Malnutrition and Sarcopenia in Patients with Neuroendocrine Tumors: A Comprehensive Review of Evidence
Abstract
1. Introduction
1.1. Background
1.2. Malnutrition, Cachexia, and Sarcopenia in NETs
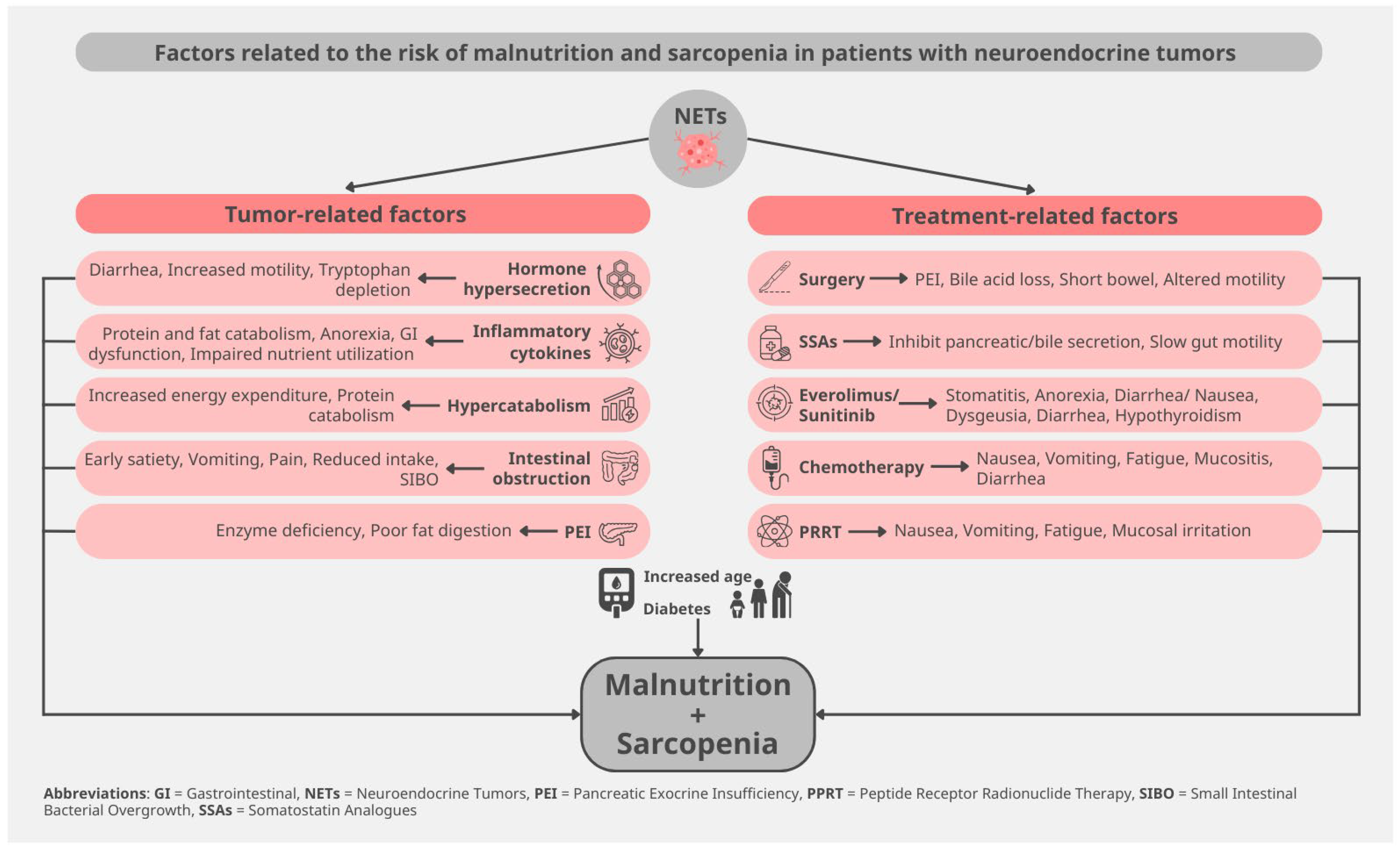
2. Pathophysiology of Malnutrition and Sarcopenia in NETs
2.1. Tumor-Related Mechanisms
2.2. Treatment-Related Mechanisms
2.3. Host-Related Factors
3. Study Selection and Quality Appraisal
4. Epidemiology and Clinical Significance
4.1. Prevalence of Nutrition-Related Problems
4.2. Nutritional Status, Prognosis, and Treatment Tolerance
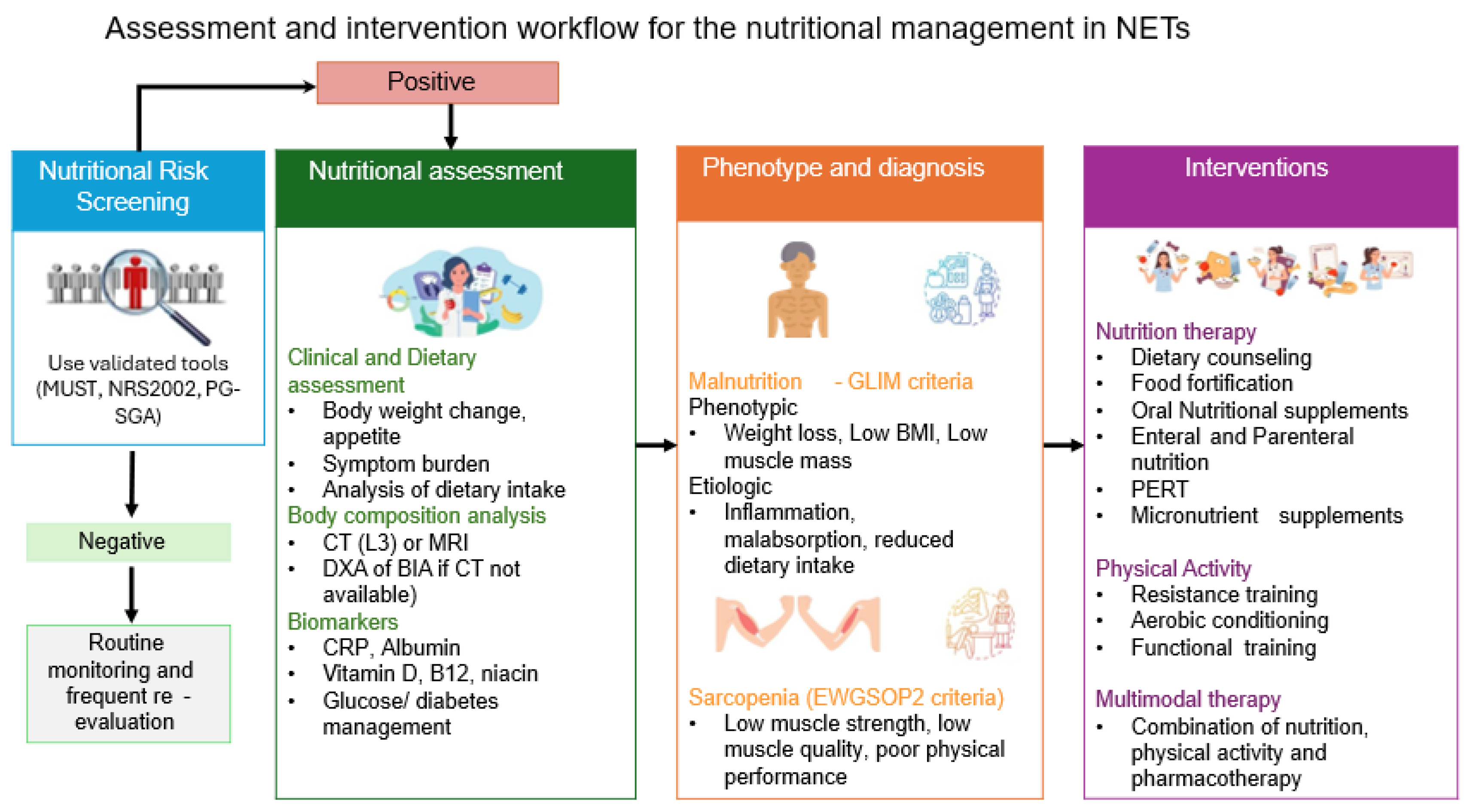
5. Assessment and Diagnosis
5.1. Nutritional Screening—Assessment
5.2. Body Composition Analysis
5.3. Biomarkers
6. Nutritional and Metabolic Interventions
6.1. Dietary Interventions
6.2. Exercise and Physical Activity
6.3. Multimodal Interventions
7. Emerging and Experimental Interventions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NENs | Neuroendocrine neoplasms |
| GEP | Gastroenteropancreatic |
| BP | Bronchopulmonary |
| NETs | Neuroendocrine tumors |
| NECs | Neuroendocrine carcinomas |
| QoL | Quality of life |
| PRRT | Peptide receptor radionuclide therapy |
| BMI | Body mass index |
| GHSR1a | Growth hormone secretagogue receptor 1-a |
| GHSR1b | Growth hormone secretagogue receptor 1-b |
| GI | Gastrointestinal |
| SIBO | Small intestine bacterial overgrowth |
| PEI | Pancreatic exocrine insufficiency |
| 5-HIAA | 5-hydroxy-indoleacetic acid |
| SSAs | Somatostatin analogs |
| mTOR | Mechanistic target of rapamycin |
| CT | Computed tomography |
| GLIM | Global leadership initiative on malnutrition |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| NRS-2002 | Nutritional Risk Screening 2002 |
| MUST | Malnutrition Universal Screening Tool |
| PG-SGA | Patient-Generated Subjective Global Assessment |
| MRI | Magnetic resonance imaging |
| DEXA | Dual-energy X-ray absorptiometry |
| BIA | Bioelectrical impedance analysis |
| CRP | C-reactive protein |
| IGF-1 | Insulin-like growth factor 1 |
| PERT | Pancreatic enzyme replacement therapy |
| ONS | Oral nutritional supplements |
| MENAC | Multimodal-Exercise, Nutrition and Anti-inflammatory Medication for Cachexia |
References
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef] [PubMed]
- Ramage, J.; Srirajaskanthan, R. Epidemiology and Health Impacts of Neuroendocrine Tumours. Neuroendocrinology 2025, 1, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Wallace, K.; Halperin, D.M.; Maxwell, J.; Kunz, P.; Singh, S.; Chasen, B.; Yao, J.C. Epidemiology of Neuroendocrine Neoplasms in the US. JAMA Netw. Open 2025, 8, e2515798. [Google Scholar] [CrossRef]
