SIRT6 in Cancer: Mechanistic Insights into Its Dual Roles in Cancer Biology and Implications for Precision Therapeutic Development
Abstract
1. Introduction
1.1. SIRT6 Is a DNA Damage Sensor and Guarder of Genomic Stability
1.2. SIRT6 Is a Central Regulator of Metabolic Reprogramming
1.3. SIRT6 Is a Master Epigenetic Regulator of Cancer Progression
2. The Dual Roles of SIRT6 in Different Cancer Types
2.1. SIRT6 in Non-Small Cell Lung Cancer (NSCLC)
2.2. SIRT6 in Breast Cancer
2.3. SIRT6 in Ovarian Cancer (OC)
2.4. SIRT6 in Head and Neck Cancer (HNSCC)
2.5. SIRT6 in Thyroid Cancer
2.6. SIRT6 in Pancreatic Cancer
2.7. SIRT6 in Gastric Cancer
2.8. SIRT6 in Liver Cancer
2.9. SIRT6 in Colorectal Cancer
2.10. SIRT6 in Malignant Tumors of the Urinary System
2.11. SIRT6 in Skin Cancer
2.12. SIRT6 in Osteosarcoma
2.13. SIRT6 in Leukemia
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Gong, H.; Chen, H.; Xiao, P.; Huang, N.; Han, X.; Zhang, J.; Yang, Y.; Li, T.; Zhao, T.; Tai, H.; et al. miR-146a impedes the anti-aging effect of AMPK via NAMPT suppression and NAD+/SIRT inactivation. Signal Transduct. Target. Ther. 2022, 7, 66. [Google Scholar] [CrossRef]
- Shen, H.; Qi, X.; Hu, Y.; Wang, Y.; Zhang, J.; Liu, Z.; Qin, Z. Targeting sirtuins for cancer therapy: Epigenetics modifications and beyond. Theranostics 2024, 14, 6726–6767. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Brosens, J.J.; Lam, E.W. Interplay between SIRT proteins and tumour suppressor transcription factors in chemotherapeutic resistance of cancer. Drug Resist. Updates 2011, 14, 35–44. [Google Scholar] [CrossRef]
- Aventaggiato, M.; Vernucci, E.; Barreca, F.; Russo, M.A.; Tafani, M. Sirtuins’ control of autophagy and mitophagy in cancer. Pharmacol. Ther. 2021, 221, 107748. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Remuzzi, G.; Benigni, A. Sirtuins in kidney health and disease. Nat. Rev. Nephrol. 2024, 20, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lin, H. Understanding the Function of Mammalian Sirtuins and Protein Lysine Acylation. Annu. Rev. Biochem. 2021, 90, 245–285. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Liu, H.; Zhang, W.; Zhou, J. Emerging roles of SIRT6 in human diseases and its modulators. Med. Res. Rev. 2021, 41, 1089–1137. [Google Scholar] [CrossRef]
- Korotkov, A.; Seluanov, A.; Gorbunova, V. Sirtuin 6: Linking longevity with genome and epigenome stability. Trends Cell Biol. 2021, 31, 994–1006. [Google Scholar] [CrossRef]
- Nahalkova, J. On the interface of aging, cancer, and neurodegeneration with SIRT6 and L1 retrotransposon protein interaction network. Ageing Res. Rev. 2024, 101, 102496. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, P.; Ge, J.; Li, H. SIRT6 in Aging, Metabolism, Inflammation and Cardiovascular Diseases. Aging Dis. 2022, 13, 1787–1822. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Afrose, A.; Rahman, M.R.; Chowdhury, R.; Nirzhor, S.S.R.; Khan, R.I.; Kabir, M.T. A Comprehensive Analysis into the Therapeutic Application of Natural Products as SIRT6 Modulators in Alzheimer’s Disease, Aging, Cancer, Inflammation, and Diabetes. Int. J. Mol. Sci. 2021, 22, 4180. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, C.; Tan, J.; Dou, C.; Chen, Y. Modulation of SIRT6 activity acts as an emerging therapeutic implication for pathological disorders in the skeletal system. Genes Dis. 2023, 10, 864–876. [Google Scholar] [CrossRef]
- Chen, R.R.; Li, Y.Y.; Wu, J.W.; Wang, Y.; Song, W.; Shao, D.; Gao, W.; Yu, H. SIRT6 in health and diseases: From molecular mechanisms to therapeutic prospects. Pharmacol. Res. 2025, 221, 107984. [Google Scholar] [CrossRef]
- Li, Y.; Jin, J.; Wang, Y. SIRT6 Widely Regulates Aging, Immunity, and Cancer. Front. Oncol. 2022, 12, 861334. [Google Scholar] [CrossRef]
- You, Y.; Liang, W. SIRT1 and SIRT6: The role in aging-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166815. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Li, T.; Liu, M.; Wang, Y.; Ma, H.; Mu, N.; Wang, H. SIRT6 in Senescence and Aging-Related Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 641315. [Google Scholar] [CrossRef] [PubMed]
