Enhancing Stability and Bioavailability of Peptidylglycine Alpha-Amidating Monooxygenase in Circulation for Clinical Use
Abstract
1. Introduction
2. Materials and Methods
2.1. PAM Constructs and PEGylation
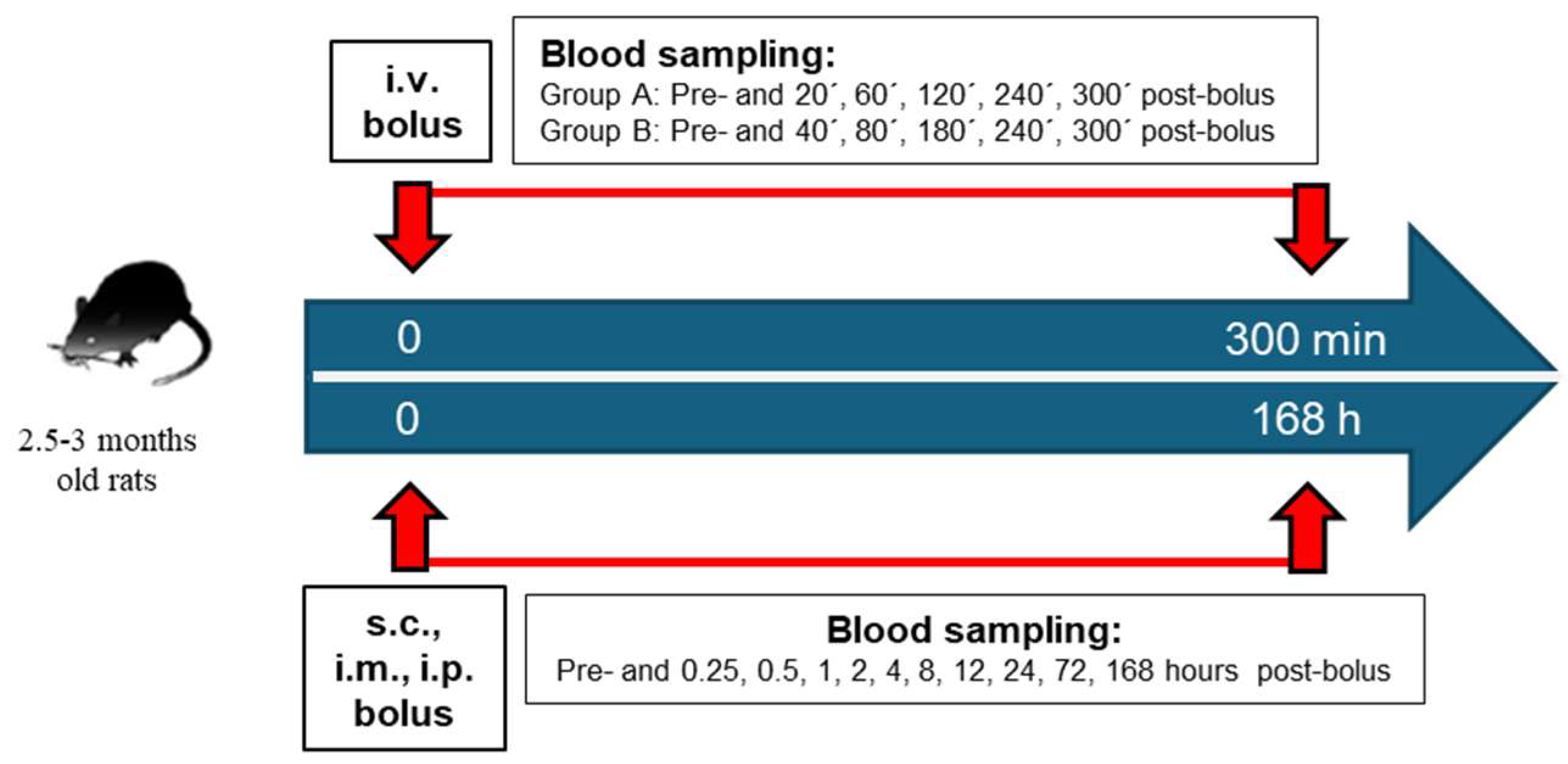
2.2. PAM In Vivo Pharmacokinetics
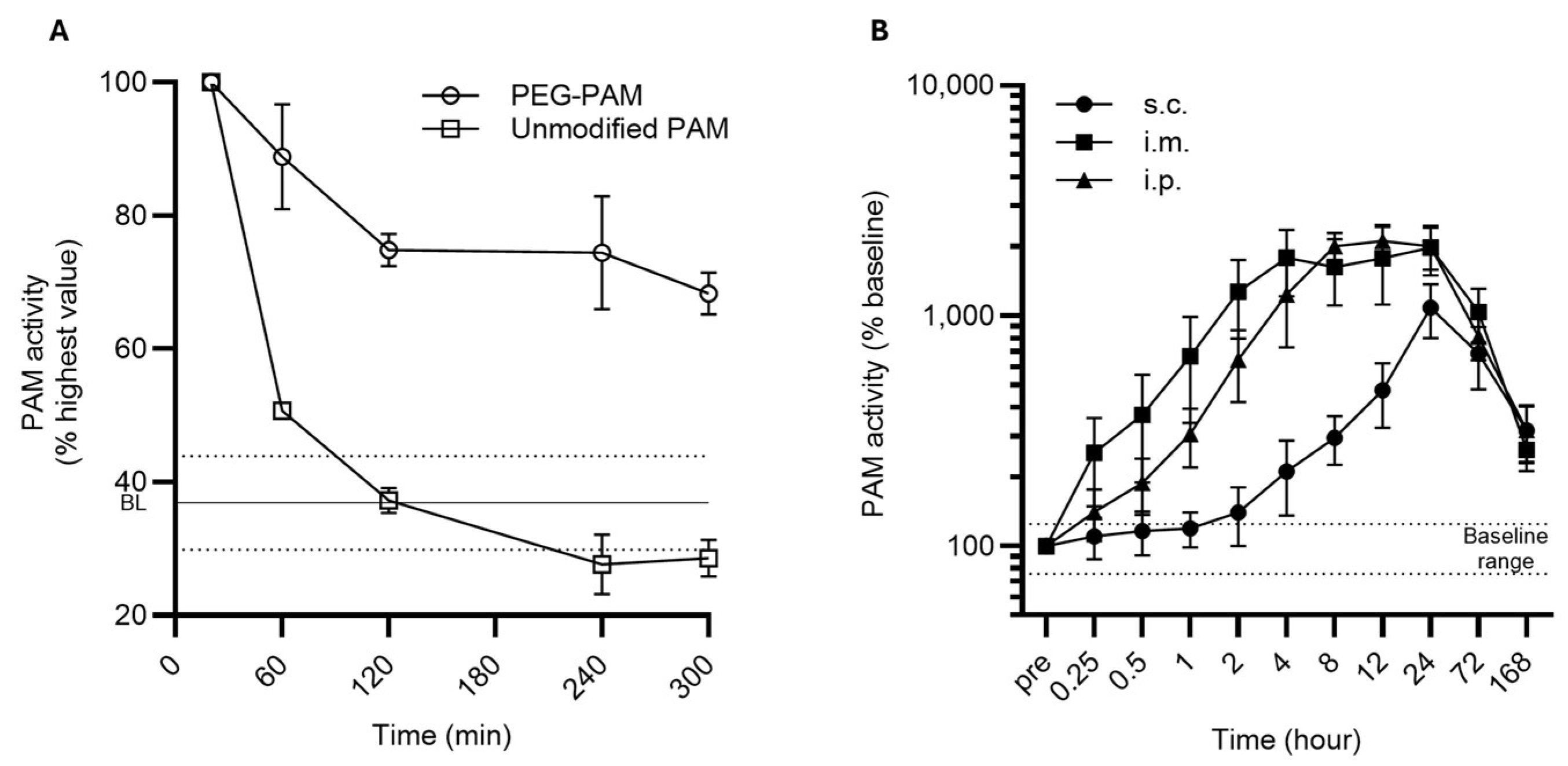
3. Results
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAM | peptidylglycine alpha-amidating monooxygenase |
| ADM | adrenomedullin |
| CGRP | calcitonin gene-related peptide |
| NPY | neuropeptide Y |
| VIP | vasoactive intestinal peptide |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| T2D | type 2 diabetes |
| COPD | chronic obstructive pulmonary disease |
| CV | coefficient of variation |
| AUC | area under the curve |
| Cmax | maximum analyte concentration in plasma |
| Tmax | time to reach maximum analyte concentration |
| BL | baseline |
| i.v. | intravenous |
| i.m. | intramuscular |
| i.p. | intraperitoneal |
References
- Kumar, D.; Mains, R.E.; Eipper, B.A. 60 YEARS OF POMC: From POMC and α-MSH to PAM, molecular oxygen, copper, and vitamin C. J. Mol. Endocrinol. 2016, 56, T63–T76. [Google Scholar] [CrossRef]
- Vishwanatha, K.S.; Mains, R.E.; Eipper, B.A. Peptidylglycine Amidating Monoxygenase (PAM). In Handbook of Biologically Active Peptides; Academic Press: Cambridge, MA, USA, 2013; Volume 3, pp. 1780–1788. [Google Scholar] [CrossRef]
- Eipper, B.A.; Mains, R.E. Peptide α-Amidation. Annu. Rev. Physiol. 1988, 50, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Eipper, B.A.; Stoffers, D.A.; Mains, R.E. The biosynthesis of neuropeptides: Peptide alpha-amidation. Annu. Rev. Neurosci. 1992, 15, 57–85. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rey, E.; Chorny, A.; Fernandez-Martin, A.; Varela, N.; Delgado, M. Vasoactive intestinal peptide family as a therapeutic target for Parkinson’s disease. Expert Opin. Ther. Targets 2005, 9, 923–929. [Google Scholar] [CrossRef]
- White, C.M.; Ji, S.; Cai, H.; Maudsley, S.; Martin, B. Therapeutic Potential of Vasoactive Intestinal Peptide and its Receptors in Neurological Disorders. CNS Neurol. Disord. Drug Targets 2012, 9, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Ponery, A.S.; Sharma, A.K.; El-Sharkawy, T.; Donáth, T. Diabetes mellitus is associated with a decrease in vasoactive intestinal polypeptide content of gastrointestinal tract of rat. Arch. Physiol. Biochem. 