On-Tissue Chemical Derivatization for Mass Spectrometry Imaging of Fatty Acids with Enhanced Detection Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. MALDI-MS Spotting Experiments
2.3. Sample Preparation for MALDI-MS Imaging
2.4. MALDI-MS Imaging Data Acquisition and Analysis
2.5. LC-MS/MS Experiments
3. Results and Discussion
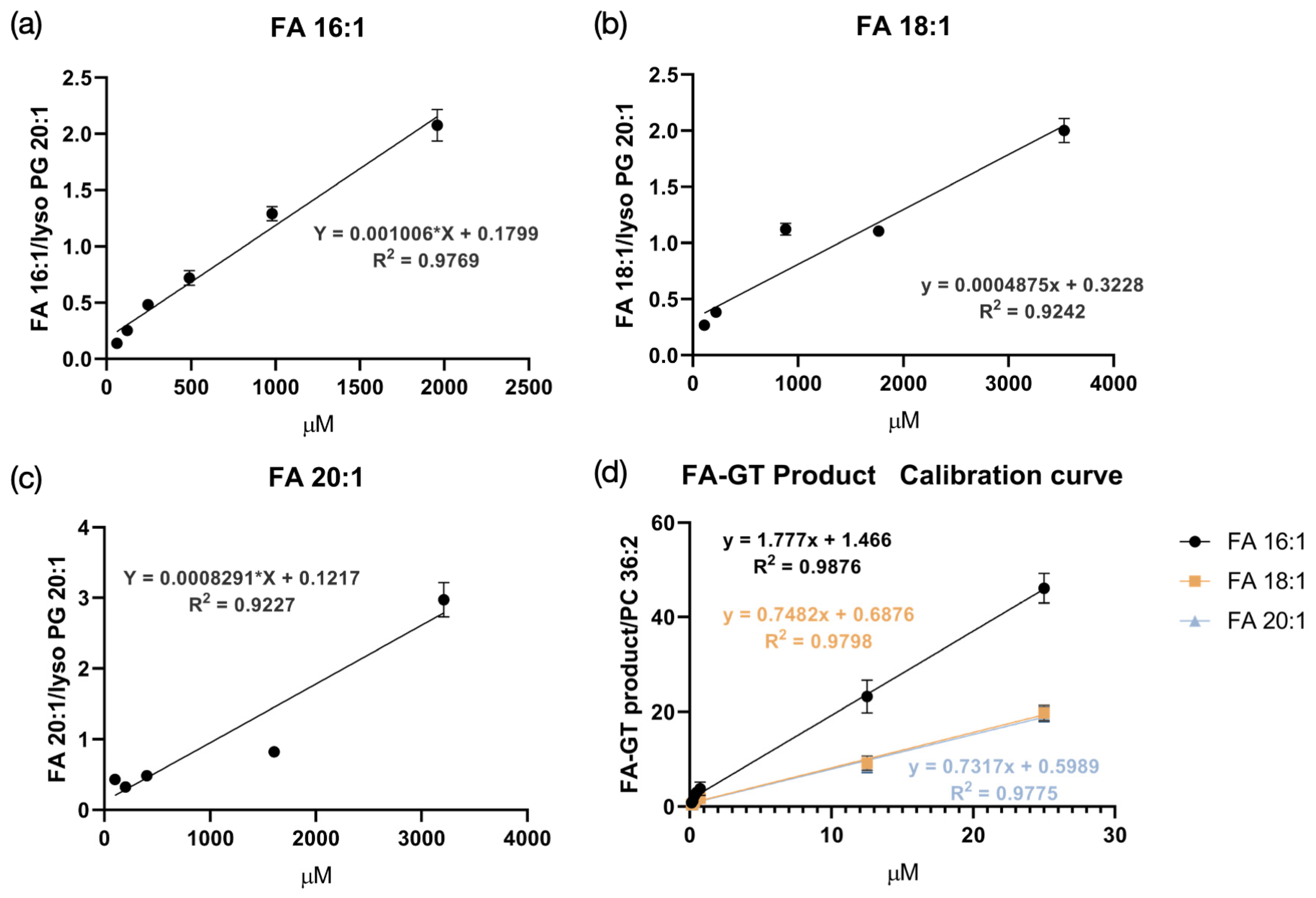
3.1. Improvement in Detection Sensitivity in Positive Mode with GT Reaction
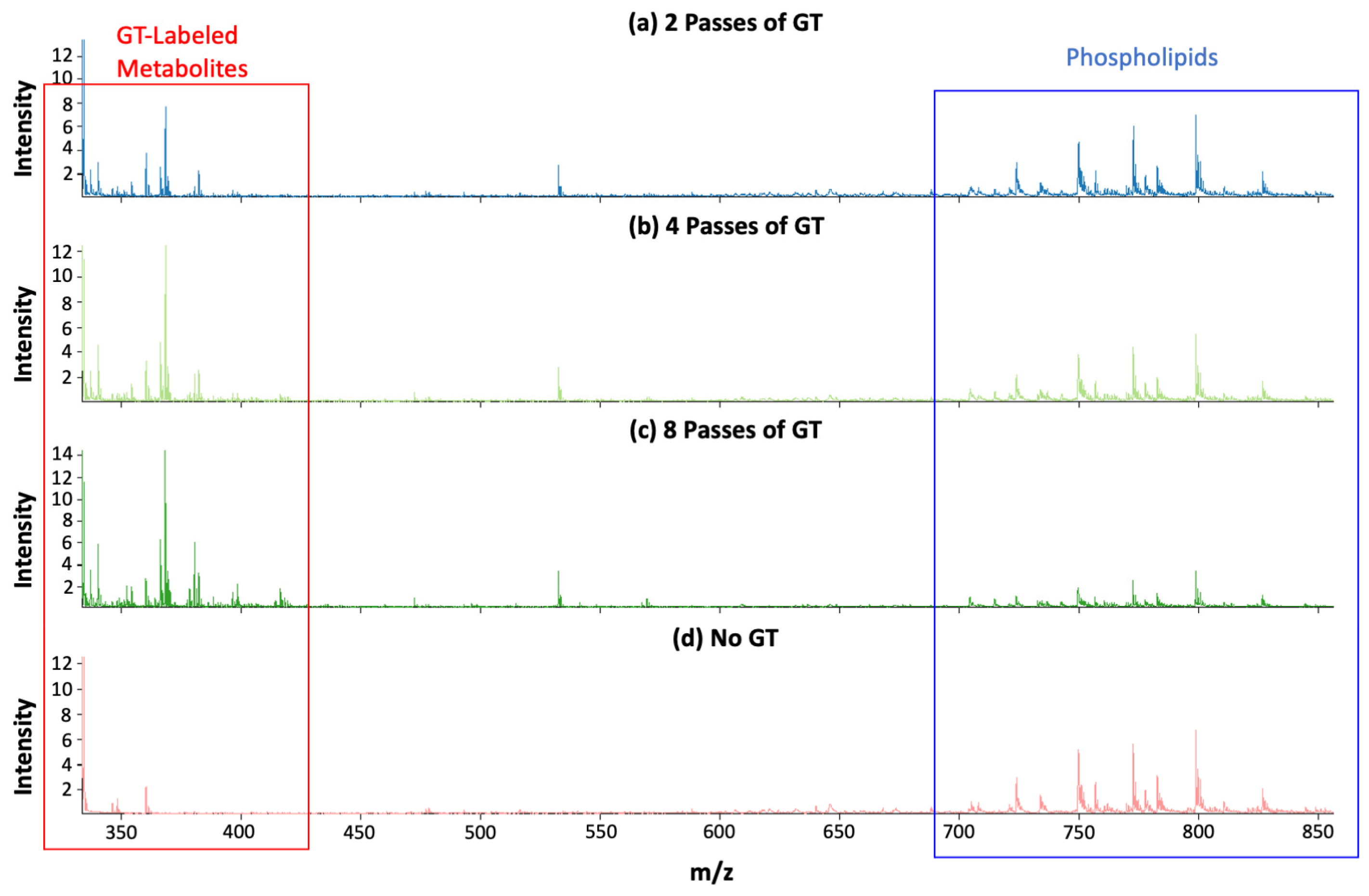
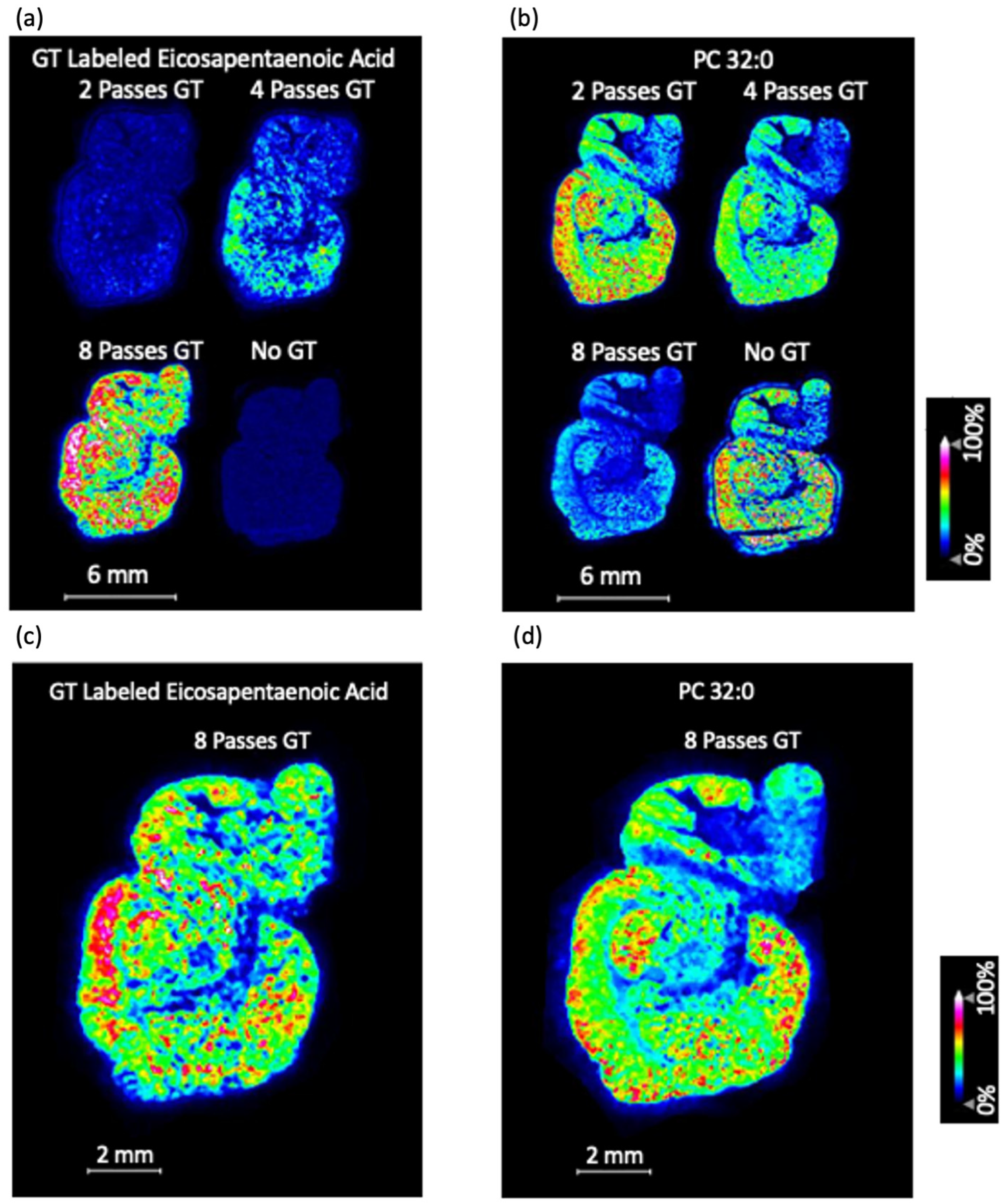
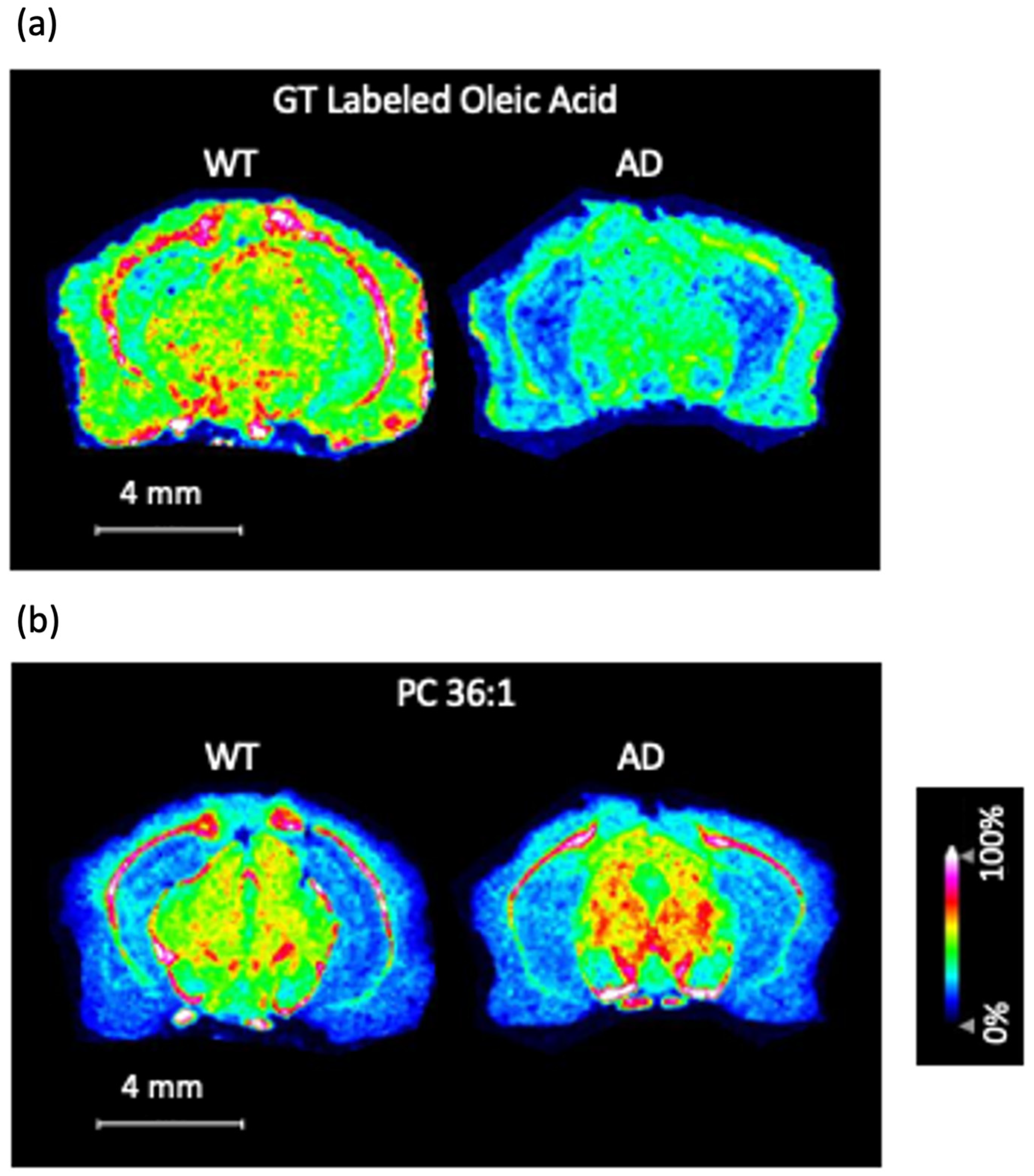
3.2. MALDI-MS Imaging of Mouse Brain Tissue Sections Using the GT Method
3.3. LC-MS/MS Analysis of GT-Labeled FA Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Shamim, A.; Mahmood, T.; Ahsan, F.; Kumar, A.; Bagga, P. Lipids: An insight into the neurodegenerative disorders. Clin. Nutr. Exp. 2018, 20, 1–19. [Google Scholar] [CrossRef]
- Ebert, D.; Haller, R.G.; Walton, M.E. Energy Contribution of Octanoate to Intact Rat Brain Metabolism Measured by 13C Nuclear Magnetic Resonance Spectroscopy. J. Neurosci. 2003, 23, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.M. Effects of fatty acid unsaturation numbers on membrane fluidity and α-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 2011, 58, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Giménez, E.; Toscani, A.M. Fatty acid signaling mechanisms in neural cells: Fatty acid receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef]
- Bagga, D.; Wang, L.; Farias-Eisner, R.; Glaspy, J.A.; Reddy, S.T. Differential effects of prostaglandin derived from ω-6 and ω-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc. Natl. Acad. Sci. USA 2003, 100, 1751–1756. [Google Scholar] [CrossRef]
