Kinetics of Circulating Progenitor Cells and Chemotactic Factors in Full-Term Neonates with Encephalopathy: Indications of Participation in the Endogenous Regenerative Process
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Neonatal Characteristics
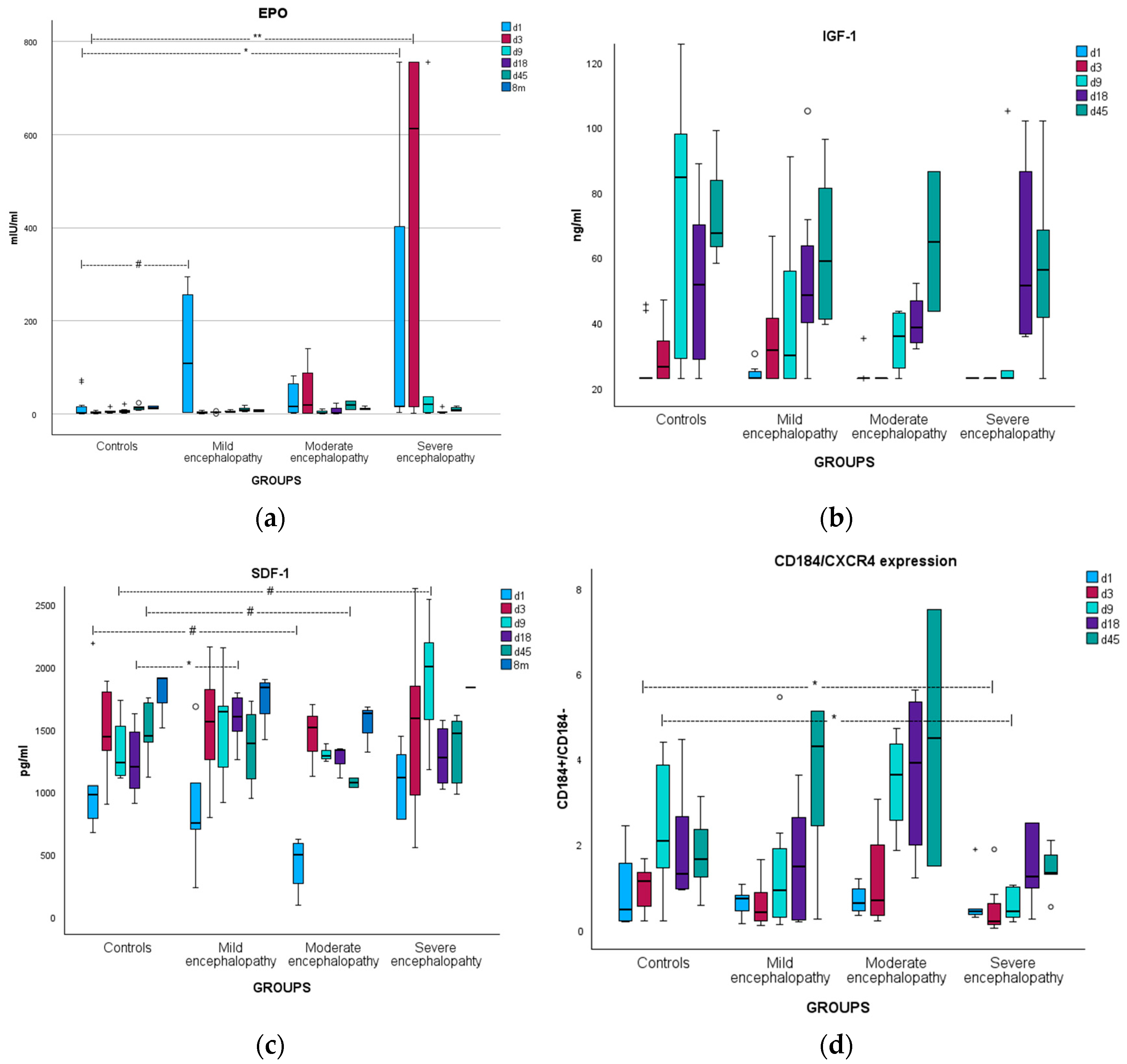
3.2. Erythropoietin
3.2.1. Kinetics
3.2.2. Correlations
3.3. Insulin-like Growth Factor-1
3.3.1. Kinetics
3.3.2. Correlations
3.4. Stromal Cell-Derived Factor-1
3.4.1. Kinetics
3.4.2. Correlations
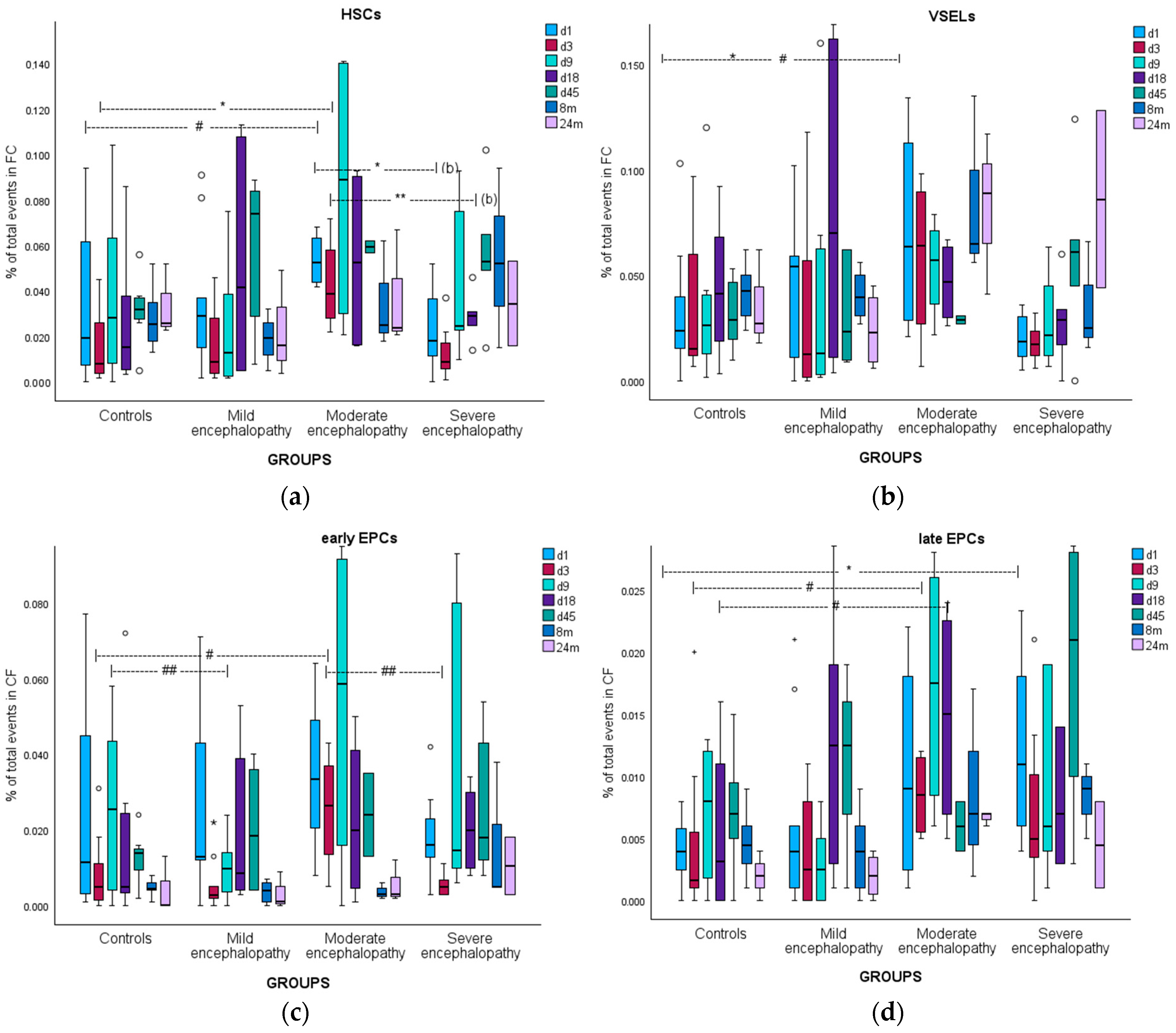
3.5. Hematopoietic Stem Cells
3.5.1. Kinetics
3.5.2. Correlations
3.6. Very Small Embryonic-like Stem Cells
3.6.1. Kinetics
3.6.2. Correlations
3.7. Early Endothelial Progenitor Cells
3.7.1. Kinetics
3.7.2. Correlations