2001, 109, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Neves, J.; de Almeida, L.P.; Cavadas, C. Neuropeptide Y (NPY) as a therapeutic target for neurodegenerative diseases. Neurobiol. Dis. 2016, 95, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Zhao, Y.; Zhu, J.; Liu, K. Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases. Front. Neurosci. 2019, 13, 869. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Liang, W.; Baxter, L.C.; Yin, J.; Tang, Z.; Beach, T.G.; Caselli, R.J.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase–activating polypeptide is reduced in Alzheimer disease. Neurology 2014, 82, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Ashizuka, S.; Kuroishi, N.; Nakashima, K.; Inatsu, H.; Kita, T.; Kitamura, K. Adrenomedullin: A novel therapy for intractable Crohn’s disease with a loss of response to infliximab. Intern. Med. 2019, 58, 1573–1576. [Google Scholar] [CrossRef]
- Kita, T.; Kitamura, K. Translational studies of adrenomedullin and related peptides regarding cardiovascular diseases. Hypertens. Res. 2022, 45, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.K.; Cheung, T.T.; Cheung, B.M.Y. Adrenomedullin and cardiovascular diseases. JRSM Cardiovasc. Dis. 2012, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Washida, K.; Yoshimoto, T.; Saito, S. Adrenomedullin: A vasoactive agent for sporadic and hereditary vascular cognitive impairment. Cereb. Circ. Cogn. Behav. 2021, 2, 100007. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Saito, S.; Omae, K.; Tanaka, K.; Kita, T.; Kitamura, K.; Fukuma, K.; Washida, K.; Abe, S.; Ishiyama, H.; et al. Efficacy and safety of adrenomedullin for acute ischemic stroke (AMFIS): A phase 2, randomized, double-blinded, placebo-controlled, clinical trial. eClinicalMedicine 2024, 77, 102901. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Varela, N.; Gonzalez-Rey, E. Vasoactive intestinal peptide protects against β-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia 2008, 56, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lee, D.; Sung, Y.K. Prospect of vasoactive intestinal peptide therapy for COPD/PAH and asthma: A review. Respir. Res. 2011, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Gomariz, R.; Martinez, C.; Abad, C.; Leceta, J.; Delgado, M. Immunology of VIP: A Review and Therapeutical Perspectives. Curr. Pharm. Des. 2001, 7, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Vaczy, A.; Rubio-Beltrán, A.E.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain 2018, 19, 19. [Google Scholar] [CrossRef]
