Chronic β-Blockade and Systemic Homeostasis: Molecular Integration of Cardiorenal and Immune Pathways, a Narrative Review
Abstract
1. Introduction
2. Methods
3. Synthesis of Literature
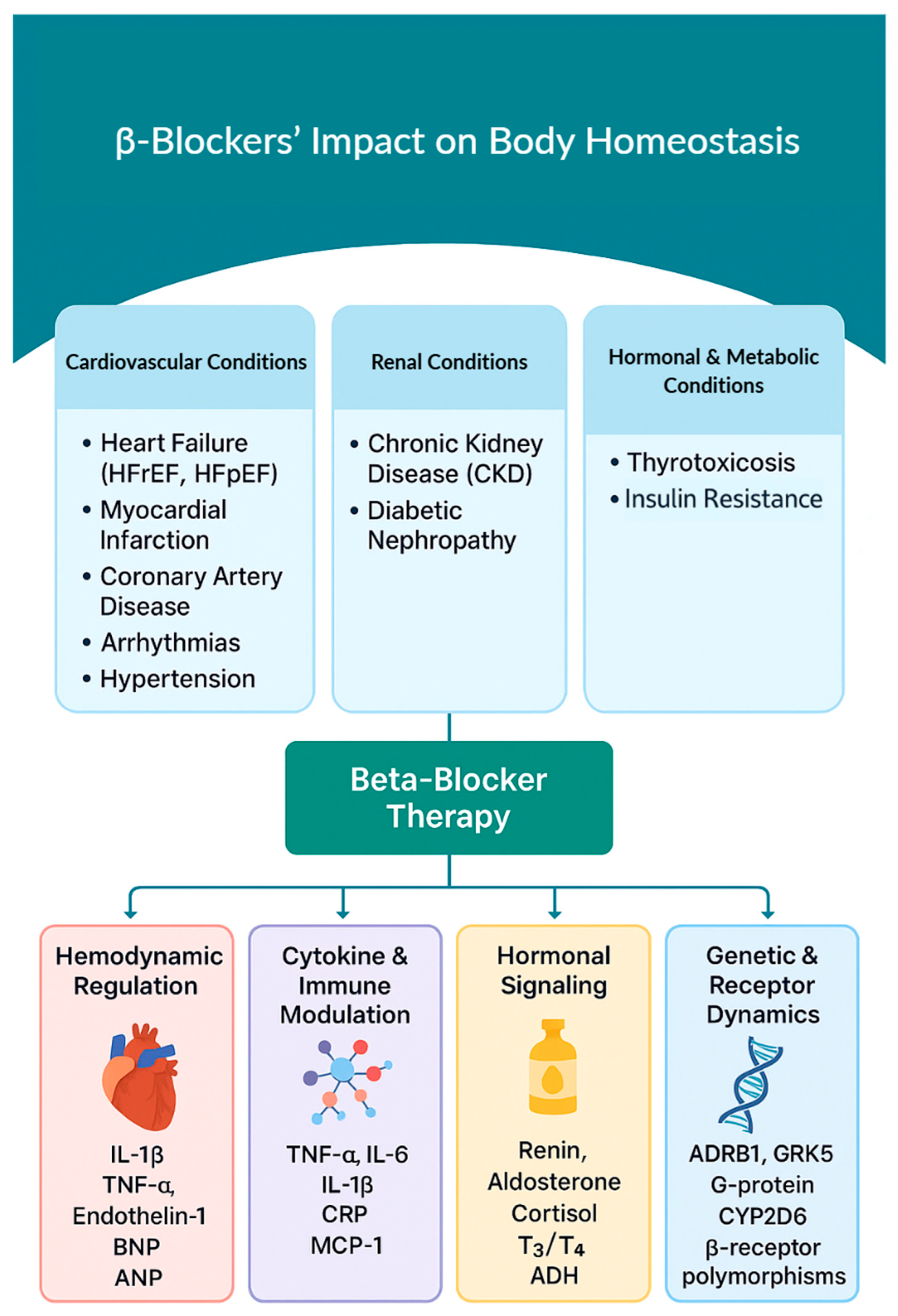
4. Disease-Specific Effects of β-Blockers
4.1. β-Blockers in Cardiovascular Disease
4.1.1. Heart Failure (HF)
4.1.2. Post-Myocardial Infarction (MI)
4.1.3. Acute and Chronic Coronary Diseases
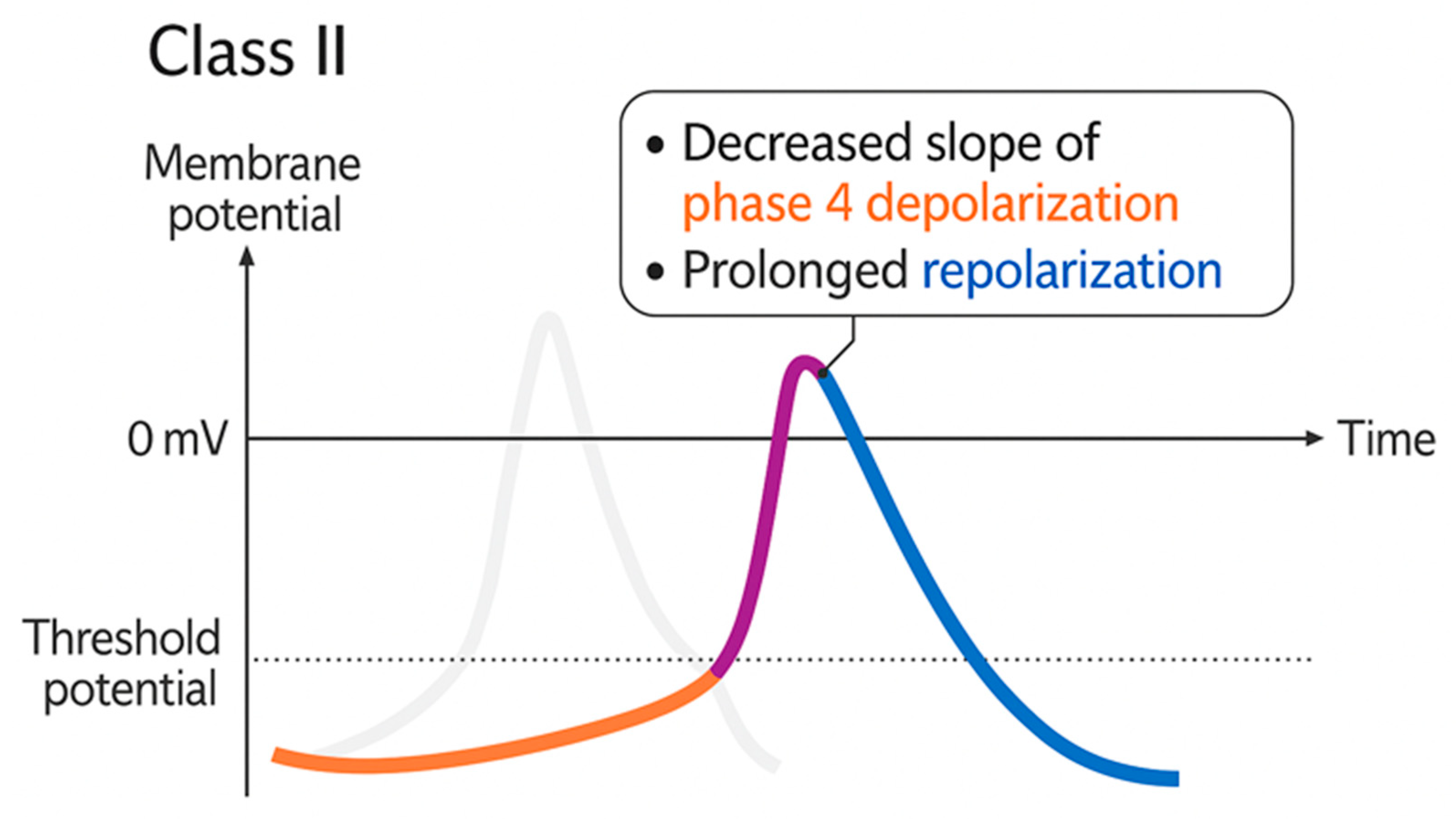
4.1.4. Arrhythmias
4.2. β-Blockers and Renal System Dysfunction
4.2.1. Chronic Kidney Disease (CKD)
4.2.2. Diabetic Nephropathy (DN)
4.3. Hormonal and Metabolic Effects of β-Blockers
5. Biochemical Pathways and Cytokine Interactions in BBs Therapy
5.1. β-Adrenergic Receptor Interactions
5.1.1. β1 Receptor (Cardiac & Renal)
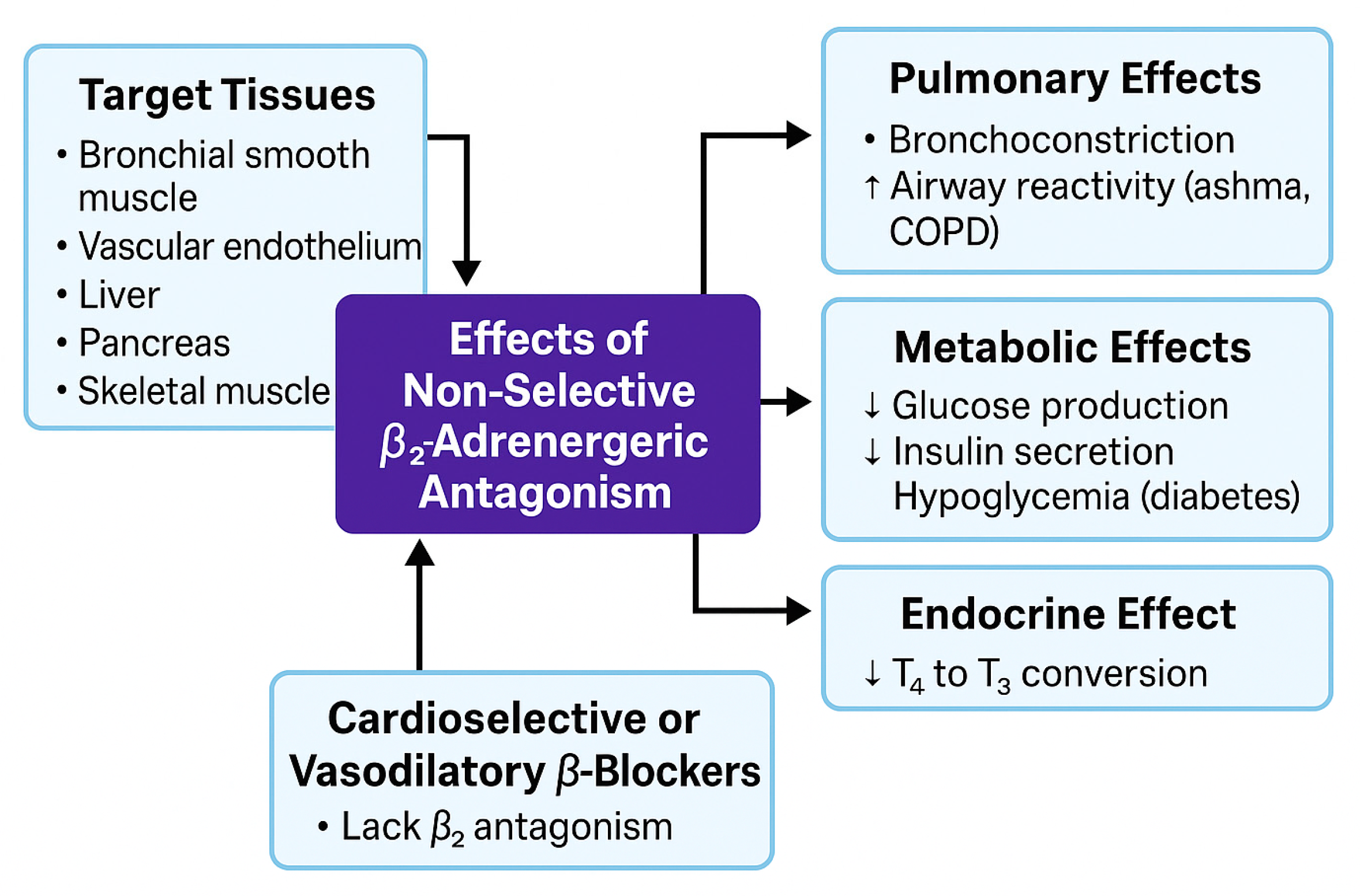
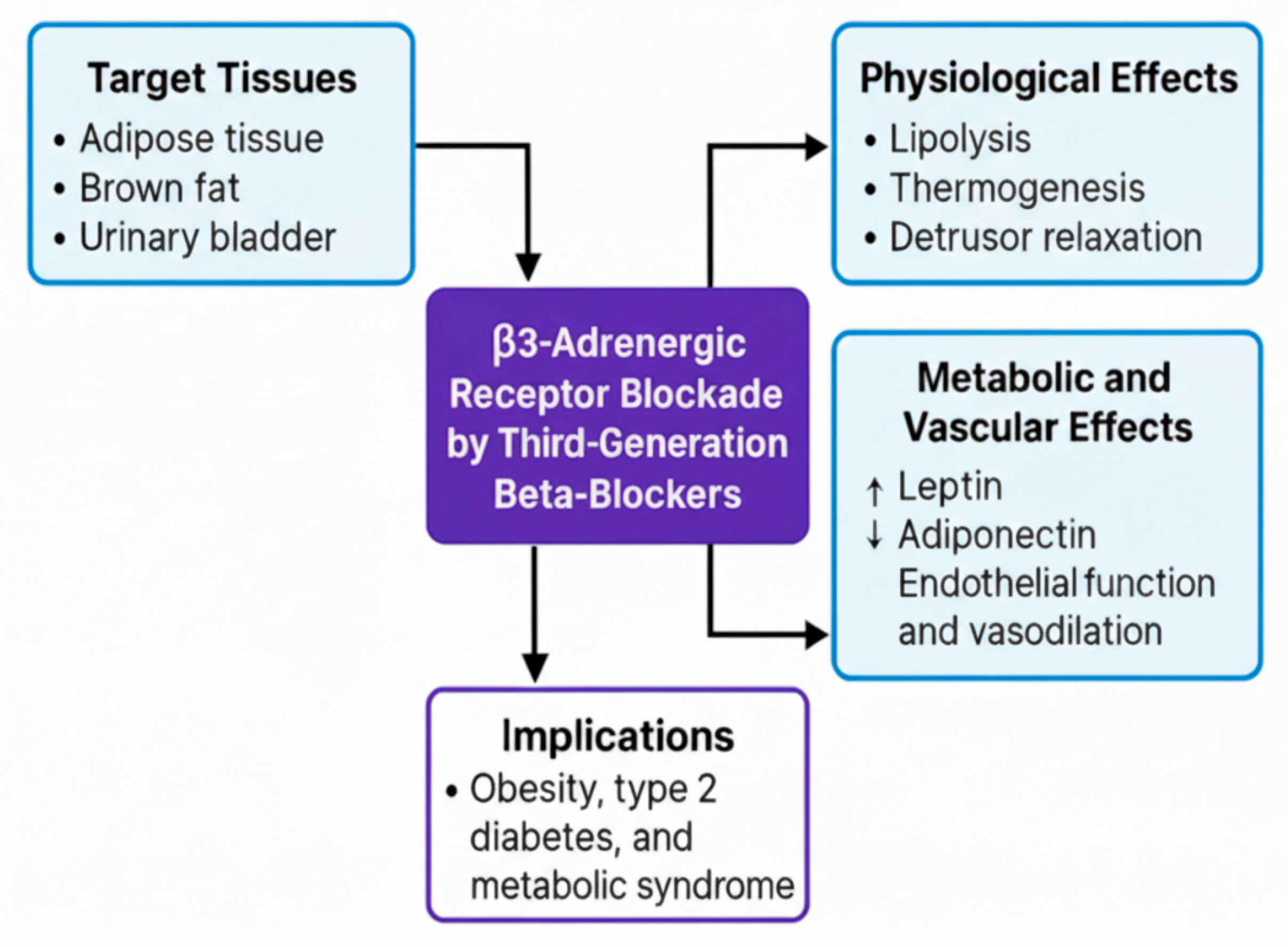
5.1.2. β2 Receptor (Vascular, Pulmonary, Metabolic)
6. Discussion
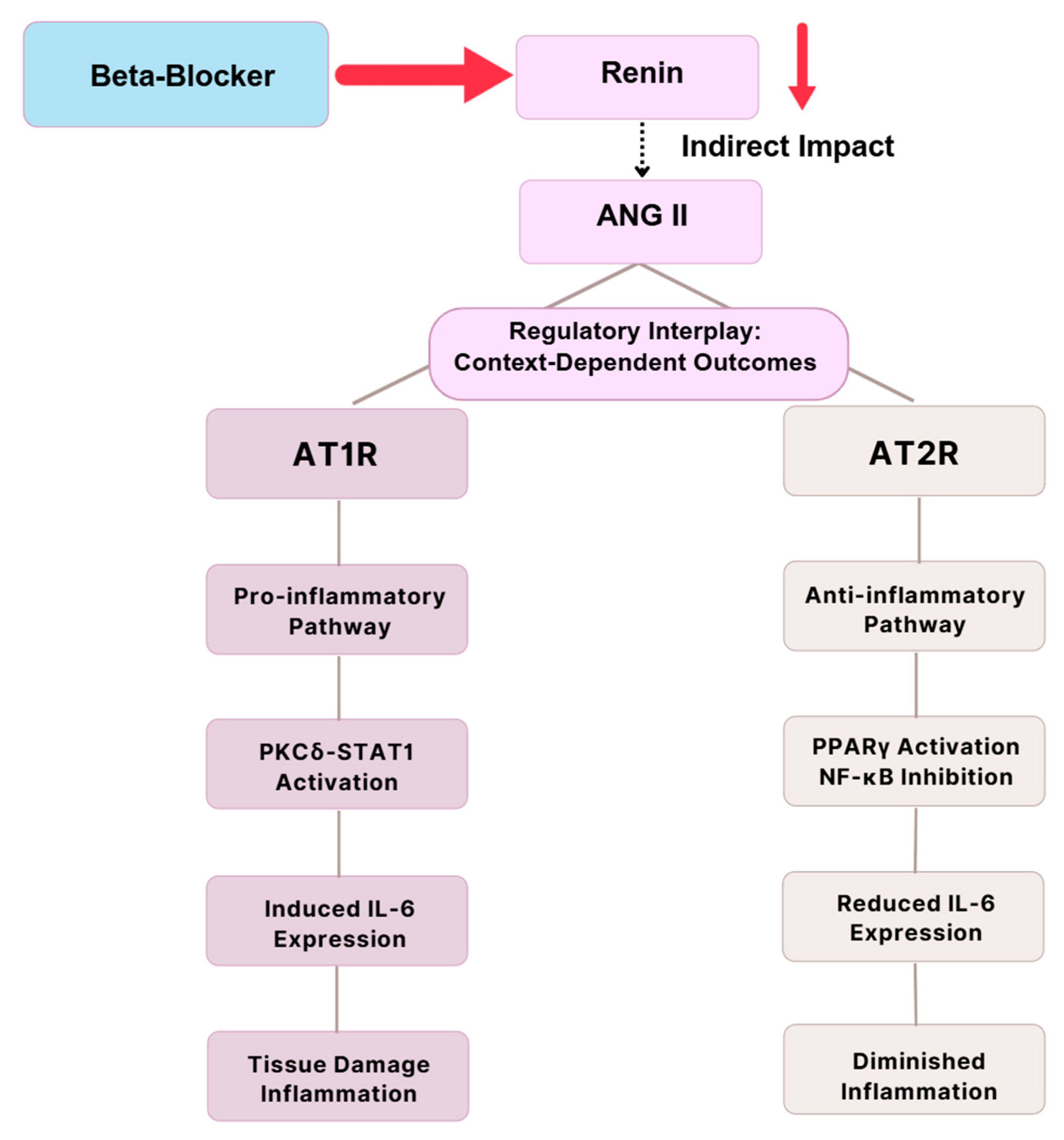
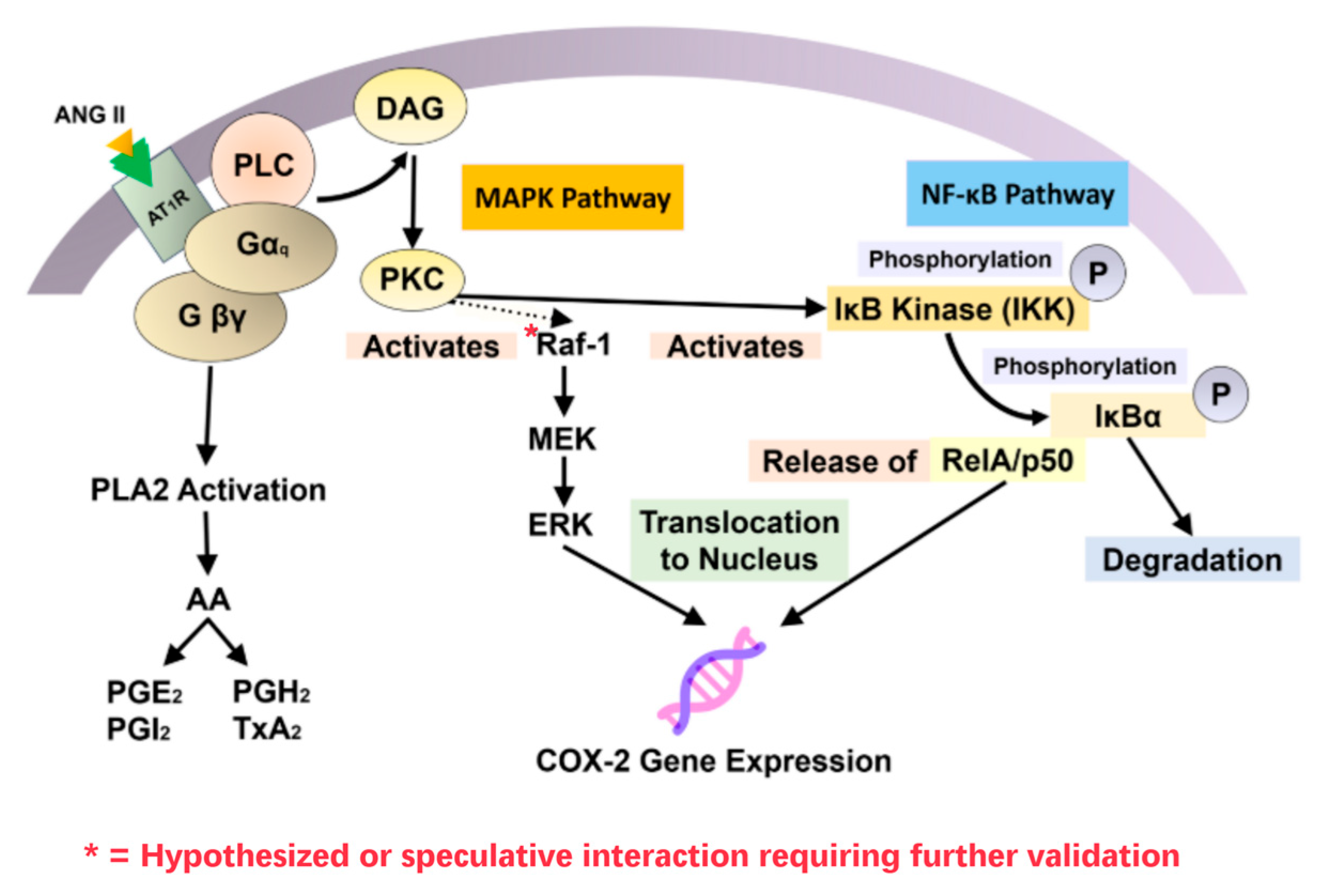
6.1. RAAS and Immune Modulation
6.2. Nitric Oxide and Vascular Homeostasis
6.3. Immune Remodeling and Macrophage Polarization
6.3.1. M1 Pro-Inflammatory Signaling
6.3.2. M2 Reparative and Fibrotic Roles
6.3.3. CD206+ Macrophages and Macrophage-to-Myofibroblast Transition (MMT)
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| ACE | Angiotensin-Converting Enzyme |
| ACS | Acute Coronary Syndrome |
| ACTH | Adrenocorticotropic Hormone |
| ADHF | Acute Decompensated Heart Failure |
| ADRB1 | Beta-1 Adrenergic Receptor |
| ADRB2 | Beta-2 Adrenergic Receptor |
| AIMAH | ACTH-Independent Macronodular Adrenal Hyperplasia |
| AKI | Acute Kidney Injury |
| AMPK | AMP-Activated Protein Kinase |
| ANP | Atrial Natriuretic Peptide |
| ANG II | Angiotensin II |
| APOE | Apolipoprotein E |
| AT1R | Angiotensin II Type 1 Receptor |
| AT2R | Angiotensin II Type 2 Receptor |
| BBs | Beta-Blockers |
