Protective Effects of Bromelain in Testicular Torsion-Detorsion: Reducing Inflammation, Oxidative Stress, and Apoptosis While Enhancing Sperm Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals and Experimental Design
2.3. Measurement of Antioxidants Enzymes (SOD, GSH) and Lipid Peroxidation (MDA)
2.4. Measurement of Pro-Inflammatory Cytokines
2.5. Immunoblotting
2.6. Epididymal Spermatozoon Density Measurement
2.7. Spermatozoon Motility Assessment
2.8. Abnormal Spermatozoon Rate Determination
2.9. Histological Procedures Evaluation of Testicular Tissue
2.10. Statistical Analyses
3. Results
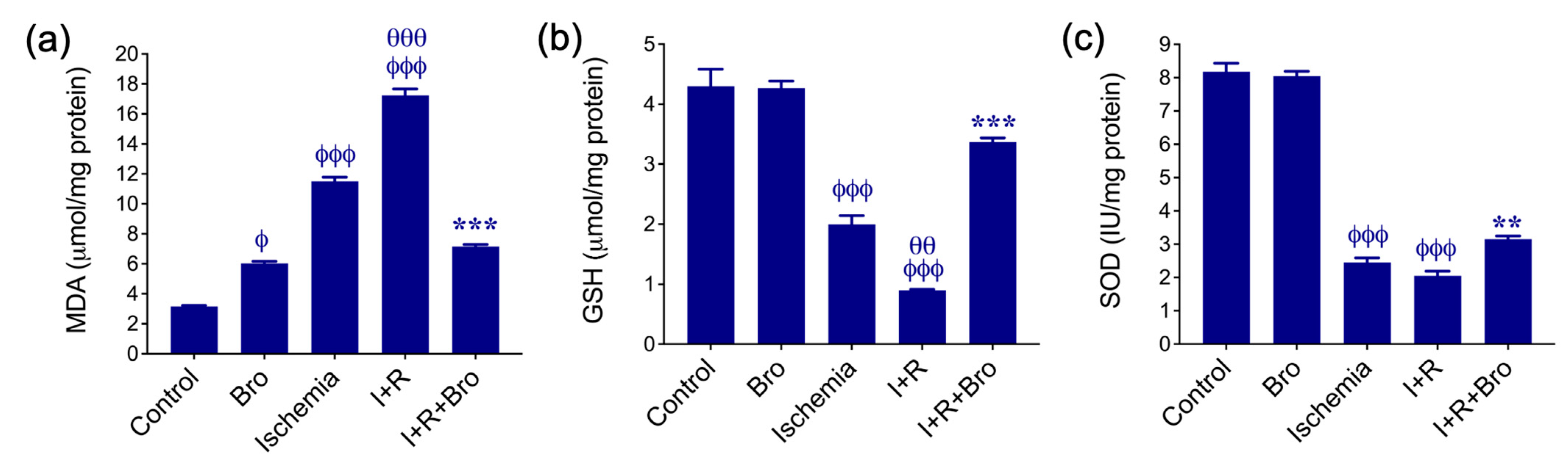
3.1. Effect of Bro on Antioxidant Enzymes and Lipid Peroxidation in I/R-Induced Testis Injury
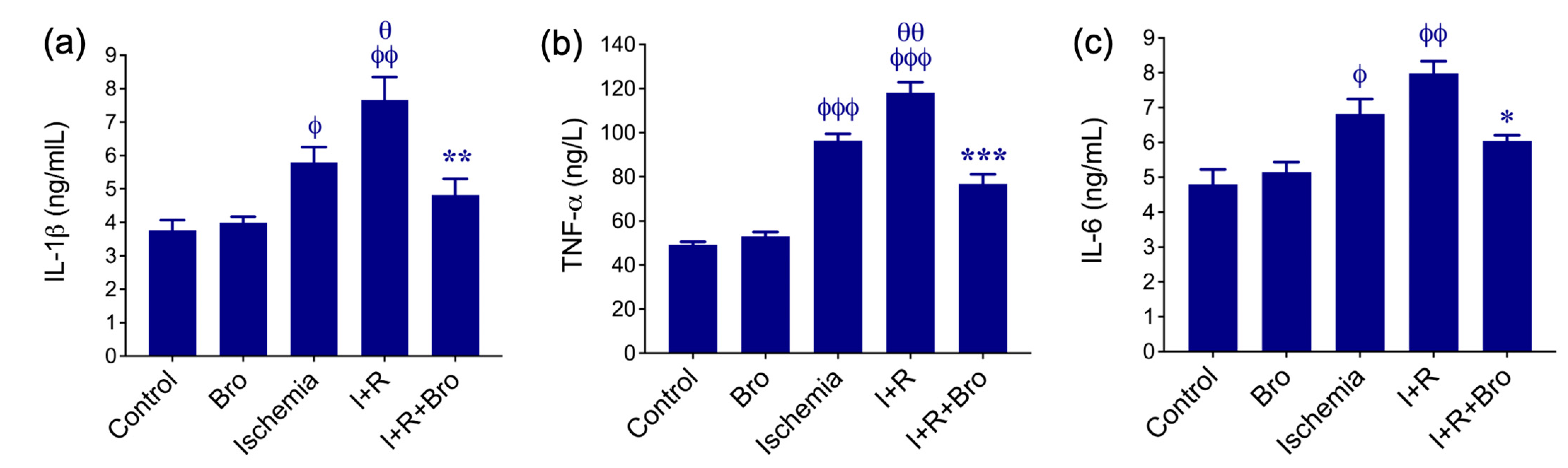
3.2. Effect of Bro on Pro-Inflammatory Cytokines Production in I/R-Induced Testis Injury
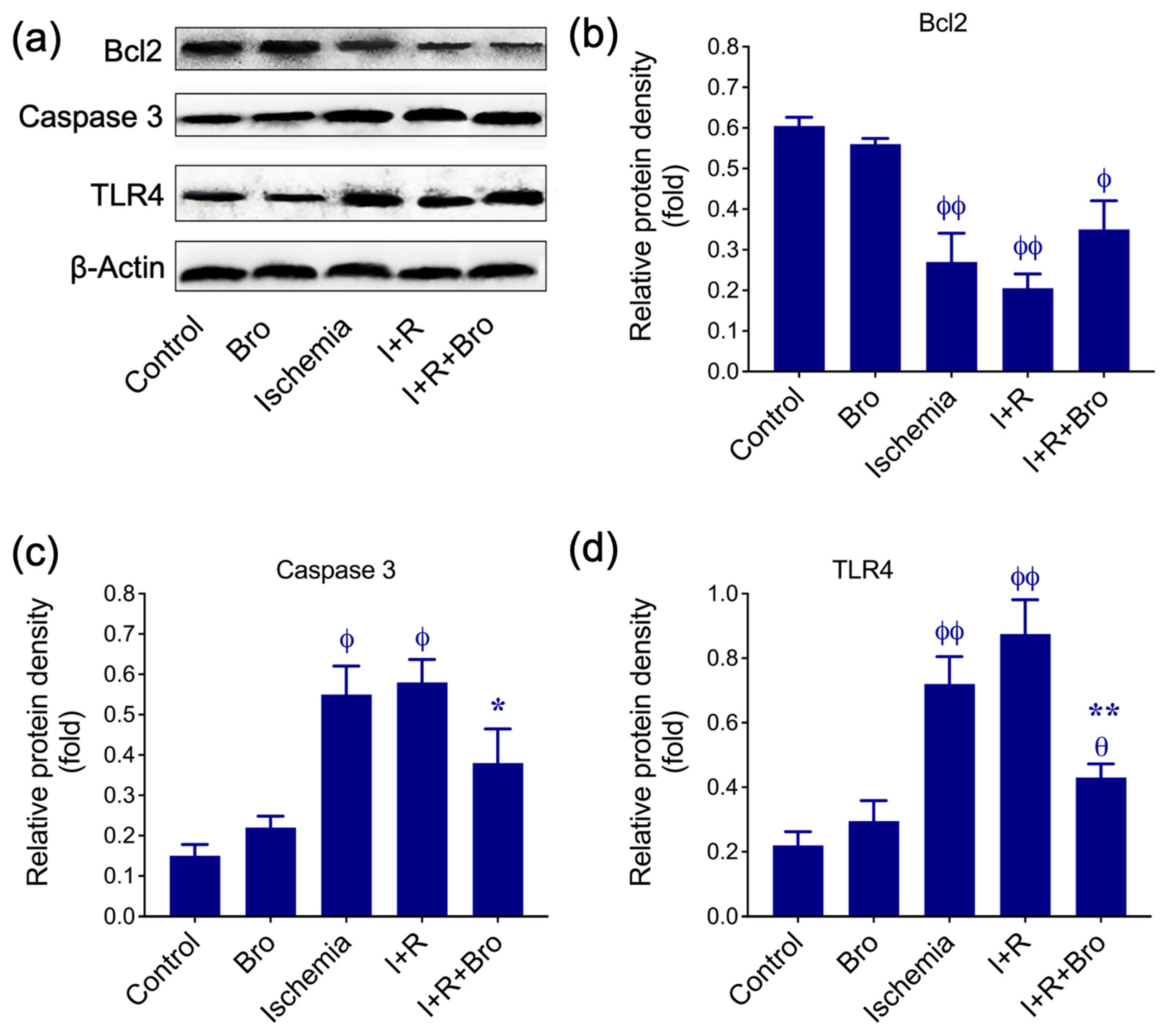
3.3. Effect of Bro on Apoptosis and Inflammatory Biomarkers in I/R-Induced Testis Injury
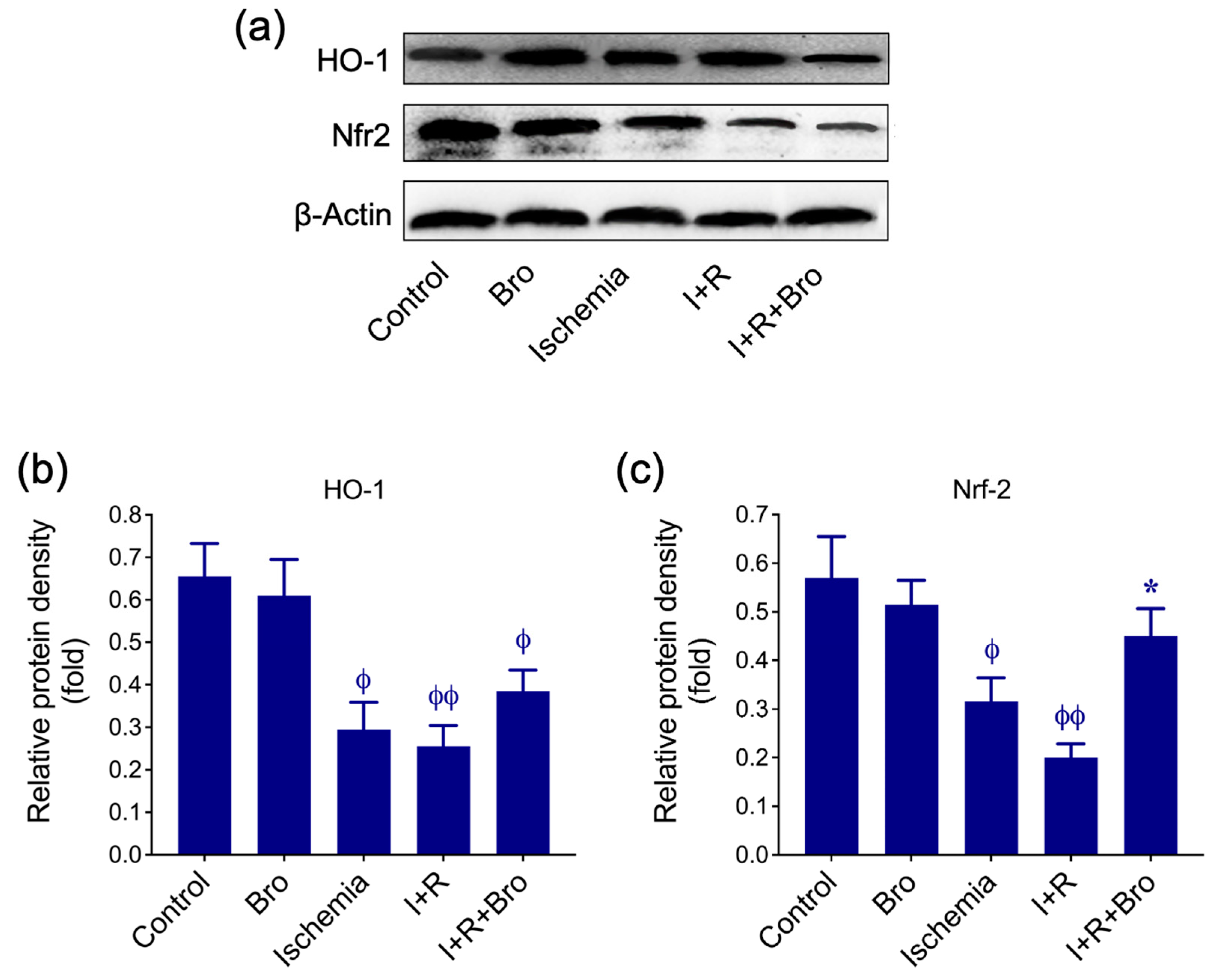
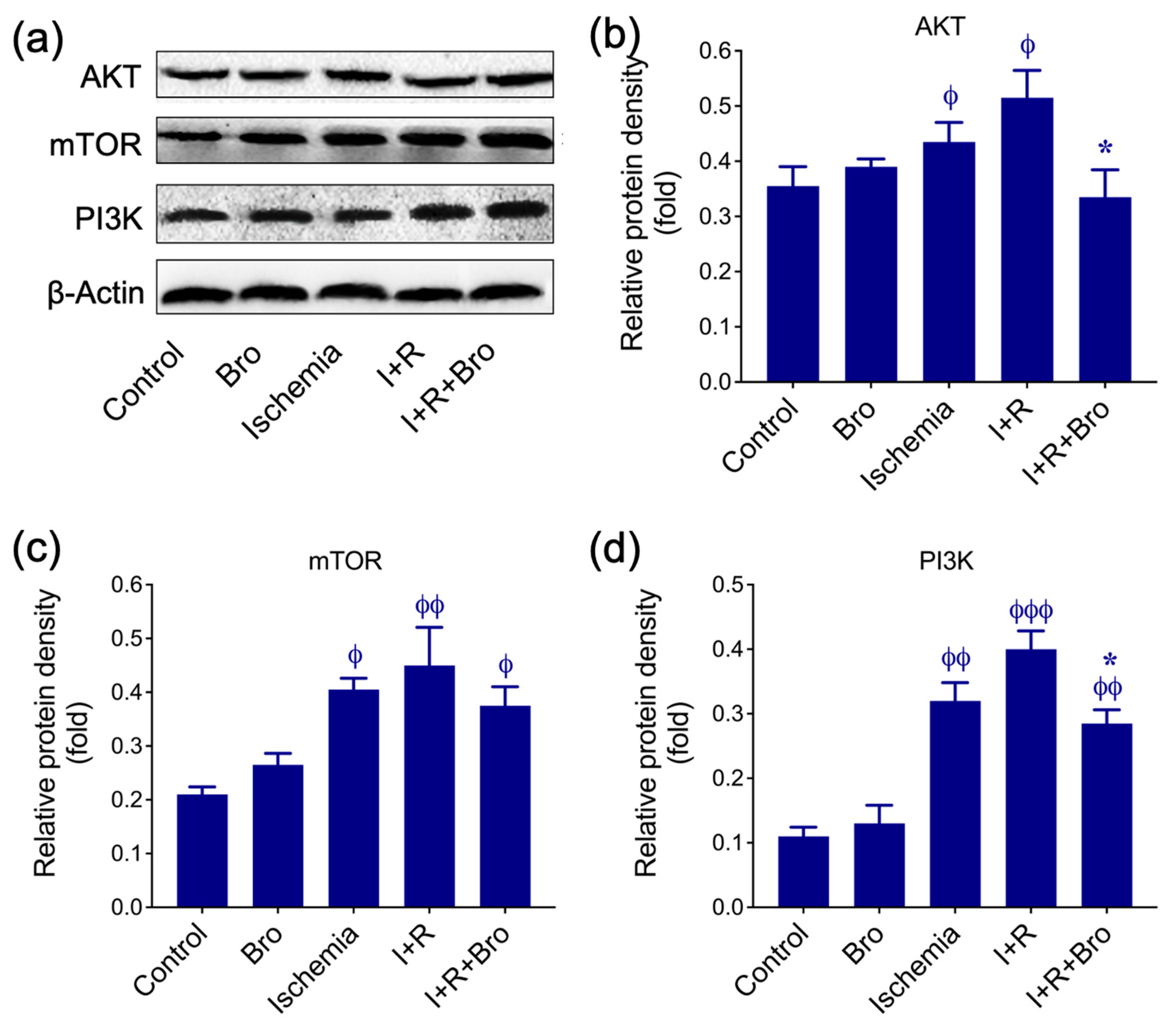
3.4. Bro Treatment Restores Nrf2 and HO-1 Levels in I/R-Induced Testis Injury
3.5. Bro Treatment Promotes Cell Survival Under I/R-Induced Testis Injury
3.6. Effect of Bro on Spermatologic Examinations in I/R-Induced Testis Injury
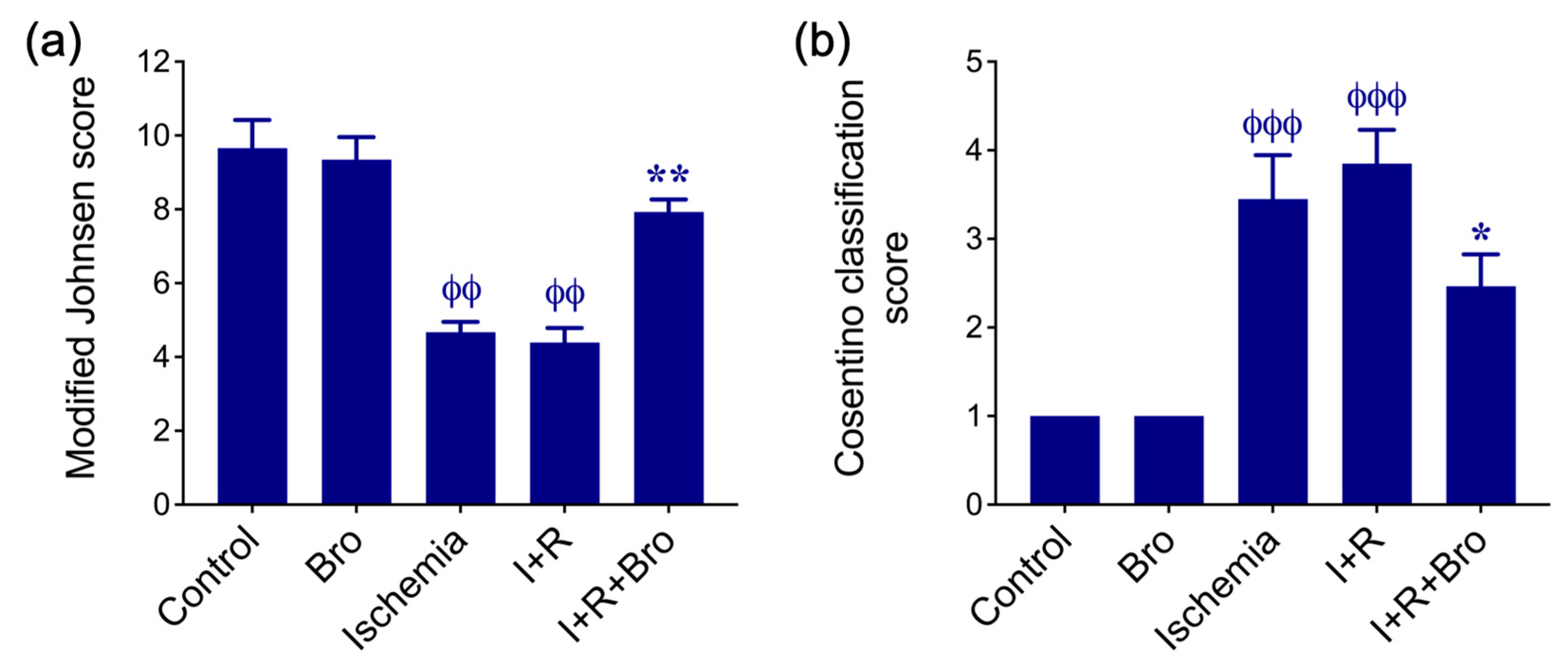
3.7. Histologic Evaluation of Testicular Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Minas, A.; Mahmoudabadi, S.; Gamchi, N.S.; Antoniassi, M.P.; Alizadeh, A.; Bertolla, R.P. Testicular torsion in vivo models: Mechanisms and treatments. Andrology 2023, 11, 1267–1285. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Campbell, C.; Omoyinmi, E.; Hong, Y.; Al Obaidi, M.; Sebire, N.; Brogan, P.A. Testicular ischemia in deficiency of adenosine deaminase 2 (DADA2). Pediatr. Rheumatol. 2019, 17, 39. [Google Scholar] [CrossRef]