- DToth, D.; Reglodi, D.; Schwieters, L.; Tamas, A. Role of endocrine PACAP in age-related diseases. Front. Endocrinol. 2023, 14, 1118927. [Google Scholar] [CrossRef]
- Reglodi, D.; Helyes, Z.; Nemeth, J.; Vass, R.A.; Tamas, A. PACAP as a Potential Biomarker: Alterations of PACAP Levels in Human Physiological and Pathological Conditions. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Current Topics in Neurotoxicity; Springer: Berlin/Heidelberg, Germany, 2016; Volume 11, pp. 815–832. [Google Scholar] [CrossRef]
- Yang, R.; Jiang, X.; Ji, R.; Meng, L.; Liu, F.; Chen, X.; Xin, Y. Therapeutic potential of PACAP for neurodegenerative diseases. Cell. Mol. Biol. Lett. 2015, 20, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Gábriel, R.; Pöstyéni, E.; Dénes, V. Neuroprotective Potential of Pituitary Adenylate Cyclase Activating Polypeptide in Retinal Degenerations of Metabolic Origin. Front. Neurosci. 2019, 13, 1031. [Google Scholar] [CrossRef]
- Ciranna, L.; Costa, L. Pituitary Adenylate Cyclase-Activating Polypeptide Modulates Hippocampal Synaptic Transmission and Plasticity: New Therapeutic Suggestions for Fragile X Syndrome. Front. Cell. Neurosci. 2019, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide pituitary adenylate cyclase-activating polypeptide (PACAP) slows down Alzheimer’s disease-like pathology in amyloid precursor protein-transgenic mice. FASEB J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Tang, Z.; Yin, J.-X.; Beach, T.; Reiman, E.; Shi, J. PACAP Deficit in Alzheimer’s Disease and Protection Against Beta-Amyloid Toxicity (I11-1.010). Neurology 2014, 82 (Suppl. S10). [Google Scholar] [CrossRef]
- Lutz, T.A.; Meyer, U. Amylin at the interface between metabolic and neurodegenerative disorders. Front. Neurosci. 2015, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xue, X.; Wang, E.; Wallack, M.; Na, H.; Hooker, J.M.; Kowall, N.; Tao, Q.; Stein, T.D.; Wolozin, B.; et al. Amylin receptor ligands reduce the pathological cascade of Alzheimer’s disease. Neuropharmacology 2017, 119, 170–181. [Google Scholar] [CrossRef]
- Nagata, S.; Yamasaki, M.; Kitamura, K. Anti-Inflammatory Effects of PEGylated Human Adrenomedullin in a Mouse DSS-Induced Colitis Model. Drug Dev. Res. 2017, 78, 129–134. [Google Scholar] [CrossRef]