| BNP | B-Type Natriuretic Peptide |
| BP | Blood Pressure |
| CAD | Coronary Artery Disease |
| CD206 | Mannose Receptor (Marker of M2 Macrophages) |
| CHF | Congestive Heart Failure |
| CKD | Chronic Kidney Disease |
| COL4A1 | Collagen Type IV Alpha 1 Chain |
| CO | Cardiac Output |
| COPD | Chronic Obstructive Pulmonary Disease |
| COX-2 | Cyclooxygenase-2 |
| CPVT | Catecholaminergic Polymorphic Ventricular Tachycardia |
| CRH | Corticotropin-Releasing Hormone |
| CRP | C-Reactive Protein |
| CNS | Central Nervous System |
| CV | Cardiovascular |
| CYP2D6 | Cytochrome P450 2D6 |
| DCM | Diabetic Cardiomyopathy |
| DN | Diabetic Nephropathy |
| ECG | Electrocardiogram |
| ECM | Extracellular Matrix |
| eNOS | Endothelial Nitric Oxide Synthase |
| FEV1 | Forced Expiratory Volume in 1 s |
| FFA | Free Fatty Acids |
| FVC | Forced Vital Capacity |
| GAL3 | Galectin-3 |
| GFR | Glomerular Filtration Rate |
| GLUT1/4 | Glucose Transporter 1/4 |
| GRK5 | G Protein-Coupled Receptor Kinase 5 |
| HDL | High-Density Lipoprotein |
| HF | Heart Failure |
| HFmrEF | Heart Failure with Mildly Reduced Ejection Fraction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HR | Heart Rate |
| HPA axis | Hypothalamic–Pituitary–Adrenal Axis |
| IL-1β | Interleukin-1 Beta |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-12p70 | Interleukin-12 subunit p70 |
| IL-13 | Interleukin-13 |
| IL-17 | Interleukin-17 |
| iNOS | Inducible Nitric Oxide Synthase |
| IRF1 | Interferon Regulatory Factor 1 |
| ISA | Intrinsic Sympathomimetic Activity |
| JAK | Janus Kinase |
| LDL | Low-Density Lipoprotein |
| LQTS | Long QT Syndrome |
| LVEF | Left Ventricular Ejection Fraction |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MI | Myocardial Infarction |
| ML | Machine Learning |
| MIP-1β | Macrophage Inflammatory Protein-1 Beta |
| M1 | Classically Activated (Pro-inflammatory) Macrophages |