- Arena, S.; Iacona, R.; Antonuccio, P.; Russo, T.; Salvo, V.; Gitto, E.; Impellizzeri, P.; Romeo, C. Medical perspective in testicular ischemia-reperfusion injury. Exp. Ther. Med. 2017, 13, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Almarzouq, D.; Al-Maghrebi, M. NADPH oxidase-mediated testicular oxidative imbalance regulates the TXNIP/NLRP3 inflammasome axis activation after ischemia reperfusion injury. Antioxidants 2023, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Kazaz, I.O.; Kerimoglu, G.; Demir, E.A.; Colak, F.; Biyik, A.F.; Cansever, Y.; Mentese, A. Propolis ameliorates ischemia/reperfusion-induced testicular damage by reducing oxidative stress. Rev. Int. Androl. 2023, 21, 100364. [Google Scholar] [CrossRef] [PubMed]
- Gezer, R.; Tuncer, M.C.; Kasirga, Z.; Deveci, E.; Aşır, F. Investigation of the effect of quercetin in experimental ischemia-reperfusion injury model in rat testicle. J. Drug Deliv. Ther. 2023, 13, 60–65. [Google Scholar] [CrossRef]
- Nia, V.M.; Rezaei, N.; Sayyad, M.S.; Seyedabadi, M.; Amiri, F.T.; Shaki, F. The protective effects of citrulline on testicular injury induced by torsion and detorsion in adult male rats: An experimental study. J. Reprod. Infertil. 2024, 25, 201. [Google Scholar]
- Wei, S.-M.; Huang, Y.-M. Baicalein alleviates testicular ischemia-reperfusion injury in a rat model of testicular torsion-detorsion. Oxid. Med. Cell. Longiv. 2022, 2022, 1603469. [Google Scholar] [CrossRef] [PubMed]