3.8. Late Endothelial Progenitor Cells
3.8.1. Kinetics
3.8.2. Correlations
3.9. Brain Injury Biomarkers
3.9.1. Correlations with Chemoattractants
3.9.2. Correlations with CPCs
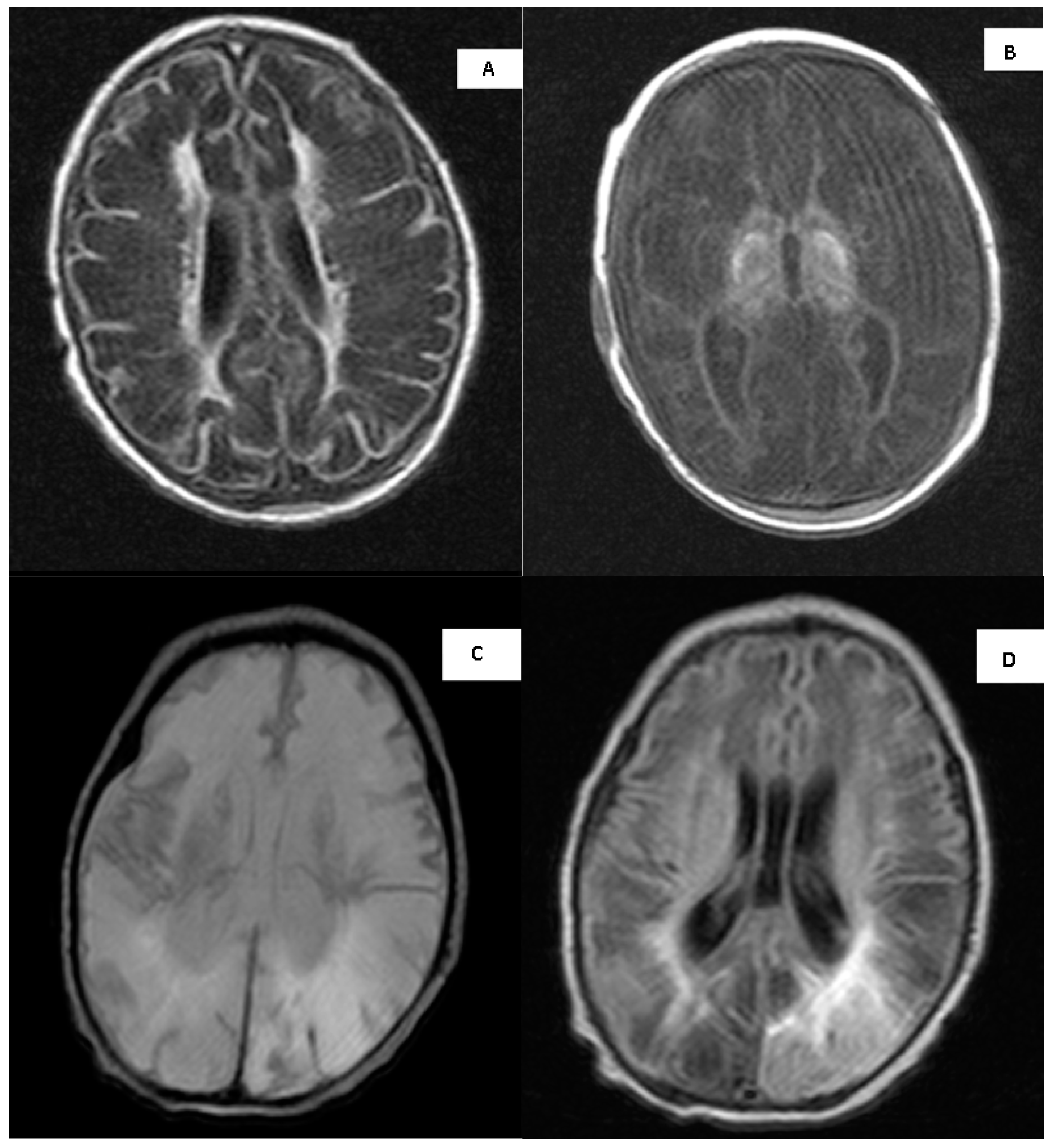
3.10. MRI Imaging
3.11. Long-Term Neurodevelopmental Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- S100B(d1) with EPO(d3) (rho = 0.312/p = 0.08)
- S100B(d1) with EPO(d9) (rho = 0.427/p = 0.06)
- S100B(d3) with EPO(d9) (rho = 0.506/p < 0.05)
- S100B(d9) with EPO(d45) (rho = 0.426/p = 0.078)
- S100B(d18) with EPO(d45) (rho = 0.617/p = 0.077)
- S100B(d1) with SDF-1(d9) (rho = 0.486/p < 0.05)
- S100B(d3) with SDF-1(d9) (rho = 0.567/p < 0.01)
- S100B(d9) with SDF-1(d18) (rho = 0.642/p < 0.005).
- S100B(d1) with IGF-1(d9) (rho = −0.472/p < 0.05).