- von der Hardt, K.; Kandler, M.; Chada, M.; Cubra, A.; Schoof, E.; Amann, K.; Rascher, W.; Dötsch, J. Brief adrenomedullin inhalation leads to sustained reduction of pulmonary artery pressure. Eur. Respir. J. 2004, 24, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Geven, C.; Kox, M.; Pickkers, P. Adrenomedullin and adrenomedullin-targeted therapy as treatment strategies relevant for sepsis. Front. Immunol. 2018, 9, 292. [Google Scholar] [CrossRef]
- Merkler, D.J.; Hawley, A.J.; Eipper, B.A.; Mains, R.E. Peptidylglycine α-amidating monooxygenase as a therapeutic target or biomarker for human diseases. Br. J. Pharmacol. 2022, 179, 3306–3324. [Google Scholar] [CrossRef]
- Kapuscinski, M.; Green, M.; Sinha, S.N.; Shepherd, J.J.; Shulkes, A. Peptide α-amidation activity in human plasma: Relationship to gastrin processing. Clin. Endocrinol. 1993, 39, 51–58. [Google Scholar] [CrossRef]
- Sheng, B.; Wei, H.; Li, Z.; Wei, H.; Zhao, Q. PAM variants were associated with type 2 diabetes mellitus risk in the Chinese population. Funct. Integr. Genom. 2022, 22, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, S.K.; Raimondo, A.; Hastoy, B.; Sengupta, S.; Dai, X.-Q.; Bautista, A.; Censin, J.; Payne, A.J.; Umapathysivam, M.M.; Spigelman, A.F.; et al. Type 2 diabetes risk alleles in PAM impact insulin release from human pancreatic β-cells. Nat. Genet. 2018, 50, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Giontella, A.; Åkerlund, M.; Bronton, K.; Fava, C.; Lotta, L.A.; Baras, A.; Overton, J.D.; Jones, M.; Bergmann, A.; Kaufmann, P.; et al. Deficiency of Peptidylglycine-alpha-amidating Monooxygenase, a Cause of Sarcopenic Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2024. [Google Scholar] [CrossRef]
- Wand, G.S.; May, C.; May, V.; Whitehouse, P.J.; Rapoport, S.I.; Eipper, B.A. Alzheimer’s disease: Low levels of peptide alpha-amidation activity in brain and CSF. Neurology 1987, 37, 1057. [Google Scholar] [CrossRef]
- Bäck, N.; Luxmi, R.; Powers, K.G.; Mains, R.E.; Eipper, B.A. Peptidylglycine α-amidating monooxygenase is required for atrial secretory granule formation. Proc. Natl. Acad. Sci. USA 2020, 117, 17820–17831. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Bergmann, A.; Melander, O. Novel insights into peptide amidation and amidating activity in the human circulation. Sci. Rep. 2021, 11, 15791. [Google Scholar] [CrossRef]
