Mitofilin–mtDNA Axis Mediates Chronic Lead Exposure-Induced Synaptic Plasticity Impairment of Hippocampal and Cognitive Deficits
Abstract
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Animals and Cell
2.3. Cell Culture and Exposure to Pb
2.4. Animals and Treatments [25]
2.5. Graphite Furnace Atomic Absorption Spectrometry (AAS)
2.6. Brain Stereolocalization
2.7. Morris Water Maze
2.8. Open Field Test
2.9. Novel Object Recognition Test
2.10. Cellular Immunofluorescence
2.11. Transmission Electron Microscope
2.12. Golgi Staining [27]
2.13. Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
2.14. Western Blot
2.15. MDA and ROS Detection
2.16. Statistical Analysis
3. Results
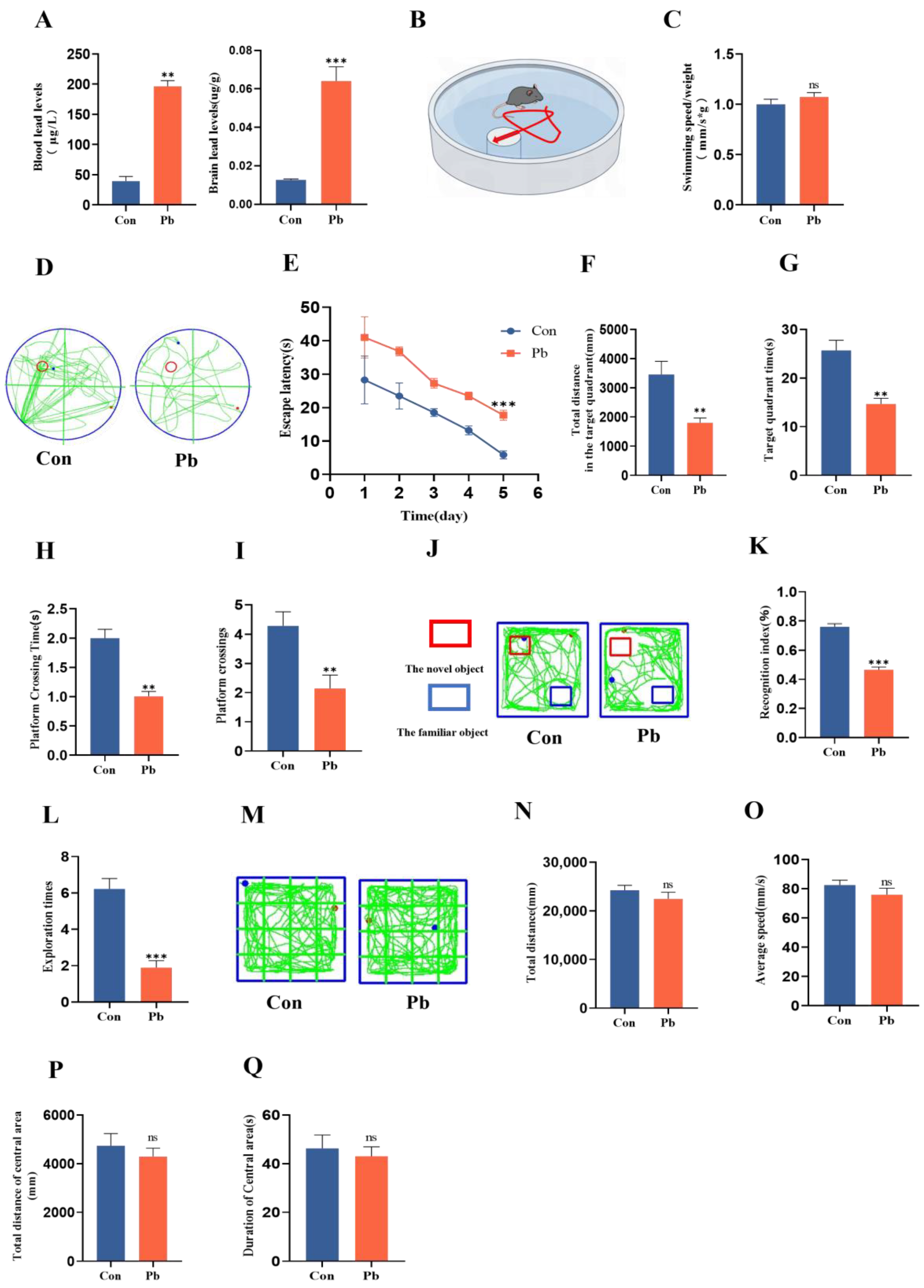
3.1. The Chronic Lead Exposure Impaired the Spatial Memory and Learning of Mice
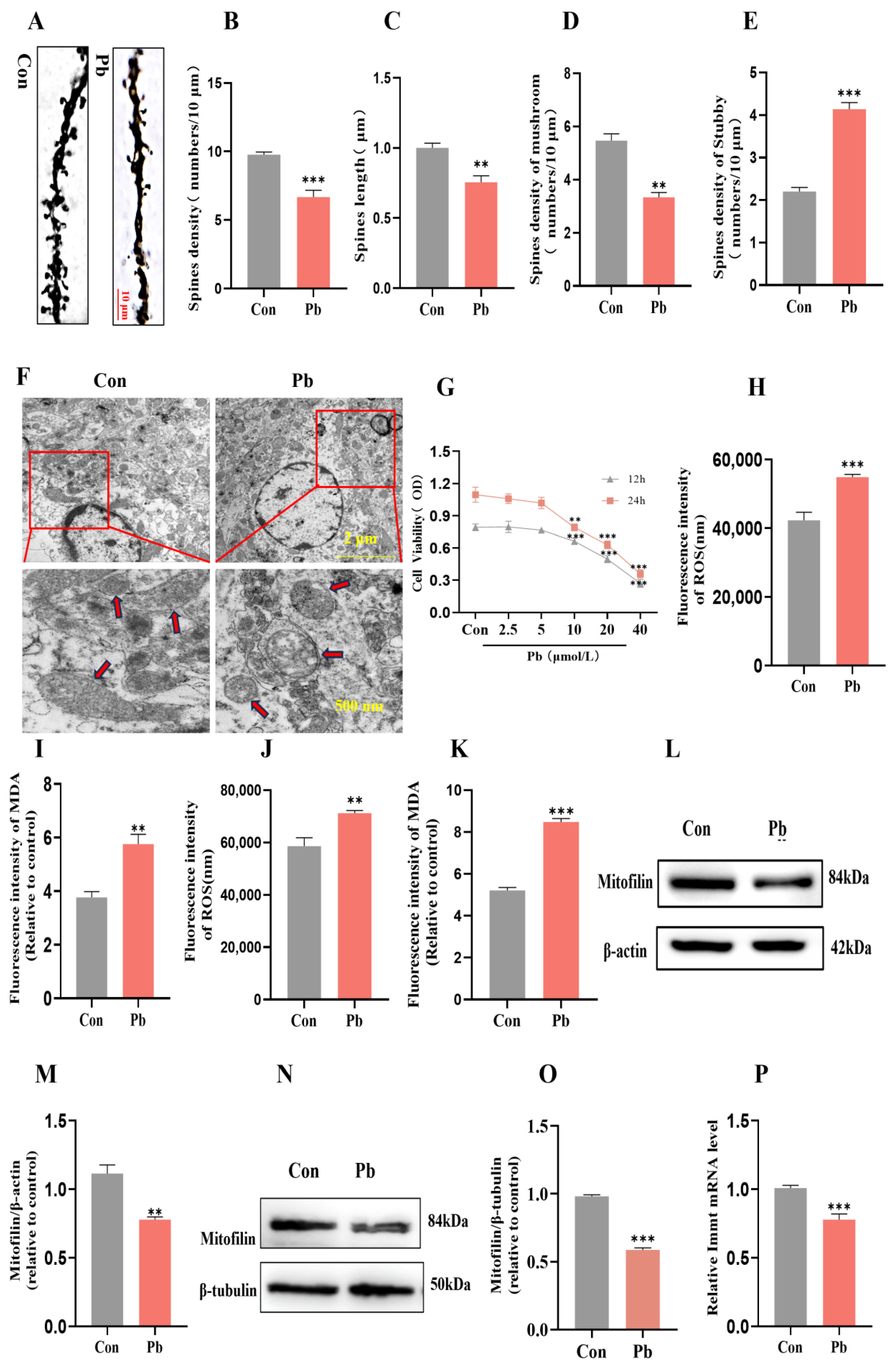
3.2. The Effects of Chronic Lead Exposure on Neuronal Dendritic Spine Plasticity and Mitochondrial Structure and Function
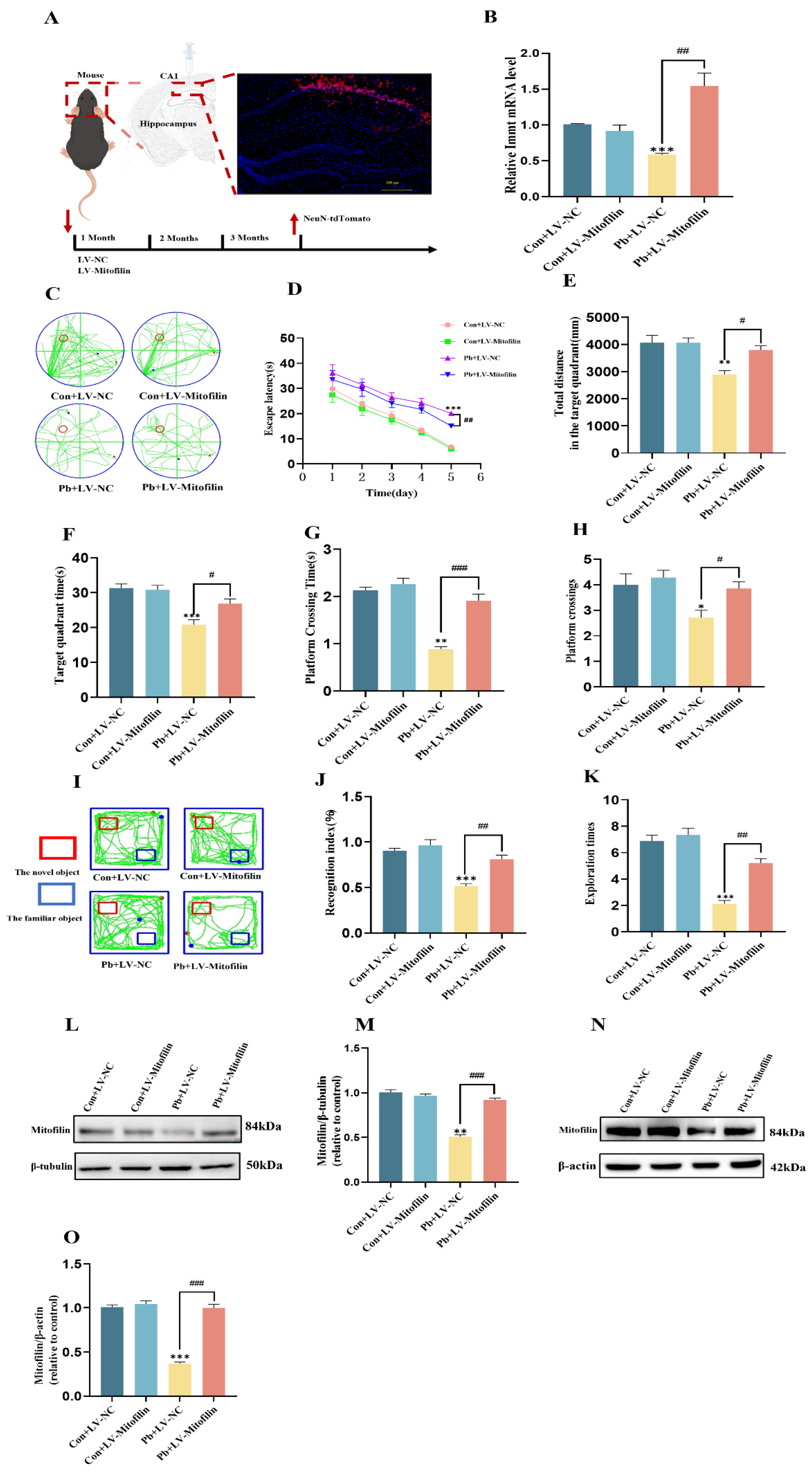
3.3. Overexpression of Mitofilin Improves Learning and Memory in Mice by Affecting the Expression of Mitofilin After Chronic Lead Exposure
3.4. A Mitochondrial Structural Integrity Improvement by Overexpressing Mitofilin Protects Neuronal Dendritic Spines from Lead-Induced Plasticity Damage
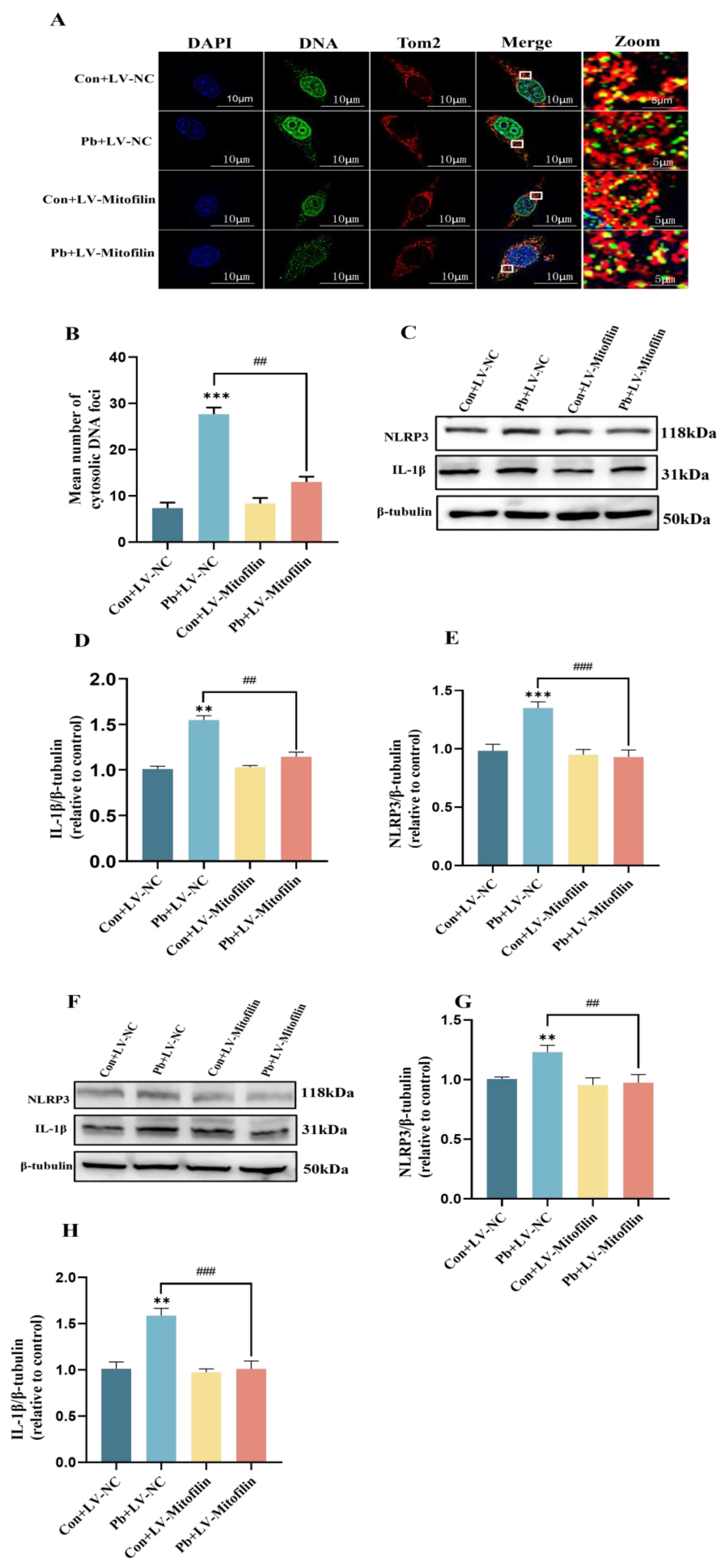
3.5. Inhibition of the Release of mtDNA by Mitofilin Overexpression Diminishes the Resultant Damage to Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramírez, O.D.; Esquivel, D.F.; Ayala, T.B.; Pineda, B.; Manzo, S.G.; Quino, J.M.; Mora, P.C.; de la Cruz, V.P. Cognitive Impairment Induced by Lead Exposure during Lifespan: Mechanisms of Lead Neurotoxicity. Toxics 2021, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Boskabady, M.; Marefati, N.; Farkhondeh, T.; Shakeri, F.; Farshbaf, A.; Boskabady, M.H. