The Role of Connections Between Cellular and Tissue Mechanical Elements and the Importance of Applied Energy in Mechanotransduction in Cancerous Tissue
Abstract
1. Introduction
2. Types of Forces Acting on Cells and Tissues That Alter Cell and Tissue Energy Storage, Transmission, and Dissipation
3. Tensile Forces: How Do They Affect Cells and Tissues?
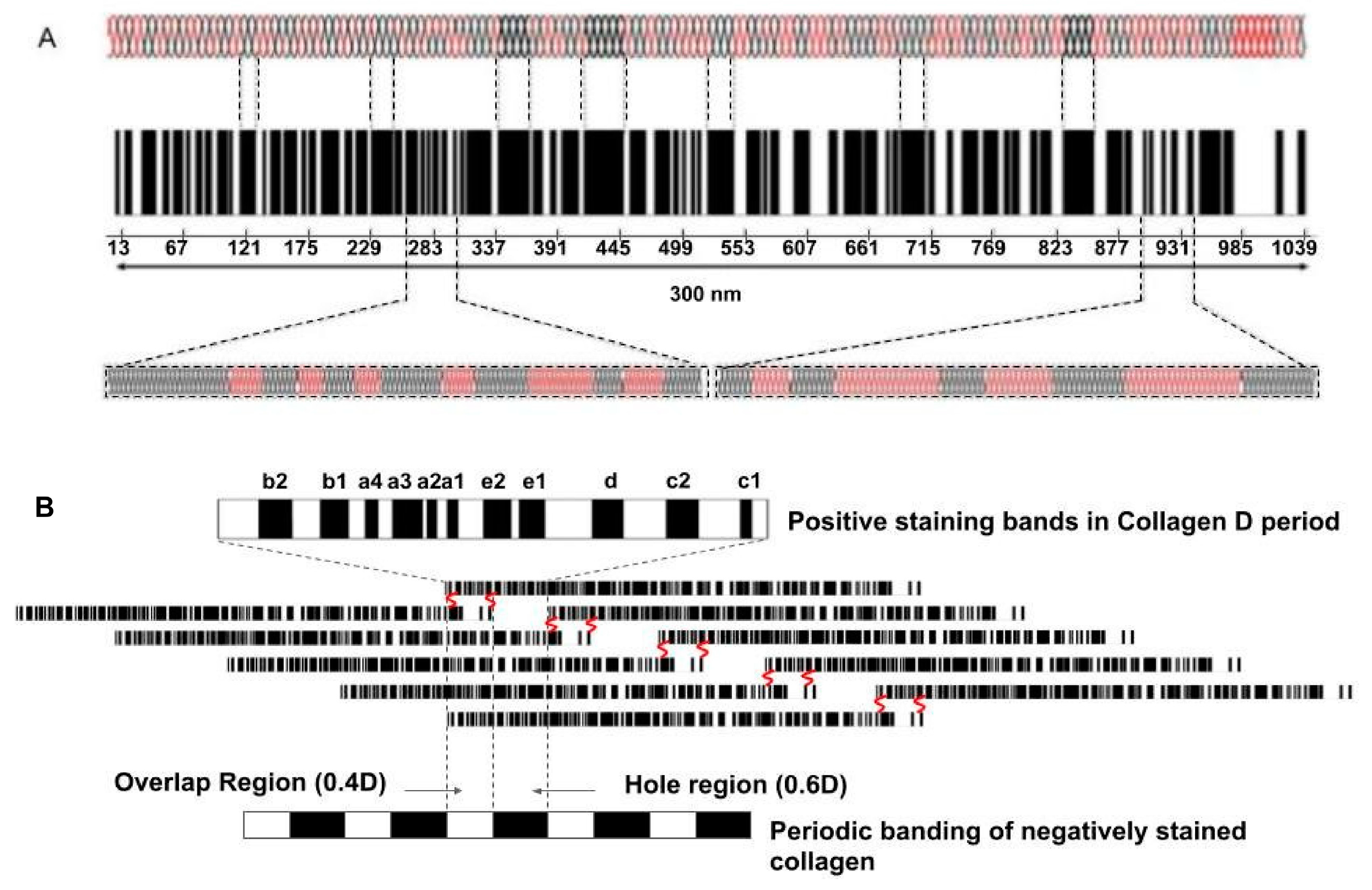
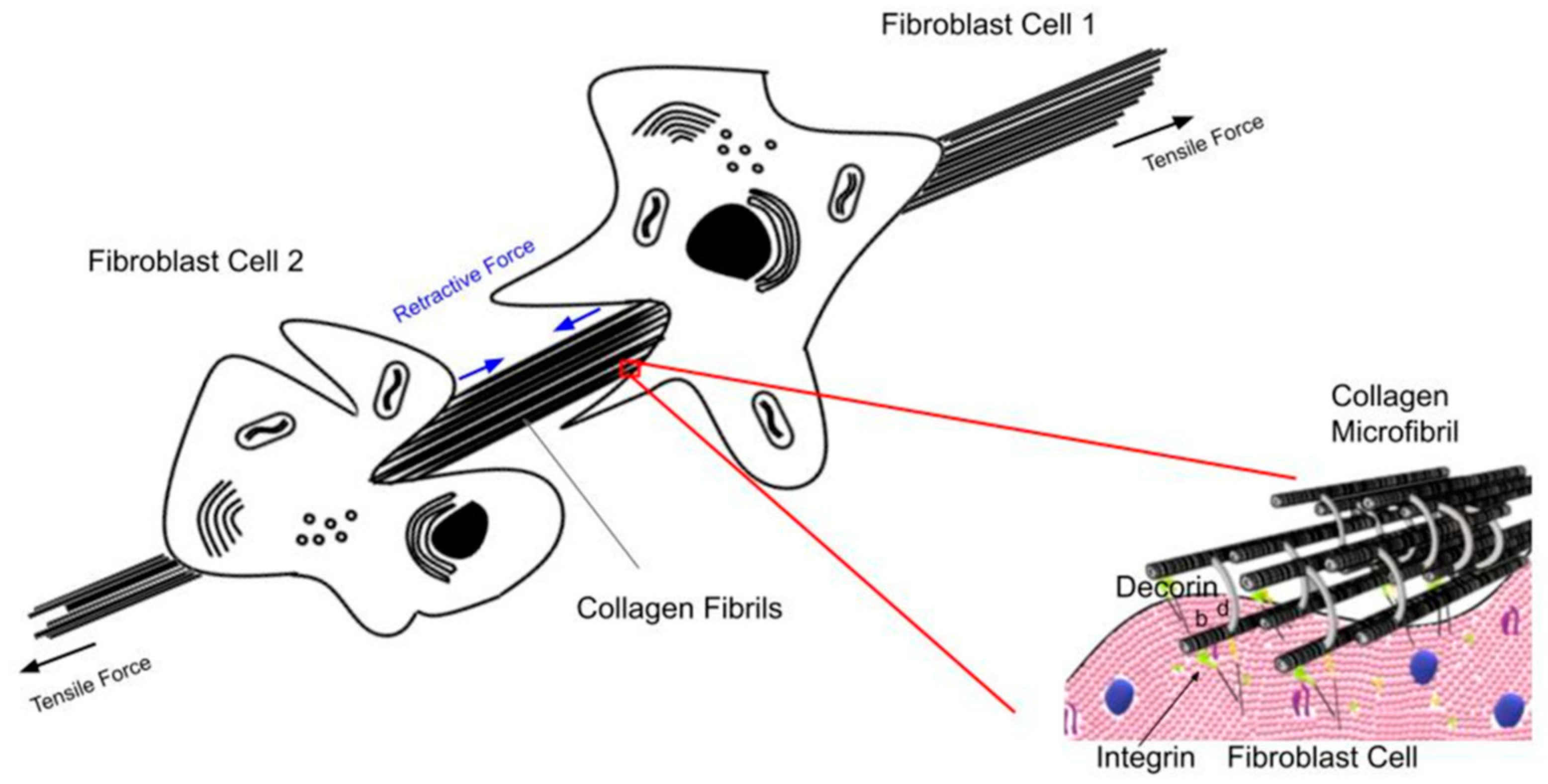
4. How Do Cellular and Macromolecular Size and Shape Affect Interstitial Fluid Behavior as Well as Tissue Stiffness?
5. Cellular Molecules and Assemblies Involved in Mechanotransduction
6. Cells Involved in Mechanotransduction
7. Cellular Junctions Involved in Mechanotransduction
8. Cellular Structures Involved in Mechanotransduction
9. Signaling Pathways Involved in Mechanotransduction
10. Molecules Involved in Integrin-Mediated Mechanotransduction Include FAK, Talin, and Vinculin
11. Role of Nuclear Membrane in Mechanotransduction
12. Role of Epithelial–Mesenchymal Transition (EMT) and Extracellular Matrix (ECM) in Mechanotransduction
13. Summary of the Effects of Mechanical Forces and Energy Storage on Cell–Cell and Cell–Matrix Interactions on Mechanotransduction
14. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McBride, D.J.; Trelstad, R.L.; Silver, F.H. Structural and Mechanical Assessment of Developing Chick Tendon. Int. J. Biol. Macromol. 1988, 10, 194–200. [Google Scholar] [CrossRef]
- Silver, F.H. Mechanosensing and Mechanochemical Transduction in Extracellular Matrix; Springer: New York, NY, USA, 2006. [Google Scholar]
- Silver, F.H.; Deshmukh, T. Do tensile and shear forces exerted on cells influence mechanotransduction through stored energy considerations? Biocell 2024, 48, 525–540. [Google Scholar] [CrossRef]
- Freeman, J.W.; Silver, F.H. Elastic energy storage in unimineralized and mineralized extracellular matrices (ECMs): A comparison between molecular modeling and experimental measurements. J. Theor. Biol. 2004, 229, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H. Engineering of Human Tissues and Implants: An Evolutionary Approach to Implant Design; Taylor & Francis: London, UK, 2024; ISBN 9781032399027. [Google Scholar]
- Macdonald, A.G.; Fraser, P.J. The transduction of very small hydrostatic pressures. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1999, 122, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.M.; Wu, Z.; Guan, K.L. Insights into recent findings and clinical application of YAP and TAZ in cancer. Nat. Rev. Cancer 2023, 23, 512–525. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, S.S.; Fassler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhao, W.; Combs, C.A.; Zhang, F.; Knutson, J.R.; Lilly, M.A.; Xu, H. Mechanical stimulation from the surrounding tissue activates mitochondrial energy metabolism in Drosophila differentiating germ cells. Dev. Cell. 2023, 58, 2249–2260.e9. [Google Scholar] [CrossRef]