- Kazak, F.; Akcakavak, G.; Alakus, I.; Alakus, H.; Kirgiz, O.; Karatas, O.; Deveci, M.Z.Y.; Coskun, P. Proanthocyanidin alleviates testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Tissue Cell 2024, 89, 102459. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hsia, C.C.; Hu, P.A.; Yeh, C.H.; Chen, C.T.; Peng, C.L.; Wang, C.H.; Lee, T.S. Bromelain ameliorates atherosclerosis by activating the TFEB-mediated autophagy and antioxidant pathways. Antioxidants 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, E.; Aazami, M.H.; Aghamiri, S.M.R.; Satari, A.; Hosseinzadeh, S.; Lemoigne, Y.; Heidarian, E. Bromelain-based chemo-herbal combination effect on human cancer cells: In-vitro study on AGS and MCF7 proliferation and apoptosis. Curr. Issues Pharm. Med. Sci. 2020, 33, 155–161. [Google Scholar] [CrossRef]
- Pezzani, R.; Jimenez-Garcia, M.; Capo, X.; Sönmez Gürer, E.; Sharopov, F.; Rachel, T.Y.L.; Ntieche Woutouoba, D.; Rescigno, A.; Peddio, S.; Zucca, P. Anticancer properties of bromelain: State-of-the-art and recent trends. Front. Oncol. 2023, 12, 1068778. [Google Scholar] [CrossRef]
- Khazaeel, K.; Rad, O.R.; Jamshidian, J.; Tabandeh, M.R.; Mohammadi, G.; Atashfaraz, A. Effect of bromelain on sperm quality, testicular oxidative stress and expression of oestrogen receptors in BISPHENOL-A treated male mice. Andrologia 2022, 54, e14584. [Google Scholar] [CrossRef] [PubMed]
- Eraky, S.M.; Ramadan, N.M.; Abo El-Magd, N.F. Ameliorative effects of bromelain on aluminum-induced Alzheimer’s disease in rats through modulation of TXNIP pathway. Int. J. Biol. Macromol. 2023, 227, 1119–1131. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Placer, Z.A.; Cushman, L.L.; Johnson, B.C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal. Biochem. 