Integrating Network Pharmacology, Molecular Docking, and Experimental Validation: Andrographolide Attenuates Acute Liver Injury via the NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Network Pharmacology Methodology
2.3. Molecular Docking Methodology
2.4. Animal Experiments and Grouping
2.5. Hepatic Index
2.6. Hematoxylin-Eosin (H&E) Staining
2.7. Immunohistochemistry
2.8. Serum Biochemical Analysis
2.9. Oxidative Stress Assessment
2.10. Enzyme-Linked Immunosorbent Assay
2.11. Cell Culture
2.12. Cell Viability Assay
2.13. Lactate Dehydrogenase Release Assay
2.14. Hoechst 33342/PI Double Staining
2.15. Flow Cytometry
2.16. Western Blotting
2.17. Real-Time Quantitative Reverse Transcription PCR
2.18. Data Analysis
3. Results
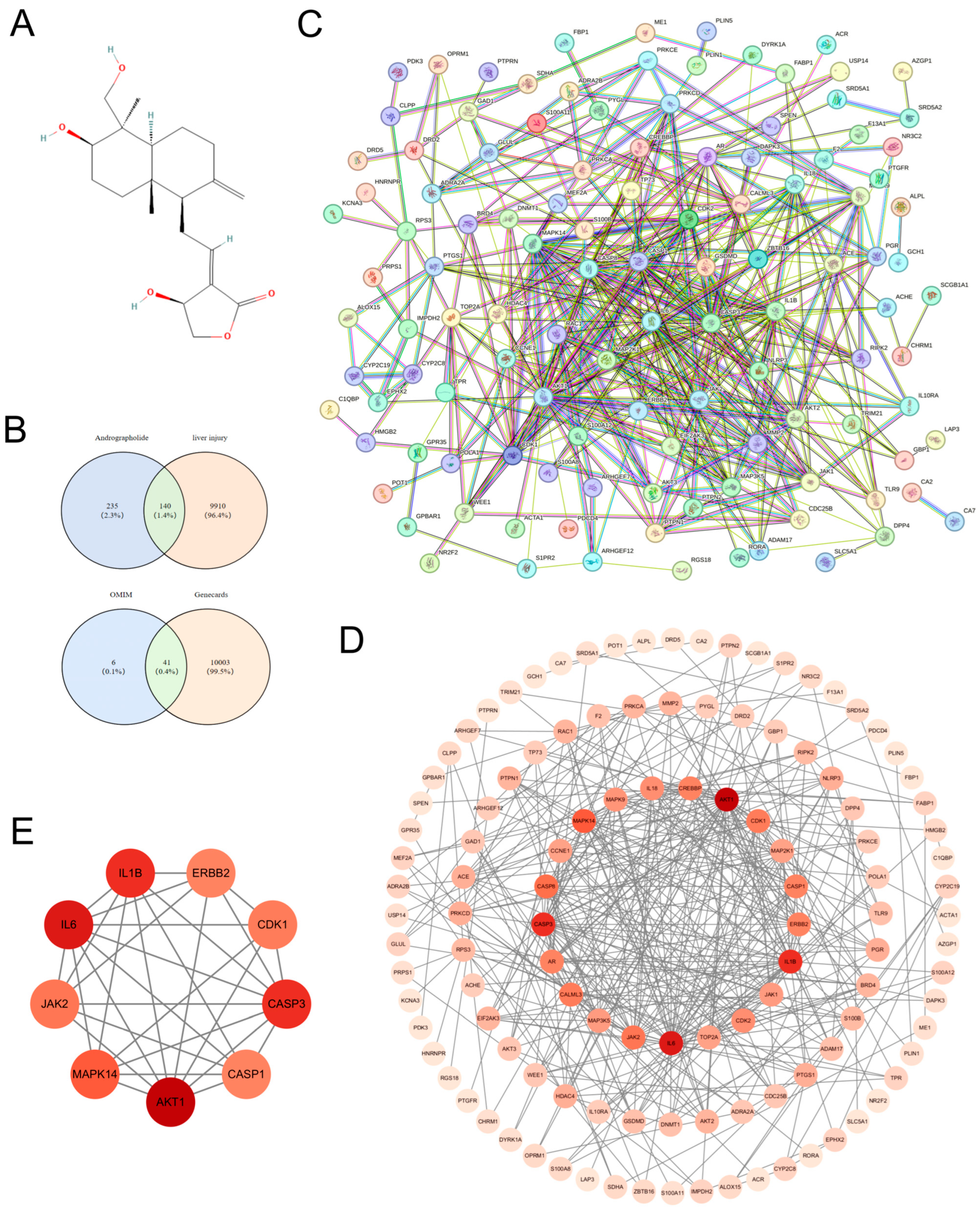
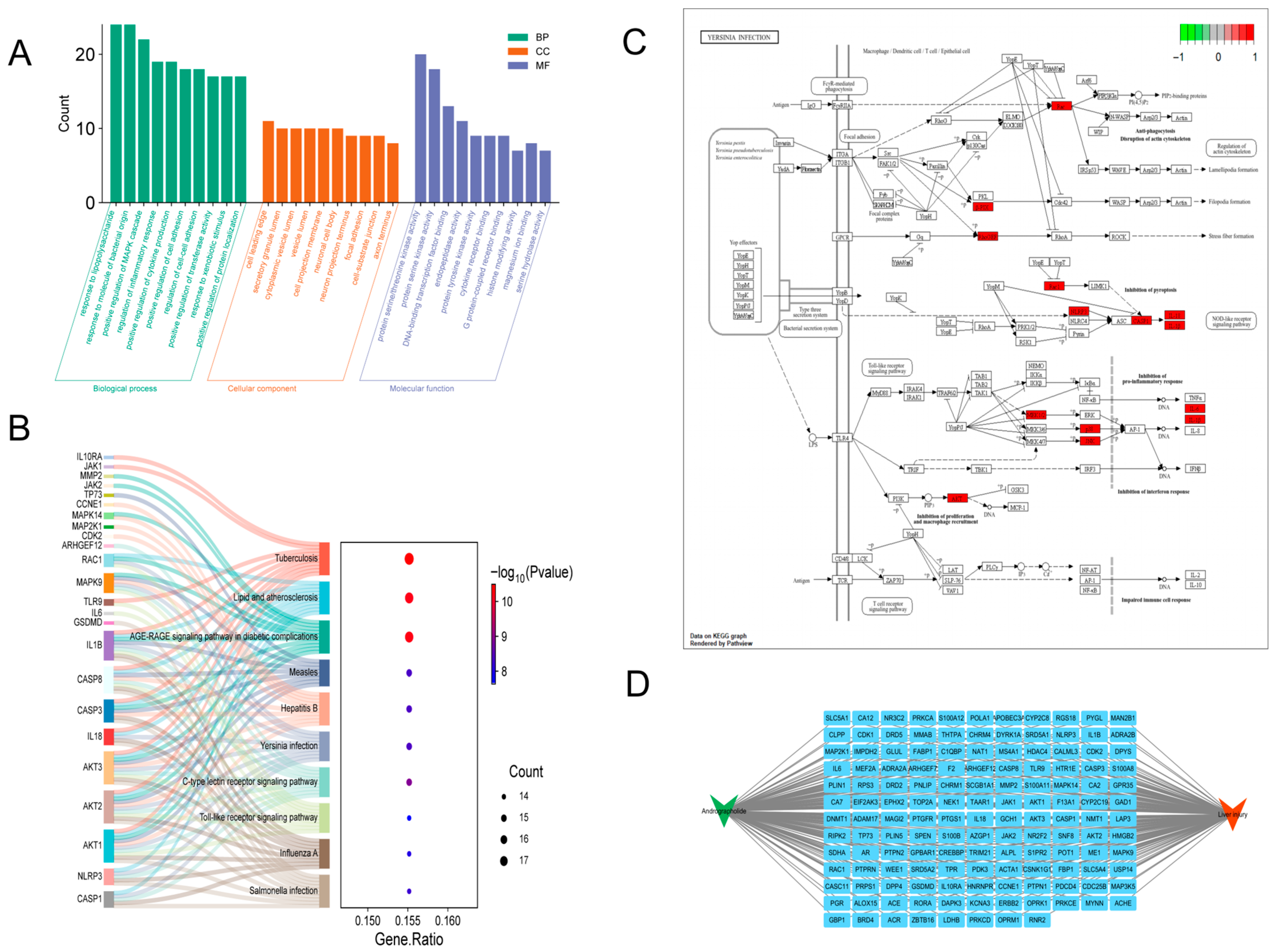
3.1. Network Pharmacology-Based Mechanistic Insights into Andrographolide Intervention in Acute Liver Injury
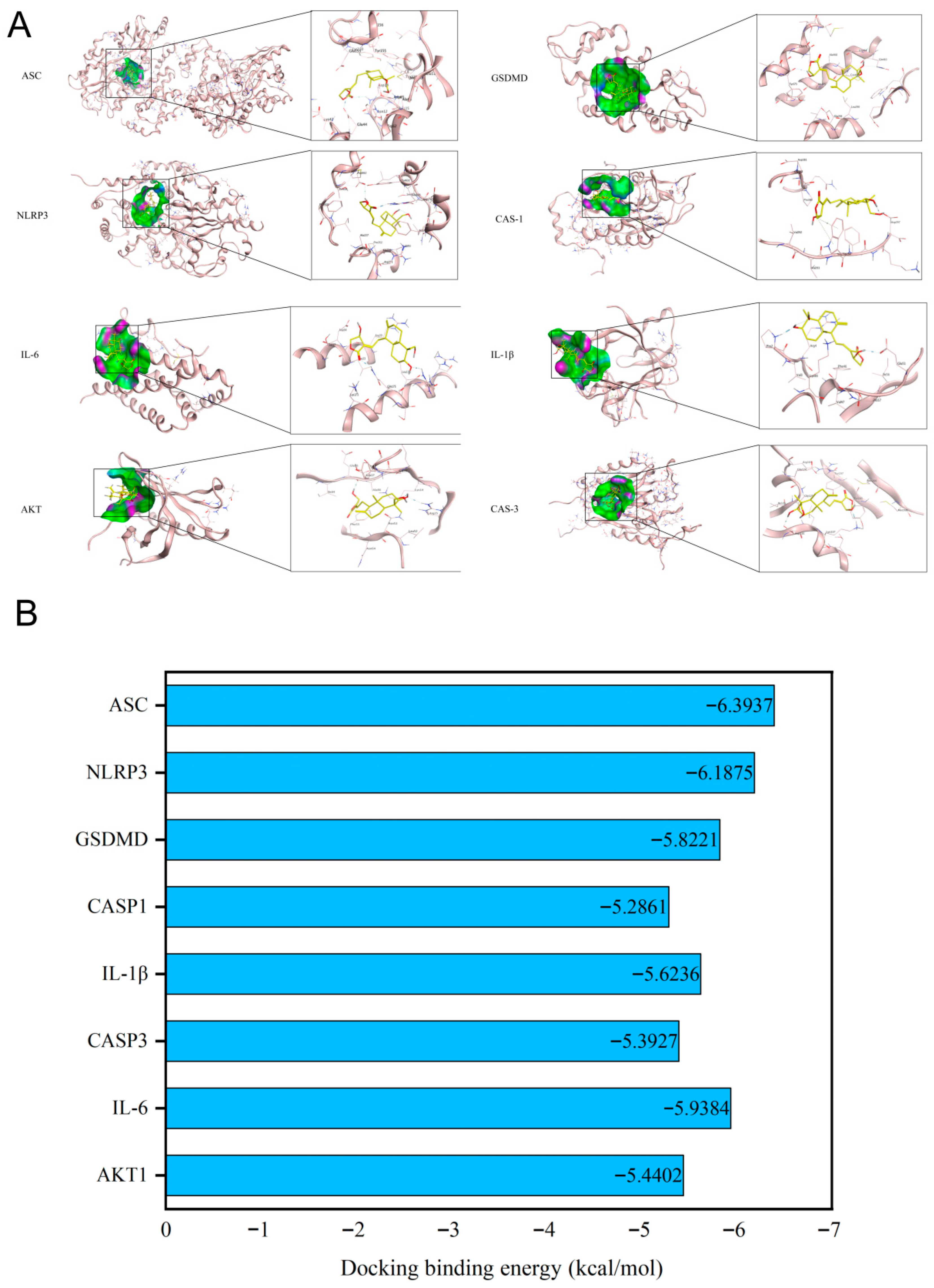
3.2. Validation of Binding Affinity Between Andrographolide and Core Targets via Molecular Docking
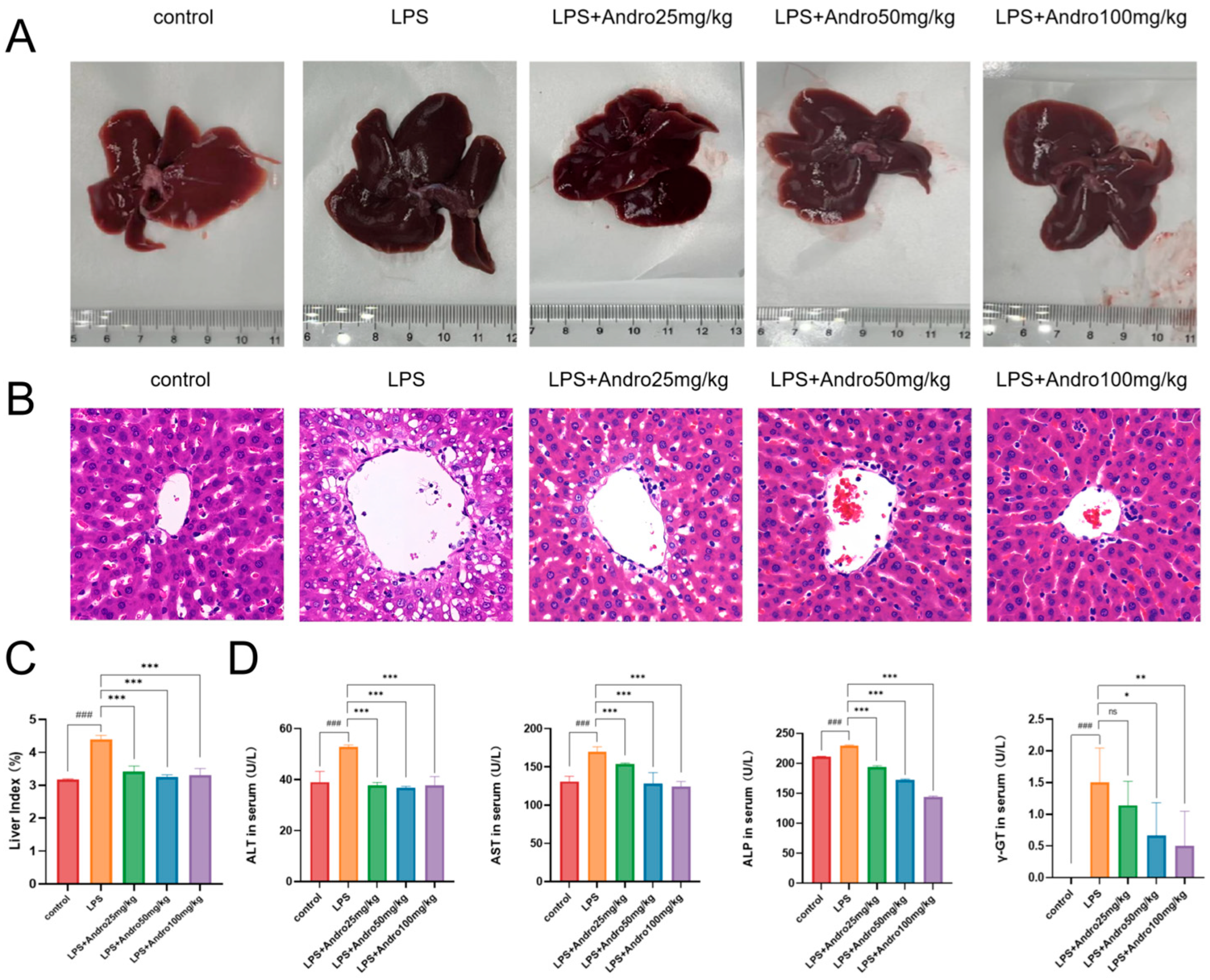
3.3. Protective Effect of Andrographolide Against Lipopolysaccharide-Induced Acute Liver Injury in Rats
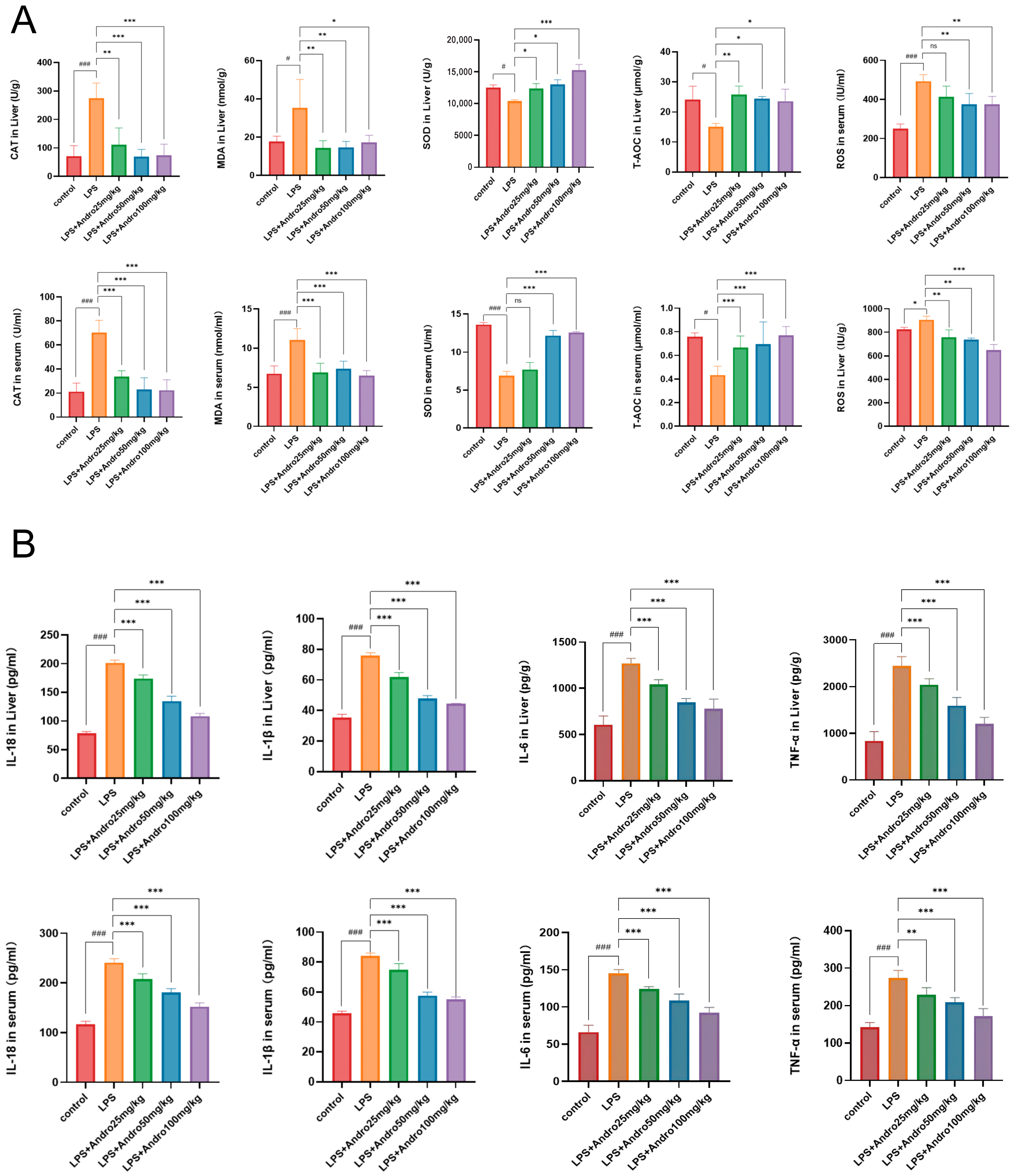
3.4. Regulatory Effects of Andrographolide on Oxidative Stress and Inflammatory Response in LPS-Induced Acute Liver Injury in Rats
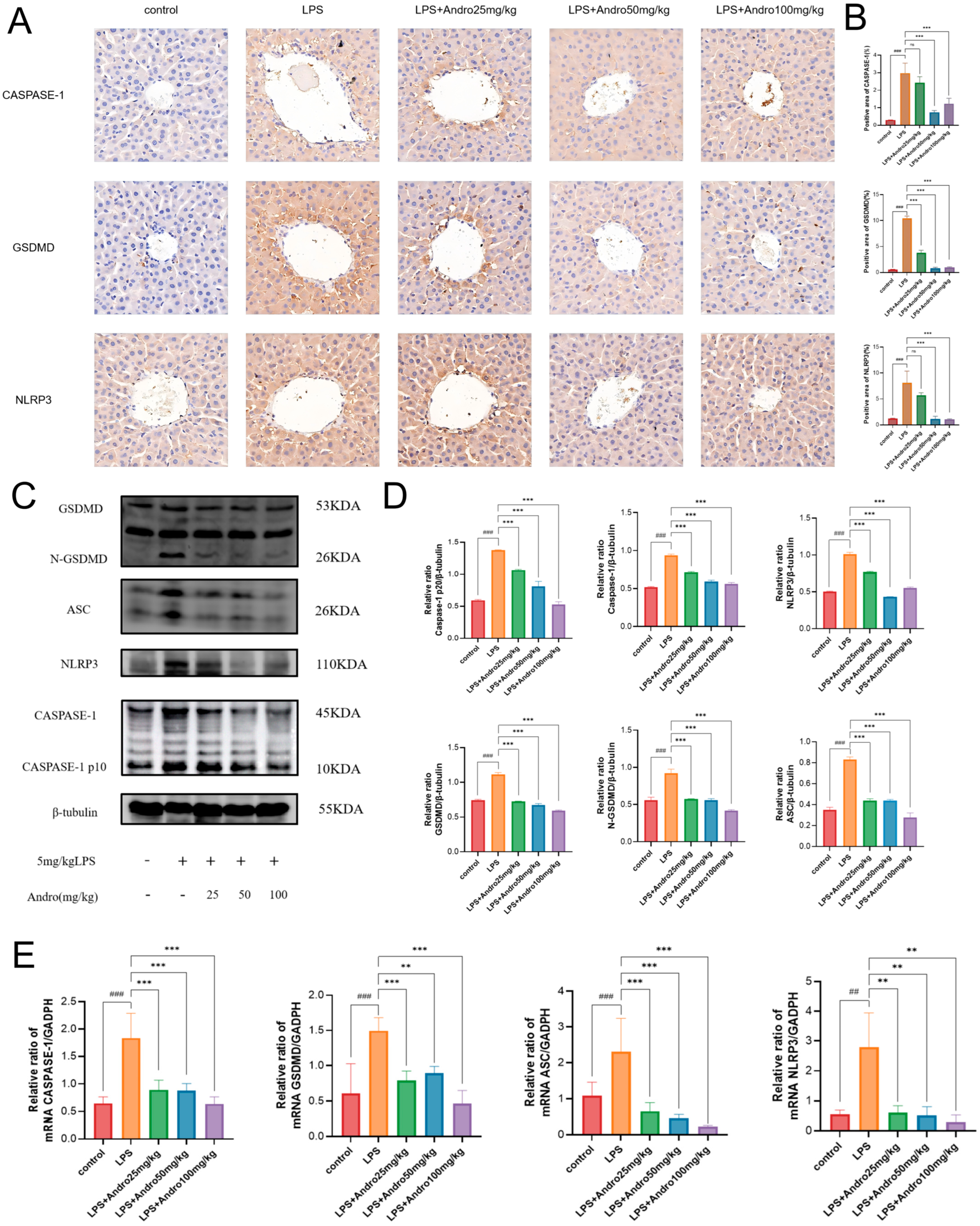
3.5. Andrographolide Suppresses Pyroptosis in Rats with LPS-Induced Acute Liver Injury
3.6. Andrographolide Ameliorates LPS-Induced Injury in BRL-3A HepatA Hepatocytes In Vitro
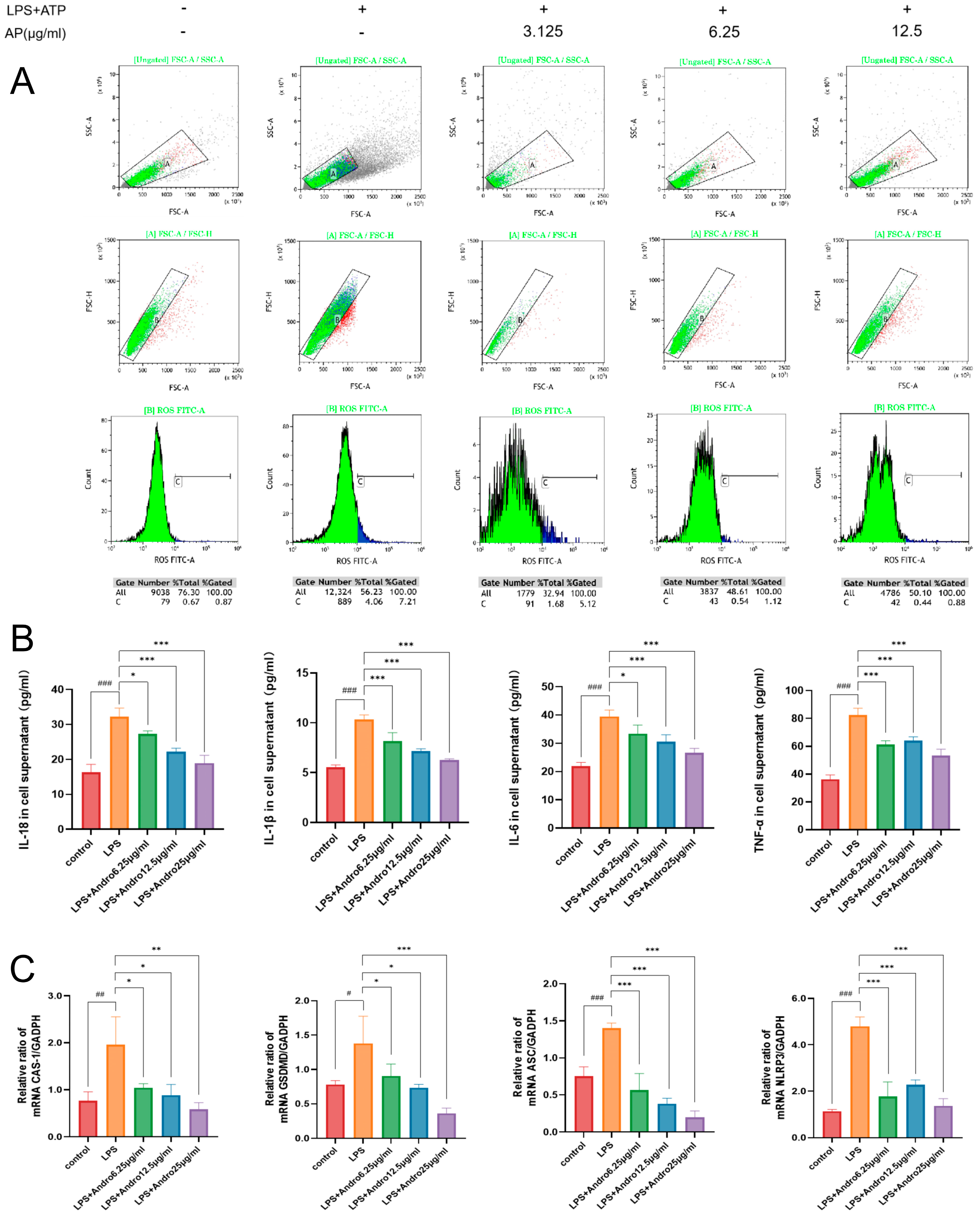
3.7. Inhibition of Pyroptosis by Andrographolide in BRL-3A Cells In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | Acute Liver Injury |
| Andro | Andrographolide |
| LPS | Lipopolysaccharide |
| NLRP3 | NACHT, LRR and PYD domains-containing protein 3 |
| GSDMD | Gasdermin D |
| CASPASE1 | Cysteinyl aspartate specific proteinase 1 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| AKT1 | AKT Serine/Threonine Kinase 1 |
| IL-18 | Interleukin-18 |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 Beta |
| TNF-α | Tumor Necrosis Factor-Alpha |
| WB | Western Blot |
| HE | Hematoxylin and Eosin |
| MAPK14 | Mitogen-Activated Protein Kinase 14 |
| CDK1 | Cyclin-Dependent Kinase 1 |
| JAK2 | Janus Kinase 2 |
| ERBB2 | Erb-B2 Receptor Tyrosine Kinase 2 |
References