- Fraenkel, M.; Faggiano, A.; Valk, G.D. Epidemiology of Neuroendocrine Tumors. Front. Horm. Res. 2015, 44, 1–23. [Google Scholar]
- Uhlig, J.; Nie, J.; Gibson, J.; Cecchini, M.; Stein, S.; Lacy, J.; Kunz, P.; Kim, H.S. Epidemiology, treatment and outcomes of gastroenteropancreatic neuroendocrine neoplasms. Sci. Rep. 2024, 14, 30536. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Hofland, J.; Kaltsas, G.; de Herder, W.W. Advances in the Diagnosis and Management of Well-Differentiated Neuroendocrine Neoplasms. Endocr. Rev. 2020, 41, 371–403. [Google Scholar] [CrossRef]
- Clement, D.; Tesselaar, M.E.T.; van Leerdam, M.E.; Srirajaskanthan, R.; Ramage, J.K. Nutritional and vitamin status in patients with neuroendocrine neoplasms. World J. Gastroenterol. 2019, 25, 1171–1184. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet. Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer (Oxf. Engl. 1990) 2016, 57, 58–67. [Google Scholar] [CrossRef]
- Massironi, S.; Panzuto, F.; Zilli, A.; Rinzivillo, M.; Ciliberto, A.; Romano, E.; Danese, S.; Laviano, A. Nutritional aspects in neuroendocrine neoplasms. bridging the gap between dietary interventions and cancer care strategies: A scoping review. J. Endocrinol. Investig. 2025, 48, 269–281. [Google Scholar] [CrossRef]
- del Olmo-García, M.; Hernandez-Rienda, L.; Garcia-Carbonero, R.; Hernando, J.; Custodio, A.; Anton-Pascual, B.; Gomez, M.; Palma Milla, S.; Suarez, L.; Bellver, M.; et al. Nutritional status and quality of life of patients with advanced gastroenteropancreatic neuroendocrine neoplasms in Spain: The NUTRIGETNE (GETNE-S2109) study. Oncologist 2025, 30, oyae343. [Google Scholar] [CrossRef]
- Grozinsky-Glasberg, S.; Hofland, J.; Alband, S.; de Lima, Y.C.; Croitoru, A.; Geilvoet, W.; Igaz, P.; Kos-Kudła, B.; Krejs, G.J.; Laviano, A.; et al. Controversies in NEN: An ENETS position statement on nutritional support in neuroendocrine neoplasms. J. Neuroendocrinol. 2025, 37, e70062. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.; Grozinsky-Glasberg, S.; Oleinikov, K.; Kos-Kudła, B.; Kogut, A.; Srirajaskanthan, R.; Pizanias, M.; Poulia, K.A.; Ferreira, C.; et al. The effect of prophylactic surgery in survival and HRQoL in appendiceal NEN. Endocrine 2020, 70, 178–186. [Google Scholar] [CrossRef]