- Chase, T.N.; Foster, N.L.; Fedio, P.; Brooks, R.; Mansi, L.; di Chiro, G. Regional cortical dysfunction in Alzheimer’s disease as determined by positron emission tomography. Ann. Neurol. 1984, 15, 170–174. [Google Scholar] [CrossRef]
- Foster, N.L.; Chase, T.N.; Fedio, P.; Patronas, N.J.; Brooks, R.A.; Chiro, G.D. Alzheimer’s disease: Focal cortical changes shown by positron emission tomography. Neurology 1983, 33, 961. [Google Scholar] [CrossRef]
- Geuze, E.; Vermetten, E.; Bremner, J.D. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol. Psychiatry 2005, 10, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.; Mena, E.; Subramaniam, R.M. Brain PET in the Diagnosis of Alzheimer’s Disease. Clin. Nucl. Med. 2014, 39, e413–e426. [Google Scholar] [CrossRef]
- Hu, T.; Zhu, Q.; Hu, Y.; Kamal, G.M.; Feng, Y.; Manyande, A.; Wang, J.; Xu, F. Qualitative and Quantitative Analysis of Regional Cerebral Free Fatty Acids in Rats Using the Stable Isotope Labeling Liquid Chromatography–Mass Spectrometry Method. Molecules 2020, 25, 5163. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Bazinet, R.P. ACSL6 is critical for maintaining brain DHA levels. Proc. Natl. Acad. Sci. USA 2018, 115, 12343–12345. [Google Scholar] [CrossRef] [PubMed]
- Rioux, F.M.; Innis, S.M. Oleic Acid (18:1) in Plasma, Liver and Brain Myelin Lipid of Piglets Fed from Birth with Formulas Differing in 18:1 Content. J. Nutr. 1992, 122, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, R.M.; Farmer, T.B.; Gile, J. Molecular Imaging of Biological Samples: Localization of Peptides and Proteins Using MALDI-TOF MS. Anal. Chem. 1997, 69, 4751–4760. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Michno, W.; Brinet, D.; Iacone, Y.; Zanni, G.; Blennow, K.; Zetterberg, H.; Hanrieder, J. Histology-Compatible MALDI Mass Spectrometry Based Imaging of Neuronal Lipids for Subsequent Immunofluorescent Staining. Anal. Chem. 2017, 89, 4685–4694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, X.; Vu, N.Q.; Li, G.; Li, Z.; Shi, Y.; Li, M.; Wang, B.; Welham, N.V.; Patankar, M.S.; et al. On-Tissue Derivatization with Girard’s Reagent P Enhances N-Glycan Signals for Formalin-Fixed Paraffin-Embedded Tissue Sections in MALDI Mass Spectrometry Imaging. Anal. Chem. 2020, 92, 13361–13368. [Google Scholar] [CrossRef]
- Hulme, H.; Fridjonsdottir, E.; Gunnarsdottir, H.; Vallianatou, T.; Zhang, X.; Wadensten, H.; Shariatgorji, R.; Nilsson, A.; Bezard, E.; Svenningsson, P.; et al. Simultaneous mass spectrometry imaging of multiple neuropeptides in the brain and alterations induced by experimental parkinsonism and L-DOPA therapy. Neurobiol. Dis. 2020, 137, 104738. [Google Scholar] [CrossRef]
- Spraggins, J.M.; Rizzo, D.G.; Moore, J.L.; Rose, K.L.; Hammer, N.D.; Skaar, E.P.; Caprioli, R.M. MALDI FTICR IMS of intact proteins: Using mass accuracy to link protein images with proteomics data. J. Am. Soc. Mass Spectrom. 2015, 26, 947–985. [Google Scholar] [CrossRef]
- Cornett, D.S.; Frappier, S.L.; Caprioli, R.M. MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal. Chem. 2008, 80, 5648–5653. [Google Scholar] [CrossRef]