- Affandi, T.; Haas, A.; Ohm, A.M.; Wright, G.M.; Black, J.C.; Reyland, M.E. PKCδ Regulates Chromatin Remodeling and DNA Repair through SIRT6. Mol. Cancer Res. MCR 2024, 22, 181–196. [Google Scholar] [CrossRef]
- Hou, T.; Cao, Z.; Zhang, J.; Tang, M.; Tian, Y.; Li, Y.; Lu, X.; Chen, Y.; Wang, H.; Wei, F.Z.; et al. SIRT6 coordinates with CHD4 to promote chromatin relaxation and DNA repair. Nucleic Acids Res. 2020, 48, 2982–3000. [Google Scholar] [CrossRef]
- Chen, F.; Xu, W.; Tang, M.; Tian, Y.; Shu, Y.; He, X.; Zhou, L.; Liu, Q.; Zhu, Q.; Lu, X.; et al. hnRNPA2B1 deacetylation by SIRT6 restrains local transcription and safeguards genome stability. Cell Death Differ. 2025, 32, 382–396. [Google Scholar] [CrossRef]
- Wang, H.; Feng, K.; Wang, Q.; Deng, H. Reciprocal interaction between SIRT6 and APC/C regulates genomic stability. Sci. Rep. 2021, 11, 14253. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Sun, X.; Yu, J.; Qian, Z.; Wu, L.; Xu, X.; Wan, X.; Jiang, Y.; Zhang, J.; et al. The SIRT6 activator MDL-800 improves genomic stability and pluripotency of old murine-derived iPS cells. Aging Cell 2020, 19, e13185. [Google Scholar] [CrossRef]
- Simon, M.; Yang, J.; Gigas, J.; Earley, E.J.; Hillpot, E.; Zhang, L.; Zagorulya, M.; Tombline, G.; Gilbert, M.; Yuen, S.L.; et al. A rare human centenarian variant of SIRT6 enhances genome stability and interaction with Lamin A. EMBO J. 2022, 41, e110393. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, H.; Zhuang, H.; Dong, G.; Gao, K.; Liu, L.; Zhou, H.; Nie, Y.; Wang, J.; Liu, L. SIRT6 mitigates doxorubicin-induced cardiomyopathy via amelioration of mitochondrial dysfunction: A mechanistic study implicating the activation of the Nrf-2/FUNDC1 signaling axis. Int. J. Med. Sci. 2025, 22, 1640–1657. [Google Scholar] [CrossRef]
- Song, M.Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.H. Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, X.; Guo, X.; Liu, H.; Ma, R.; Wang, Y.; Liang, Y.; Sun, Y.; Wang, M.; Zhao, R.; et al. Low Glucose-Induced Overexpression of HOXC-AS3 Promotes Metabolic Reprogramming of Breast Cancer. Cancer Res. 2022, 82, 805–818. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Yan, J.; Huang, J.; Shen, Y.; He, H.; Dou, H. SIRT6 deficiency impairs the deacetylation and ubiquitination of UHRF1 to strengthen glycolysis and lactate secretion in bladder cancer. Cell Biosci. 2024, 14, 153. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, Y.; Kang, M.; Feng, M.; Ren, Y.; Dai, H.; Wang, Y.; Wang, Y.; Tang, B. USP48 Is Upregulated by Mettl14 to Attenuate Hepatocellular Carcinoma via Regulating SIRT6 Stabilization. Cancer Res. 2021, 81, 3822–3834. [Google Scholar] [CrossRef]
- Pang, K.; Huang, J.; Zhang, S.; Guan, Y.; Zou, N.; Kang, J.; Du, H.; Zhao, D.; Abramochkin, D.V.; Chen, H.; et al. Translocation of SIRT6 promotes glycolysis reprogramming to exacerbate diabetic angiopathy. Redox Biol. 2025, 85, 103736. [Google Scholar] [CrossRef]
- Arif, T.; Shteinfer-Kuzmine, A.; Shoshan-Barmatz, V. Decoding Cancer through Silencing the Mitochondrial Gatekeeper VDAC1. Biomolecules 2024, 14, 1304. [Google Scholar] [CrossRef]
- Tao, Z.; Jin, Z.; Wu, J.; Cai, G.; Yu, X. Sirtuin family in autoimmune diseases. Front. Immunol. 2023, 14, 1186231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Y.; Ni, D.; Huang, Z.; Wei, J.; Feng, L.; Su, J.C.; Wei, Y.; Ning, S.; Yang, X.; et al. Targeting a cryptic allosteric site of SIRT6 with small-molecule inhibitors that inhibit the migration of pancreatic cancer cells. Acta Pharm. Sin. B 2022, 12, 876–889. [Google Scholar] [CrossRef] [PubMed]
- Dindi, U.M.R.; Al-Ghamdi, S.; Alrudian, N.A.; Dayel, S.B.; Abuderman, A.A.; Saad Alqahtani, M.; Bahakim, N.O.; Ramesh, T.; Vilwanathan, R. Ameliorative inhibition of sirtuin 6 by imidazole derivative triggers oxidative stress-mediated apoptosis associated with Nrf2/Keap1 signaling in non-small cell lung cancer cell lines. Front. Pharmacol. 2023, 14, 1335305. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, X.; Zhao, S.; Zhong, X.; Huang, D.; Feng, R.; Li, P.; Fang, Z.; Hu, Y.; Zhang, Z.; et al. SIRT6 deficiency in endothelial cells exacerbates oxidative stress by enhancing HIF1α accumulation and H3K9 acetylation at the Ero1α promoter. Clin. Transl. Med. 2023, 13, e1377. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Wang, J.; Yu, Y.; Li, F.; Meng, L.; Chen, M.; Yang, Q.; Xu, Z.; Sun, J.; Zhuo, W.; et al. The histone deacetylase SIRT6 promotes glycolysis through the HIF-1α/HK2 signaling axis and induces erlotinib resistance in non-small cell lung cancer. Apoptosis 2022, 27, 883–898. [Google Scholar] [CrossRef]