1966, 16, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Yokoi, K.; Uthus, E.O.; Nielsen, F.H. Nickel deficiency diminishes sperm quantity and movement in rats. Biol. Trace Elem. Res. 2003, 93, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Koca, R.H.; Hürkul, M.M.; Kurt, S.; Köroğlu, A. Wistar Albino Sıçanlarda Michauxia campanuloides L’Hér.’in Bazı Sperma Parametreleri Üzerine Etkisi. Atatürk Univ. Vet. Bilim. Derg. 2020, 15, 138–144. [Google Scholar]
- Johnsen, S.G. Testicular biopsy score count–a method for registration of spermatogenesis in human testes: Normal values and results in 335 hypogonadal males. Horm. Res. Pediatr. 1970, 1, 2–25. [Google Scholar] [CrossRef]
- Cosentino, M.J.; Nishida, M.; Rabinowitz, R.; Cockett, A.T. Histological changes occurring in the contralateral testes of prepubertal rats subjected to various durations of unilateral spermatic cord torsion. J. Urol. 1985, 133, 906–911. [Google Scholar] [CrossRef]
- Demir, S.; Kazaz, I.O.; Mungan, S.A.; Alemdar, N.T.; Perolli, N.; Demir, E.A.; Mentese, A. Usnic acid alleviates testicular ischemia/reperfusion injury in rats by modulating endoplasmic reticulum stress. Reprod. Toxicol. 2024, 128, 108654. [Google Scholar] [CrossRef] [PubMed]
- Ganjiani, V.; Bigham-Sadegh, A.; Ahmadi, N.; Divar, M.-R.; Meimandi-Parizi, A.; Asude, M. The potential prophylactic and therapeutic impacts of niacin on ischemia/reperfusion injury of testis. J. Pediatr. Urol. 2024, 20, 281.e1–281.e7. [Google Scholar] [CrossRef]
- Wei, S.M.; Huang, Y.M. Protective effect of allicin on ischemia-reperfusion injury caused by testicular torsion-detorsion in rats. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 2817–2826. [Google Scholar]