- Baijnath, S.; Kaya, I.; Nilsson, A.; Shariatgorji, R.; Andrén, P.E. Advances in spatial mass spectrometry enable in-depth neuropharmacodynamics. Trends Pharmacol. Sci. 2022, 43, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, A.R.; DeLaney, K.; Johnson, J.; Li, L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal. Chem. 2018, 90, 240–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Comi, T.J.; Li, B.; Rubakhin, S.S.; Sweedler, J.V. On-Tissue Derivatization via Electrospray Deposition for Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging of Endogenous Fatty Acids in Rat Brain Tissues. Anal. Chem. 2016, 88, 5988–5995. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, Y.J.; Zhang, J.; Sun, T.Q.; Guo, Y.L. Derivatization strategy for simultaneous molecular imaging of phospholipids and low-abundance free fatty acids in thyroid cancer tissue sections. Anal. Chem. 2019, 91, 4070–4076. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, W.; Geng, Y.; Wang, X. On-Tissue Derivatization Strategy for Mass Spectrometry Imaging of Carboxyl-Containing Metabolites in Biological Tissues. Anal. Chem. 2020, 92, 12126–12131. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Schembri, L.S.; Nilsson, A.; Shariatgorji, R.; Baijnath, S.; Zhang, X.; Bezard, E.; Svenningsson, P.; Odell, L.R.; Andrén, P.E. On-Tissue Chemical Derivatization for Comprehensive Mapping of Brain Carboxyl and Aldehyde Metabolites by MALDI-MS Imaging. J. Am. Soc. Mass Spectrom. 2023, 34, 836–846. [Google Scholar] [CrossRef]
- Shimma, S.; Kumada, H.O.; Taniguchi, H.; Konno, A.; Yao, I.; Furuta, K.; Matsuda, T.; Ito, S. Microscopic visualization of testosterone in mouse testis by use of imaging mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 7607–7615. [Google Scholar] [CrossRef] [PubMed]