- Lemmer, P.; Sowa, J.; Bulut, Y.; Strnad, P.; Canbay, A. Mechanisms and aetiology-dependent treatment of acute liver failure. Liver Int. 2025, 45, e15739. [Google Scholar] [CrossRef]
- Li, M.; Yang, Q.; Gao, J.; Liu, X.; Shi, J.; Guo, W.; Zhang, Y.; Yu, Q.; Sun, X.; Zhang, S. Nanotuner targeting mitochondrial redox and iron homeostasis imbalance for the treatment of acute liver injury. Theranostics 2025, 15, 9131–9158. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kong, X.; Qiao, J.; Wei, J. Decoding Parkinson’s Disease: The interplay of cell death pathways, oxidative stress, and therapeutic innovations. Redox Biol. 2025, 85, 103787. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Carpentier, A.C.; Wang, D. MASH: The nexus of metabolism, inflammation, and fibrosis. J. Clin. Investig. 2025, 135, e186420. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, J.-M.; Lu, X.-L.; Lin, X.-Y.; Hong, M.-Z.; Weng, S.; Pan, J.-S. Global burden of adult non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) has been steadily increasing over the past decades and is expected to persist in the future. Transl. Gastroenterol. Hepatol. 2024, 9, 33. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rolph, T.; Knott, M.; Dubourg, J. FGF21 agonists: An emerging therapeutic for metabolic dysfunction-associated steatohepatitis and beyond. J. Hepatol. 2024, 81, 562–576. [Google Scholar] [CrossRef]
- Roy, A.; Ghoshal, U.C.; Kulkarni, A.V.; Lohia, K.; Tiwary, I.; Tiwari, S.; Tewari, A.; Sonthalia, N.; Goenka, M.K. Determinants, profile and outcomes of hepatitis A virus–associated severe acute liver injury in adults. Indian J. Gastroenterol. 2024, 43, 505–512. [Google Scholar] [CrossRef]
- Yoshimura, R.; Tanaka, M.; Kurokawa, M.; Nakamura, N.; Goya, T.; Imoto, K.; Kohjima, M.; Fujiu, K.; Iwami, S.; Ogawa, Y. Stratifying and predicting progression to acute liver failure during the early phase of acute liver injury. PNAS Nexus 2025, 4, pgaf004. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, H.; Chun, C.; Li, X.; Xu, S.; Zhao, Y. Self-assembled FGF21 nanoparticles alleviate drug-induced acute liver injury. Front. Pharmacol. 2022, 13, 1084799. [Google Scholar] [CrossRef]
- Cui, K.; Liu, C.-H.; Teng, X.; Chen, F.; Xu, Y.; Zhou, S.; Yang, Q.; Du, L.; Ma, Y.; Bai, L. Association Between Artificial Liver Support System and Prognosis in Hepatitis B Virus-Related Acute-on-Chronic Liver Failure. Infect. Drug Resist. 2025, 18, 113–126. [Google Scholar] [CrossRef]