- Feng, S.; Li, Y.; Huang, H.; Huang, H.; Duan, Y.; Yuan, Z.; Zhu, W.; Mei, Z.; Luo, L.; Yan, P. Isoorientin reverses lung cancer drug resistance by promoting ferroptosis via the SIRT6/Nrf2/GPX4 signaling pathway. Eur. J. Pharmacol. 2023, 954, 175853. [Google Scholar] [CrossRef]
- Subramani, P.; Nagarajan, N.; Mariaraj, S.; Vilwanathan, R. Knockdown of sirtuin6 positively regulates acetylation of DNMT1 to inhibit NOTCH signaling pathway in non-small cell lung cancer cell lines. Cell. Signal. 2023, 105, 110629. [Google Scholar] [CrossRef]
- Xiong, L.; Tan, B.; Lei, X.; Zhang, B.; Li, W.; Liu, D.; Xia, T. SIRT6 through PI3K/Akt/mTOR signaling pathway to enhance radiosensitivity of non-Small cell lung cancer and inhibit tumor progression. IUBMB Life 2021, 73, 1092–1102. [Google Scholar] [CrossRef]
- Chen, S.; Yang, H.; Hu, Z.; Jin, J.; Xiong, X.; Zhang, Z.; Xie, C. Deacetylation by SIRT6 increases the stability of GILZ to suppress NSCLC cell migration and invasion. Cell. Signal. 2024, 124, 111414. [Google Scholar] [CrossRef]
- Zhao, K.; Zheng, M.; Su, Z.; Ghosh, S.; Zhang, C.; Zhong, W.; Ho, J.W.K.; Jin, G.; Zhou, Z. MOF-mediated acetylation of SIRT6 disrupts SIRT6-FOXA2 interaction and represses SIRT6 tumor-suppressive function by upregulating ZEB2 in NSCLC. Cell Rep. 2023, 42, 112939. [Google Scholar] [CrossRef]
- Andreani, C.; Bartolacci, C.; Persico, G.; Casciaro, F.; Amatori, S.; Fanelli, M.; Giorgio, M.; Galié, M.; Tomassoni, D.; Wang, J.; et al. SIRT6 promotes metastasis and relapse in HER2-positive breast cancer. Sci. Rep. 2023, 13, 22000. [Google Scholar] [CrossRef]
- Becherini, P.; Caffa, I.; Piacente, F.; Damonte, P.; Vellone, V.G.; Passalacqua, M.; Benzi, A.; Bonfiglio, T.; Reverberi, D.; Khalifa, A.; et al. SIRT6 enhances oxidative phosphorylation in breast cancer and promotes mammary tumorigenesis in mice. Cancer Metab. 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Li, B.; Li, Y.; Aman, S.; Xia, K.; Yang, Y.; Ahmad, B.; Wu, H. Acetylation of ELF5 suppresses breast cancer progression by promoting its degradation and targeting CCND1. NPJ Precis. Oncol. 2021, 5, 20. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef]
- Fan, G.; Yu, B.; Tang, L.; Zhu, R.; Chen, J.; Zhu, Y.; Huang, H.; Zhou, L.; Liu, J.; Wang, W.; et al. TSPAN8+ myofibroblastic cancer-associated fibroblasts promote chemoresistance in patients with breast cancer. Sci. Transl. Med. 2024, 16, eadj5705. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Wang, Y.; Shang, X.; Yan, Z.; Li, S.; Bao, W. Cisplatin-induced PANDAR-Chemo-EVs contribute to a more aggressive and chemoresistant ovarian cancer phenotype through the SRSF9-SIRT4/SIRT6 axis. J. Gynecol. Oncol. 2024, 35, e13. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hah, Y.S.; Ryu, S.; Cheon, S.Y.; Won, S.J.; Lee, J.S.; Hwa, J.S.; Seo, J.H.; Chang, H.W.; Kim, S.W.; et al. MDM2-dependent Sirt1 degradation is a prerequisite for Sirt6-mediated cell death in head and neck cancers. Exp. Mol. Med. 2021, 53, 422–431. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, R.; Wei, X.; Yu, W.; Min, Z.; Ye, M. The SIRT6-Autophagy-Warburg Effect Axis in Papillary Thyroid Cancer. Front. Oncol. 2020, 10, 1265. [Google Scholar] [CrossRef]
- Lian, F.; Dong, D.; Pu, J.; Yang, G.; Yang, J.; Yang, S.; Wang, Y.; Zhao, B.; Lu, M. Ubiquitin-specific peptidase 10 attenuates the ferroptosis to promote thyroid cancer malignancy by facilitating GPX4 via elevating SIRT6. Environ. Toxicol. 2024, 39, 1129–1139. [Google Scholar] [CrossRef]
