0.78 |
| “Day” × “Treatment” | F4,100 = 1.28, p = 0.28 | F4,100 = 6.6, p < 0.001 |
| “Day” × “Genotype” × “Treatment” | F4,100 = 1.7, p = 0.16 | F4,100 = 2.46, p = 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khotskin, N.V.; Komleva, P.D.; Arefieva, A.B.; Moskaliuk, V.S.; Khotskina, A.; Alhalabi, G.; Izyurov, A.E.; Sinyakova, N.A.; Sherbakov, D.; Kulikova, E.A.; et al. The C1473G Mutation in the Mouse Tph2 Gene: From Molecular Mechanism to Biological Consequences. Biomolecules 2025, 15, 461. https://doi.org/10.3390/biom15040461
Khotskin NV, Komleva PD, Arefieva AB, Moskaliuk VS, Khotskina A, Alhalabi G, Izyurov AE, Sinyakova NA, Sherbakov D, Kulikova EA, et al. The C1473G Mutation in the Mouse Tph2 Gene: From Molecular Mechanism to Biological Consequences. Biomolecules. 2025; 15(4):461. https://doi.org/10.3390/biom15040461
Chicago/Turabian StyleKhotskin, Nikita V., Polina D. Komleva, Alla B. Arefieva, Vitalii S. Moskaliuk, Anna Khotskina, Ghofran Alhalabi, Arseniy E. Izyurov, Nadezhda A. Sinyakova, Daniil Sherbakov, Elizabeth A. Kulikova, and et al. 2025. "The C1473G Mutation in the Mouse Tph2 Gene: From Molecular Mechanism to Biological Consequences" Biomolecules 15, no. 4: 461. https://doi.org/10.3390/biom15040461
APA StyleKhotskin, N. V., Komleva, P. D., Arefieva, A. B., Moskaliuk, V. S., Khotskina, A., Alhalabi, G., Izyurov, A. E., Sinyakova, N. A., Sherbakov, D., Kulikova, E. A., Bazovkina, D. V., & Kulikov, A. V. (2025). The C1473G Mutation in the Mouse Tph2 Gene: From Molecular Mechanism to Biological Consequences. Biomolecules, 15(4), 461. https://doi.org/10.3390/biom15040461