- Jothimani, D.; Marannan, N.K.; Rela, M. Acute liver failure and liver transplantation. Indian J. Gastroenterol. 2025, 44, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Chen, Y.; Ding, M.; Duan, Z. Prognostic Factors Related to the Mortality Rate of Acute-on-Chronic Liver Failure Patients. Diabetes Metab. Syndr. Obes. 2021, 14, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, Y.; Wang, K.; Mo, J.; Weng, Z.; Jiang, H.; Jin, C. Stem cell exosomes: New hope and future potential for relieving liver fibrosis. Clin. Mol. Hepatol. 2025, 31, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, J.; Wu, R.; Li, Y.; Zhang, C. Targeting pyroptosis for cancer immunotherapy: Mechanistic insights and clinical perspectives. Mol. Cancer 2025, 24, 131. [Google Scholar] [CrossRef]
- Liu, Y.; Stockwell, B.R.; Jiang, X.; Gu, W. p53-regulated non-apoptotic cell death pathways and their relevance in cancer and other diseases. Nat. Rev. Mol. Cell Biol. 2025, 26, 600–614. [Google Scholar] [CrossRef]
- Bai, Y.; Pan, Y.; Liu, X. Mechanistic insights into gasdermin-mediated pyroptosis. Nat. Rev. Mol. Cell Biol. 2025, 26, 501–521. [Google Scholar] [CrossRef]
- Xu, X.-D.; Chen, J.-X.; Zhu, L.; Xu, S.-T.; Jiang, J.; Ren, K. The emerging role of pyroptosis-related inflammasome pathway in atherosclerosis. Mol. Med. 2022, 28, 160. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, C.; Lin, C. Pyroptosis as a double-edged sword: The pathogenic and therapeutic roles in inflammatory diseases and cancers. Life Sci. 2023, 318, 121498. [Google Scholar] [CrossRef]
- Zhao, P.; Yin, S.; Qiu, Y.; Sun, C.; Yu, H. Ferroptosis and pyroptosis are connected through autophagy: A new perspective of overcoming drug resistance. Mol. Cancer 2025, 24, 23. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Su, W.; Zhang, H.; Wei, X.; Ma, X.; Gong, S.; Qu, G.; Zhang, L.; Xu, H.; et al. The mitochondria-targeted antioxidant MitoQ ameliorates inorganic arsenic-induced DCs/Th1/Th2/Th17/Treg differentiation partially by activating PINK1-mediated mitophagy in murine liver. Ecotoxicol. Environ. Saf. 2024, 277, 116350. [Google Scholar] [CrossRef]