- Silver, F.H.; Kelkar, N.; Deshmukh, T. Molecular Basis for Mechanical Properties of ECMs: Proposed Role of Fibrillar Collagen and Proteoglycans in Tissue Biomechanics. Biomolecules 2021, 11, 1018. [Google Scholar] [CrossRef]
- Silver, F.H.; Freeman, J.; Seehra, G.P. Collagen self-assembly development of matrix mechanical properties. J. Biomechanics 2003, 36, 1529–1553. [Google Scholar] [CrossRef]
- Starr, D.A.; Fridolfsson, H.N. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu. Rev. Cell Dev. Biol. 2010, 26, 421–444. [Google Scholar] [CrossRef]
- Kerch, G. Distribution of tightly and loosely bound water in biological macromolecules and age-related diseases. Int. J. Biol. Macromol. 2018, 118 Pt A, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Christiansen, D.L. Biomaterials Science and Biocompatibility; Springer: New York, NY, USA, 1999. [Google Scholar]
- Walsh, D.J.; Guironnet, D. Macromolecules with programmable shape, size, and chemistry. Proc. Natl. Acad. Sci. USA 2019, 116, 1538–1542. [Google Scholar] [CrossRef] [PubMed]
- Yanga, X.; Bib, D.; Czajkowskic, M.; Merkelc, M.; Manniung, L.; Marchettic, M.C. Correlating cell shape and cellular stress in motile confluent tissues. Proc. Natl. Acad. Sci. USA 2017, 114, 12663–12668. [Google Scholar] [CrossRef]
- Rubin, B.K.; Thornton, D.J. Dropping acid: Why is cystic fibrosis mucus abnormal? Eur. Respir. J. 2018, 52, 1802057. [Google Scholar] [CrossRef]
- Fritton, S.P.; Weinbaum, S. Fluid and solute transport in bone: Flow-induced mechanotransduction. Annu. Rev. Fluid. Mech. 2009, 41, 347–374. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 26, 821–828. [Google Scholar] [CrossRef]
- Li, X.; Dao, M.; Lykotrafitis, G.; Karniadakis, G.E. Biomechanics and biorheology of red blood cells in sickle cell anemia. J. Biomech. 2017, 50, 34–41. [Google Scholar] [CrossRef]
- Brückner, B.R.; Janshoff, A. Importance of integrity of cell-cell junctions for the mechanics of confluent MDCK II cell. Sci. Rep. 2018, 8, 14117. [Google Scholar] [CrossRef]
- Silver, F.H.; Ebrahimi, A.; Snowhill, P.B. Viscoelastic properties of self-assembled type I collagen fibers: Molecular basis of elastic and viscous behaviors. Connect. Tissue Res. 2002, 43, 569–580. [Google Scholar] [CrossRef]
- Van Damme, T.; Colige, A.; Syx, D.; Giunta, C.; Lindert, U.; Rohrbach, M.; Aryani, O.; Alanay, Y.; Simsek-Kiper, P.Ö.; Kroes, H.Y.; et al. Expanding the clinical and mutational spectrum of the Ehlers–Danlos syndrome, dermatosparaxis type. Genet. Med. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Houdusse, A.; Sweeney, H.L. How Myosin Generates Force on Actin Filaments. Trends Biochem. Sci. 2016, 41, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Hernandez, I.; Cantelli, G.; Bruce, F.; Sanz-Moreno, V. Rho, ROCK and actomyosin contractility in metastasis as drug targets. F1000Research 2016, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Karantza, V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011, 30, 127–138. [Google Scholar] [CrossRef]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef]