- Maasberg, S.; Knappe-Drzikova, B.; Vonderbeck, D.; Jann, H.; Weylandt, K.H.; Grieser, C.; Pascher, A.; Schefold, J.C.; Pavel, M.; Wiedenmann, B.; et al. Malnutrition Predicts Clinical Outcome in Patients with Neuroendocrine Neoplasia. Neuroendocrinology 2017, 104, 11–25. [Google Scholar] [CrossRef]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Brown, S.; Leerdam, M.V.; Tesselaar, M.; Ramage, J.; Srirajaskanthan, R. Sarcopenia and Neuroendocrine Neoplasms. Curr. Oncol. Rep. 2024, 26, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, Y.; Alzas Teomiro, C.; León Idougourram, S.; Molina Puertas, M.J.; Calañas Continente, A.; Serrano Blanch, R.; Castaño, J.P.; Gálvez Moreno, M.; Gahete, M.D.; Luque, R.M.; et al. Sarcopenia and Ghrelin System in the Clinical Outcome and Prognosis of Gastroenteropancreatic Neuroendocrine Neoplasms. Cancers 2021, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Ramage, J.; Srirajaskanthan, R. Update on Pathophysiology, Treatment, and Complications of Carcinoid Syndrome. J. Oncol. 2020, 2020, 8341426. [Google Scholar] [CrossRef]
- Artale, S.; Barzaghi, S.; Grillo, N.; Maggi, C.; Lepori, S.; Butti, C.; Bovio, A.; Barbarini, L.; Colombo, A.; Zanlorenzi, L.; et al. Role of Diet in the Management of Carcinoid Syndrome: Clinical Recommendations for Nutrition in Patients with Neuroendocrine Tumors. Nutr. Cancer 2022, 74, 2–11. [Google Scholar] [CrossRef]
- Altieri, B.; Barrea, L.; Modica, R.; Muscogiuri, G.; Savastano, S.; Colao, A.; Faggiano, A. Nutrition and neuroendocrine tumors: An update of the literature. Rev. Endocr. Metab. Disord. 2018, 19, 159–167. [Google Scholar] [CrossRef]
- Laing, E.; Kiss, N.; Michael, M.; Krishnasamy, M. Nutritional Complications and the Management of Patients with Gastroenteropancreatic Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 430–442. [Google Scholar] [CrossRef]
- Angelousi, A.; Koffas, A.; Grozinsky-Glasberg, S.; Gertner, J.; Kassi, E.; Alexandraki, K.; Caplin, M.E.; Kaltsas, G.; Toumpanakis, C. Diagnostic and Management Challenges in Vasoactive Intestinal Peptide Secreting Tumors: A Series of 15 Patients. Pancreas 2019, 48, 934–942. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Grozinsky-Glasberg, S. Emerging therapies for advanced insulinomas and glucagonomas. Endocr. Relat. Cancer 2023, 30, e230020. [Google Scholar] [CrossRef]
- de Herder, W.W.; Hofland, J. Insulinoma. In Endotext; Copyright © 2000–2025; Feingold, K.R., Ahmed, S.F., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Barrea, L.; Verde, L.; Annunziata, G.; Camajani, E.; Caprio, M.; Sojat, A.S.; Marina, L.V.; Guarnotta, V.; Colao, A.; Muscogiuri, G. Role of Mediterranean diet in endocrine diseases: A joint overview by the endocrinologist and the nutritionist. J. Endocrinol. Investig. 2024, 47, 17–33. [Google Scholar] [CrossRef]