- Song, N.; Guan, X.; Zhang, S.; Wang, Y.; Wang, X.; Lu, Z.; Chong, D.; Wang, J.Y.; Yu, R.; Yu, W.; et al. Discovery of a pyrrole-pyridinimidazole derivative as novel SIRT6 inhibitor for sensitizing pancreatic cancer to gemcitabine. Cell Death Dis. 2023, 14, 499. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Tang, Y.; Wang, Y.; Guan, X.; Yu, W.; Jiang, T.; Lu, L.; Gu, Y. A SIRT6 Inhibitor, Marine-Derived Pyrrole-Pyridinimidazole Derivative 8a, Suppresses Angiogenesis. Mar. Drugs 2023, 21, 517. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chen, S.L.; Peng, S.L.; Tsai, Y.L.; Chang, Z.M.; Chang, V.H.; Ch′ang, H.J. Upregulating sirtuin 6 ameliorates glycolysis, EMT and distant metastasis of pancreatic adenocarcinoma with krüppel-like factor 10 deficiency. Exp. Mol. Med. 2021, 53, 1623–1635. [Google Scholar] [CrossRef]
- Kartha, N.; Gianopulos, J.E.; Schrank, Z.; Cavender, S.M.; Dobersch, S.; Kynnap, B.D.; Wallace-Povirk, A.; Wladyka, C.L.; Santana, J.F.; Kim, J.C.; et al. Sirtuin 6 is required for the integrated stress response and resistance to inhibition of transcriptional cyclin-dependent kinases. Sci. Transl. Med. 2023, 15, eabn9674. [Google Scholar] [CrossRef]
- Liu, W.; Yang, L.J.; Liu, Y.L.; Yuan, D.S.; Zhao, Z.M.; Wang, Q.; Yan, Y.; Pan, H.F. Dynamic characterization of intestinal metaplasia in the gastric corpus mucosa of Atp4a-deficient mice. Biosci. Rep. 2020, 40, BSR20181881. [Google Scholar] [CrossRef]
- Seo, J.H.; Ryu, S.; Cheon, S.Y.; Lee, S.J.; Won, S.J.; Yim, C.D.; Lee, H.J.; Hah, Y.S.; Park, J.J. Sirt6-Mediated Cell Death Associated with Sirt1 Suppression in Gastric Cancer. Cancers 2024, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, S.; Li, Q.; Zhang, H.; Xiao, W.; Chen, Y. miR-338-3p Targets SIRT6 to Inhibit Liver Cancer Malignancy and Paclitaxel Resistance. Drug Dev. Res. 2025, 86, e70089. [Google Scholar] [CrossRef]
- Han, L.; Jia, L.; Zan, Y. Long intergenic noncoding RNA smad7 (Linc-smad7) promotes the epithelial-mesenchymal transition of HCC by targeting the miR-125b/SIRT6 axis. Cancer Med. 2020, 9, 9123–9137. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, J.; Zhang, X.; Feng, Y.; Lei, J.H.; Xu, X.; Chen, Q.; Deng, C.X. Sirt6 ablation in the liver causes fatty liver that increases cancer risky by upregulating Serpina12. EMBO Rep. 2024, 25, 1361–1386. [Google Scholar] [CrossRef]
- Li, F.; Yu, W.; Zhou, X.; Hou, J.; Gao, Y.; Zhang, J.; Gao, X. SIRT6 Inhibits Anoikis of Colorectal Cancer Cells by Down-Regulating NDRG1. Int. J. Mol. Sci. 2024, 25, 5585. [Google Scholar] [CrossRef]
- Qiu, B.; Li, S.; Li, M.; Wang, S.; Mu, G.; Chen, K.; Wang, M.; Zhu, W.G.; Wang, W.; Wang, J.; et al. KAT8 acetylation-controlled lipolysis affects the invasive and migratory potential of colorectal cancer cells. Cell Death Dis. 2023, 14, 164. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Song, X.; Fang, K.; Chang, X. A high proportion of CD38 (high) CD16 (low) NK cells in colorectal cancer can interrupt immune surveillance and favor tumor growth. Cancer Immunol. Immunother. CII 2025, 74, 263. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Hu, B.; Si, Z.; Yang, H.; Xie, J. Sirtuin 6 is a negative regulator of the anti-tumor function of natural killer cells in murine inflammatory colorectal cancer. Mol. Immunol. 2023, 158, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gu, L.; Lin, X.; Liu, C.; Lu, B.; Cui, K.; Zhou, F.; Zhao, Q.; Prochownik, E.V.; Fan, C.; et al. Dynamic Regulation of ME1 Phosphorylation and Acetylation Affects Lipid Metabolism and Colorectal Tumorigenesis. Mol. Cell 2020, 77, 138–149.e135. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Gao, Q. Transfer of microRNA-25 by colorectal cancer cell-derived extracellular vesicles facilitates colorectal cancer development and metastasis. Mol. Therapy Nucleic Acids 2021, 23, 552–564. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Luo, G.; Jiang, M.; Liu, C.; Zhang, Y.; Zhang, L. SIRT6 suppresses colon cancer growth by inducing apoptosis and autophagy through transcriptionally down-regulating Survivin. Mitochondrion 2024, 78, 101932. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Li, J.; Jiang, M.; Guo, S.; Yang, G.; Zhang, L.; Wang, F.; Yi, S.; Wang, J.; et al. Inhibition of AKT induces p53/SIRT6/PARP1-dependent parthanatos to suppress tumor growth. Cell Commun. Signal. CCS 2022, 20, 93. [Google Scholar] [CrossRef]
- Shao, J.; Shi, T.; Chen, L.; Wang, X.; Yu, H.; Feng, N.; Wang, X. AF9 targets acetyl-modified STAT6 to diminish purine metabolism and accelerate cell apoptosis during metastasis. Cell Death Differ. 2023, 30, 1695–1709. [Google Scholar] [CrossRef]