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ. Int. 2018, 120, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Gyasi, E. Sources of lead exposure in various countries. Rev. Environ. Health 2019, 34, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Chibowska, K.; Baranowska-Bosiacka, I.; Falkowska, A.; Gutowska, I.; Goschorska, M.; Chlubek, D. Effect of Lead (Pb) on Inflammatory Processes in the Brain. Int. J. Mol. Sci. 2016, 17, 2140. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Fu, H.; Han, X.; Hu, X.; Gu, H.; Chen, Y.; Wei, Q.; Hu, Q. Role of synaptic structural plasticity in impairments of spatial learning and memory induced by developmental lead exposure in Wistar rats. PLoS ONE 2014, 9, e115556. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Choi, H.; Hwang, U.-K.; Kang, J.-C.; Kang, Y.J.; Kim, K.I.; Kim, J.-H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Preston, A.R.; Eichenbaum, H. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 2013, 23, R764–R773. [Google Scholar] [CrossRef]
- Liu, J.-H.; Zhang, M.; Wang, Q.; Wu, D.-Y.; Jie, W.; Hu, N.-Y.; Lan, J.-Z.; Zeng, K.; Li, S.-J.; Li, X.-W.; et al. Distinct roles of astroglia and neurons in synaptic plasticity and memory. Mol. Psychiatry 2022, 27, 873–885. [Google Scholar] [CrossRef]
- Ma, S.; Zuo, Y. Synaptic modifications in learning and memory—A dendritic spine story. Semin. Cell Dev. Biol. 2022, 125, 84–90. [Google Scholar] [CrossRef]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Bakulski, K.M.; Seo, Y.A.; Hickman, R.C.; Brandt, D.; Vadari, H.S.; Hu, H.; Park, S.K. Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J. Alzheimers Dis. 2020, 76, 1215–1242. [Google Scholar] [CrossRef] [PubMed]
- Shvachiy, L.; Geraldes, V.; Amaro-Leal, Â.; Rocha, I. Intermittent low-level lead exposure provokes anxiety, hypertension, autonomic dysfunction and neuroinflammation. Neurotoxicology 2018, 69, 307–319. [Google Scholar] [CrossRef]
- Parithathvi, A.; Choudhari, N.; Dsouza, H.S. Prenatal and early life lead exposure induced neurotoxicity. Hum. Exp. Toxicol. 2024, 43, 9603271241285523. [Google Scholar] [CrossRef]