- Cobice, D.F.; Livingstone, D.E.W.; MacKay, C.L.; Goodwin, R.J.A.; Smith, L.B.; Walker, B.R.; Andrew, R. Spatial Localization and Quantitation of Androgens in Mouse Testis by Mass Spectrometry Imaging. Anal. Chem. 2016, 88, 10362–10367. [Google Scholar] [CrossRef]
- Barré, F.P.Y.; Flinders, B.; Garcia, J.P.; Jansen, I.; Huizing, L.R.S.; Porta, T.; Creemers, L.B.; Heeren, R.M.A.; Cillero-Pastor, B. Derivatization strategies for the detection of triamcinolone acetonide in cartilage by using matrix-assisted laser desorption/ionization mass spectrometry imaging. Anal. Chem. 2016, 88, 12051–12059. [Google Scholar] [CrossRef]
- Song, W.-S.; Park, H.-G.; Kim, S.-M.; Jo, S.-H.; Kim, B.-G.; Theberge, A.B.; Kim, Y.-G. Chemical derivatization-based LC–MS/MS method for quantitation of gut microbial short-chain fatty acids. J. Ind. Eng. Chem. 2020, 83, 297–302. [Google Scholar] [CrossRef]
- Sládková, K.; Houška, J.; Havel, J. Laser desorption ionization of red phosphorus clusters and their use for mass calibration in time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 3114–3118. [Google Scholar] [CrossRef] [PubMed]
- Szájli, E.; Fehér, T.; Medzihradszky, K.F. Investigating the Quantitative Nature of MALDI-TOF MS. Mol. Cell. Proteom. 2008, 7, 2410–2418. [Google Scholar] [CrossRef]
- Tobias, F.; Hummon, A.B. Considerations for MALDI-Based Quantitative Mass Spectrometry Imaging Studies. J. Proteome Res. 2020, 19, 3620–3630. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Jurcic, K.; Wang, J.S.H.; Whitehead, S.N.; Yeung, K.K.C. 1,6-Diphenyl-1,3,5-hexatriene (DPH) as a Novel Matrix for MALDI MS Imaging of Fatty Acids, Phospholipids, and Sulfatides in Brain Tissues. Anal. Chem. 2017, 89, 12828–12836. [Google Scholar] [CrossRef] [PubMed]