| M2 | Alternatively Activated (Anti-inflammatory/Reparative) Macrophages |
| MMP-2 | Matrix Metalloproteinase-2 |
| MMT | Macrophage-to-Myofibroblast Transition |
| MOD | Myocardial Oxygen Demand |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| NO | Nitric Oxide |
| NOS | Nitric Oxide Synthase |
| nNOS | Neuronal Nitric Oxide Synthase |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-Kinase |
| PKC | Protein Kinase C |
| PLA2 | Phospholipase A2 |
| PM | Poor Metabolizers |
| PPARγ | Peroxisome Proliferator-Activated Receptor Gamma |
| PVR | Peripheral Vascular Resistance |
| RAAS | Renin–Angiotensin–Aldosterone System |
| RBF | Renal Blood Flow |
| RCT | Randomized Controlled Trial |
| ROS | Reactive Oxygen Species |
| SCD | Sudden Cardiac Death |
| Smad3 | Mothers Against Decapentaplegic Homolog 3 |
| SOD2 | Superoxide Dismutase 2 |
| SNS | Sympathetic Nervous System |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| T3 | Triiodothyronine |
| T4 | Thyroxine |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TIMP-2 | Tissue Inhibitor of Metalloproteinases-2 |
| TNF-α | Tumor Necrosis Factor Alpha |
| UM | Ultra-rapid Metabolizers |
| α-SMA | Alpha-Smooth Muscle Actin |
References
- Wołowiec, Ł.; Grześk, G.; Osiak, J.; Wijata, A.; Mędlewska, M.; Gaborek, P.; Banach, J.; Wołowiec, A.; Głowacka, M. Beta-Blockers in Cardiac Arrhythmias–Clinical Pharmacologist’s Point of View. Front. Pharmacol. 2023, 13, 1043714. [Google Scholar] [CrossRef]
- Taddei, S.; Tsabedze, N.; Tan, R.-S. β-Blockers Are Not All the Same: Pharmacologic Similarities and Differences, Potential Combinations and Clinical Implications. Curr. Med. Res. Opin. 2024, 40, 15–23. [Google Scholar] [CrossRef]
- Ibanez, B.; Latini, R.; Rossello, X.; Dominguez-Rodriguez, A.; Fernández-Vazquez, F.; Pelizzoni, V.; Sánchez, P.L.; Anguita, M.; Barrabés, J.A.; Raposeiras-Roubín, S.; et al. Beta-Blockers after Myocardial Infarction without Reduced Ejection Fraction. N. Engl. J. Med. 2025, 393, 1889–1900. [Google Scholar] [CrossRef]