- Kadono, K.; Kageyama, S.; Nakamura, K.; Hirao, H.; Ito, T.; Kojima, H.; Dery, K.J.; Li, X.; Kupiec-Weglinski, J.W. Myeloid Ikaros–SIRT1 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed mouse and human liver. J. Hepatol. 2021, 76, 896–909. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Qi, M.; Guo, J.; Rong, Q.; Tang, J.; Wu, Y.; Ma, X.; Huang, L. Molecular cloning and functional characterization of multiple NADPH-cytochrome P450 reductases from Andrographis paniculata. Int. J. Biol. Macromol. 2017, 102, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Huang, L.; Hou, Y.; Pang, P.; Zhou, Y.; Zhang, X.; Long, Y.; Li, H.; Muhetaer, H.; Zhang, M.; et al. Molecular mechanisms of andrographolide-induced kidney injury and senescence via SIRT3 inhibition. Toxicol. Appl. Pharmacol. 2025, 498, 117306. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Halihaman, B.; Han, Y.; Zhou, J.; Liu, C.; Bai, H.; Ding, X. New insight into the epidemiological trends of respiratory syncytial virus infection and the underlying anti-respiratory syncytial virus mechanisms of andrographolide: Integrating Global Burden of Disease database, network pharmacological analysis, and in vitro experiments. Microbiol. Spectr. 2025, e0234125. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Su, J.; Cheng, Y.; Zhang, G.; Zheng, C.; Xiao, J.; Leung, G.P.-H.; Li, J.; Zhou, G.-C. Design and synthesis of lactam analogs of andrographolide and discovery of their anticancer activity as dual EGFR and VEGFR2 inhibitors. Eur. J. Med. Chem. 2025, 299, 118042. [Google Scholar] [CrossRef]
- He, W.; Sun, J.; Zhang, Q.; Li, Y.; Fu, Y.; Zheng, Y.; Jiang, X. Andrographolide exerts anti-inflammatory effects in Mycobacterium tuberculosis-infected macrophages by regulating the Notch1/Akt/NF-κB axis. J. Leukoc. Biol. 2020, 108, 1747–1764. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Sun, Q.; Hua, T.; Bai, J.; Zhang, Q.; Liu, Q.; Ni, X. TXNIP-NLRP3-GSDMD axis-mediated inflammation and pyroptosis of islet β-cells is involved in cigarette smoke-induced hyperglycemia, which is alleviated by andrographolide. Environ. Toxicol. 2024, 39, 1415–1428. [Google Scholar] [CrossRef]
- Mishra, K. Andrographolide: Regulating the Master Regulator NF-κB. Indian J. Clin. Biochem. 2021, 36, 117–119. [Google Scholar] [CrossRef]
- Shen, J.; Xu, Q.; Chen, L.; Chang, X.; Shen, R.; Zhao, Z.; Zhu, L.; Wu, Y.; Hou, X. Andrographolide inhibits infectious bronchitis virus-induced apoptosis, pyroptosis, and inflammation. Antivir. Ther. 2023, 28, 13596535231207499. [Google Scholar] [CrossRef]