- Aktypis, C.; Yavropoulou, M.P.; Efstathopoulos, E.; Polichroniadi, D.; Poulia, K.A.; Papatheodoridis, G.; Kaltsas, G. Bone and muscle mass characteristics in patients with gastroenteropancreatic neuroendocrine neoplasms. Endocrine 2025, 88, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Daskalakis, K.; Tsoli, M.; Grossman, A.B.; Kaltsas, G.A. Endocrinological Toxicity Secondary to Treatment of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Trends Endocrinol. Metab. TEM 2020, 31, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Xue, Z.; Lu, J.; He, Q.L.; Zheng, Z.F.; Xu, B.B.; Xie, J.W.; Li, P.; Xu, Y.; Lin, J.X.; et al. Effect of sarcopenia on short- and long-term outcomes in patients with gastric neuroendocrine neoplasms after radical gastrectomy: Results from a large, two-institution series. BMC Cancer 2020, 20, 1002. [Google Scholar] [CrossRef] [PubMed]
- Alexandraki, K.I.; Angelousi, A.; Chatzellis, E.; Chrisoulidou, A.; Kalogeris, N.; Kanakis, G.; Savvidis, C.; Vassiliadi, D.; Spyroglou, A.; Kostopoulos, G.; et al. The Role of Somatostatin Analogues in the Control of Diarrhea and Flushing as Markers of Carcinoid Syndrome: A Systematic Review and Meta-Analysis. J. Pers. Med. 2023, 13, 304. [Google Scholar] [CrossRef]
- Romano, E.; Polici, M.; Marasco, M.; Lerose, F.; Dell’Unto, E.; Nardacci, S.; Zerunian, M.; Iannicelli, E.; Rinzivillo, M.; Laghi, A.; et al. Sarcopenia in Patients with Advanced Gastrointestinal Well-Differentiated Neuroendocrine Tumors. Nutrients 2024, 16, 2224. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Cantone, M.C.; Guarnotta, V.; Mazzilli, R.; Verde, L.; Vetrani, C.; Colao, A.; Faggiano, A. Neuroendocrine Tumors: A Comprehensive Review on Nutritional Approaches. Cancers 2022, 14, 4402. [Google Scholar] [CrossRef]
- Ranallo, N.; Iamurri, A.P.; Foca, F.; Liverani, C.; De Vita, A.; Mercatali, L.; Calabrese, C.; Spadazzi, C.; Fabbri, C.; Cavaliere, D.; et al. Prognostic and Predictive Role of Body Composition in Metastatic Neuroendocrine Tumor Patients Treated with Everolimus: A Real-World Data Analysis. Cancers 2022, 14, 3231. [Google Scholar] [CrossRef]
- Chan, D.L.; Clarke, S.J.; Engel, A.; Diakos, C.I.; Pavlakis, N.; Roach, P.J.; Bailey, D.L.; Bauer, J.; Findlay, M. Computed tomography (CT)-defined sarcopenia and myosteatosis are prevalent in patients with neuroendocrine neoplasms (NENs) treated with peptide receptor radionuclide therapy (PRRT). Eur. J. Clin. Nutr. 2022, 76, 143–149. [Google Scholar] [CrossRef]
- Spyroglou, A.; Bramis, K.; Alexandraki, K. Neuroendocrine neoplasms: Evolving and future treatments. Curr. Opin. Endocr. Metab. Res. 2021, 19, 15–21. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef]