- El-Demerdash, F.M.; Hussien, D.M.; Ghanem, N.F.; Al-Farga, A.M. Bromelain modulates liver injury, hematological, molecular, and biochemical perturbations induced by aluminum via oxidative stress inhibition. BioMed Res. Int. 2022, 2022, 5342559. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, F.M.; Baghdadi, H.H.; Ghanem, N.F.; Mhanna, A.B.A. Nephroprotective role of bromelain against oxidative injury induced by aluminium in rats. Environ. Toxicol. Pharmacol. 2020, 80, 103509. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Prescient indices of activity: The application of functional system sensitivity to measurement of drug effect. Trends Pharmacol. Sci. 2019, 40, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, E.; Özkanlar, S.; Yeşildağ, A.; Kara, A.; Kumar, K.S. Syringic acid loaded silver nanoparticles protects ovarian ischemia-reperfusion injury in rats by inhibiting endoplasmic reticulum stress-mediated apoptosis. J. Drug Deliv. Sci. Technol. 2024, 99, 105944. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Volos, L.; OM, O.G.; Veselyi, M.Y.; Veselyy, S.; Lavrov, D.; Hladkiy, O.; Usenko, T. Histopathological changes and immunohistochemical characteristics of the testicle in children with subcompensated ischemia during acute torsion. Med. Sci. Ukr. 2023, 19, 37–45. [Google Scholar] [CrossRef]
- Schanaider, A.; Aiex, C.; Errico, G. Immunological effects of acute testicular torsion on the contralateral testis in rats. Eur. J. Pediatr. Surg. 2011, 21, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Tsounapi, P.; Saito, M.; Dimitriadis, F.; Shimizu, S.; Kinoshita, Y.; Shomori, K.; Satoh, I.; Satoh, K. Protective effect of sivelestat, a neutrophil elastase inhibitor, on ipsilateral and contralateral testes after unilateral testicular ischaemia–reperfusion injury in rats. BJU Int. 2011, 107, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Karaguzel, E.; Kadihasanoglu, M.; Kutlu, O. Mechanisms of testicular torsion and potential protective agents. Nat. Rev. Urol. 2014, 11, 391–399. [Google Scholar] [CrossRef]