- Thomason, M.E.; Hect, J.L.; Rauh, V.A.; Trentacosta, C.; Wheelock, M.D.; Eggebrecht, A.T.; Espinoza-Heredia, C.; Burt, S.A. Prenatal lead exposure impacts cross-hemispheric and long-range connectivity in the human fetal brain. Neuroimage 2019, 191, 186–192. [Google Scholar] [CrossRef]
- Zou, R.-X.; Gu, X.; Huang, C.; Wang, H.-L.; Chen, X.-T. Chronic Pb exposure impairs learning and memory abilities by inhibiting excitatory projection neuro-circuit of the hippocampus in mice. Toxicology 2024, 502, 153717. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ouyang, L.; Li, Q.; Yang, S.; Liu, S.; Yu, H.; Jia, Q.; Rao, S.; Xie, J.; Du, G.; et al. Hippocampal LIMK1-mediated Structural Synaptic Plasticity in Neurobehavioral Deficits Induced by a Low-dose Heavy Metal Mixture. Mol. Neurobiol. 2023, 60, 6029–6042. [Google Scholar] [CrossRef]
- Wang, T.; Guan, R.-L.; Liu, M.-C.; Shen, X.-F.; Chen, J.Y.; Zhao, M.-G.; Luo, W.-J. Lead Exposure Impairs Hippocampus Related Learning and Memory by Altering Synaptic Plasticity and Morphology During Juvenile Period. Mol. Neurobiol. 2016, 53, 3740–3752. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Chen, G.; Li, W.; Kepp, O.; Zhu, Y.; Chen, Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Todorova, V.; Blokland, A. Mitochondria and Synaptic Plasticity in the Mature and Aging Nervous System. Curr. Neuropharmacol. 2017, 15, 166–173. [Google Scholar] [CrossRef]
- Feng, Y.; Madungwe, N.B.; Bopassa, J.C. Mitochondrial inner membrane protein, Mic60/mitofilin in mammalian organ protection. J. Cell Physiol. 2019, 234, 3383–3393. [Google Scholar] [CrossRef]
- Hessenberger, M.; Zerbes, R.M.; Rampelt, H.; Kunz, S.; Xavier, A.H.; Purfürst, B.; Lilie, H.; Pfanner, N.; van der Laan, M.; Daumke, O. Regulated membrane remodeling by Mic60 controls formation of mitochondrial crista junctions. Nat. Commun. 2017, 8, 15258. [Google Scholar] [CrossRef] [PubMed]