- NSE(d1) with EPO(d3) (rho = 0.554/p < 0.005)
- NSE(d3) with EPO(d3) (rho = 0.467/p < 0.01)
- NSE(d3) with SDF-1(d9) (rho = 0.486/p < 0.05)
- NSE(d9) with SDF-1(d9) (rho = 0.467/p < 0.05)
- NSE(d1) with IGF-1(d3) (rho = −0.387/p < 0.05)
- NSE(d3) with IGF-1(d3) (rho = −0.391/p < 0.05)
- NSE(d9) with IGF-1(d9) (rho = −0.457/p < 0.05)
Appendix B
| Patient | Basal Ganglia Injury Score | Parasagittal Injury Score | Total Injury Score |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 2 | 0 | 2 |
| 3 | 1 | 0 | 1 |
| 4 | 3 | 0 | 3 |
| 5 | 4 | 5 | 9 |
| 6 | 3 | 5 | 8 |
| 7 | 2 | 0 | 2 |
| 8 | 0 | 0 | 0 |
| 9 | 3 | 0 | 3 |
References
- Volpe, J.; Inder, T.; Darras, B.; de Vries, L.; du Plessis, A.; Neil, J.; Perlman, J. Volpe’s Neurology of the Newborn, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323428767. [Google Scholar]
- Committee on Fetus and Newborn. Hypothermia and Neonatal Encephalopathy. Pediatrics 2014, 133, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, T.; Petit, I. Current Understanding of Stem Cell Mobilization: The Roles of Chemokines, Proteolytic Enzymes, Adhesion Molecules, Cytokines, and Stromal Cells. Exp. Hematol. 2002, 30, 973–981. [Google Scholar] [CrossRef]
- Efstathiou, N.; Soubasi, V.; Koliakos, G.; Kyriazis, G.; Zafeiriou, D.I.; Slavakis, A.; Kantziou, K.; Pozotou, T.; Chatzizisi, O.; Drosou-Agakidou, V. Mobilization of Circulating Progenitor Cells Following Brain Injury in Premature Neonates Could Be Indicative of an Endogenous Repair Process. A Pilot Study. Hippokratia 2015, 19, 141–147. [Google Scholar] [PubMed]
- van Velthoven, C.T.J.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Mesenchymal Stem Cell Treatment after Neonatal Hypoxic-Ischemic Brain Injury Improves Behavioral Outcome and Induces Neuronal and Oligodendrocyte Regeneration. Brain Behav. Immun. 2010, 24, 387–393. [Google Scholar] [CrossRef]
- Park, K.I.; Hack, M.; Ourednik, J.; Yandava, B.; Flax, J.D.; Stieg, P.E.; Gullans, S.; Jensen, F.E.; Sidman, R.L.; Ourednik, V.; et al. Acute Injury Directs the Migration, Proliferation, and Differentiation of Solid Organ Stem Cells: Evidence from the Effect of Hypoxia-Ischemia in the CNS on Clonal “Reporter” Neural Stem Cells. Exp. Neurol. 2006, 199, 156–178. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.T.; Bartley, J.H.; Wimborne, H.J.C.; Walker, A.L.; Hess, D.C.; Hill, W.D.; Carroll, J.E. The Neuroblast and Angioblast Chemotaxic Factor SDF-1 (CXCL12) Expression Is Briefly up Regulated by Reactive Astrocytes in Brain Following Neonatal Hypoxic-Ischemic Injury. BMC Neurosci. 2005, 6, 63. [Google Scholar] [CrossRef]
- Hill, W.D.; Hess, D.C.; Martin-Studdard, A.; Carothers, J.J.; Zheng, J.; Hale, D.; Maeda, M.; Fagan, S.C.; Carroll, J.E.; Conway, S.J. SDF-1 (CXCL12) Is Upregulated in the Ischemic Penumbra Following Stroke: Association with Bone Marrow Cell Homing to Injury. J. Neuropathol. Exp. Neurol. 2004, 63, 84–96. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Zhou, G.-Q. SDF-1alpha/CXCR4-Mediated Migration of Systemically Transplanted Bone Marrow Stromal Cells towards Ischemic Brain Lesion in a Rat Model. Brain Res. 2008, 1195, 104–112. [Google Scholar] [CrossRef]
- Aicher, A.; Zeiher, A.M.; Dimmeler, S. Mobilizing Endothelial Progenitor Cells. Hypertension 2005, 45, 321–325. [Google Scholar] [CrossRef]
- Aiuti, A.; Webb, I.J.; Bleul, C.; Springer, T.; Gutierrez-Ramos, J.C. The Chemokine SDF-1 Is a Chemoattractant for Human CD34+ Hematopoietic Progenitor Cells and Provides a New Mechanism to Explain the Mobilization of CD34+ Progenitors to Peripheral Blood. J. Exp. Med. 1997, 185, 111–120. [Google Scholar] [CrossRef]
- Vannucci, R.C.; Vannucci, S.J. A Model of Perinatal Hypoxic-Ischemic Brain Damage. Ann. N. Y. Acad. Sci. 1997, 835, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Ikeda, T.; Hayashi, T.; Sato, K.; Nagata, T.; Nagano, I.; Shoji, M.; Ikenoue, T.; Abe, K. Temporal Profile of Neural Stem Cell Proliferation in the Subventricular Zone after Ischemia/Hypoxia in the Neonatal Rat Brain. Neurol. Res. 2006, 28, 461–468. [Google Scholar] [CrossRef]
- Muñoz-Elías, G.; Woodbury, D.; Black, I.B. Marrow Stromal Cells, Mitosis, and Neuronal Differentiation: Stem Cell and Precursor Functions. Stem Cells 2003, 21, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Yasuhara, T.; Maki, M.; Matsukawa, N.; Masuda, T.; Yu, S.J.; Ali, M.; Yu, G.; Xu, L.; Kim, S.U.; et al. Neural Progenitor NT2N Cell Lines from Teratocarcinoma for Transplantation Therapy in Stroke. Prog. Neurobiol. 2008, 85, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.C.; Borlongan, C. V Stem Cells and Neurological Diseases. Cell Prolif. 2008, 41 (Suppl S1), 94–114. [Google Scholar] [CrossRef]
- Carroll, J.E.; Borlongan, C. V Adult Stem Cell Therapy for Acute Brain Injury in Children. CNS Neurol. Disord. Drug Targets 2008, 7, 361–369. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Hadman, M.; Sanberg, C.D.; Sanberg, P.R. Central Nervous System Entry of Peripherally Injected Umbilical Cord Blood Cells Is Not Required for Neuroprotection in Stroke. Stroke 2004, 35, 2385–2389. [Google Scholar] [CrossRef]
- Wang, M.; Crisostomo, P.R.; Herring, C.; Meldrum, K.K.; Meldrum, D.R. Human Progenitor Cells from Bone Marrow or Adipose Tissue Produce VEGF, HGF, and IGF-I in Response to TNF by a P38 MAPK-Dependent Mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R880–R884. [Google Scholar] [CrossRef]
- Wang, F.; Maeda, N.; Yasuhara, T.; Kameda, M.; Tsuru, E.; Yamashita, T.; Shen, Y.; Tsuda, M.; Date, I.; Sagara, Y. The Therapeutic Potential of Human Umbilical Cord Blood Transplantation for Neonatal Hypoxic-Ischemic Brain Injury and Ischemic Stroke. Acta Med. Okayama 2012, 66, 429–434. [Google Scholar]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W., II.; Park, W.S. Mesenchymal Stem Cells for Bronchopulmonary Dysplasia: Phase 1 Dose-Escalation Clinical Trial. J. Pediatr. 2014, 164, 966–972.e6. [Google Scholar] [CrossRef]