- Kotecha, D.; Gill, S.K.; Flather, M.D.; Holmes, J.; Packer, M.; Rosano, G.; Böhm, M.; McMurray, J.J.V.; Wikstrand, J.; Anker, S.D.; et al. Impact of Renal Impairment on Beta-Blocker Efficacy in Patients with Heart Failure. J. Am. Coll. Cardiol. 2019, 74, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Abbott, K.C.; Trespalacios, F.C.; Agodoa, L.Y.; Taylor, A.J.; Bakris, G.L. β-Blocker Use in Long-Term Dialysis Patients. Arch. Intern. Med. 2004, 164, 2465. [Google Scholar] [CrossRef] [PubMed]
- Schwäble Santamaria, A.; Grassi, M.; Meeusen, J.W.; Lieske, J.C.; Scott, R.; Robertson, A.; Schiffer, E. Performance of Nuclear Magnetic Resonance-Based Estimated Glomerular Filtration Rate in a Real-World Setting. Bioengineering 2023, 10, 717. [Google Scholar] [CrossRef]
- Wang, D.; Jose, P.; Wilcox, C.S. Β1 Receptors Protect the Renal Afferent Arteriole of Angiotensin-Infused Rabbits from Norepinephrine-Induced Oxidative Stress. J. Am. Soc. Nephrol. 2006, 17, 3347–3354. [Google Scholar] [CrossRef]
- Suarez-Roca, H.; Mamoun, N.; Sigurdson, M.I.; Maixner, W. Baroreceptor Modulation of the Cardiovascular System, Pain, Consciousness, and Cognition. In Comprehensive Physiology; Wiley: Hoboken, NJ, USA, 2021; pp. 1373–1423. [Google Scholar]
- Castaño-Amores, C.; Antúnez-Rodríguez, A.; Pozo-Agundo, A.; García-Rodríguez, S.; Martínez-González, L.J.; Dávila-Fajardo, C.L. Genetic Polymorphisms in ADRB1, ADRB2 and CYP2D6 Genes and Response to Beta-Blockers in Patients with Acute Coronary Syndrome. Biomed. Pharmacother. 2023, 169, 115869. [Google Scholar] [CrossRef]