- Kaufmann, P.; Ilina, Y.; Press, M.; Bergmann, A. Sandwich immunoassay for adrenomedullin precursor and its practical application. Sci. Rep. 2024, 14, 28091. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Kato, J.; Kawamoto, M.; Tanaka, M.; Chino, N.; Kangawa, K.; Eto, T. The intermediate form of glycine-extended adrenomedullin is the major circulating molecular form in human plasma. Biochem. Biophys. Res. Commun. 1998, 244, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Tsuruda, T.; Kita, T.; Kitamura, K.; Eto, T. Adrenomedullin: A Protective Factor for Blood Vessels. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2480–2487. [Google Scholar] [CrossRef]
- Müller, T.; Finan, B.; Bloom, S.; D’Alessio, D.; Drucker, D.; Flatt, P.; Fritsche, A.; Gribble, F.; Grill, H.; Habener, J.; et al. Glucagon-Like Peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Manecka, D.L.; Boukhzar, L.; Falluel-Morel, A.; Lihrmann, I.; Anouar, Y. PACAP signaling in neuroprotection. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 549–561. [Google Scholar] [CrossRef]
- Scherbaum, W.A. The role of amylin in the physiology of glycemic control. Exp. Clin. Endocrinol. Diabetes 1998, 106, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.J.; Dong, M.; Harikumar, K.G.; Miller, L.J. Cholecystokinin-induced satiety, a key gut servomechanism that is affected by the membrane microenvironment of this receptor. Int. J. Obes. Suppl. 2016, 6, S22–S27. [Google Scholar] [CrossRef] [PubMed]
- Den Ouden, D.T.; Meinders, A.E. Vasopressin: Physiology and clinical use in patients with vasodilatory shock: A review. Neth. J. Med. 2005, 63, 4–13. [Google Scholar] [PubMed]
- Jang, M.; Park, J.; Kho, H.; Chung, S.; Chung, J. Plasma and saliva levels of nerve growth factor and neuropeptides in chronic migraine patients. Oral Dis. 2011, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ailanen, L.; Ruohonen, S.T.; Vähätalo, L.H.; Tuomainen, K.; Eerola, K.; Salomäki-Myftari, H.; Röyttä, M.; Laiho, A.; Ahotupa, M.; Gylling, H.; et al. The metabolic syndrome in mice overexpressing neuropeptide Y in noradrenergic neurons. J. Endocrinol. 2017, 234, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, M.; Di Mise, A.; Tamma, G.; Valenti, G. Vasopressin–aquaporin-2 pathway: Recent advances in understanding water balance disorders. F1000Research 2019, 8, 149. [Google Scholar] [CrossRef]
- Fossmark, R.; Qvigstad, G.; Martinsen, T.C.; Hauso, Ø.; Waldum, H.L. Animal Models to Study the Role of Long-Term Hypergastrinemia in Gastric Carcinogenesis. J. Biomed. Biotechnol. 2011, 2011, 975479. [Google Scholar] [CrossRef]