- Cotten, C.M.; Murtha, A.P.; Goldberg, R.N.; Grotegut, C.A.; Smith, P.B.; Goldstein, R.F.; Fisher, K.A.; Gustafson, K.E.; Waters-Pick, B.; Swamy, G.K.; et al. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. J. Pediatr. 2014, 164, 973–979.e1. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.M.; Song, A.W.; Case, L.E.; Mikati, M.A.; Gustafson, K.E.; Simmons, R.; Goldstein, R.; Petry, J.; McLaughlin, C.; Waters-Pick, B.; et al. Effect of Autologous Cord Blood Infusion on Motor Function and Brain Connectivity in Young Children with Cerebral Palsy: A Randomized, Placebo-Controlled Trial. Stem Cells Transl. Med. 2017, 6, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Cotten, M.; Tan, S.; Kurtzberg, J.; Cairo, M.S. Rescuing the Neonatal Brain from Hypoxic Injury with Autologous Cord Blood. Bone Marrow Transplant. 2013, 48, 890–900. [Google Scholar] [CrossRef]
- van Velthoven, C.T.J.; Kavelaars, A.; Heijnen, C.J. Mesenchymal Stem Cells as a Treatment for Neonatal Ischemic Brain Damage. Pediatr. Res. 2012, 71, 474–481. [Google Scholar] [CrossRef]
- Daval, J.-L.; Pourié, G.; Grojean, S.; Lièvre, V.; Strazielle, C.; Blaise, S.; Vert, P. Neonatal Hypoxia Triggers Transient Apoptosis Followed by Neurogenesis in the Rat CA1 Hippocampus. Pediatr. Res. 2004, 55, 561–567. [Google Scholar] [CrossRef]
- Ong, J.; Plane, J.M.; Parent, J.M.; Silverstein, F.S. Hypoxic-Ischemic Injury Stimulates Subventricular Zone Proliferation and Neurogenesis in the Neonatal Rat. Pediatr. Res. 2005, 58, 600–606. [Google Scholar] [CrossRef]
- Antonitsis, P.; Anastasiadis, K.; Koliakos, G.; Vaitsopoulou, C.; Kouzi-Koliakou, K.; Doumas, A.; Argiriadou, H.; Tossios, P. Intramyocardial Implantation of Autologous Bone Marrow-Derived Stem Cells Combined with Coronary Artery Bypass Grafting in Patients with Ischemic Cardiomyopathy: A Pilot Study. Hippokratia 2012, 16, 366–370. [Google Scholar] [PubMed]
- Anastasiadis, K.; Antonitsis, P.; Doumas, A.; Koliakos, G.; Argiriadou, H.; Vaitsopoulou, C.; Tossios, P.; Papakonstantinou, C.; Westaby, S. Stem Cells Transplantation Combined with Long-Term Mechanical Circulatory Support Enhances Myocardial Viability in End-Stage Ischemic Cardiomyopathy. Int. J. Cardiol. 2012, 155, e51–e53. [Google Scholar] [CrossRef]
- Branco, L.G.S.; Gargaglioni, L.H.; Barros, R.C.H. Anapyrexia during Hypoxia. J. Therm. Biol. 2006, 31, 82–89. [Google Scholar] [CrossRef]
- Steiner, A.A.; Branco, L.G.S. Hypoxia-Induced Anapyrexia: Implications and Putative Mediators. Annu. Rev. Physiol. 2002, 64, 263–288. [Google Scholar] [CrossRef]
- Garg, A.; Saili, A.; Nangia, S. Role of Anapyrexia in Perinatal Asphyxia in Term Neonates: An Open Labeled Randomized Controlled Trial; Pediatric Academic Societies: Washington, DC, USA, 2013; p. board 379. [Google Scholar]
- Lin, K.K.; Goodell, M.A. Detection of Hematopoietic Stem Cells by Flow Cytometry. Methods Cell Biol. 2011, 103, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Verina, T.; Fatemi, A.; Johnston, M.V.; Comi, A.M. Pluripotent Possibilities: Human Umbilical Cord Blood Cell Treatment after Neonatal Brain Injury. Pediatr. Neurol. 2013, 48, 346–354. [Google Scholar] [CrossRef]
- Bowie, M.B.; McKnight, K.D.; Kent, D.G.; McCaffrey, L.; Hoodless, P.A.; Eaves, C.J. Hematopoietic Stem Cells Proliferate until after Birth and Show a Reversible Phase-Specific Engraftment Defect. J. Clin. Investig. 2006, 116, 2808–2816. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a Novel Hierarchy of Endothelial Progenitor Cells Using Human Peripheral and Umbilical Cord Blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Hirschi, K.K.; Ingram, D.A.; Yoder, M.C. Assessing Identity, Phenotype, and Fate of Endothelial Progenitor Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1584–1595. [Google Scholar] [CrossRef]
- Lapidot, T.; Dar, A.; Kollet, O. How Do Stem Cells Find Their Way Home? Blood 2005, 106, 1901–1910. [Google Scholar] [CrossRef]
- Nervi, B.; Link, D.C.; DiPersio, J.F. Cytokines and Hematopoietic Stem Cell Mobilization. J. Cell. Biochem. 2006, 99, 690–705. [Google Scholar] [CrossRef]
- Papayannopoulou, T.; Scadden, D.T. Stem-Cell Ecology and Stem Cells in Motion. Blood 2008, 111, 3923–3930. [Google Scholar] [CrossRef]
- Dar, A.; Kollet, O.; Lapidot, T. Mutual, Reciprocal SDF-1/CXCR4 Interactions between Hematopoietic and Bone Marrow Stromal Cells Regulate Human Stem Cell Migration and Development in NOD/SCID Chimeric Mice. Exp. Hematol. 2006, 34, 967–975. [Google Scholar] [CrossRef]
- Taguchi, A.; Soma, T.; Tanaka, H.; Kanda, T.; Nishimura, H.; Yoshikawa, H.; Tsukamoto, Y.; Iso, H.; Fujimori, Y.; Stern, D.M.; et al. Administration of CD34+ Cells after Stroke Enhances Neurogenesis via Angiogenesis in a Mouse Model. J. Clin. Investig. 2004, 114, 330–338. [Google Scholar] [CrossRef]
- Hennemann, B.; Ickenstein, G.; Sauerbruch, S.; Luecke, K.; Haas, S.; Horn, M.; Andreesen, R.; Bogdahn, U.; Winkler, J. Mobilization of CD34+ Hematopoietic Cells, Colony-Forming Cells and Long-Term Culture-Initiating Cells into the Peripheral Blood of Patients with an Acute Cerebral Ischemic Insult. Cytotherapy 2008, 10, 303–311. [Google Scholar] [CrossRef]
- Taguchi, A.; Matsuyama, T.; Moriwaki, H.; Hayashi, T.; Hayashida, K.; Nagatsuka, K.; Todo, K.; Mori, K.; Stern, D.M.; Soma, T.; et al. Circulating CD34-Positive Cells Provide an Index of Cerebrovascular Function. Circulation 2004, 109, 2972–2975. [Google Scholar] [CrossRef] [PubMed]
- Dunac, A.; Frelin, C.; Popolo-Blondeau, M.; Chatel, M.; Mahagne, M.H.; Philip, P.J.M. Neurological and Functional Recovery in Human Stroke Are Associated with Peripheral Blood CD34+ Cell Mobilization. J. Neurol. 2007, 254, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Palange, P.; Testa, U.; Huertas, A.; Calabrò, L.; Antonucci, R.; Petrucci, E.; Pelosi, E.; Pasquini, L.; Satta, A.; Morici, G.; et al. Circulating Haemopoietic and Endothelial Progenitor Cells Are Decreased in COPD. Eur. Respir. J. 2006, 27, 529–541. [Google Scholar] [CrossRef]
- Fadini, G.P.; Schiavon, M.; Cantini, M.; Baesso, I.; Facco, M.; Miorin, M.; Tassinato, M.; de Kreutzenberg, S.V.; Avogaro, A.; Agostini, C. Circulating Progenitor Cells Are Reduced in Patients with Severe Lung Disease. Stem Cells 2006, 24, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Boilson, B.A.; Kiernan, T.J.; Harbuzariu, A.; Nelson, R.E.; Lerman, A.; Simari, R.D. Circulating CD34+ Cell Subsets in Patients with Coronary Endothelial Dysfunction. Nat. Clin. Pract. Cardiovasc. Med. 2008, 5, 489–496. [Google Scholar] [CrossRef]