- Li, J.; Yan, Z.; Wang, Q.; Wei, S.; Liu, Q.; Liu, T.; Hu, Z. Pretreatment with remote ischemic conditioning attenuates testicular damage after testicular ischemia and reperfusion injury in rats. PLoS ONE 2023, 18, e0287987. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Güler, M.C.; Tanyeli, A.; Akdemir, F.N.E.; Eraslan, E.; Şebin, S.Ö.; Erdoğan, D.G.; Nacar, T. An Overview of Ischemia–Reperfusion Injury: Review on Oxidative Stress and Inflammatory Response. Eurasian J. Med. 2022, 54, S62. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Ng, M.Y.; Liao, Y.-W.; Maekawa, S.; Lin, T.; Yu, C.-C. Bromelain inhibits the inflammation and senescence effect in diabetic periodontitis: A preliminary in vitro study. J. Dent. Sci. 2023, 18, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.; Harder, Y.; Schmauss, D.; Menger, M.D.; Laschke, M.W. Bromelain protects critically perfused musculocutaneous flap tissue from necrosis. Biomedicines 2022, 10, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Glaviano, A.; Foo, A.S.; Lam, H.Y.; Yap, K.C.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.-m.; Zhou, J. The role of PI3K/AKT signaling pathway in myocardial ischemia-reperfusion injury. Int. Immunopharmacol. 2023, 123, 110714. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, S.M.; Jinga, M.; Miricescu, D.; Stefani, C.; Nica, R.I.; Stanescu-Spinu, I.-I.; Vacaroiu, I.A.; Greabu, M.; Nica, S. mTOR Dysregulation, Insulin Resistance, and Hypertension. Biomedicines 2024, 12, 1802. [Google Scholar] [CrossRef] [PubMed]