- Wu, M.; Li, Y.; Liu, D.; Wen, W.; Min, Y.; Su, Q.; An, Z.; Yang, X. Time trends and health inequalities of cirrhosis caused by metabolic Dysfunction-associated steatotic liver disease from 1990 to 2021: A global burden of disease study. J. Endocrinol. Investig. 2025, 48, 2599–2614. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, C.; Wei, R.; Li, R.; Li, Z.; Zhang, K.; Zhao, X.; Ma, Q. TRPV1/cPLA2/AA pathway contributes to ferroptosis-mediated acute liver injury in heatstroke. Int. Immunopharmacol. 2024, 138, 112539. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Long, L.-Z.; Zhang, X.-Y.; Yao, T.-T.; Cheng, X.-Y.; Yu, P.; Zou, L.-J.; He, Y.-J.; Jiang, M.; Meng, J. Ammonium tetrathiomolybdate ameliorates heat stroke-induced murine liver injury by activating the Nrf2/HO-1 signaling pathway and inhibiting oxidative stress. Free. Radic. Biol. Med. 2025, 242, 535–548. [Google Scholar] [CrossRef]
- Pu, X.; Lu, C.; Yang, X.; He, H.; Chen, X.; Wang, R.; Li, B.; Chen, S.; Zhang, Y.; Wang, W.; et al. Unveiling the hepatoprotective mechanisms of Desmodium heterocarpon (L.) DC: Novel flavonoid identification and Keap1/Nrf2 pathway activation. Phytomedicine 2025, 136, 156323. [Google Scholar] [CrossRef]
- Mao, Y.; Dai, Z.; Zhang, Q.; Peng, F.; Li, Q.; Cui, H.; Liu, Y. Advances of Chinese herbal medicine-derived polysaccharides as carrier-free and carrier agents in anti-melanoma therapy. J. Mater. Chem. B 2025, 13, 11508–11524. [Google Scholar] [CrossRef]
- Li, Y.; Huang, L.; Li, J.; Li, S.; Lv, J.; Zhong, G.; Gao, M.; Yang, S.; Han, S.; Hao, W. Targeting TLR4 and regulating the Keap1/Nrf2 pathway with andrographolide to suppress inflammation and ferroptosis in LPS-induced acute lung injury. Chin. J. Nat. Med. 2024, 22, 914–928. [Google Scholar] [CrossRef]
- Kongsomros, S.; Boonyarattanasoonthorn, T.; Phongphaew, W.; Kasorndorkbua, C.; Sunyakumthorn, P.; Im-Erbsin, R.; Lugo-Roman, L.A.; Kongratanapasert, T.; Paha, J.; Manopwisedjaroen, S.; et al. In vivo evaluation of Andrographis paniculata and Boesenbergia rotunda extract activity against SARS-CoV-2 Delta variant in Golden Syrian hamsters: Potential herbal alternative for COVID-19 treatment. J. Tradit. Complement. Med. 2024, 14, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Sui, B.; Wang, X.; Wang, W.; Li, L.; Xie, H. The effects and mechanisms of the anti-COVID-19 traditional Chinese medicine, Dehydroandrographolide from Andrographis paniculata (Burm.f.) Wall, on acute lung injury by the inhibition of NLRP3-mediated pyroptosis. Phytomedicine 2023, 114, 154753. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, Z.; Zhang, J.; Wang, K.; Zhao, Q.; Chen, M.; Yan, S.; Guo, Q.; Ma, Y.; Ji, L. Andrographolide attenuated MCT-induced HSOS via regulating NRF2-initiated mitochondrial biogenesis and antioxidant response. Cell Biol. Toxicol. 2023, 39, 3269–3285. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Lian, W.; Bai, Y.; Wang, L.; Zhao, F.; Li, H.; Wang, D.; Pang, Q. TMT-based quantitative proteomics reveals the targets of andrographolide on LPS-induced liver injury. BMC Vet.-Res. 2023, 19, 199. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Chen, H.-W.; Lii, C.-K.; Jhuang, J.-H.; Huang, C.-S.; Li, M.-L.; Yao, H.-T. A Diterpenoid, 14-Deoxy-11, 12-Didehydroandrographolide, in Andrographis paniculata Reduces Steatohepatitis and Liver Injury in Mice Fed a High-Fat and High-Cholesterol Diet. Nutrients 2020, 12, 523. [Google Scholar] [CrossRef]
- Ali, S.K.; Makeen, H.A.; Khuwaja, G.; Alhazmi, H.A.; Sharma, M.; Koty, A.; Mazahirul, I.; Parveen, H.; Mohammed, A.; Mukhtar, S.; et al. Assessment of the Phytochemical Profile, Antioxidant Capacity, and Hepatoprotective Effect of Andrographis paniculata against CCl 4-Induced Liver Dysfunction in Wistar Albino Rats. Medicina 2023, 59, 1260. [Google Scholar] [CrossRef]
- Wertman, R.S.; Yost, W.; Herrmann, B.I.; Bourne, C.M.; Sorobetea, D.; Go, C.K.; Saller, B.S.; Groß, O.; Scott, P.; Rongvaux, A.; et al. Distinct sequential death complexes regulate pyroptosis and IL-1β release in response to Yersinia blockade of immune signaling. Sci. Adv. 2024, 10, eadl3629. [Google Scholar] [CrossRef] [PubMed]
- Wertman, R.S.; Go, C.K.; Saller, B.S.; Groß, O.; Scott, P.; Brodsky, I.E. Sequentially activated death complexes regulate pyroptosis and IL-1β release in response to Yersinia blockade of immune signaling. bioRxiv 2023. [Google Scholar] [CrossRef]
- Bliska, J.B.; Brodsky, I.E.; Mecsas, J. Role of the Yersinia pseudotuberculosis Virulence Plasmid in Pathogen-Phagocyte Interactions in Mesenteric Lymph Nodes. EcoSal Plus 2021, 9, eESP00142021. [Google Scholar] [CrossRef] [PubMed]
- Exconde, P.M.; Hernandez-Chavez, C.; Bourne, C.M.; Richards, R.M.; Bray, M.B.; Lopez, J.L.; Srivastava, T.; Egan, M.S.; Zhang, J.; Yoo, W.; et al. The tetrapeptide sequence of IL-18 and IL-1β regulates their recruitment and activation by inflammatory caspases. Cell Rep. 2023, 42, 113581. [Google Scholar] [CrossRef]