- Arjonen, A.; Kaukonen, R.; Mattila, E.; Rouhi, P.; Högnäs, G.; Sihto, H.; Miller, B.W.; Morton, J.P.; Bucher, E.; Taimen, P.; et al. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J. Clin. Investig. 2014, 124, 1069–1082. [Google Scholar] [CrossRef]
- Li, Y.R.; Yang, W.X. Myosins as fundamental components during tumorigenesis: Diverse and indispensable. Oncotarget 2016, 7, 46785–46812. [Google Scholar] [CrossRef]
- Hinck, L.; Näthke, I. Changes in cell and tissue organization in cancer of the breast and colon. Curr. Opin. Cell Biol. 2014, 26, 87–95. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tolls and targets in cancer therapy. Nat. Rev. Drug Discov. 2020, 21, 799–820. [Google Scholar] [CrossRef]
- Bates, M.E.; Libring, S.; Reinhart-King, C.A. Forces exerted and transduced by cancer-associated fibroblasts during cancer progression. Biol. Cell. 2023, 115, e2200104. [Google Scholar] [CrossRef]
- Schmitter, C.; Di-Luoffo, M.; Guillermet-Guibert, J. Transducing compressive forces into cellular outputs in cancer and beyond. Life Sci. Alliance 2023, 6, e202201862. [Google Scholar] [CrossRef] [PubMed]
- Ansardamavandi, A.; Tafazzoli-Shadpour, M. The functional cross talk between cancer cells and cancer associated fibroblasts from a cancer mechanics perspective. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119103. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Razipour, S.E.; McCain, M.L. Featured Article: TGF-β1 dominates extracellular matrix rigidity for inducing differentiation of human cardiac fibroblasts to myofibroblasts. Exp. Biol. Med. 2018, 243, 601–612. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshioka, Y.; Ochiya, T. Intercellular crosstalk between cancer cells and cancer-associated fibroblasts via extracellular vesicles. Cancer Cell Int. 2022, 22, 367. [Google Scholar] [CrossRef] [PubMed]
- Barbazan, J.; Pérez-González, C.; Gómez-González, M.; Dedenon, M.; Richon, S.; Latorre, E.; Serra, M.; Mariani, P.; Descroix, S.; Sens, P.; et al. Cancer-associated fibroblasts actively compress cancer cells and modulate mechanotransduction. Nat. Commun. 2023, 14, 6966. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Gaggar, S.; Gögenur, I. Cancer-Associated Fibroblasts and Tumor-Associated Macrophages in Cancer and Cancer Immunotherapy. Front. Oncol. 2021, 11, 668731. [Google Scholar] [CrossRef]
- Tomasetti, C.; Marchionni, L.; Nowak, M.A.; Parmigiani, G.; Vogelstein, B. Only three driver gene mutations are needed for the development of lung and colorectal cancers. Proc. Natl. Acad. Sci. USA 2015, 112, 118–123. [Google Scholar] [CrossRef]