- Tarasenko, D.; Barbot, M.; Jans, D.C.; Kroppen, B.; Sadowski, B.; Heim, G.; Möbius, W.; Jakobs, S.; Meinecke, M. The MICOS component Mic60 displays a conserved membrane-bending activity that is necessary for normal cristae morphology. J. Cell Biol. 2017, 216, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Liang, S.-C.; Diao, K.-Y.; Wang, Q.; He, Y. Mitofilin Mitigates Myocardial Damage in Acute Myocardial Infarction by Regulating Pyroptosis of Cardiomyocytes. Front. Cardiovasc. Med. 2022, 9, 823591. [Google Scholar] [CrossRef] [PubMed]
- Van Laar, V.S.; Otero, P.A.; Hastings, T.G.; Berman, S.B. Potential Role of Mic60/Mitofilin in Parkinson’s Disease. Front. Neurosci. 2018, 12, 898. [Google Scholar] [CrossRef]
- Banerjee, R.; Rai, A.; Iyer, S.M.; Narwal, S.; Tare, M. Animal models in the study of Alzheimer’s disease and Parkinson’s disease: A historical perspective. Anim. Model. Exp. Med. 2022, 5, 27–37. [Google Scholar] [CrossRef]
- Huang, D.; Chen, L.; Ji, Q.; Xiang, Y.; Zhou, Q.; Chen, K.; Zhang, X.; Zou, F.; Zhang, X.; Zhao, Z.; et al. Lead aggravates Alzheimer’s disease pathology via mitochondrial copper accumulation regulated by COX17. Redox Biol. 2024, 69, 102990. [Google Scholar] [CrossRef]
- Zaqout, S.; Kaindl, A.M. Golgi-Cox Staining Step by Step. Front. Neuroanat. 2016, 10, 38. [Google Scholar] [CrossRef]
- Jeong, Y.; Huh, N.; Lee, J.; Yun, I.; Lee, J.W.; Lee, I.; Jung, M.W. Role of the hippocampal CA1 region in incremental value learning. Sci. Rep. 2018, 8, 9870. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Briguglio, J.J.; Cohen, J.D.; Romani, S.; Lee, A.K. The Statistical Structure of the Hippocampal Code for Space as a Function of Time, Context, and Value. Cell 2020, 183, 620–635.e22. [Google Scholar] [CrossRef]
- Mijalkov, M.; Volpe, G.; Fernaud-Espinosa, I.; DeFelipe, J.; Pereira, J.B.; Merino-Serrais, P. Dendritic spines are lost in clusters in Alzheimer’s disease. Sci. Rep. 2021, 11, 12350. [Google Scholar] [CrossRef]