- Morgan, R.L.; Thayer, K.A.; Santesso, N.; Holloway, A.C.; Blain, R.; Eftim, S.E.; Goldstone, A.E.; Ross, P.; Ansari, M.; Akl, E.A.; et al. A risk of bias instrument for non-randomized studies of exposures: A users’ guide to its application in the context of GRADE. Environ. Int. 2019, 122, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Perelmuter, M.; Buch, A.; Rabinowich, A.; Izkhakov, E.; Greenman, Y.; Wolf, I.; Geva, R.; Osher, E. Clinical implications of measuring muscle mass by computed tomography in neuroendocrine tumor patients. Endocr. Abstr. 2025, 110, 491. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, Z.; Zhu, Y.; Zhang, L.; Zhang, L.; Wan, H. Prevalence of malnutrition comparing NRS2002, MUST, and PG-SGA with the GLIM criteria in adults with cancer: A multi-center study. Nutrition 2021, 83, 111072. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Leerdam, M.E.V.; de Jong, S.; Weickert, M.O.; Ramage, J.K.; Tesselaar, M.E.T.; Srirajaskanthan, R. Prevalence of Sarcopenia and Impact on Survival in Patients with Metastatic Gastroenteropancreatic Neuroendocrine Tumours. Cancers 2023, 15, 782. [Google Scholar] [CrossRef]
- Celik, E.; Suzan, V.; Samanci, N.S.; Suzan, A.A.; Karadag, M.; Sahin, S.; Aslan, M.S.; Yavuzer, H.; Demirci, N.S.; Doventas, A.; et al. Sarcopenia assessment by new EWGSOP2 criteria for predicting chemotherapy dose-limiting toxicity in patients with gastrointestinal tract tumors. Eur. Geriatr. Med. 2022, 13, 267–274. [Google Scholar] [CrossRef]
- Srirajaskanthan, R.; Pavel, M.; Kulke, M.; Clement, D.; Houchard, A.; Keeber, L.; Weickert, M.O. Weight Maintenance up to 48 Weeks in Patients With Carcinoid Syndrome Treated With Telotristat Ethyl: Pooled Data From the Open-Label Extensions of the Phase III Clinical Trials TELESTAR and TELECAST. Clin. Ther. 2021, 43, 1779–1785. [Google Scholar] [CrossRef]
- Papadopoulos, E.; Irving, B.A.; Brown, J.C.; Heymsfield, S.B.; Sattar, S.; Alibhai, S.M.H.; Williams, G.R.; Dunne, R.F. Sarcopenia and Cachexia in Older Patients with Cancer: Pathophysiology, Diagnosis, Impact on Outcomes, and Management Strategies. Drugs Aging 2025, 42, 1113–1142. [Google Scholar] [CrossRef]
- Borys, K.; Haubold, J.; Keyl, J.; Bali, M.A.; De Angelis, R.; Boni, K.B.; Coquelet, N.; Kohnke, J.; Baldini, G.; Kroll, L.; et al. Leveraging Sarcopenia index by automated CT body composition analysis for pan cancer prognostic stratification. NPJ Digit. Med. 2025, 8, 611. [Google Scholar] [CrossRef]
- Kroll, L.; Mathew, A.; Baldini, G.; Hosch, R.; Koitka, S.; Kleesiek, J.; Rischpler, C.; Haubold, J.; Fuhrer, D.; Nensa, F.; et al. CT-derived body composition analysis could possibly replace DXA and BIA to monitor NET-patients. Sci. Rep. 2022, 12, 13419. [Google Scholar] [CrossRef]