- Zhang, Y.; Matkovich, S.J.; Duan, X.; Gold, J.I.; Koch, W.J.; Dorn, G.W. Nuclear Effects of G-Protein Receptor Kinase 5 on Histone Deacetylase 5–Regulated Gene Transcription in Heart Failure. Circ. Heart Fail. 2011, 4, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Liggett, S.B.; Cresci, S.; Kelly, R.J.; Syed, F.M.; Matkovich, S.J.; Hahn, H.S.; Diwan, A.; Martini, J.S.; Sparks, L.; Parekh, R.R.; et al. A GRK5 Polymorphism That Inhibits β-Adrenergic Receptor Signaling Is Protective in Heart Failure. Nat. Med. 2008, 14, 510–517. [Google Scholar] [CrossRef]
- Luzum, J.A.; Peterson, E.; Li, J.; She, R.; Gui, H.; Liu, B.; Spertus, J.A.; Pinto, Y.M.; Williams, L.K.; Sabbah, H.N.; et al. Race and Beta-Blocker Survival Benefit in Patients with Heart Failure: An Investigation of Self-Reported Race and Proportion of African Genetic Ancestry. J. Am. Heart Assoc. 2018, 7, e007956. [Google Scholar] [CrossRef]
- Bruck, H.; Poller, U.; Lüssenhop, H.; Pönicke, K.; Temme, T.; Heusch, G.; Philipp, T.; Brodde, O.-E. B2-Adrenoceptor-Mediated Intrinsic Sympathomimetic Activity of Carteolol: An in Vivo Study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 370, 361–368. [Google Scholar] [CrossRef]
- Nyberg, G.; Wilhelmsson, C.; Vedin, A. Intrinsic Sympathomimetic Activity of Penbutolol. Eur. J. Clin. Pharmacol. 1979, 16, 381–386. [Google Scholar] [CrossRef]
- Schweda, F.; Friis, U.; Wagner, C.; Skott, O.; Kurtz, A. Renin Release. Physiology 2007, 22, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A. Renin Release: Sites, Mechanisms, and Control. Annu. Rev. Physiol. 2011, 73, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Luft, F.C. Angiotensin, Inflammation, Hypertension, and Cardiovascular Disease. Curr. Hypertens. Rep. 2001, 3, 61–67. [Google Scholar] [CrossRef]
- Dong, J.; Li, D.; Kang, L.; Luo, C.; Wang, J. Insights into Human ENOS, NNOS and INOS Structures and Medicinal Indications from Statistical Analyses of Their Interactions with Bound Compounds. Biophys. Rep. 2023, 9, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Gul, R.; Alsalman, N.; Alfadda, A.A. Inhibition of ENOS Partially Blunts the Beneficial Effects of Nebivolol on Angiotensin II-Induced Signaling in H9c2 Cardiomyoblasts. Curr. Issues Mol. Biol. 2022, 44, 2139–2152. [Google Scholar] [CrossRef]