- Nebeling, F.C.; Poll, S.; Justus, L.C.; Steffen, J.; Keppler, K.; Mittag, M.; Fuhrmann, M. Microglial motility is modulated by neuronal activity and correlates with dendritic spine plasticity in the hippocampus of awake mice. Elife 2023, 12, e83176. [Google Scholar] [CrossRef]
- Borczyk, M.; Śliwińska, M.A.; Caly, A.; Bernas, T.; Radwanska, K. Neuronal plasticity affects correlation between the size of dendritic spine and its postsynaptic density. Sci. Rep. 2019, 9, 1693. [Google Scholar] [CrossRef]
- Gautam, M.; Genç, B.; Helmold, B.; Ahrens, A.; Kuka, J.; Makrecka-Kuka, M.; Günay, A.; Koçak, N.; Aguilar-Wickings, I.R.; Keefe, D.; et al. SBT-272 improves TDP-43 pathology in ALS upper motor neurons by modulating mitochondrial integrity, motility, and function. Neurobiol. Dis. 2023, 178, 106022. [Google Scholar] [CrossRef]
- Mishra, E.; Thakur, M.K. Mdivi-1 Rescues Memory Decline in Scopolamine-Induced Amnesic Male Mice by Ameliorating Mitochondrial Dynamics and Hippocampal Plasticity. Mol. Neurobiol. 2023, 60, 5426–5449. [Google Scholar] [CrossRef] [PubMed]
- Sayehmiri, F.; Motamedi, F.; Batool, Z.; Naderi, N.; Shaerzadeh, F.; Zoghi, A.; Rezaei, O.; Khodagholi, F.; Pourbadie, H.G. Mitochondrial plasticity and synaptic plasticity crosstalk; in health and Alzheimer’s disease. CNS Neurosci. Ther. 2024, 30, e14897. [Google Scholar] [CrossRef]
- Yu, T.; Dohl, J.; Chen, Y.; Gasier, H.G.; Deuster, P.A. Astaxanthin but not quercetin preserves mitochondrial integrity and function, ameliorates oxidative stress, and reduces heat-induced skeletal muscle injury. J. Cell Physiol. 2019, 234, 13292–13302. [Google Scholar] [CrossRef]
- Sánchez-Pérez, P.; Mata, A.; Torp, M.-K.; López-Bernardo, E.; Heiestad, C.M.; Aronsen, J.M.; Molina-Iracheta, A.; Jiménez-Borreguero, L.J.; García-Roves, P.; Costa, A.S.; et al. Energy substrate metabolism, mitochondrial structure and oxidative stress after cardiac ischemia-reperfusion in mice lacking UCP3. Free Radic. Biol. Med. 2023, 205, 244–261. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Shi, J.; He, Q.; Lui, K.; Liu, Y.; Huang, Y.; Sheikh, M.S. CHCM1/CHCHD6, novel mitochondrial protein linked to regulation of mitofilin and mitochondrial cristae morphology. J. Biol. Chem. 2012, 287, 7411–7426. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Yu, H.; Liu, S.; Wan, H.; Fu, S.; Liu, S.; Yang, J.; Zhang, Z.; Huang, H.; Li, Q.; et al. Mitochondrial cristae architecture protects against mtDNA release and inflammation. Cell Rep. 2022, 41, 111774. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.S.; Hong, Z.; Wu, W.; Xiong, S.; Zhong, M.; Gao, X.; Rehman, J.; Malik, A.B. mtDNA Activates cGAS Signaling and Suppresses the YAP-Mediated Endothelial Cell Proliferation Program to Promote Inflammatory Injury. Immunity 2020, 52, 475–486.e5. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, D.; Shah, S.Z.A.; Zhang, X.; Lai, M.; Yang, D.; Wu, X.; Guan, Z.; Li, J.; Zhao, H.; et al. OPA1 overexpression ameliorates mitochondrial cristae remodeling, mitochondrial dysfunction, and neuronal apoptosis in prion diseases. Cell Death Dis. 2019, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Wang, D.; Cao, Z.; Chen, J.; Zhang, J. The role of NLRP3 in lead-induced neuroinflammation and possible underlying mechanism. Environ. Pollut. 2021, 287, 117520. [Google Scholar] [CrossRef]
- Karri, V.; Schuhmacher, M.; Kumar, V. Heavy metals (Pb, Cd, As and MeHg) as risk factors for cognitive dysfunction: A general review of metal mixture mechanism in brain. Environ. Toxicol. Pharmacol. 2016, 48, 203–213. [Google Scholar] [CrossRef]
- Ferreira, M.; Aragão, W.A.B.; Bittencourt, L.O.; Puty, B.; Dionizio, A.; de Souza, M.P.C.; Buzalaf, M.A.R.; de Oliveira, E.H.; Crespo-Lopez, M.E.; Lima, R.R. Fluoride exposure during pregnancy and lactation triggers oxidative stress and molecular changes in hippocampus of offspring rats. Ecotoxicol. Environ. Saf. 2021, 208, 111437. [Google Scholar] [CrossRef]