- Jensen, G.L.; Cederholm, T.; Ballesteros-Pomar, M.D.; Blaauw, R.; Correia, M.; Cuerda, C.; Evans, D.C.; Fukushima, R.; Gautier, J.B.O.; Gonzalez, M.C.; et al. Guidance for assessment of the inflammation etiologic criterion for the GLIM diagnosis of malnutrition: A modified Delphi approach. JPEN. J. Parenter. Enter. Nutr. 2024, 48, 145–154. [Google Scholar] [CrossRef]
- Park, S.Y.; Hwang, B.O.; Song, N.Y. The role of myokines in cancer: Crosstalk between skeletal muscle and tumor. BMB Rep. 2023, 56, 365–373. [Google Scholar] [CrossRef] [PubMed]
- de Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Gonçalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in treatment-naïve patients with cancer-associated cachexia. Clin. Nutr. 2021, 40, 2443–2455. [Google Scholar] [CrossRef] [PubMed]
- Zandee, W.T.; Merola, E.; Poczkaj, K.; de Mestier, L.; Klümpen, H.J.; Geboes, K.; de Herder, W.W.; Munir, A. Evaluation of multidisciplinary team decisions in neuroendocrine neoplasms: Impact of expert centres. Eur. J. Cancer Care 2022, 31, e13639. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Kadaj-Lipka, R.; Monica, M.; Stożek-Tutro, A.; Ryś, P.; Rydzewska, G. Pancreatic Enzyme Replacement Therapy in Pancreatic Exocrine Insufficiency-Real-World’s Dosing and Effectiveness: A Systematic Review. Dig. Dis. Sci. 2025, 70, 2270–2284. [Google Scholar] [CrossRef]
- Orsso, C.E.; Caretero, A.; Poltronieri, T.S.; Arends, J.; de van der Schueren, M.A.; Kiss, N.; Laviano, A.; Prado, C.M. Effects of high-protein supplementation during cancer therapy: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2024, 120, 1311–1324. [Google Scholar] [CrossRef]
- Delsoglio, M.; Capener, R.; Smith, T.R.; Donald, M.; Hubbard, G.P.; Stratton, R.J. High-protein oral nutritional supplement use in patients with cancer reduces complications and length of hospital stay: A systematic review and meta-analysis. Front. Nutr. 2025, 12, 1654637. [Google Scholar] [CrossRef]
- Heywood, R.; McCarthy, A.L.; Skinner, T.L. Efficacy of Exercise Interventions in Patients With Advanced Cancer: A Systematic Review. Arch. Phys. Med. Rehabil. 2018, 99, 2595–2620. [Google Scholar] [CrossRef]
- Cormie, P.; Atkinson, M.; Bucci, L.; Cust, A.; Eakin, E.; Hayes, S.; McCarthy, S.; Murnane, A.; Patchell, S.; Adams, D. Clinical Oncology Society of Australia position statement on exercise in cancer care. Med. J. Aust. 2018, 209, 184–187. [Google Scholar] [CrossRef]
- Neuroendocrine Tumor Research Foundation. Exercise While Living with a Neuroendocrine Tumor. Available online: https://netrf.org/old-for-patients/living-with-nets/exercise/ (accessed on 12 December 2025).