- Chavez, M.; Hudson, L. Dynamics of cell-cell junctions in squamous cell carcinoma. Cancer Res. 2007, 67, 2045. [Google Scholar]
- Martin, T.A.; Jiang, W.G. Loss of tight junction barrier function and its role in cancer metastasis. Biochim. Biophys. Acta 2009, 1788, 872–891. [Google Scholar] [CrossRef]
- Xu, Q.R.; Du, X.H.; Huang, T.T.; Zheng, Y.C.; Li, Y.L.; Huang, D.Y.; Dai, H.Q.; Li, E.M.; Fang, W.K. Role of Cell-Cell Junctions in Oesophageal Squamous Cell Carcinoma. Biomolecules 2022, 12, 1378. [Google Scholar] [CrossRef]
- Lee, D.K.; Oh, J.; Park, H.W.; Gee, H.Y. Anchorage Dependence and Cancer Metastasis. J. Korean Med. Sci. 2024, 39, e156. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, C.; Kiel, C. Cell Adhesion Molecules in Normal Skin and Melanoma. Biomolecules 2021, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Dusek, R.L.; Attardi, L.D. Desmosomes: New perpetrators in tumour suppression. Nat. Rev. Cancer 2011, 11, 317–323. [Google Scholar] [CrossRef]
- Takeichi, M. Cadherins in cancer: Implications for invasion and metastasis. Curr. Opin. cell Biol. 1993, 5, 806–811. [Google Scholar] [CrossRef]
- Price, A.J.; Cost, A.L.; Ungewiß, H.; Waschke, J.; Dunn, A.R.; Grashoff, C. Mechanical loading of desmosomes depends on the magnitude and orientation of external stress. Nat. Commun. 2018, 9, 5284. [Google Scholar] [CrossRef]
- Legan, P.K.; Collins, J.E.; Garrod, D.R. The molecular biology of desmosomes and hemidesmosomes: “what’s in a name”? Bioessays 1992, 14, 385–393. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, L.; Gray, A.; Srivastava, A.K.; Li, C.; Zhang, G.; Cui, T. The role of desmosomes in carcinogenesis. Onco Targets Ther. 2017, 10, 4059–4063. [Google Scholar] [CrossRef]
- Maziveyi, M.; Alahari, S.K. Cell matrix adhesions in cancer: The proteins that form the glue. Oncotarget 2017, 8, 48471–48487. [Google Scholar] [CrossRef]
- Salvador, E.; Burek, M.; Förster, C.Y. Tight Junctions and the Tumor Microenvironment. Curr. Pathobiol. Rep. 2016, 4, 135–145. [Google Scholar] [CrossRef]
- Yayan, J.; Franke, K.J.; Berger, M.; Windisch, W.; Rasche, K. Adhesion, metastasis, and inhibition of cancer cells: A comprehensive review. Mol. Biol. Rep. 2024, 51, 165. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamb, C.A.; Vanzulli, S.I.; Lanari, C. Hormone receptors in breast cancer: More than estrogen receptors. Medicina (B Aires) 2019, 79, 540–545. (In English) [Google Scholar] [PubMed]
- Karska, J.; Kowalski, S.; Saczko, J.; Moisescu, M.G.; Kulbacka, J. Mechanosensitive Ion Channels and Their Role in Cancer Cells. Membranes 2023, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Otero-Sobrino, Á.; Blanco-Carlón, P.; Navarro-Aguadero, M.Á.; Gallardo, M.; Martínez-López, J.; Velasco-Estévez, M. Mechanosensitive Ion Channels: Their Physiological Importance and Potential Key Role in Cancer. Int. J. Mol. Sci. 2023, 24, 13710. [Google Scholar] [CrossRef]