- Taylor, C.M.; Kordas, K.; Golding, J.; Emond, A.M. Effects of low-level prenatal lead exposure on child IQ at 4 and 8 years in a UK birth cohort study. Neurotoxicology 2017, 62, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ayyalasomayajula, N.; Bandaru, L.J.M.; Chetty, C.S.; Dixit, P.K.; Challa, S. Mitochondria-Mediated Moderation of Apoptosis by EGCG in Cytotoxic Neuronal Cells Induced by Lead (Pb) and Amyloid Peptides. Biol. Trace Elem. Res. 2022, 200, 3582–3593. [Google Scholar] [CrossRef]
- Bandaru, L.; Ayyalasomayajula, N.; Murumulla, L.; Challa, S. Mechanisms associated with the dysregulation of mitochondrial function due to lead exposure and possible implications on the development of Alzheimer’s disease. Biometals 2022, 35, 1–25. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Xu, Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int. J. Environ. Res. Public Health 2020, 17, 4878. [Google Scholar] [CrossRef]
- Kochan, S.; Malo, M.C.; Jevtic, M.; Jahn-Kelleter, H.M.; Wani, G.A.; Ndoci, K.; Pérez-Revuelta, L.; Gaedke, F.; Schäffner, I.; Lie, D.C.; et al. Enhanced mitochondrial fusion during a critical period of synaptic plasticity in adult-born neurons. Neuron 2024, 112, 1997–2014.e6. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Mercaldo, V.; Jacob, A.D.; Kramer, E.; Mocle, A.; Ramsaran, A.I.; Tran, L.; Rashid, A.J.; Park, S.; Insel, N.; et al. Higher-order interactions between hippocampal CA1 neurons are disrupted in amnestic mice. Nat. Neurosci. 2024, 27, 1794–1804. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimers Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tian, R.; Han, H.; Slone, J.; Wang, C.; Ke, X.; Zhang, T.; Li, X.; He, Y.; Liao, P.; et al. PINK1-mediated Drp1(S616) phosphorylation modulates synaptic development and plasticity via promoting mitochondrial fission. Signal Transduct. Target. Ther. 2022, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, Y.; Luo, Q.; Zeng, W.; Shao, X.; Li, L.; Wang, Q.; Wang, D.; Zhang, Y.; Diao, H.; et al. Mitochondrial damage and activation of the cytosolic DNA sensor cGAS-STING pathway lead to cardiac pyroptosis and hypertrophy in diabetic cardiomyopathy mice. Cell Death Discov. 