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Agirman, G.; Hsiao, E.Y. SnapShot: The microbiota-gut-brain axis. Cell 2021, 184, 2524–2524.e1. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a more comprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310. [Google Scholar] [CrossRef]
- Xu, Y.; He, B. The gut-muscle axis: A comprehensive review of the interplay between physical activity and gut microbiota in the prevention and treatment of muscle wasting disorders. Front. Microbiol. 2025, 16, 1695448. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Geng, J.; Bao, J.; Tang, Y.; Liu, M.; Yu, H.; Han, Y.; Huang, W.; Zhou, S. Two ghrelin receptor agonists for adults with malnutrition: A systematic review and meta-analysis. Nutr. J. 2016, 15, 97. [Google Scholar] [CrossRef] [PubMed]
- Wetzlich, B.; Nyakundi, B.B.; Yang, J. Therapeutic applications and challenges in myostatin inhibition for enhanced skeletal muscle mass and functions. Mol. Cell. Biochem. 2025, 480, 1535–1553. [Google Scholar] [CrossRef]
- Smith, R.C.; Lin, B.K. Myostatin inhibitors as therapies for muscle wasting associated with cancer and other disorders. Curr. Opin. Support. Palliat. Care 2013, 7, 352–360. [Google Scholar] [CrossRef]
- Salinari, A.; Machì, M.; Armas Diaz, Y.; Cianciosi, D.; Qi, Z.; Yang, B.; Ferreiro Cotorruelo, M.S.; Villar, S.G.; Dzul Lopez, L.A.; Battino, M.; et al. The Application of Digital Technologies and Artificial Intelligence in Healthcare: An Overview on Nutrition Assessment. Diseases 2023, 11, 97. [Google Scholar] [CrossRef]
- Castano, J.P.; Dattani, M.T.; Grozinsky-Glasberg, S.; Karavitaki, N.; Pavel, M.E.; Andoniadou, C.; Alexandraki, K.; Capatina, C.; Cerbone, M.; Ferone, D.; et al. EndoCompass project: Research roadmap for pituitary and neuroendocrine tumor endocrinology. Eur. J. Endocrinol. 2025, 193, ii84–ii96. [Google Scholar] [CrossRef]
| Tool/Framework | Type | Primary Scope | Key Components | Main Strength | Key Limitation | Use in NETs/Oncology |
|---|---|---|---|---|---|---|
| NRS2002 | Screening tool | Nutritional risk screening | Recent weight loss, BMI, reduced intake, disease severity, and age adjustment | Simple, quick, validated, hospitalized, and oncology patients, good agreement with GLIM | Does not directly assess body composition or sarcopenia; less sensitive to micronutrient deficiencies | Commonly used at diagnosis and during treatment in cancer and NET cohorts to identify patients at risk of malnutrition, who need nutritional assessment |
| MUST | Screening tool | Nutritional risk screening | BMI, unintentional weight loss, and acute disease effect | Simple, quick, and easy to apply in outpatient/ clinic settings; widely used in cancer | May underestimate risk in patients with fluid retention or preserved BMI, but muscle loss | Applied in some NET studies and general oncology clinics, especially in ambulatory settings |
| PG-SGA | Assessment tool | Comprehensive nutritional assessment | Symptoms, dietary intake, weight change, functional impact, physical exam | Oncology specific; high sensitivity for malnutrition; guides targeted intervention | Requires more time and trained staff | Frequently used as a reference for malnutrition diagnosis and for monitoring response to nutrition support in cancer and selected NET cohorts |
| GLIM criteria | Diagnostic criteria | Diagnostic framework for malnutrition | Phenotypic (weight loss, low BMI, reduced muscle mass) plus etiologic (reduced intake, inflammation/disease burden) criteria | International consensus; links screening to diagnosis; adaptable across settings | Requires body composition or surrogate; interpretation of inflammation markers (CRP, albumin) can be challenging in cancer | Increasingly applied in oncology and beginning to be used in NET studies to quantify malnutrition burden. |