- Khamidova, N.; Pergande, M.R.; Pathmasiri, K.C.; Khan, R.; Mohr, J.T.; Cologna, S.M. DBDA Matrix Increases Ion Abundance of Fatty Acids and Sulfatides in MALDI-TOF and Mass Spectrometry Imaging Studies. J. Am. Soc. Mass Spectrom. 2023, 34, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Mougan, I. Fatty Acid Composition of Human Brain Phospholipids During Normal Development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jordan, J.D.; Zhang, Q. Myelin Pathology in Alzheimer’s Disease: Potential Therapeutic Opportunities. Aging Dis. 2024, 15, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Trépanier, M.O.; Hildebrand, K.D.; Nyamoya, S.D.; Amor, S.; Bazinet, R.P.; Kipp, M. Phosphatidylcholine 36:1 concentration decreases along with demyelination in the cuprizone animal model and in post-mortem multiple sclerosis brain tissue. J. Neurochem. 2018, 145, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.J.; Post, S.N.J.J.; Woods, A.S. A minimalist approach to MALDI imaging of glycerophospholipids and sphingolipids in rat brain sections. Int. J. Mass Spectrom. 2008, 278, 143–149. [Google Scholar] [CrossRef]
- Martínez-Gardeazabal, J.; González de San Román, E.; Moreno-Rodríguez, M.; Llorente-Ovejero, A.; Manuel, I.; Rodríguez-Puertas, R. Lipid mapping of the rat brain for models of disease. Biochim. Biophys. Acta—Biomembr. 2017, 1859, 1548–1557. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, M.; Shi, X.; Liu, Y.; Li, Z.; Jagodinsky, J.C.; Ma, M.; Welham, N.V.; Morris, Z.S.; Li, L. Quantification and molecular imaging of fatty acid isomers from complex biological samples by mass spectrometry. Chem. Sci. 2021, 12, 8115–8122. [Google Scholar] [CrossRef]