- Marini, M.; Titiz, M.; Souza Monteiro de Araújo, D.; Geppetti, P.; Nassini, R.; De Logu, F. TRP Channels in Cancer: Signaling Mechanisms and Translational Approaches. Biomolecules 2023, 13, 1557. [Google Scholar] [CrossRef]
- Gargalionis, A.N.; Basdra, E.K.; Papavassiliou, A.G. Polycystins and Mechanotransduction in Human Disease. Int. J. Mol. Sci. 2019, 20, 2182. [Google Scholar] [CrossRef]
- Whitmarsh, A.J. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim. Biophys. Acta. 2007, 1773, 1285–1298. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G. The roles of integrins in cancer. Fac. Rev. 2021, 10, 45. [Google Scholar] [CrossRef]
- Desgrosellier, J.; Cheresh, D. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef]
- Yang, L.; Zheng, L.; Chng, W.J.; Ding, J.L. Comprehensive Analysis of ERK1/2 Substrates for Potential Combination Immunotherapies. Trends Pharmacol. Sci. 2019, 40, 899–910. [Google Scholar] [CrossRef]
- Bubici, C.; Papa, S. JNK signalling in cancer: In need of new, smarter therapeutic targets. Br. J. Pharmacol. 2014, 171, 24–37. [Google Scholar] [CrossRef]
- Kamaraju, A.K.; Roberts, A.B. Role of Rho/ROCK and p38 MAP Kinase Pathways in Transforming Growth Factor-β-mediated Smad-dependent Growth Inhibition of Human Breast Carcinoma Cells in Vivo. J. Biol. Chem. 2005, 280, 1024–1036. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.-H.; Lee, E.J.; Kim, Y.K.; Hong, Y.; Choi, Y.; Ryu, M.-J.; Woo, J.; Cho, Y.; Ahn, D.J.; Yang, Y.; et al. Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer. Nat. Commun. 2018, 9, 2165. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes. Cancer. 2011, 2, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Oxford, G.; Theodorescu, D. The role of Ras superfamily proteins in bladder cancer progression. J. Urol. 2003, 170, 1987–1993. [Google Scholar] [CrossRef]
- Reddy, S.; Yang, W.; Taylor, D.G.; Shen, X.-Q.; Oxender, D.; Kust, G.; Leff, T. Mitogen-activated protein kinase regulates transcription of the ApoCIII gene. Involvement of the orphan nuclear receptor HNF4. J. Biol. Chem. 1999, 274, 33050–33056. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Rovida, E. Impact of ERK5 on the Hallmarks of Cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Xiao, Y.; Dong, J. The Hippo Signaling Pathway in Cancer: A Cell Cycle Perspective. Cancers 2021, 13, 6214. [Google Scholar] [CrossRef]
- Harvey, K.; Zhang, X.; Thomas, D. The Hippo pathway and human cancer. Nat. Rev. Cancer 2013, 13, 246–257. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Palla, M.; Scarpato, L.; Di Trolio, R.; Ascierto, P.A. Sonic hedgehog pathway for the treatment of inflammatory diseases: Implications and opportunities for future research. J. Immunother. Cancer. 2022, 10, e004397. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of β-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Yeomanson, D.; Bryant, H.E. PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J. Pediatr. Hematol. Oncol. 2015, 37, 245–251. [Google Scholar] [CrossRef]