- Ratajczak, M.; Zuba-Surma, E. Very Small Embryonic-like Stem Cells: Characterization, Developmental Origin, and Biological Significance. Experimental 2008, 36, 742–751. [Google Scholar] [CrossRef][Green Version]
- Kucia, M.; Zhang, Y.P.; Reca, R.; Wysoczynski, M.; Machalinski, B.; Majka, M.; Ildstad, S.T.; Ratajczak, J.; Shields, C.B.; Ratajczak, M.Z. Cells Enriched in Markers of Neural Tissue-Committed Stem Cells Reside in the Bone Marrow and Are Mobilized into the Peripheral Blood Following Stroke. Leukemia 2006, 20, 18–28. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Kim, C.H.; Wojakowski, W.; Janowska-Wieczorek, A.; Kucia, M.; Ratajczak, J. Innate Immunity as Orchestrator of Stem Cell Mobilization. Leuk. Off. J. Leuk. Soc. Am. Leuk. Res. Fund UK 2010, 24, 1667–1675. [Google Scholar] [CrossRef]
- Paczkowska, E.; Kucia, M.; Koziarska, D.; Halasa, M.; Safranow, K.; Masiuk, M.; Karbicka, A.; Nowik, M.; Nowacki, P.; Ratajczak, M.Z.; et al. Clinical Evidence That Very Small Embryonic-like Stem Cells Are Mobilized into Peripheral Blood in Patients after Stroke. Stroke J. Cereb. Circ. 2009, 40, 1237–1244. [Google Scholar] [CrossRef]
- Kotowski, M.; Safranow, K.; Kawa, P.; Lewandowska, J.; Patrycja, K.; Dziedziejko, V.; Paczkowska, E.; Czajka, R.; Celewicz, Z.; Rudnicki, J. Circulating Hematopoietic Stem Cell Count Is a Valuable Predictor of Prematurity Complications in Preterm Newborns. BMC Pediatr. 2012, 12, 148. [Google Scholar] [CrossRef]
- Machalinska, A.; Modrzejewska, M.; Kotowski, M.; Dziedziejko, V.; Kucia, M.; Kawa, M.; Safranow, K.; Baskiewicz-Masiuk, M.; Modrzejewska, A.; Karczewicz, D.; et al. Circulating Stem Cell Populations in Preterm Infants: Implications for the Development of Retinopathy of Prematurity. Arch. Ophthalmol. 2010, 128, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; van der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Human Endothelial Progenitor Cells. Cold Spring Harb. Perspect. Med. 2012, 2, a006692. [Google Scholar] [CrossRef] [PubMed]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine. Stem Cells 2011, 29, 1650–1655. [Google Scholar] [CrossRef]
- Hunting, C.B.; Noort, W.A.; Zwaginga, J.J. Circulating Endothelial (Progenitor) Cells Reflect the State of the Endothelium: Vascular Injury, Repair and Neovascularization. Vox Sang. 2005, 88, 1–9. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Mervis, C.F.; Maxey, A.M.; Markham, N.E.; Abman, S.H. Hyperoxia Reduces Bone Marrow, Circulating, and Lung Endothelial Progenitor Cells in the Developing Lung: Implications for the Pathogenesis of Bronchopulmonary Dysplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L1073–L1084. [Google Scholar] [CrossRef]
- Bui, K.C.T.; Weems, M.; Biniwale, M.; George, A.; Zielinska, E.; Azen, C.G.; Durand, M.; Abdel-Azim, H. Circulating Hematopoietic and Endothelial Progenitor Cells in Newborn Infants: Effects of Gestational Age, Postnatal Age and Clinical Stress in the First 3 Weeks of Life. Early Hum. Dev. 2013, 89, 411–418. [Google Scholar] [CrossRef]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical Reevaluation of Endothelial Progenitor Cell Phenotypes for Therapeutic and Diagnostic Use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef]
- Fadini, G.P. An Underlying Principle for the Study of Circulating Progenitor Cells in Diabetes and Its Complications. Diabetologia 2008, 51, 1091–1094. [Google Scholar] [CrossRef]
- Umemura, T.; Soga, J.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Jitsuiki, D.; Nishioka, K.; Goto, C.; Teragawa, H.; Yoshizumi, M.; et al. Aging and Hypertension Are Independent Risk Factors for Reduced Number of Circulating Endothelial Progenitor Cells. Am. J. Hypertens. 2008, 21, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Adams, V.; Lenk, K.; Linke, A.; Lenz, D.; Erbs, S.; Sandri, M.; Tarnok, A.; Gielen, S.; Emmrich, F.; Schuler, G.; et al. Increase of Circulating Endothelial Progenitor Cells in Patients with Coronary Artery Disease after Exercise-Induced Ischemia. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 684–690. [Google Scholar] [CrossRef]
- Yip, H.-K.; Tsai, T.-H.; Lin, H.-S.; Chen, S.-F.; Sun, C.-K.; Leu, S.; Yuen, C.-M.; Tan, T.-Y.; Lan, M.-Y.; Liou, C.-W.; et al. Effect of Erythropoietin on Level of Circulating Endothelial Progenitor Cells and Outcome in Patients after Acute Ischemic Stroke. Crit. Care 2011, 15, R40. [Google Scholar] [CrossRef] [PubMed]
- Paviotti, G.; Fadini, G.P.; Boscaro, E.; Agostini, C.; Avogaro, A.; Chiandetti, L.; Baraldi, E.; Filippone, M. Endothelial Progenitor Cells, Bronchopulmonary Dysplasia and Other Short-Term Outcomes of Extremely Preterm Birth. Early Hum. Dev. 2011, 87, 461–465. [Google Scholar] [CrossRef]
- Mund, J.A.; Estes, M.L.; Yoder, M.C.; Ingram, D.A.; Case, J. Flow Cytometric Identification and Functional Characterization of Immature and Mature Circulating Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1045–1053. [Google Scholar] [CrossRef]
- Baker, C.D.; Balasubramaniam, V.; Mourani, P.M.; Sontag, M.K.; Black, C.P.; Ryan, S.L.; Abman, S.H. Cord Blood Angiogenic Progenitor Cells Are Decreased in Bronchopulmonary Dysplasia. Eur. Respir. J. 2012, 40, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Lucke, C.; Fichtlscherer, S.; Aicher, A.; Tschöpe, C.; Schultheiss, H.-P.; Zeiher, A.M.; Dimmeler, S. Quantification of Circulating Endothelial Progenitor Cells Using the Modified ISHAGE Protocol. PLoS ONE 2010, 5, e13790. [Google Scholar] [CrossRef]
- Safranow, K.; Kotowski, M.; Lewandowska, J.; Machalińska, A.; Dziedziejko, V.; Czajka, R.; Celewicz, Z.; Rudnicki, J.; Machaliński, B. Circulating Endothelial Progenitor Cells in Premature Infants: Is There an Association with Premature Birth Complications? J. Perinat. Med. 2012, 40, 455–462. [Google Scholar] [CrossRef]