- Weber, J.; Sachse, J.; Bergmann, S.; Sparwaßer, A.; Struck, J.; Bergmann, A. Sandwich Immunoassay for Bioactive Plasma Adrenomedullin. J. Appl. Lab. Med. 2017, 2, 222–233. [Google Scholar] [CrossRef] [PubMed]
| Route | Parameter | PEG-PAM | Unmodified PAM | |
|---|---|---|---|---|
| Variable | Units | |||
| i.v. | Animals | n/group | 6 | 6 |
| T1/2 * | min | 218.2 | 42.3 | |
| Cmax * | Units | 18.3 × 103 | 27.9 × 103 | |
| AUC * | h*Units | 80.9 × 103 | 38.3 × 103 | |
| BL activity | Units (mean + SD) | 12.7 × 103 ± 2.8 × 103 | 12.7 × 103 ± 2.3 × 103 | |
| i.m. | Animals | n/group | 6 | |
| Cmax | Units | 93.9 × 103 | ||
| Tmax | h | 24 | ||
| AUC | h*Units | 6.5 × 106 | ||
| BL activity | Units (mean ± SD) | 7.2 × 103 ± 1.8 × 103 | ||
| 7d post-bolus activity | 13,615 ± 1370 | |||
| i.p. | Animals | n/group | 6 | |
| Cmax | Units | 104.6 × 103 | ||
| Tmax | h | 12 | ||
| AUC | h*Units | 7.0 × 106 | ||
| BL activity | Units (mean ± SD) | 5.7 × 103 ± 0.8 × 103 | ||
| 7d post-bolus activity | 16.2 × 103 ± 2.0 × 103 | |||
| s.c. | Animals | n/group | 6 | |
| Cmax | Units | 50.2 × 103 | ||
| Tmax | h | 24 | ||
| AUC | h*Units | 4.2 × 106 | ||
| BL activity | Units (mean ± SD) | 5.4 × 103 ± 1.2 × 103 | ||
| 7d post-bolus activity | 16.3 × 103 ± 2.1 × 103 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilina, Y.; Kaufmann, P.; Press, M.; Uba, T.I.; Bergmann, A. Enhancing Stability and Bioavailability of Peptidylglycine Alpha-Amidating Monooxygenase in Circulation for Clinical Use. Biomolecules 2025, 15, 224. https://doi.org/10.3390/biom15020224
Ilina Y, Kaufmann P, Press M, Uba TI, Bergmann A. Enhancing Stability and Bioavailability of Peptidylglycine Alpha-Amidating Monooxygenase in Circulation for Clinical Use. Biomolecules. 2025; 15(2):224. https://doi.org/10.3390/biom15020224
Chicago/Turabian StyleIlina, Yulia, Paul Kaufmann, Michaela Press, Theo Ikenna Uba, and Andreas Bergmann. 2025. "Enhancing Stability and Bioavailability of Peptidylglycine Alpha-Amidating Monooxygenase in Circulation for Clinical Use" Biomolecules 15, no. 2: 224. https://doi.org/10.3390/biom15020224
APA StyleIlina, Y., Kaufmann, P., Press, M., Uba, T. I., & Bergmann, A. (2025). Enhancing Stability and Bioavailability of Peptidylglycine Alpha-Amidating Monooxygenase in Circulation for Clinical Use. Biomolecules, 15(2), 224. https://doi.org/10.3390/biom15020224