- Fu, D.; Hu, Z.; Xu, X.; Dai, X.; Liu, Z. Key signal transduction pathways and crosstalk in cancer: Biological and therapeutic opportunities. Transl. Oncol. 2022, 26, 101510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Chuang, H.H.; Zhen, Y.Y.; Tsai, Y.C.; Chuang, C.H.; Hsiao, M.; Huang, M.S.; Yang, C.J. FAK in cancer: From mechanisms to therapeutic strategies. Int. J. Mol. Sci. 2022, 23, 1726. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, D. Targeting Src family kinases in anti-cancer therapies: Turning promise into triumph. Trends Pharmacol Sci. 2012, 33, 122–128. [Google Scholar] [CrossRef]
- Azizi, L.; Cowell, A.R.; Mykuliak, V.V.; Goult, B.T.; Turkki, P.; Hytönen, V.P. Cancer associated talin point mutations disorganise cell adhesion and migration. Sci. Rep. 2021, 11, 347. [Google Scholar] [CrossRef]
- Metsiou, D.N.; Deligianni, D.; Giannopoulou, E.; Kalofonos, H.; Koutras, A.; Athanassiou, G. Adhesion strength and anti-tumor agents regulate vinculin of breast cancer cells. Front. Oncol. 2022, 12, 811508. [Google Scholar] [CrossRef]
- Chowdhury, D.; Mistry, A.; Maity, D.; Bhatia, R.; Priyadarshi, S.; Wadan, S.; Chakraborty, S.; Haldar, S. Pan-cancer analyses suggest kindlin-associated global mechanochemical alterations. Commun. Biol. 2024, 7, 372. [Google Scholar] [CrossRef]
- Luxton, G.W.; Starr, D.A. KASHing up with the nucleus: Novel functional roles of KASH proteins at the cytoplasmic surface of the nucleus. Curr. Opin. Cell Biol. 2014, 28, 69–75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yerima, G.; Domkam, N.; Ornowski, J.; Jahed, Z.; Mofrad, M.R.K. Force transmission and SUN-KASH higher-order assembly in the LINC complex models. Biophys. J. 2023, 122, 4582–4597. [Google Scholar] [CrossRef] [PubMed]
- Stroud, M.J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiomyopathy. Biophys. Rev. 2018, 10, 1033–1051. [Google Scholar] [CrossRef]
- Bernard, F.; Lepesant, J.A.; Guichet, A. Nucleus positioning within Drosophila egg chamber. Semin. Cell Dev. Biol. 2018, 82, 25–33. [Google Scholar] [CrossRef]
- Chiarini, F.; Paganelli, F.; Balestra, T.; Capanni, C.; Fazio, A.; Manara, M.C.; Landuzzi, L.; Petrini, S.; Evangelisti, C.; Lollini, P.-L.; et al. Lamin A and the LINC complex act as potential tumor suppressors in Ewing Sarcoma. Cell Death Dis. 2022, 13, 346. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. The role of inner nuclear membrane proteins in tumourigenesis and as potential targets for cancer therapy. Cancer Metastasis Rev. 2022, 41, 953–963. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428, Erratum in J. Clin. Investig. 2010, 120, 1786. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Mikhailova, T.; Li, X.; Porter, B.A.; Bah, A.; Kotula, L. Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition. Cell Commun Signal 2021, 19, 67. [Google Scholar] [CrossRef]