- Iwai, M.; Cao, G.; Yin, W.; Stetler, R.A.; Liu, J.; Chen, J. Erythropoietin Promotes Neuronal Replacement through Revascularization and Neurogenesis after Neonatal Hypoxia/Ischemia in Rats. Stroke J. Cereb. Circ. 2007, 38, 2795–2803. [Google Scholar] [CrossRef]
- Zawada, W.M.; Zastrow, D.J.; Clarkson, E.D.; Adams, F.S.; Bell, K.P.; Freed, C.R. Growth Factors Improve Immediate Survival of Embryonic Dopamine Neurons after Transplantation into Rats. Brain Res. 1998, 786, 96–103. [Google Scholar] [CrossRef]
- Gonzalez, F.F.; Ferriero, D.M. Neuroprotection in the Newborn Infant. Clin. Perinatol. 2009, 36, 859–880. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; McPherson, R.J.; Bauer, L.A.; Ledbetter, K.J.; Gleason, C.A.; Mayock, D.E. A Phase I/II Trial of High-Dose Erythropoietin in Extremely Low Birth Weight Infants: Pharmacokinetics and Safety. Pediatrics 2008, 122, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Fauchère, J.-C.; Dame, C.; Vonthein, R.; Koller, B.; Arri, S.; Wolf, M.; Bucher, H.U. An Approach to Using Recombinant Erythropoietin for Neuroprotection in Very Preterm Infants. Pediatrics 2008, 122, 375–382. [Google Scholar] [CrossRef]
- Bierer, R.; Peceny, M.C.; Hartenberger, C.H.; Ohls, R.K. Erythropoietin Concentrations and Neurodevelopmental Outcome in Preterm Infants. Pediatrics 2006, 118, e635–e640. [Google Scholar] [CrossRef]
- He, J.-S.; Huang, Z.-L.; Yang, H.; Weng, K.-Z.; Zhu, S.-B. Early Use of Recombinant Human Erythropoietin Promotes Neurobehavioral Development in Preterm Infants. Zhongguo Dang Dai Er Ke Za Zhi 2008, 10, 586–588. [Google Scholar]
- Zhu, C.; Kang, W.; Xu, F.; Cheng, X.; Zhang, Z.; Jia, L.; Ji, L.; Guo, X.; Xiong, H.; Simbruner, G.; et al. Erythropoietin Improved Neurologic Outcomes in Newborns With Hypoxic-Ischemic Encephalopathy. Pediatrics 2009, 124, e218–e226. [Google Scholar] [CrossRef] [PubMed]
- Juul, S.E.; Stallings, S.A.; Christensen, R.D. Erythropoietin in the Cerebrospinal Fluid of Neonates Who Sustained CNS Injury. Pediatr. Res. 1999, 46, 543. [Google Scholar] [CrossRef]
- Bahlmann, F.H.; De Groot, K.; Spandau, J.-M.; Landry, A.L.; Hertel, B.; Duckert, T.; Boehm, S.M.; Menne, J.; Haller, H.; Fliser, D. Erythropoietin Regulates Endothelial Progenitor Cells. Blood 2004, 103, 921–926. [Google Scholar] [CrossRef]
- Meister, B.; Maurer, H.; Simma, B.; Kern, H.; Ulmer, H.; Hittmair, A.; Fink, F.M. The Effect of Recombinant Human Erythropoietin on Circulating Hematopoietic Progenitor Cells in Anemic Premature Infants. Stem Cells 1997, 15, 359–363. [Google Scholar] [CrossRef]
- Lin, S.; Fan, L.W.; Pang, Y.; Rhodes, P.G.; Mitchell, H.J.; Cai, Z. IGF-1 Protects Oligodendrocyte Progenitor Cells and Improves Neurological Functions Following Cerebral Hypoxia-Ischemia in the Neonatal Rat. Brain Res. 2005, 1063, 15–26. [Google Scholar] [CrossRef]
- Lin, S.; Fan, L.-W.; Rhodes, P.G.; Cai, Z. Intranasal Administration of IGF-1 Attenuates Hypoxic-Ischemic Brain Injury in Neonatal Rats. Exp. Neurol. 2009, 217, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Brywe, K.G.; Mallard, C.; Gustavsson, M.; Hedtjärn, M.; Leverin, A.L.; Wang, X.; Blomgren, K.; Isgaard, J.; Hagberg, H. IGF-I Neuroprotection in the Immature Brain after Hypoxia-Ischemia, Involvement of Akt and GSK3β? Eur. J. Neurosci. 2005, 21, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Du, Z.; Zhao, L.; Feng, D.; Wei, G.; He, Y.; Tan, J.; Lee, W.-H.; Hampel, H.; Dodel, R.; et al. IFATS Collection: The Conditioned Media of Adipose Stromal Cells Protect against Hypoxia-Ischemia-Induced Brain Damage in Neonatal Rats. Stem Cells 2009, 27, 478–488. [Google Scholar] [CrossRef]
- Beck, K.D.; Powell-Braxton, L.; Widmer, H.R.; Valverde, J.; Hefti, F. Igf1 Gene Disruption Results in Reduced Brain Size, CNS Hypomyelination, and Loss of Hippocampal Granule and Striatal Parvalbumin-Containing Neurons. Neuron 1995, 14, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Bennet, L.; George, S.; Wu, D.; Waldvogel, H.J.; Gluckman, P.D.; Faull, R.L.; Crosier, P.S.; Gunn, A.J. Insulin-like Growth Factor-1 Reduces Postischemic White Matter Injury in Fetal Sheep. J. Cereb. Blood Flow Metab. 2001, 21, 493–502. [Google Scholar] [CrossRef]
- Langford, K.; Nicoiaides, K.; Miell, J.P. Maternal and Fetal Insulin-like Growth Factors and Their Binding Proteins in the Second and Third Trimesters of Human Pregnancy. Hum. Reprod. 1998, 13, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Wilson, D.M.; Liu, F.; Nagashima, R.; Rosenfeld, R.G.; Hintz, R.L. Levels of Insulin-like Growth Factors I and II in Human Cord Blood. J. Clin. Endocrinol. Metab. 1983, 57, 609–612. [Google Scholar] [CrossRef]
- Hansen-Pupp, I.; Löfqvist, C.; Polberger, S.; Niklasson, A.; Fellman, V.; Hellström, A.; Ley, D. Influence of Insulin-like Growth Factor i and Nutrition during Phases of Postnatal Growth in Very Preterm Infants. Pediatr. Res. 2011, 69, 448–453. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Zuba-Surma, E.; Kucia, M.; Reca, R.; Wojakowski, W.; Ratajczak, J. The Pleiotropic Effects of the SDF-1-CXCR4 Axis in Organogenesis, Regeneration and Tumorigenesis. Leukemia 2006, 20, 1915–1924. [Google Scholar] [CrossRef]
- Shen, L.H.; Li, Y.; Chen, J.; Zacharek, A.; Gao, Q.; Kapke, A.; Lu, M.; Raginski, K.; Vanguri, P.; Smith, A.; et al. Therapeutic Benefit of Bone Marrow Stromal Cells Administered 1 Month after Stroke. J. Cereb. Blood Flow Metab. 2007, 27, 6–13. [Google Scholar] [CrossRef]
- Chang, Y.; Shyu, W.; SZ, L.; Li, H. Regenerative Therapy against Stroke. Jpn. J. Neurosurg. 2007, 16, 481–489. [Google Scholar]
- Wojakowski, W.; Tendera, M.; Kucia, M.; Zuba-Surma, E.; Paczkowska, E.; Ciosek, J.; Hałasa, M.; Król, M.; Kazmierski, M.; Buszman, P.; et al. Mobilization of Bone Marrow-Derived Oct-4+ SSEA-4+ Very Small Embryonic-like Stem Cells in Patients with Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2009, 53, 1–9. [Google Scholar] [CrossRef]