- Fu, W.; Li, H.; Fu, H.; Zhao, S.; Shi, W.; Sun, M.; Li, Y. The SIRT3 and SIRT6 Promote Prostate Cancer Progression by Inhibiting Necroptosis-Mediated Innate Immune Response. J. Immunol. Res. 2020, 2020, 8820355. [Google Scholar] [CrossRef]
- Han, Q.; Xie, Q.R.; Li, F.; Cheng, Y.; Wu, T.; Zhang, Y.; Lu, X.; Wong, A.S.T.; Sha, J.; Xia, W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 2021, 11, 6526–6541. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Wang, X.; Zhang, D.; Zhang, L.; Cheng, J.; Zhang, Q.; Hua, Z.; Miao, X.; Shi, J. Expression analysis and biological regulation of silencing regulatory protein 6 (SIRT6) in cutaneous squamous cell carcinoma. An. Bras. Dermatol. 2024, 99, 535–545. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, J.; Li, L.; Tan, L.; Shi, P.; Zhang, J.; Zhong, X.; Ge, L.; Wu, Z.; Cui, H. FOXO3a-SIRT6 axis suppresses aerobic glycolysis in melanoma. Int. J. Oncol. 2020, 56, 728–742. [Google Scholar] [CrossRef]
- Hussein, U.K.; Ahmed, A.G.; Song, Y.; Kim, K.M.; Moon, Y.J.; Ahn, A.R.; Park, H.S.; Ahn, S.J.; Park, S.H.; Kim, J.R.; et al. CK2α/CSNK2A1 Induces Resistance to Doxorubicin through SIRT6-Mediated Activation of the DNA Damage Repair Pathway. Cells 2021, 10, 1770. [Google Scholar] [CrossRef]
- Zhang, P.; Brinton, L.T.; Williams, K.; Sher, S.; Orwick, S.; Tzung-Huei, L.; Mims, A.S.; Coss, C.C.; Kulp, S.K.; Youssef, Y.; et al. Targeting DNA Damage Repair Functions of Two Histone Deacetylases, HDAC8 and SIRT6, Sensitizes Acute Myeloid Leukemia to NAMPT Inhibition. Clin. Cancer Res. 2021, 27, 2352–2366. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Li, Y.; Liang, Q.; Xie, J.; Li, X.; Fang, J. SIRT6-PARP1 is involved in HMGB1 polyADP-ribosylation and acetylation and promotes chemotherapy-induced autophagy in leukemia. Cancer Biol. Ther. 2020, 21, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Liu, Y.; Chen, L.; Luo, Y.; Cui, Y.; Zhang, N.; Liu, P.; Zhou, M.; Xie, Y. α-Hederin inhibits the growth of lung cancer A549 cells in vitro and in vivo by decreasing SIRT6 dependent glycolysis. Pharm. Biol. 2021, 59, 11–20. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Wang, F.; Guo, Y.; Ren, Y.; Low, V.; Cho, S.; Liu, Q.; Qiu, Y.; Li, X.; et al. SIRT6 Ameliorates Cancer Cachexia-Associated Adipose Wasting by Suppressing TNFR2 Signalling in Mice. J. Cachexia Sarcopenia Muscle 2025, 16, e13734. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, J.; Zhang, L.; Zhao, Q.; Zhao, S.; Jiang, S. MitoAMPK inhibits the Warburg effect by MZF1-SIRT6 with glycosis related genes in NSCLC. J. Cell. Mol. Med. 2024, 28, e70053. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Vilwanathan, R. Silencing Sirtuin 6 induces cell cycle arrest and apoptosis in non-small cell lung cancer cell lines. Genomics 2020, 112, 3703–3712. [Google Scholar] [CrossRef]
- Jing, N.; Zhang, K.; Chen, X.; Liu, K.; Wang, J.; Xiao, L.; Zhang, W.; Ma, P.; Xu, P.; Cheng, C.; et al. ADORA2A-driven proline synthesis triggers epigenetic reprogramming in neuroendocrine prostate and lung cancers. J. Clin. Investig. 2023, 133, e168670. [Google Scholar] [CrossRef]
- Shang, J.L.; Ning, S.B.; Chen, Y.Y.; Chen, T.X.; Zhang, J. MDL-800, an allosteric activator of SIRT6, suppresses proliferation and enhances EGFR-TKIs therapy in non-small cell lung cancer. Acta Pharmacol. Sin. 2021, 42, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Tenhunen, J.; Kučera, T.; Huovinen, M.; Küblbeck, J.; Bisenieks, E.; Vigante, B.; Ogle, Z.; Duburs, G.; Doležal, M.; Moaddel, R.; et al. Screening of SIRT6 inhibitors and activators: A novel activator has an impact on breast cancer cells. Biomed. Pharmacother. 2021, 138, 111452. [Google Scholar] [CrossRef]
- Liang, C.; Wang, S.; Feng, D.; Wang, S.; Zheng, C.; Qu, Y.; Wang, W.; Ma, Y.; Li, H.; Yang, H.; et al. Structure-Guided Discovery of Subtype Selective SIRT6 Inhibitors with a β-Carboline Skeleton for the Treatment of Breast Cancer. J. Med. Chem. 2024, 67, 21975–22001. [Google Scholar] [CrossRef]
- Hong, O.Y.; Jang, H.Y.; Lee, Y.R.; Jung, S.H.; Youn, H.J.; Kim, J.S. Inhibition of cell invasion and migration by targeting matrix metalloproteinase-9 expression via sirtuin 6 silencing in human breast cancer cells. Sci. Rep. 2022, 12, 12125. [Google Scholar] [CrossRef]