- Gul, R.; Alsalman, N.; Bazighifan, A.; Alfadda, A.A. Comparative Beneficial Effects of Nebivolol and Nebivolol/Valsartan Combination against Mitochondrial Dysfunction in Angiotensin II-Induced Pathology in H9c2 Cardiomyoblasts. J. Pharm. Pharmacol. 2021, 73, 1520–1529. [Google Scholar] [CrossRef]
- Esplugues, J.V. NO as a Signalling Molecule in the Nervous System. Br. J. Pharmacol. 2002, 135, 1079–1095. [Google Scholar] [CrossRef]
- Toma, T.; Miyakawa, N.; Arakaki, Y.; Watanabe, T.; Nakahara, R.; Ali, T.F.S.; Biswas, T.; Todaka, M.; Kondo, T.; Fujita, M.; et al. An Antifibrotic Compound That Ameliorates Hyperglycaemia and Fat Accumulation in Cell and HFD Mouse Models. Diabetologia 2024, 67, 2568–2584. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Jiang, H.; Pan, J.; Huang, X.-R.; Wang, Y.-C.; Huang, H.-F.; To, K.-F.; Nikolic-Paterson, D.J.; Lan, H.-Y.; Chen, J.-H. Macrophage-to-Myofibroblast Transition Contributes to Interstitial Fibrosis in Chronic Renal Allograft Injury. J. Am. Soc. Nephrol. 2017, 28, 2053–2067. [Google Scholar] [CrossRef]
- Bai, W.; Liu, Z.-Q.; He, P.-Y.; Muhuyati. The Role of IL-6, IL-10, TNF-α and PD-1 Expression on CD4 T Cells in Atrial Fibrillation. Heliyon 2023, 9, e18818. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, H.; Zhou, L.; Yang, Y.; Chen, R. Research Progress on Macrophages in Cardiovascular Diseases. J. Cardiothorac. Surg. 2025, 20, 307. [Google Scholar] [CrossRef] [PubMed]
- Baltogiannis, G.G.; Lysitsas, D.N.; di Giovanni, G.; Ciconte, G.; Sieira, J.; Conte, G.; Kolettis, T.M.; Chierchia, G.-B.; de Asmundis, C.; Brugada, P. CPVT: Arrhythmogenesis, Therapeutic Management, and Future Perspectives. A Brief Review of the Literature. Front. Cardiovasc. Med. 2019, 6, 92. [Google Scholar] [CrossRef]