- Jebur, A.B.; El-Demerdash, F.M.; Kang, W. Bromelain from Ananas comosus stem attenuates oxidative toxicity and testicular dysfunction caused by aluminum in rats. J. Trace Elem. Med. Biol. 2020, 62, 126631. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.; Sönmez, M.G.; Ecer, G.; Kılınç, F.; Kocabaş, R.; Atılgan, A.E.; Oltulu, P.; Balasar, M. The effect of intratesticular dexpanthenol on experimentally-induced testicular ischaemia/reperfusion injury. J. Pediatr. Urol. 2021, 17, 440.e1–440.e7. [Google Scholar] [CrossRef] [PubMed]
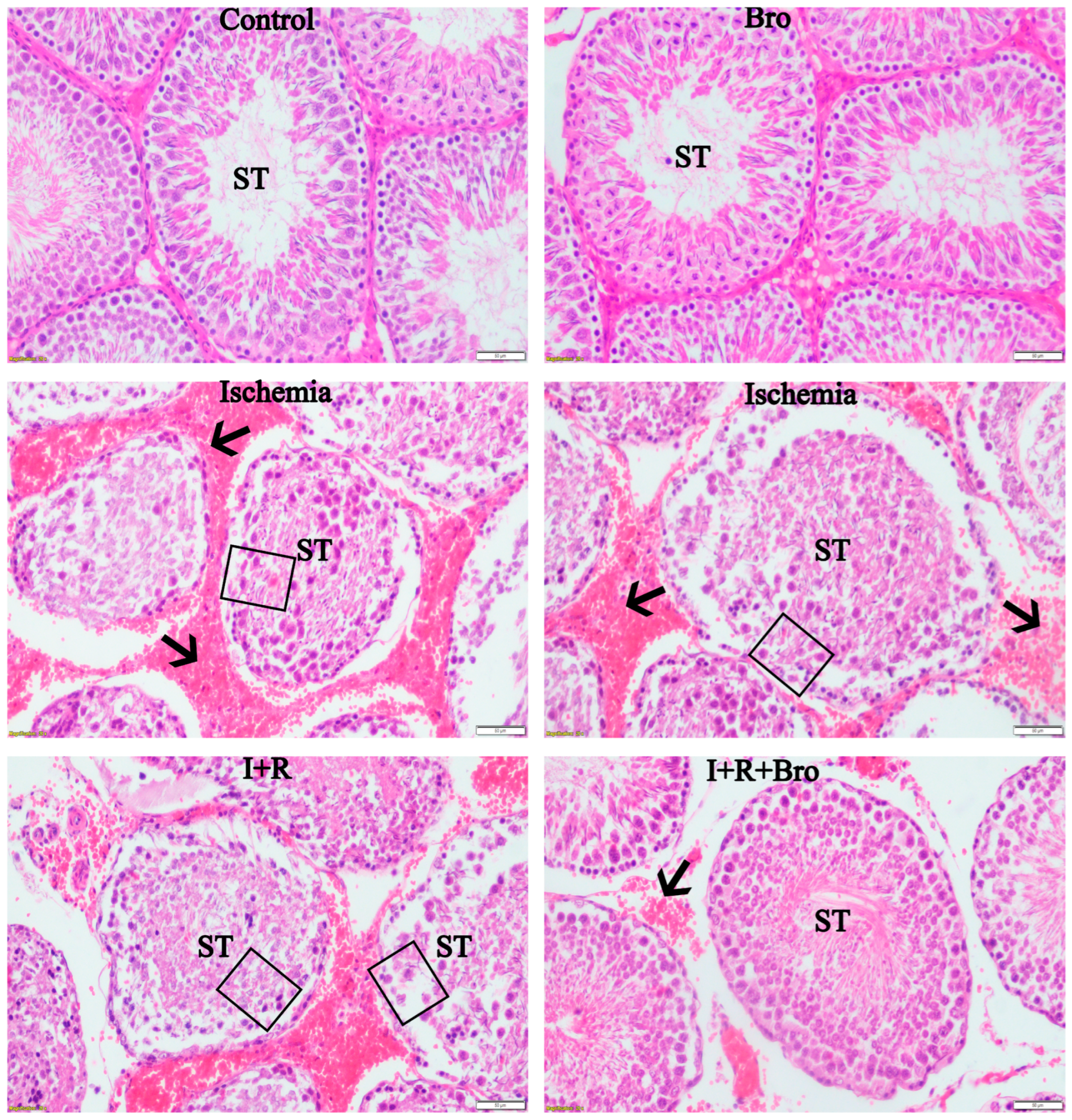
| Grade | Criteria |
|---|---|
| Grade 1 | Normal germinal cells in the testicles |
| Grade 2 | Mild hemorrhage interstitial edema, or less regular, densely packed seminiferous tubules |
| Grade 3 | Widespread hemorrhage an irregular pyknotic nucleus, and less pronounced seminiferous tubule borders |
| Grade 4 | Tightly packed seminiferous tubules with germ cell coagulation necrosis |
| Parameters | Control | Bro | Ischemia | I+R | I+R+Bro |
|---|---|---|---|---|---|
| Total Motility (%) | 72.22 ± 11.08 | 76.12 ± 3.33 | 44.66 ± 28.73 ϕ | 12.85 ± 17.47 ϕ,θ | 60.00 ± 28.08 ϕ,* |
| Concentration (×106/cauda) | 82.25 ± 7.47 | 84.33 ± 3.50 | 63.50 ± 6.51 ϕ | 47.92 ± 7.21 ϕ,θ | 83.33 ± 4.16 ϕ,* |
| Abnormal Head Spermatozoon | 4.20 ± 0.83 | 4.16 ± 1.32 | 5.00 ± 2.82 | 5.42 ± 2.07 | 5.66 ± 2.08 |
| Abnormal Tail Spermatozoon | 9.20 ± 3.34 | 10.16 ± 4.11 | 10.21 ± 3.27 | 14.28 ± 3.77 | 8.66 ± 2.08 |
| Abnormal Total Spermatozoon | 13.40 ± 3.91 | 14.33 ± 5.20 | 15.21 ± 5.89 | 19.71 ± 5.64 | 14.33 ± 4.16 |
| Right Testis (gr) | 1.48 ± 0.14 | 1.43 ± 0.09 | 1.36 ± 0.05 | 1.49 ± 0.17 | 1.42 ± 0.05 |
| Left Testis (gr) | 1.68 ± 0.12 | 1.71 ± 0.16 | 1.37 ± 0.40 ϕ | 1.45 ± 0.10 ϕ,θ | 1.54 ± 0.20 ϕ,* |
| Right Epidydimis (gr) | 0.62 ± 0.11 | 0.60 ± 0.08 | 0.45 ± 0.13 | 0.50 ± 0.06 | 0.45 ± 0.05 |