- Fu, X.; Hong, W.; Li, S.; Chen, Z.; Zhou, W.; Dai, J.; Deng, X.; Zhou, H.; Li, B.; Ran, P. Wood smoke particulate matter (WSPM2.5) induces pyroptosis through both Caspase-1/IL-1β/IL-18 and ATP/P2Y-dependent mechanisms in human bronchial epithelial cells. Chemosphere 2022, 307, 135726. [Google Scholar] [CrossRef]
- Evavold, C.L.; Hafner-Bratkovič, I.; Devant, P.; D’andrea, J.M.; Ngwa, E.M.; Boršić, E.; Doench, J.G.; LaFleur, M.W.; Sharpe, A.H.; Thiagarajah, J.R.; et al. Control of gasdermin D oligomerization and pyroptosis by the Ragulator-Rag-mTORC1 pathway. Cell 2021, 184, 4495–4511.e4419. [Google Scholar] [CrossRef]
- Du, G.; Healy, L.B.; David, L.; Walker, C.; El-Baba, T.J.; Lutomski, C.A.; Goh, B.; Gu, B.; Pi, X.; Devant, P.; et al. ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature 2024, 630, 437–446. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, W.; Zhang, Y.; Yu, Q.; Zeng, M.; Gan, J.; Zhang, W.; Jiang, X.; Li, H. Focus on the role of mitochondria in NLRP3 inflammasome activation: A prospective target for the treatment of ischemic stroke (Review). Int. J. Mol. Med. 2022, 49, 74. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 inflammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Frank, M.G.; Fonken, L.K.; Watkins, L.R.; Maier, S.F. Acute stress induces chronic neuroinflammatory, microglial and behavioral priming: A role for potentiated NLRP3 inflammasome activation. Brain Behav. Immun. 2020, 89, 32–42. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Z.-W.; Li, J.; Li, A.-H.; Huo, T.-G. Insight into the regulation of NLRP3 inflammasome activation by mitochondria in liver injury and the protective role of natural products. Biomed. Pharmacother. 2022, 156, 113968. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Z.; Zhou, M.; Zhang, Q.; Feng, J. Fisetin Attenuates Zinc Overload-Induced Hepatotoxicity in Mice via Autophagy-Dependent Nrf2 Activation. Int. J. Mol. Sci. 2025, 26, 4978. [Google Scholar] [CrossRef] [PubMed]
- Kuklin, A.; Slabber, C.F.; Tortola, L.; Kwan, C.L.; Liebisch, G.; Kondylis, V.; Mair, F.; Kopf, M.; Weber, A.; Werner, S. An Nrf2-NF-κB Crosstalk Controls Hepatocyte Proliferation in the Normal and Injured Liver. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101480. [Google Scholar] [CrossRef] [PubMed]