- Shetty, S.; Kumari, S. Fatty acids and their role in type-2 diabetes (Review). Exp. Ther. Med. 2021, 22, 706. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Alves, B.d.S.; Schimith, L.E.; da Cunha, A.B.; Dora, C.L.; Hort, M.A. Omega-3 polyunsaturated fatty acids and Parkinson’s disease: A systematic review of animal studies. J. Neurochem. 2024, 168, 1655–1683. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebbini, M.; Wang, Z.; Zhang, H.; Lu, K.H.; Huang, P.; Kaminsky, C.J.; Puglielli, L.; Li, L. On-Tissue Chemical Derivatization for Mass Spectrometry Imaging of Fatty Acids with Enhanced Detection Sensitivity. Biomolecules 2025, 15, 366. https://doi.org/10.3390/biom15030366
Ebbini M, Wang Z, Zhang H, Lu KH, Huang P, Kaminsky CJ, Puglielli L, Li L. On-Tissue Chemical Derivatization for Mass Spectrometry Imaging of Fatty Acids with Enhanced Detection Sensitivity. Biomolecules. 2025; 15(3):366. https://doi.org/10.3390/biom15030366
Chicago/Turabian StyleEbbini, Malik, Zicong Wang, Hua Zhang, Kelly H. Lu, Penghsuan Huang, Cameron J. Kaminsky, Luigi Puglielli, and Lingjun Li. 2025. "On-Tissue Chemical Derivatization for Mass Spectrometry Imaging of Fatty Acids with Enhanced Detection Sensitivity" Biomolecules 15, no. 3: 366. https://doi.org/10.3390/biom15030366
APA StyleEbbini, M., Wang, Z., Zhang, H., Lu, K. H., Huang, P., Kaminsky, C. J., Puglielli, L., & Li, L. (2025). On-Tissue Chemical Derivatization for Mass Spectrometry Imaging of Fatty Acids with Enhanced Detection Sensitivity. Biomolecules, 15(3), 366. https://doi.org/10.3390/biom15030366