- Drukała, J.; Paczkowska, E.; Kucia, M.; Młyńska, E.; Krajewski, A.; Machaliński, B.; Madeja, Z.; Ratajczak, M.Z. Stem Cells, Including a Population of Very Small Embryonic-like Stem Cells, Are Mobilized into Peripheral Blood in Patients after Skin Burn Injury. Stem Cell Rev. 2012, 8, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, N. Biomarkers in Neonatal Brain Injury: Interpreting Research into Clinical Practice; Springer: Cham, Switzerland, 2022; pp. 1–47. [Google Scholar]
- Efstathiou, N.; Slavakis, A.; Drossou, V.; Kantziou, K.; Dermetzoglou, V.; Soubasi, V. Can We Delineate Brain Injury in Full-Term Neonates Using Serum Biomarkers? Brain Inj. 2021, 35, 821–830. [Google Scholar] [CrossRef]
- Sarnat, H.B.; Sarnat, M.S. Neonatal Encephalopathy Following Fetal Distress. A Clinical and Electroencephalographic Study. Arch. Neurol. 1976, 33, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Gazzolo, D.; Marinoni, E.; di Iorio, R.; Lituania, M.; Bruschettini, P.L.; Michetti, F. Circulating S100beta Protein Is Increased in Intrauterine Growth-Retarded Fetuses. Pediatr. Res. 2002, 51, 215–219. [Google Scholar] [CrossRef]
- Loukovaara, M.; Teramo, K.; Alfthan, H.; Hämäläinen, E.; Stefanovic, V.; Andersson, S. Amniotic Fluid S100B Protein and Erythropoietin in Pregnancies at Risk for Fetal Hypoxia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 142, 115–118. [Google Scholar] [CrossRef]
- Florio, P.; Marinoni, E.; Di Iorio, R.; Bashir, M.; Ciotti, S.; Sacchi, R.; Bruschettini, M.; Lituania, M.; Serra, G.; Michetti, F.; et al. Urinary S100B Protein Concentrations Are Increased in Intrauterine Growth-Retarded Newborns. Pediatrics 2006, 118, e747–e754. [Google Scholar] [CrossRef]
- Netto, C.B.O.; Portela, L.V.; Ferreira, C.T.; Kieling, C.; Matte, U.; Felix, T.; Da Silveira, T.R.; Souza, D.O.; Gonçalves, C.A.; Giugliani, R. Ontogenetic Changes in Serum S100B in Down Syndrome Patients. Clin. Biochem. 2005, 38, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Miller, S.P.; Bartha, A.; Newton, N.; Hamrick, S.E.G.; Mukherjee, P.; Glenn, O.A.; Xu, D.; Partridge, J.C.; Ferriero, D.M.; et al. MR Imaging, MR Spectroscopy, and Diffusion Tensor Imaging of Sequential Studies in Neonates with Encephalopathy. Am. J. Neuroradiol. 2006, 27, 533–547. [Google Scholar]
- Barkovich, A.J.; Hajnal, B.L.; Vigneron, D.; Sola, A.; Partridge, J.C.; Allen, F.; Ferriero, D.M. Prediction of Neuromotor Outcome in Perinatal Asphyxia: Evaluation of MR Scoring Systems. Am. J. Neuroradiol. 1998, 19, 143–149. [Google Scholar] [PubMed]
- Albers, C.A.; Grieve, A.J. Test Review: Bayley, N. Bayley Scales of Infant and Toddler Development- Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 2007, 25, 180–190. [Google Scholar] [CrossRef]
- Li, K.; Yau, F.W.; Fok, T.F.; So, K.W.; Li, C.K.; Yuen, P.M. Haematopoietic Stem and Progenitor Cells in Human Term and Preterm Neonatal Blood. Vox Sang. 2001, 80, 162–169. [Google Scholar] [CrossRef]
- Qi, Y.; Qian, L.; Sun, B.; Chen, C.; Cao, Y. Circulating CD34+ Cells Are Elevated in Neonates with Respiratory Distress Syndrome. Inflamm. Res. 2010, 59, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Bizzarro, M.J.; Bhandari, V.; Krause, D.S.; Smith, B.R.; Gross, I. Circulating Stem Cells in Extremely Preterm Neonates. Acta Paediatr. 2007, 96, 521–525. [Google Scholar] [CrossRef]
- Borghesi, A.; Garofoli, F.; Cabano, R.; Tzialla, C.; Bollani, L.; Stronati, M. Circulating Endothelial Progenitor Cells and Diseases of the Preterm Infant. Minerva Pediatr. 2010, 62, 21–23. [Google Scholar]
- Efstathiou, N.; Soubasi, V.; Koliakos, G.; Kantziou, K.; Kyriazis, G.; Slavakis, A.; Dermentzoglou, V.; Michalettou, I.; Drosou-Agakidou, V. Beyond Brain Injury Biomarkers: Chemoattractants and Circulating Progenitor Cells as Biomarkers of Endogenous Rehabilitation Effort in Preterm Neonates with Encephalopathy. Front. Pediatr. 2023, 11, 1151787. [Google Scholar] [CrossRef]
- Ingram, D.A.; Lien, I.Z.; Mead, L.E.; Estes, M.; Prater, D.N.; Derr-Yellin, E.; Dimeglio, L.A.; Haneline, L.S. In Vitro Hyperglycemia or a Diabetic Intrauterine Environment Reduces Neonatal Endothelial Colony-Forming Cell Numbers and Function. Diabetes 2008, 57, 724–731. [Google Scholar] [CrossRef]
- Kwon, J.-Y.; Maeng, Y.-S.; Kwon, Y.-G.; Kim, Y.-H.; Kang, M.-H.; Park, Y.-W. Decreased Endothelial Progenitor Cells in Umbilical Cord Blood in Severe Preeclampsia. Gynecol. Obstet. Investig. 2007, 64, 103–108. [Google Scholar] [CrossRef]
- Borghesi, A.; Massa, M.; Campanelli, R.; Bollani, L.; Tzialla, C.; Figar, T.A.; Ferrari, G.; Bonetti, E.; Chiesa, G.; de Silvestri, A.; et al. Circulating Endothelial Progenitor Cells in Preterm Infants with Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2009, 180, 540–546. [Google Scholar] [CrossRef]
- Spiegel, A.; Kalinkovich, A.; Shivtiel, S.; Kollet, O.; Lapidot, T. Stem Cell Regulation via Dynamic Interactions of the Nervous and Immune Systems with the Microenvironment. Cell Stem Cell 2008, 3, 484–492. [Google Scholar] [CrossRef]
- Lapidot, T.; Kollet, O. The Brain-Bone-Blood Triad: Traffic Lights for Stem-Cell Homing and Mobilization. Hematol. Educ. Program Am. Soc. Hematol. 2010, 2010, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manegold, G.; Meyer-Monard, S.; Tichelli, A.; Pauli, D.; Holzgreve, W.; Troeger, C. Cesarean Section Due to Fetal Distress Increases the Number of Stem Cells in Umbilical Cord Blood. Transfusion 2008, 48, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.T.H.; Scherjon, S.A.; Van Beckhoven, J.M.; Brand, A.; Kanhai, H.H.H.; Hermans, J.M.H.; Falkenburg, J.H.F. Association of Stress during Delivery with Increased Numbers of Nucleated Cells and Hematopoietic Progenitor Cells in Umbilical Cord Blood. Am. J. Obstet. Gynecol. 2000, 183, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, F.; Plum, J.; Yöder, M.C.; Ingram, D.A.; Vandekerckhove, B.; Case, J. Endothelial Progenitor Cells: Identity Defined? J. Cell. Mol. Med. 2009, 13, 87–102. [Google Scholar] [CrossRef]
- Richardson, M.R.; Yoder, M.C. Endothelial Progenitor Cells: Quo Vadis? J. Mol. Cell. Cardiol. 2011, 50, 266–272. [Google Scholar] [CrossRef]
- Wood, T.; Thoresen, M. Physiological Responses to Hypothermia. Semin. Fetal Neonatal Med. 2015, 20, 87–96. [Google Scholar] [CrossRef]