- Bera, K.; Kiepas, A.; Godet, I.; Li, Y.; Mehta, P.; Ifemembi, B.; Paul, C.D.; Sen, A.; Serra, S.A.; Stoletov, K.; et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature 2022, 611, 365–373. [Google Scholar] [CrossRef]
- Bettinger, D.A.; Yager, D.R.; Diegelmann, R.F.; Cohen, I.K. The effect of TGF-beta on keloid fibroblast proliferation and collagen synthesis. Plast. Reconstr. Surg. 1996, 98, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G. Pathobiology of transforming growth factor β in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Rømer, A.M.A.; Thorseth, M.L.; Madsen, D.H. Immune Modulatory Properties of Collagen in Cancer. Front. Immunol. 2021, 12, 791453. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kajiwara, Y.; Yonemura, K.; Shinto, E.; Mochizuki, S.; Okamoto, K.; Kishi, Y.; Ueno, H. Morphological consistency of desmoplastic reactions between the primary colorectal cancer lesion and associated metastatic lesions. Virchows Arch. 2020, 477, 47–55. [Google Scholar] [CrossRef]
- Chang, Z.; Zhang, L.; Hang, J.-T.; Liu, W.; Xu, G.-K. Viscoelastic Multiscale Mechanical Indexes for Assessing Liver Fibrosis and Treatment Outcomes. Nano Lett. 2023, 23, 9618–9625. [Google Scholar] [CrossRef]


| Structural Component | Effectors of Mechanotransduction |
|---|---|
| Cell membrane | Integrins, ion channels, growth factor receptors, cadherins, catenins |
| Cell cytoskeleton | Actin, myosin, intermediate filaments, microtubules |
| Cell junctions | Focal adhesions, tight junctions, gap junctions, adherens junctions, desmosomes |
| Nucleoskeleton | LINC proteins |
| Component | Effect |
|---|---|
| Actin filaments | Change in actin–myosin interactions, actin polymerization, cell spreading, actomyosin tension at cell junctions, cancer cell migration |
| Mitochondria | Activation of mitochondria and energy production |
| Intermediate filaments | Increased cell motility of cancer cells |
| Microtubules | Increased pulling forces between cells |
| Cell | Role in Mechanotransduction |
|---|---|
| Cancer-associated fibroblasts (CAF) | Cause cellular proliferation, degrade ECM, applyand generate traction forces, deform ECM |
| Epithelial | Act as mechanosensory, trigger changes in cell behavior |
| Fibroblast | With epithelium, alter cell and tissue organizationTransition into myofibroblasts |
| Melanocytes, basal, and squamous cells | Mutations cause uncontrolled growth |
| Myofibroblast | Applies tension to ECM, contributes to ECM synthesis and stiffness |