- Thirumurthi, U.; Shen, J.; Xia, W.; LaBaff, A.M.; Wei, Y.; Li, C.W.; Chang, W.C.; Chen, C.H.; Lin, H.K.; Yu, D.; et al. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci. Signal. 2014, 7, ra71. [Google Scholar] [CrossRef]
- Wu, X.; Wang, S.; Zhao, X.; Lai, S.; Yuan, Z.; Zhan, Y.; Ni, K.; Liu, Z.; Liu, L.; Xin, R.; et al. Clinicopathological and prognostic value of SIRT6 in patients with solid tumors: A meta-analysis and TCGA data review. Cancer Cell Int. 2022, 22, 84. [Google Scholar] [CrossRef]
- Choi, J.E.; Sebastian, C.; Ferrer, C.M.; Lewis, C.A.; Sade-Feldman, M.; LaSalle, T.; Gonye, A.; Lopez, B.G.C.; Abdelmoula, W.M.; Regan, M.S.; et al. A unique subset of glycolytic tumour-propagating cells drives squamous cell carcinoma. Nat. Metab. 2021, 3, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Gong, Z.; Li, Y.; Naseem, D.F.; Li, C.; Wen, M.; Zhao, B.; Xu, Z.; Zhang, S.; Zang, R.; et al. Association of SIRT6 Expression With Risk of Pneumonitis Induced by Radiotherapy in Cancer Patients. Mol. Carcinog. 2025, 64, 1104–1118. [Google Scholar] [CrossRef]
- Hu, Z.I.; O′Reilly, E.M. Therapeutic developments in pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 7–24. [Google Scholar] [CrossRef]
- Chouhan, S.; Kumar, A.; Muhammad, N.; Usmani, D.; Khan, T.H. Sirtuins as Key Regulators in Pancreatic Cancer: Insights into Signaling Mechanisms and Therapeutic Implications. Cancers 2024, 16, 4095. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Q.; Wang, X.; Jin, J.; Wu, C.; Feng, L.; Yang, X.; Zhao, M.; Chen, Y.; Lu, S.; et al. Discovery of a potent and highly selective inhibitor of SIRT6 against pancreatic cancer metastasis in vivo. Acta Pharm. Sin. B 2024, 14, 1302–1316. [Google Scholar] [CrossRef]
- Wang, M.; Bi, C.; Li, H.; Lu, L.; Gao, T.; Huang, P.; Liu, C.; Wang, B. The emerging double-edged sword role of Sirtuins in the gastric inflammation-carcinoma sequence revealed by bulk and single-cell transcriptomes. Front. Oncol. 2022, 12, 1004726. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Ke, D.; Li, F.; Shi, R.S.; Liu, T.; Li, D.; Zhang, Q.X. A review shows that ATG10 has been identified as a potential prognostic marker and therapeutic target for cancer patients based on real-world studies. Front. Oncol. 2025, 15, 1573378. [Google Scholar] [CrossRef]
- Varghese, B.; Chianese, U.; Capasso, L.; Sian, V.; Bontempo, P.; Conte, M.; Benedetti, R.; Altucci, L.; Carafa, V.; Nebbioso, A. SIRT1 activation promotes energy homeostasis and reprograms liver cancer metabolism. J. Transl. Med. 2023, 21, 627. [Google Scholar] [CrossRef]
- Dai, L.; Lu, S.; Mao, L.; Zhong, M.; Feng, G.; He, S.; Yuan, G. A Novel Prognostic Model of Hepatocellular Carcinoma per Two NAD+ Metabolic Synthesis-Associated Genes. Int. J. Mol. Sci. 2024, 25, 10362. [Google Scholar] [CrossRef]
- Wang, M.; Lan, L.; Yang, F.; Jiang, S.; Xu, H.; Zhang, C.; Zhou, G.; Xia, H.; Xia, J. Hepatic SIRT6 deficit promotes liver tumorigenesis in the mice models. Genes Dis. 2022, 9, 789–796. [Google Scholar] [CrossRef]
- Huang, J.; Su, J.; Wang, H.; Chen, J.; Tian, Y.; Zhang, J.; Feng, T.; Di, L.; Lu, X.; Sheng, H.; et al. Discovery of Novel PROTAC SIRT6 Degraders with Potent Efficacy against Hepatocellular Carcinoma. J. Med. Chem. 2024, 67, 17319–17349. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zhu, Z.; Chen, Y.; Song, J.; Huang, Y.; Song, K.; Zhong, J.; Xu, X.; Wei, J.; Wang, C.; et al. Small-molecule activating SIRT6 elicits therapeutic effects and synergistically promotes anti-tumor activity of vitamin D3 in colorectal cancer. Theranostics 2020, 10, 5845–5864. [Google Scholar] [CrossRef]
- Wang, Q.L.; Chen, Z.; Lu, X.; Lin, H.; Feng, H.; Weng, N.; Chen, L.; Liu, M.; Long, L.; Huang, L.; et al. Methionine Metabolism Dictates PCSK9 Expression and Antitumor Potency of PD-1 Blockade in MSS Colorectal Cancer. Adv. Sci. 2025, 12, e2501623. [Google Scholar] [CrossRef]
- Seibert, T.M.; Garraway, I.P.; Plym, A.; Mahal, B.A.; Giri, V.; Jacobs, M.F.; Cheng, H.H.; Loeb, S.; Helfand, B.T.; Eeles, R.A.; et al. Genetic Risk Prediction for Prostate Cancer: Implications for Early Detection and Prevention. Eur. Urol. 2023, 83, 241–248. [Google Scholar] [CrossRef]