| Left Epidydimis (gr) | 0.61 ± 0.06 | 0.62 ± 0.09 | 0.47 ± 0.22 ϕ | 0.48 ± 0.07 ϕ | 0.50 ± 0.11 * |
| Right Cauda (gr) | 0.24 ± 0.07 | 0.27 ± 0.03 | 0.17 ± 0.03 | 0.24 ± 0.08 | 0.24 ± 0.10 |
| Vesicula Seminalis (gr) | 0.83 ± 0.12 | 0.93 ± 0.20 | 0.65 ± 0.11 ϕ | 0.62 ± 0.11 ϕ,θ | 0.63 ± 0.09 |
| Prostate (gr) | 0.22 ± 0.02 | 0.22 ± 0.18 | 0.28 ± 0.06 ϕ | 0.32 ± 0.18 ϕ,θ | 0.28 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakut, S.; Karabulut, M.; Koca, R.H.; Erbaş, E.; Özkanlar, S.; Gençer, B.T.; Kara, A.; Kumar, K.J.S. Protective Effects of Bromelain in Testicular Torsion-Detorsion: Reducing Inflammation, Oxidative Stress, and Apoptosis While Enhancing Sperm Quality. Biomolecules 2025, 15, 292. https://doi.org/10.3390/biom15020292
Yakut S, Karabulut M, Koca RH, Erbaş E, Özkanlar S, Gençer BT, Kara A, Kumar KJS. Protective Effects of Bromelain in Testicular Torsion-Detorsion: Reducing Inflammation, Oxidative Stress, and Apoptosis While Enhancing Sperm Quality. Biomolecules. 2025; 15(2):292. https://doi.org/10.3390/biom15020292
Chicago/Turabian StyleYakut, Seda, Merve Karabulut, Recep Hakkı Koca, Elif Erbaş, Seçkin Özkanlar, Berrin Tarakçı Gençer, Adem Kara, and K. J. Senthil Kumar. 2025. "Protective Effects of Bromelain in Testicular Torsion-Detorsion: Reducing Inflammation, Oxidative Stress, and Apoptosis While Enhancing Sperm Quality" Biomolecules 15, no. 2: 292. https://doi.org/10.3390/biom15020292
APA StyleYakut, S., Karabulut, M., Koca, R. H., Erbaş, E., Özkanlar, S., Gençer, B. T., Kara, A., & Kumar, K. J. S. (2025). Protective Effects of Bromelain in Testicular Torsion-Detorsion: Reducing Inflammation, Oxidative Stress, and Apoptosis While Enhancing Sperm Quality. Biomolecules, 15(2), 292. https://doi.org/10.3390/biom15020292