- Tamaki, Y.; Yaku, H.; Morimoto, T.; Inuzuka, Y.; Ozasa, N.; Yamamoto, E.; Yoshikawa, Y.; Miyake, M.; Kondo, H.; Tamura, T.; et al. Lower In-Hospital Mortality with Beta-Blocker Use at Admission in Patients with Acute Decompensated Heart Failure. J. Am. Heart Assoc. 2021, 10, e020012. [Google Scholar] [CrossRef]
- Fragasso, G. The Concept of “Heart Failure with Preserved Ejection Fraction”: Time for a Critical Reappraisal. Rev. Cardiovasc. Med. 2023, 24, 202. [Google Scholar] [CrossRef]
- Godoy, L.C.; Farkouh, M.E.; Austin, P.C.; Shah, B.R.; Qiu, F.; Jackevicius, C.A.; Wijeysundera, H.C.; Krumholz, H.M.; Ko, D.T. Association of Beta-Blocker Therapy with Cardiovascular Outcomes in Patients with Stable Ischemic Heart Disease. J. Am. Coll. Cardiol. 2023, 81, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.A.; Bennett, T.; Brown, A.M.; Wilcox, R.G.; Skene, A.M. The Effects of Acute or Chronic Ingestion of Propranolol or Metoprolol on the Metabolic and Hormonal Responses to Prolonged, Submaximal Exercise in Hypertensive Men. Br. J. Clin. Pharmacol. 1984, 17, 283–293. [Google Scholar] [CrossRef]
- Tomiyama, H.; Yamashina, A. Beta-Blockers in the Management of Hypertension and/or Chronic Kidney Disease. Int. J. Hypertens. 2014, 2014, 919256. [Google Scholar] [CrossRef]
- Abbott, J.D.; Goldberger, J.J. Β-Blocker Therapy After Myocardial Infarction: A Little Goes a Long Way. J. Am. Heart Assoc. 2023, 12, e030867. [Google Scholar] [CrossRef]
- Safi, S.; Sethi, N.J.; Korang, S.K.; Nielsen, E.E.; Feinberg, J.; Gluud, C.; Jakobsen, J.C. Beta-Blockers in Patients without Heart Failure after Myocardial Infarction. Cochrane Database Syst. Rev. 2021, 2021, CD012565. [Google Scholar] [CrossRef]
- Silvain, J.; Cayla, G.; Ferrari, E.; Range, G.; Puymirat, E.; Delarche, N.; Guedeney, P.; Cuisset, T.; Ivanes, F.; Lhermusier, T.; et al. Beta-Blocker Interruption or Continuation after Myocardial Infarction. N. Engl. J. Med. 2024, 391, 1277–1286. [Google Scholar] [CrossRef]