2022, 8, 258. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, W.; Wang, C.; Zhang, B.; Li, R.; Zhang, L.; Zhao, K.; Li, Y.; Tian, L.; Li, B.; et al. IRGM promotes the PINK1-mediated mitophagy through the degradation of Mitofilin in SH-SY5Y cells. FASEB J. 2020, 34, 14768–14779. [Google Scholar] [CrossRef] [PubMed]
- Romero-Zerbo, S.Y.; Valverde, N.; Claros, S.; Zamorano-Gonzalez, P.; Boraldi, F.; Lofaro, F.-D.; Lara, E.; Pavia, J.; Garcia-Fernandez, M.; Gago, B.; et al. New molecular mechanisms to explain the neuroprotective effects of insulin-like growth factor II in a cellular model of Parkinson’s disease. J. Adv. Res. 2025, 67, 349–359. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Hou, J.; Wang, B.; Li, Y.; Huo, X.; Wang, T.; Zou, Y.; Zheng, G. Mitofilin–mtDNA Axis Mediates Chronic Lead Exposure-Induced Synaptic Plasticity Impairment of Hippocampal and Cognitive Deficits. Biomolecules 2025, 15, 272. https://doi.org/10.3390/biom15020272
Su L, Hou J, Wang B, Li Y, Huo X, Wang T, Zou Y, Zheng G. Mitofilin–mtDNA Axis Mediates Chronic Lead Exposure-Induced Synaptic Plasticity Impairment of Hippocampal and Cognitive Deficits. Biomolecules. 2025; 15(2):272. https://doi.org/10.3390/biom15020272
Chicago/Turabian StyleSu, Lihong, Jinchao Hou, Boxuan Wang, Yuqi Li, Xiaodong Huo, Tao Wang, Yuankang Zou, and Gang Zheng. 2025. "Mitofilin–mtDNA Axis Mediates Chronic Lead Exposure-Induced Synaptic Plasticity Impairment of Hippocampal and Cognitive Deficits" Biomolecules 15, no. 2: 272. https://doi.org/10.3390/biom15020272
APA StyleSu, L., Hou, J., Wang, B., Li, Y., Huo, X., Wang, T., Zou, Y., & Zheng, G. (2025). Mitofilin–mtDNA Axis Mediates Chronic Lead Exposure-Induced Synaptic Plasticity Impairment of Hippocampal and Cognitive Deficits. Biomolecules, 15(2), 272. https://doi.org/10.3390/biom15020272