- Young, M.; Jackson-Spence, F.; Beltran, L.; Day, E.; Suarez, C.; Bex, A.; Powles, T.; Szabados, B. Renal cell carcinoma. Lancet 2024, 404, 476–491. [Google Scholar] [CrossRef]
- Chouhan, S.; Muhammad, N.; Usmani, D.; Khan, T.H.; Kumar, A. Molecular Sentinels: Unveiling the Role of Sirtuins in Prostate Cancer Progression. Int. J. Mol. Sci. 2024, 26, 183. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Ma, X.; Jiang, Z.; Duan, Y.; Lv, S.; Jing, W. Integrative analysis of Anoikis-related genes reveals that FASN is a novel prognostic biomarker and promotes the malignancy of bladder cancer via Wnt/β-catenin pathway. Heliyon 2024, 10, e34029. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Peterson, L.M.; Guzmán-Pérez, G.; Krier, C.R.; Ahmad, N. The sirtuin 6: An overture in skin cancer. Exp. Dermatol. 2020, 29, 124–135. [Google Scholar] [CrossRef]
- Abbotto, E.; Miro, C.; Piacente, F.; Salis, A.; Murolo, M.; Nappi, A.; Millo, E.; Russo, E.; Cichero, E.; Sturla, L.; et al. SIRT6 pharmacological inhibition delays skin cancer progression in the squamous cell carcinoma. Biomed. Pharmacother. 2023, 166, 115326. [Google Scholar] [CrossRef]
- Geng, A.; Tang, H.; Huang, J.; Qian, Z.; Qin, N.; Yao, Y.; Xu, Z.; Chen, H.; Lan, L.; Xie, H.; et al. The deacetylase SIRT6 promotes the repair of UV-induced DNA damage by targeting DDB2. Nucleic Acids Res. 2020, 48, 9181–9194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ha, S.H.; Moon, Y.J.; Hussein, U.K.; Song, Y.; Kim, K.M.; Park, S.H.; Park, H.S.; Park, B.H.; Ahn, A.R.; et al. Inhibition of SIRT6 potentiates the anti-tumor effect of doxorubicin through suppression of the DNA damage repair pathway in osteosarcoma. J. Exp. Clin. Cancer Res. CR 2020, 39, 247. [Google Scholar] [CrossRef]

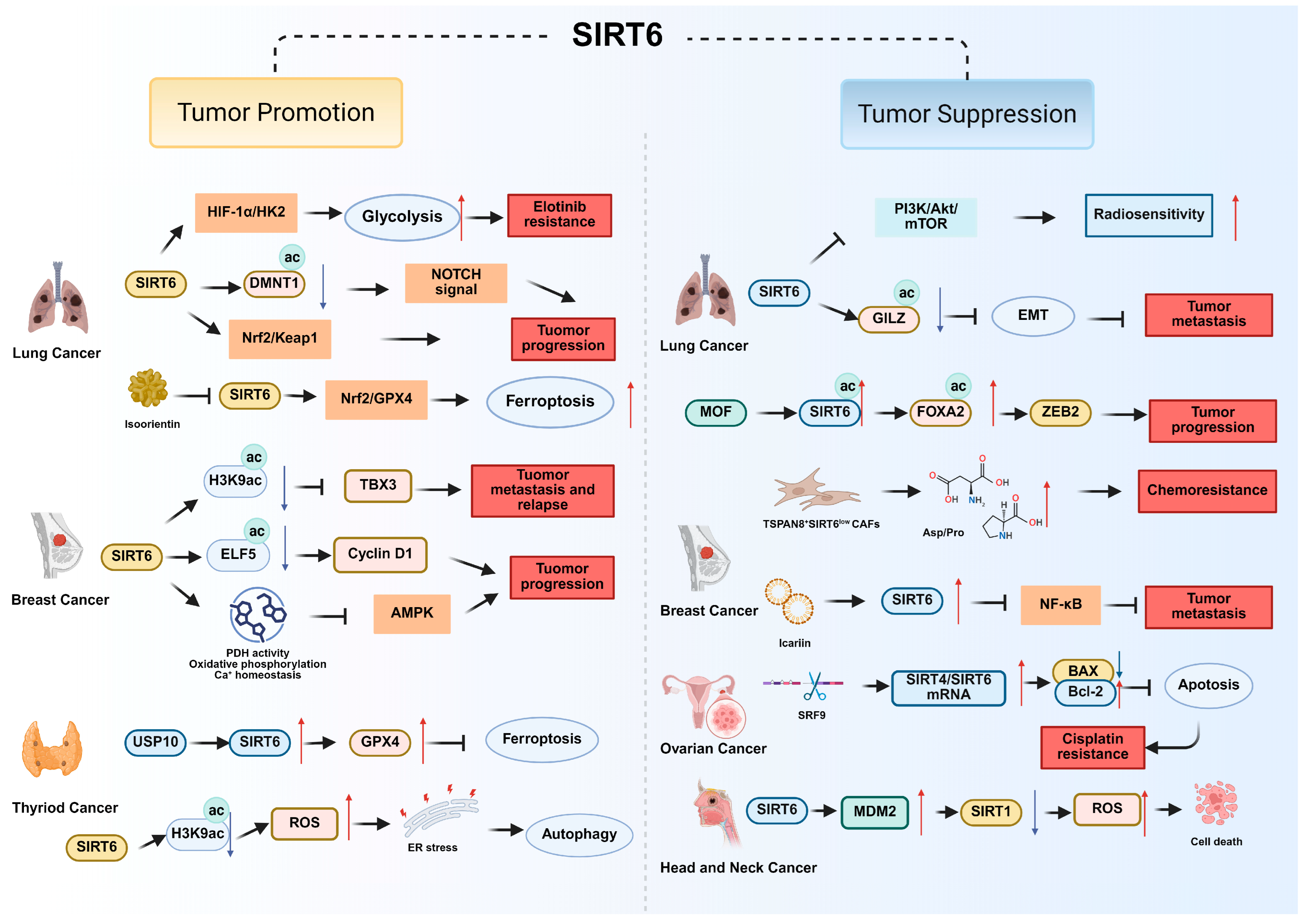

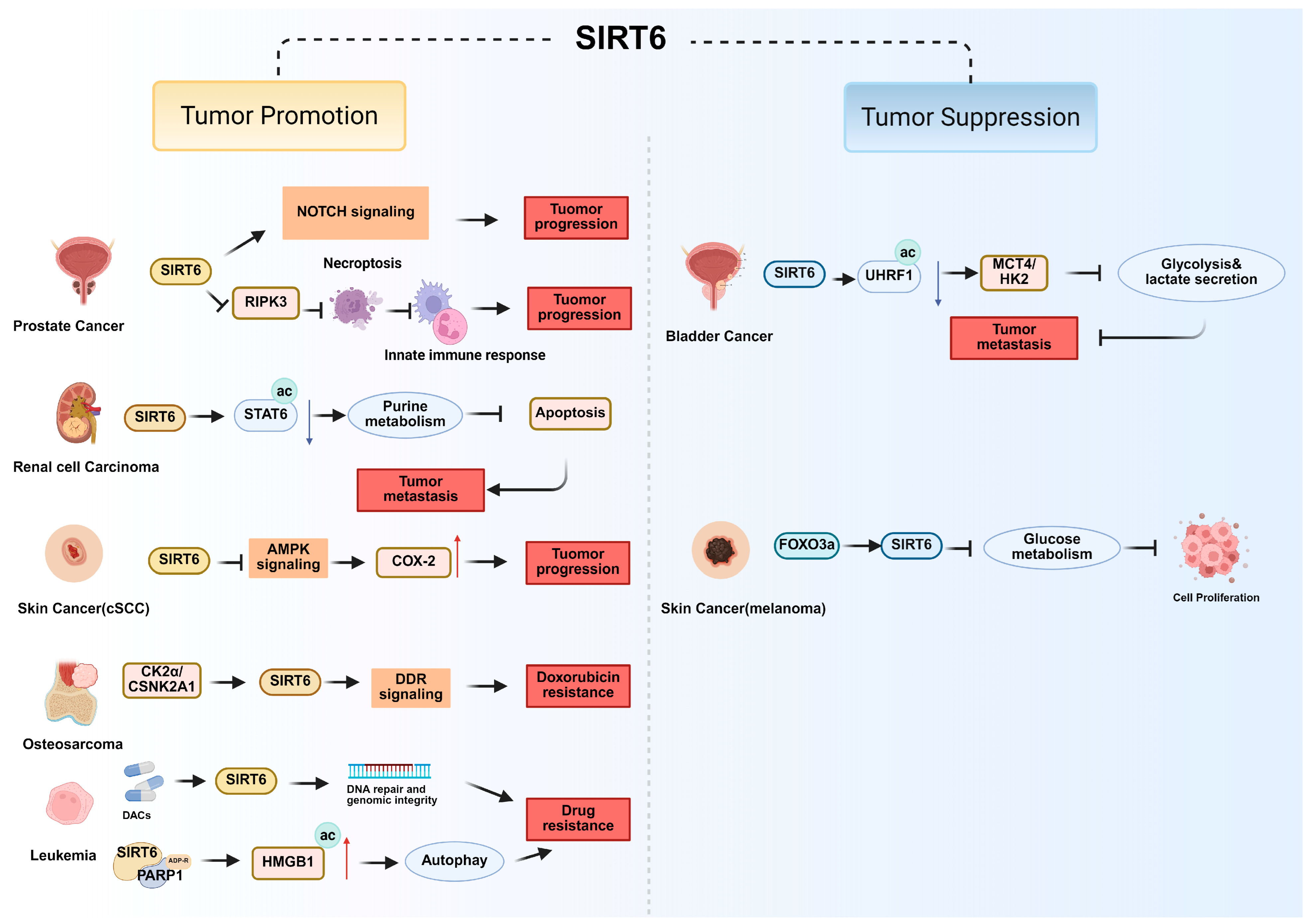
| Cancer Type | Function of SIRT6 | Pathways and Molecular Mechanism | References |
|---|---|---|---|
| Non-Small Cell Lung Cancer (NSCLC) | Tumor promotion | SIRT6 promotes erlotinib resistance by enhancing glycolysis through the HIF-1α/HK2 signaling axis. | [36] |
| SIRT6 Sensitizes cells to ferroptosis by regulating the SIRT6/Nrf2/GPX4 pathway. | [37] | ||
| SIRT6 induces oxidative stress-mediated apoptosis via the Nrf2/Keap1 pathway. | [34] | ||
| SIRT6 activates NOTCH signaling through DNMT1 acetylation to promote tumor progression. | [38] | ||
| Tumor suppression | SIRT6 inhibits PI3K/Akt/mTOR signaling to increase radiosensitivity | [39] | |
| SIRT6 deacetylates GILZ to suppress EMT and metastasis. | [40] | ||
| SIRT6 deacetylates FOXA2 to inhibit ZEB2 transcription that suppress tumor growth. | [41] | ||
| Breast Cancer | Tumor promotion | SIRT6 overexpression promotes metastasis and relapse by repressing TBX3 through H3K9ac deacetylation. | [42] |
| SIRT6 enhances PDH activity, oxidative phosphorylation and Ca2+ homeostasis to inhibit AMPK signaling that suppresses tumor growth. | [43] | ||
| SIRT6 deacetylates and stabilizes ELF5 to upregulate cyclin D1 (CCND1) and promote cell proliferation. | [44] | ||
| Tumor suppression | SIRT6 upregulated by icariin suppresses NF-κB/EMT signaling, induces redox-mediated apoptosis and suppresses tumor metastasis. | [45] | |
| Loss of SIRT6 in TSPAN8+ CAFs induces chemoresistance by promoting metabolic reprogramming and secretion of pro-tumorigenic metabolites. | [46] | ||
| Ovarian Cancer | Tumor suppression | SIRT4/SIRT6 mRNA level increases BAX/Bcl-2 expression level and induces tumor cell apoptosis, sensitizing cells to cisplatin. | [47] |
| Head and Neck Cancer | Tumor suppression | SIRT6 induces MDM2-mediated degradation of SIRT1 and accumulates of ROS, driving cancer cell death. | [48] |
| Thyroid Cancer | Tumor promotion | SIRT6 accumulates ROS then activates endoplasmic reticulum stress (ER stress) and subsequently induced autophagy by deacetylating H3K56ac | [49] |
| SIRT6 elevated by USP10 attenuates the ferroptosis to promote thyroid cancer malignancy by facilitating GPX4 | [50] | ||
| Pancreatic Cancer (PDAC) | Tumor promotion | SIRT6 activates PI3K/AKT/mTOR and ERK signaling pathways to induce gemcitabine resistance | [51] |