- Dooka, B.D.; Orish, C.N.; Ezejiofor, A.N.; Anyachor, C.P.; Umeji, T.C.; Nkpaa, K.W.; Obasi, C.N.; Cirovic, A.; Cirovic, A.; Orisakwe, O.E. Heavy metal mixture induced hippocampal toxicity involve biometal accumulation, increase in oxidative stress, inflammation, and caspase-3 activation in rats via Nrf-2/HO-1/BDNF pathway. Drug Chem. Toxicol. 2025, 48, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kwatra, M.; Panda, S.R.; Murty, U.; Naidu, V. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav. Immun. 2021, 91, 142–158. [Google Scholar] [CrossRef]
- Albornoz, A.; Pardo, B.; Apaoblaza, S.; Henriquez, C.; Ojeda, J.; Uberti, B.; Hancke, J.; Burgos, R.A.; Moran, G. Andrographolide Inhibits Expression of NLPR3 Inflammasome in Canine Mononuclear Leukocytes. Animals 2024, 14, 2036. [Google Scholar] [CrossRef]

| Primer | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| Caspase-1 | CTGGGCAAAGGGAAGACTGTAGATG | ATGATGGCAACGATGGCAGGATAC |
| GSDMD | GTGAGCCACCCTGCTATTCA | GCAGGCATCCAGGCA ATAGA |
| NLRP3 | GAGCTGGACCTCAGTGACAATGC | AGAACCAATGCGAGATCCTGACAAC |
| ASC | ATGGTTTGCTGGATGCTCTGTATGG | AAGGAACAAGTTCTTGCAGGTCAGG |
| GAPDH | ACTCTACCCACGGCAAGTTC | TGGGTTTCCCGTTGATGACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, S.; Liang, X.; Yin, L.; He, C. Integrating Network Pharmacology, Molecular Docking, and Experimental Validation: Andrographolide Attenuates Acute Liver Injury via the NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis Pathway. Biomolecules 2025, 15, 1743. https://doi.org/10.3390/biom15121743
Zhang Y, Liu S, Liang X, Yin L, He C. Integrating Network Pharmacology, Molecular Docking, and Experimental Validation: Andrographolide Attenuates Acute Liver Injury via the NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis Pathway. Biomolecules. 2025; 15(12):1743. https://doi.org/10.3390/biom15121743
Chicago/Turabian StyleZhang, Yankun, Shuanghui Liu, Xiaoxia Liang, Lizi Yin, and Changliang He. 2025. "Integrating Network Pharmacology, Molecular Docking, and Experimental Validation: Andrographolide Attenuates Acute Liver Injury via the NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis Pathway" Biomolecules 15, no. 12: 1743. https://doi.org/10.3390/biom15121743
APA StyleZhang, Y., Liu, S., Liang, X., Yin, L., & He, C. (2025). Integrating Network Pharmacology, Molecular Docking, and Experimental Validation: Andrographolide Attenuates Acute Liver Injury via the NLRP3/Caspase-1/GSDMD-Mediated Pyroptosis Pathway. Biomolecules, 15(12), 1743. https://doi.org/10.3390/biom15121743