| EWGSOP2 criteria | Diagnostic criteria | Diagnostic framework for sarcopenia | Muscle strength (grip, chair stand), muscle quantity/quality (DXA, CT, BIA, MRI), physical performance (gait speed, SPPB) | Emphasizes function plus mass; clear staging (probable, confirmed, severe) | Requires equipment and time; not routinely implemented in oncology clinics | Used as a conceptual reference in studies for NET, the full EWGSOP2 application remains rare in NET cohorts due to resource constraints. |
| CT derived skeletal muscle index | Body composition analysis | Quantification of muscle mass/ imaging-defined sarcopenia | Cross-sectional muscle area at the lumbar (often L3) or cervical (C3) level, normalized for height | Uses routine staging CT; highly reproducible; strong prognostic value in many cancers | No direct functional measure; heterogeneous cut-offs and vertebral levels; influenced by edema | Widely used in GEP-NET studies to estimate sarcopenia prevalence and associations with survival and treatment toxicity, though methods and thresholds vary |
| BIA | Body composition analysis | Bedside body composition assessment | Whole-body or segmental electrical conductivity to estimate fat-free mass, fat mass, phase angle, and extracellular water | Portable, non-invasive, rapid (<5 min), relatively inexpensive; good for serial monitoring and hydration status | Highly sensitive to hydration/fluid shifts (common in cancer/NETs with diarrhea/edema); requires standardized conditions; less accurate in extreme BMI | Used in NET research for tracking treatment effects on lean mass and phase angle; valuable for ambulatory monitoring, but needs cautious interpretation |
| DXA | Body composition analysis | Precise body composition and bone assessment | Whole-body or regional scanning for bone mineral density, lean soft tissue mass, and fat mass | Gold standard for body composition; precise ASMM; simultaneous bone assessment | Requires specialized equipment; radiation exposure (low); not bedside; limited availability in oncology settings | Applied in select NET studies for accurate sarcopenia phenotyping and osteoporosis assessment; ideal research tool, but rarely routine in NET clinics. |
| Trigger Category | Foods to Limit/Avoid | Alternatives |
|---|---|---|
| High-histamine/tyramine food items | Aged cheeses (cheddar, blue), cured meats (salami, bacon), fermented foods (sauerkraut, soy sauce) | Fresh mozzarella/cottage cheese, fresh poultry/fish, homemade broths |
| Beverages | Alcohol (red wine, beer), caffeine | Herbal teas, diluted fruit juices, and water with lemon |
| Other triggers | Chocolate, spicy foods, walnuts, yeast extracts | Carob/white chocolate, mild herbs, pumpkin seeds, fresh bread |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulia, K.A.; Spyroglou, A.; Violetis, O.; Mastorakos, G.; Alexandraki, K.I.; Papavassiliou, A.G. Malnutrition and Sarcopenia in Patients with Neuroendocrine Tumors: A Comprehensive Review of Evidence. Biomolecules 2025, 15, 1746. https://doi.org/10.3390/biom15121746
Poulia KA, Spyroglou A, Violetis O, Mastorakos G, Alexandraki KI, Papavassiliou AG. Malnutrition and Sarcopenia in Patients with Neuroendocrine Tumors: A Comprehensive Review of Evidence. Biomolecules. 2025; 15(12):1746. https://doi.org/10.3390/biom15121746
Chicago/Turabian StylePoulia, Kalliopi Anna, Ariadni Spyroglou, Odysseas Violetis, George Mastorakos, Krystallenia I. Alexandraki, and Athanasios G. Papavassiliou. 2025. "Malnutrition and Sarcopenia in Patients with Neuroendocrine Tumors: A Comprehensive Review of Evidence" Biomolecules 15, no. 12: 1746. https://doi.org/10.3390/biom15121746
APA StylePoulia, K. A., Spyroglou, A., Violetis, O., Mastorakos, G., Alexandraki, K. I., & Papavassiliou, A. G. (2025). Malnutrition and Sarcopenia in Patients with Neuroendocrine Tumors: A Comprehensive Review of Evidence. Biomolecules, 15(12), 1746. https://doi.org/10.3390/biom15121746