| SIRT6 upregulates VEGF/HIF-1α/VEGFR2 signaling and activates AKT/MEK signaling to promote angiogenesis | [52] | ||
| Tumor suppression | KLF10-SIRT6 reverses EMT, suppresses glycolysis and reduces metastasis via NF-κB & HIF-1α. | [53] | |
| SIRT6 regulates ISR via ATF4 and loss of SIRT6 defines basal subtype vulnerable to CDK7/9 inhibition. | [54] | ||
| Gastric Cancer | Tumor promotion | SIRT6 inhibits HIF1-α to adapt to the microenvironment of cell hypoxia in in Atp4a−/− mice. | [55] |
| Tumor suppression | SIRT6 suppresses SIRT1 to upregulate MDM2 and ROS expression, suppressing tumor progression. | [56] | |
| Liver Cancer (HCC) | Tumor promotion | SIRT6 is upregulated by Linc-smad7 and miR-338-3p and promotes cancer progression. | [57,58] |
| Tumor suppression | SIRT6 suppresses Serpina12 expression through histone H3K9 deacetylation and limits cancer progression. | [59] | |
| Colorectal Cancer (CRC) | Tumor promotion | SIRT6 inhibits NDRG1 repression and activates AKT signaling to support anoikis resistance. | [60] |
| SIRT6 deacetylates KAT6 to modulate lipolysis and promote CRC metastasis. | [61] | ||
| SIRT6 is upregulated in CRC-infiltrating NK cells and decreases cytotoxicity and immune evasion. | [62,63] | ||
| Tumor suppression | SIRT6 counteracts ACAT1-driven ME1 acetylation and downregulates nicotinamide adenine dinucleotide phosphate (NADPH) & lipid metabolism, thereby restraining tumorigenesis. | [64] | |
| MiR-25 downregulates SIRT6 to activate Lin28b/NRP-1 axis and promote tumor metastasis. | [65] | ||
| SIRT6 represses Survivin to induce mitochondrial apoptosis and autophagy. | [66] | ||
| SIRT6 cooperates with p53/PARP1 to promote the formation of PAR polymer and cell death upon AKT inhibition. | [67] | ||
| Bladder Cancer | Tumor suppression | SIRT6 deacetylates UHRF1 and upregulates MCT4 and HK2 expression, in turn inhibiting glycolysis, lactate secretion and tumor migration. | [28] |
| Renal Cell Carcinoma | Tumor suppression | SIRT6 deacetylates STAT6 and modulates the AF9/STAT6 signaling axis that inhibits purine metabolism and promotes apoptosis. | [68] |
| Prostate Cancer | Tumor promotion | SIRT6 inhibits necroptosis-mediated immune responses through repression of RIPK3. | [69] |
| SIRT6 promotes tumorigenesis by activating Notch signaling | [70] | ||
| Skin Cancer | Tumor promotion | SIRT6 inhibits AMPK pathway and regulates COX-2 stability to promote cutaneous squamous cell carcinoma (cSCC) progression. | [71] |
| Tumor suppression | SIRT6 regulating by FOXO3a inhibited glucose metabolism and tumor cell proliferation in melanoma. | [72] | |
| Osteosarcoma | Tumor promotion | SIRT6 regulated by CK2α activates the DNA damage repair (DDR) pathway and enhances doxorubicin resistance. | [73] |
| Leukemia | Tumor promotion | SIRT6 activated by DACs supports genomic integrity and DNA repair and protects leukemia cells. | [74] |
| SIRT6 catalyzes the monoADP-ribosylation of PARP1 and acetylates HMGB1 that promoting autophagy and drug resistance | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Han, Z.; Zhu, K.; Han, Y.; Xiao, X.; Tong, J.; Li, Y.; Xia, S. SIRT6 in Cancer: Mechanistic Insights into Its Dual Roles in Cancer Biology and Implications for Precision Therapeutic Development. Biomolecules 2025, 15, 1655. https://doi.org/10.3390/biom15121655
Feng Y, Han Z, Zhu K, Han Y, Xiao X, Tong J, Li Y, Xia S. SIRT6 in Cancer: Mechanistic Insights into Its Dual Roles in Cancer Biology and Implications for Precision Therapeutic Development. Biomolecules. 2025; 15(12):1655. https://doi.org/10.3390/biom15121655
Chicago/Turabian StyleFeng, Yanqi, Zhuoyan Han, Kunrui Zhu, Yuelin Han, Xiangtian Xiao, Jie Tong, Yiming Li, and Shu Xia. 2025. "SIRT6 in Cancer: Mechanistic Insights into Its Dual Roles in Cancer Biology and Implications for Precision Therapeutic Development" Biomolecules 15, no. 12: 1655. https://doi.org/10.3390/biom15121655
APA StyleFeng, Y., Han, Z., Zhu, K., Han, Y., Xiao, X., Tong, J., Li, Y., & Xia, S. (2025). SIRT6 in Cancer: Mechanistic Insights into Its Dual Roles in Cancer Biology and Implications for Precision Therapeutic Development. Biomolecules, 15(12), 1655. https://doi.org/10.3390/biom15121655