| Macrophage | Promotes angiogenesis, ECM remodeling, cancer cell proliferation, metastasis, immune suppression |
| Junction | Role in Cancer |
|---|---|
| Adherens | Initiate cell–cell contacts through cadherins and the cytoskeleton; mutations facilitate metastasis |
| Desmosomes | Connect cells through intermediate filaments, downregulated in cancer |
| Gap | Allow ion and small-molecule cell–cell communication, lost or reduced in cancer |
| Tight | Watertight junction, loss in cancer leading to metastasis |
| Structure | Role in Mechanotransduction |
|---|---|
| Cell membrane and associated components | Focal adhesions provide connection to ECM, growth factor and hormone receptors and membrane channels activate pathways |
| Cell junctions | Provide connections between cells and activate mechanotransduction pathways |
| Anchoring junctions | Activate integrin dependent mechanotransduction |
| Actin microfilaments, intermediate filaments, microtubules | Support the cytoskeleton and form cellular connections |
| Hormone and growth factors | Stretching and mutations activate mechanotransduction |
| Ion channels | Respond to membrane tension, influence EMT and remodeling |
| Pathway | Role in Mechanotransduction |
|---|---|
| MAPK | ERK (EGF/ERK ½), cJun, p 38, ERK 5 involved in gene transcription, cell differentiation, cytokine release, and apoptosis |
| Hippo | Limits cell proliferation, inhibits YAP/TAZ activity, YAP/TAZ activated in cancer |
| PAM (p13/AKT) | Activated in human cancers, influenced by growth factor pathways |
| RAS (ROCK) | Encode proteins involved in cell signaling and mutation, cause uncontrolled growth and invasion and cell death |
| Sonic Hedgehog | Activated in development and progression of several cancers |
| WNT/beta-catenin | Promotes differentiations of cancer stem cells that are precursors of mature cancer cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silver, F.H. The Role of Connections Between Cellular and Tissue Mechanical Elements and the Importance of Applied Energy in Mechanotransduction in Cancerous Tissue. Biomolecules 2025, 15, 457. https://doi.org/10.3390/biom15040457
Silver FH. The Role of Connections Between Cellular and Tissue Mechanical Elements and the Importance of Applied Energy in Mechanotransduction in Cancerous Tissue. Biomolecules. 2025; 15(4):457. https://doi.org/10.3390/biom15040457
Chicago/Turabian StyleSilver, Frederick H. 2025. "The Role of Connections Between Cellular and Tissue Mechanical Elements and the Importance of Applied Energy in Mechanotransduction in Cancerous Tissue" Biomolecules 15, no. 4: 457. https://doi.org/10.3390/biom15040457
APA StyleSilver, F. H. (2025). The Role of Connections Between Cellular and Tissue Mechanical Elements and the Importance of Applied Energy in Mechanotransduction in Cancerous Tissue. Biomolecules, 15(4), 457. https://doi.org/10.3390/biom15040457