- Dinleyici, E.C.; Tekin, N.; Colak, O.; Aksit, M.A. Cord Blood IGF-1 and IGFBP-3 Levels in Asphyxiated Term Newborns. Neuroendocrinol. Lett. 2006, 27, 745–747. [Google Scholar]
- Satar, M.; Ozcan, K.; Yapicioğlu, H.; Narli, N. Serum Insulin-like Growth Factor 1 and Growth Hormone Levels of Hypoxic-Ischemic Newborns. Biol. Neonate 2004, 85, 15–20. [Google Scholar] [CrossRef]
- Wan, Z.-H.; Xiao, X. Relationship between Serum Levels of Insulin-like Growth Factor I and Growth Hormone and Neonatal Hypoxic-Ischemic Encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi 2007, 9, 22–24. [Google Scholar]
- Hansen-Pupp, I.; Hövel, H.; Löfqvist, C.; Hellström-Westas, L.; Fellman, V.; Hüppi, P.S.; Hellström, A.; Ley, D. Circulatory Insulin-like Growth Factor-I and Brain Volumes in Relation to Neurodevelopmental Outcome in Very Preterm Infants. Pediatr. Res. 2013, 74, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Hansen-Pupp, I.; Hövel, H.; Hellström, A.; Hellström-Westas, L.; Löfqvist, C.; Larsson, E.M.; Lazeyras, F.; Fellman, V.; Hüppi, P.S.; Ley, D. Postnatal Decrease in Circulating Insulin-like Growth Factor-I and Low Brain Volumes in Very Preterm Infants. J. Clin. Endocrinol. Metab. 2011, 96, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.D.; Huang, Y.; Liu, D.; Hickey, R.; Krause, D.S.; Giordano, F.J. Stromal Cell-Derived Factor-1 Plays a Critical Role in Stem Cell Recruitment to the Heart after Myocardial Infarction but Is Not Sufficient to Induce Homing in the Absence of Injury. Circulation 2004, 110, 3300–3305. [Google Scholar] [CrossRef] [PubMed]

| Groups | Controls (Group 1) | Mild NE (Group 2) | Mode Rate/Severe NE (Group 3) | Comparisons between Groups (p) |
|---|---|---|---|---|
| n | 12 | 11 | 13 | |
| Gestational age (weeks) [Median (range)] | 38 (4) | 39 (4) | 38 (4) | |
| Birth weight (gr) [Mean (SD)] | 2962 (333) | 3341 (445) | 3098 (680) | 1 vs. 2 (<0.05) |
| Male gender [n (%)] | 5 (41.6%) | 9 (81.8%) | 8 (61.5%) | |
| Cesarean section [n (%)] | 6 (50%) | 5 (45.5%) | 6 (46.2%) | |
| Multiple gestation [n (%)] | 2 (16.7%) | 0 | 0 | |
| Apgar score 1 [Median (range)] | 8 (1) | 5 (7) | 1 (3) | 1 vs. 2 (<0.005) 1 vs. 3 (<0.005) 2 vs. 3 (<0.01) |
| Apgar score 5 [Median (range)] | 9 (1) | 8 (5) | 5 (3) | 1 vs. 2 (<0.005) 1 vs. 3 (<0.005) 2 vs. 3 (<0.05) |
| pH (first hours of life) [Mean (SD)] | 7.36 (0.1) | 7.3 (0.07) | 6.9 (0.1) | 1 vs. 3 (<0.01) 2 vs. 3 (<0.005) |
| SBE (mmol/l, first hours of life) [Mean (SD)] | −6.82 (3.14) | −8.47 (4.58) | −17.81 (4.96) | 1 vs. 3 (<0.005) 2 vs. 3 (<0.005) |
| Sepsis [n (%)] | 0 | 1 (9.1%) | 1 (7.7%) | |
| Death [n] | 0 | 0 | 3 |
| GROUPS | Comparisons (p) | |||
|---|---|---|---|---|
| Time | Controls (Group 1) | Mild NE (Group 2) | Patients (Group 3) | |
| d1 | 17.03 (27.20) | 133.06 (137.25) | 148.24 (284.58) [3a: 33.74 (37.1), 3b: 230.03 (358.92)] | 1–2 (0.062) 1–3 (<0.05) 1–3b (<0.05) |
| d3 | 3.3 (2.38) | 3.47 (2.52) | 308.78 (354.1) [3a: 45.26 (65.13), 3b: 440.54 (368.36)] | 1–3 (<0.005) 1–3b (<0.005) * |
| d9 | 6.23 (4.96) | 3.66 (2.03) | 94.51 (248.11) [3a: 4.68 (5.44), 3b: 139.42 (302.02] | |
| d18 | 7.23 (6.6) | 5.57 (2.49) | 6.32 (7.89) [3a: 6.88 (10.9), 3b: 5.87 (5.87)] | |
| d45 | 13,71 (5,49) | 10.02 (5.17) | 13.22 (8.02) [3a: 18.85 (12.52), 3b: 10.4 (4.83)] | |
| 8 m | 13.35 (4.31) | 6.7 (3.05) | 12.14 (3.69) [3a: 12.14 (3.69), 3b: -] | |
| GROUPS | Comparisons (p) | |||
|---|---|---|---|---|
| Time | Controls (Group 1) | Mild NE (Group 2) | Patients (Group 3) | |
| d1 | 27.82 (9.58) | 24.37 (2.54) | 24.02 (3.52) [3a: 25.43 (5.46), 3b: <23] | |
| d3 | 30.04 (8.84) | 34.26 (13.87) | <23 [3a: <23, 3b: <23] | 1–3 (0.072) |
| d9 | 75.17 (49.45) | 41.49 (25.74) | 36.04 (25.53) [3a: 34.55 (10.14), 3b: 37.04 (33.3)] | 1–3 (0.08) |
| d18 | 51.7 (23.99) | 54.83 (26.8) | 52.59 (24.88) [3a: 40.32 (8.75), 3b: 62.4 (30.16)] | |
| d45 | 74.11 (16.09) | 62.65 (24.33) | 60.23 (27.46) [3a: 64.95 (30.33), 3b: 58.34 (29.75)] | |
| GROUPS | Comparisons (p) | |||
|---|---|---|---|---|
| Time | Controls (Group 1) | Mild NE (Group 2) | Patients (Group 3) | |
| d1 | 1139.1 (605.6) | 865.2 (483.2) | 794.5 (430.3) [3a: 430.6 (235.6), 3b:1085.7 (300.2)] | 1–3a (0.065) |
| d3 | 1499.6 (359.1) | 1496 (443.4) | 1485.8 (581.6) [3a: 1447 (291.4), 3b: 1500.4 (676.9)] | |
| d9 | 1328.9 (283.8) | 1519.4 (476.5) | 1713.7 (495.6) [3a: 1304 (71.7), 3b: 1918.5 (489.8)] | 1–3b (0.064) |
| d18 | 1244.9 (262.7) | 1578.1 (216.4) | 1276.9 (199.7) [3a: 1264.2(132.5), 3b: 1286.4(260.4)] | 1–2 (<0.05) |
| d45 | 1485.9 (258) | 1362.8 (335) | 1264.6 (270.9) [3a: 1076.6 (52.2), 3b: 1339.8 (291)] | 1–3a (0.08) |
| 8m | 1777.5 (230.4) | 1719.12(265) | 1613.71 (216.26) [3a: 1540.1 (194.1), 3b: 1834.5] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Efstathiou, N.; Koliakos, G.; Kantziou, K.; Kyriazis, G.; Slavakis, A.; Drossou, V.; Soubasi, V. Kinetics of Circulating Progenitor Cells and Chemotactic Factors in Full-Term Neonates with Encephalopathy: Indications of Participation in the Endogenous Regenerative Process. Biomolecules 2025, 15, 427. https://doi.org/10.3390/biom15030427
Efstathiou N, Koliakos G, Kantziou K, Kyriazis G, Slavakis A, Drossou V, Soubasi V. Kinetics of Circulating Progenitor Cells and Chemotactic Factors in Full-Term Neonates with Encephalopathy: Indications of Participation in the Endogenous Regenerative Process. Biomolecules. 2025; 15(3):427. https://doi.org/10.3390/biom15030427
Chicago/Turabian StyleEfstathiou, Nikolaos, Georgios Koliakos, Katerina Kantziou, Georgios Kyriazis, Aristeidis Slavakis, Vasiliki Drossou, and Vasiliki Soubasi. 2025. "Kinetics of Circulating Progenitor Cells and Chemotactic Factors in Full-Term Neonates with Encephalopathy: Indications of Participation in the Endogenous Regenerative Process" Biomolecules 15, no. 3: 427. https://doi.org/10.3390/biom15030427
APA StyleEfstathiou, N., Koliakos, G., Kantziou, K., Kyriazis, G., Slavakis, A., Drossou, V., & Soubasi, V. (2025). Kinetics of Circulating Progenitor Cells and Chemotactic Factors in Full-Term Neonates with Encephalopathy: Indications of Participation in the Endogenous Regenerative Process. Biomolecules, 15(3), 427. https://doi.org/10.3390/biom15030427