- Badve, S.V.; Roberts, M.A.; Hawley, C.M.; Cass, A.; Garg, A.X.; Krum, H.; Tonkin, A.; Perkovic, V. Effects of Beta-Adrenergic Antagonists in Patients with Chronic Kidney Disease. J. Am. Coll. Cardiol. 2011, 58, 1152–1161. [Google Scholar] [CrossRef]
- Parada Barcia, J.A.; Raposeiras Roubin, S.; Fernández, D.G.; García, A.G.; Otero, C.I.; González Bermúdez, I.; Íñiguez Romo, A.; Abu-Assi, E. Comparison between Beta-blockers and Calcium Channel Blockers in Patients with Atrial Fibrillation According to Renal Function. Clin. Cardiol. 2024, 47, e24257. [Google Scholar] [CrossRef]
- Wiersinga, W.M. Propranolol and Thyroid Hormone Metabolism. Thyroid 1991, 1, 273–277. [Google Scholar] [CrossRef]
- Geffner, D.L.; Hershman, J.M. β-Adrenergic Blockade for the Treatment of Hyperthyroidism. Am. J. Med. 1992, 93, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Schurtz, G.; Mewton, N.; Lemesle, G.; Delmas, C.; Levy, B.; Puymirat, E.; Aissaoui, N.; Bauer, F.; Gerbaud, E.; Henry, P.; et al. Beta-Blocker Management in Patients Admitted for Acute Heart Failure and Reduced Ejection Fraction: A Review and Expert Consensus Opinion. Front. Cardiovasc. Med. 2023, 10, 1263482. [Google Scholar] [CrossRef]
- Joo, S.-J. Beta-Blocker Therapy in Patients with Acute Myocardial Infarction: Not All Patients Need It. Acute Crit. Care 2023, 38, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Gentile, F.; Ghionzoli, N.; Chiriacò, M.; Panichella, G.; Aimo, A.; Vergaro, G.; Giannoni, A.; Passino, C.; Emdin, M. Pathophysiological Rationale and Clinical Evidence for Neurohormonal Modulation in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2023, 9, e09. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, A.; Cestiè, C.; Ferranti, F.; Segato, C.; Prosperi, S.; Germanò, R.; Myftari, V.; Bartimoccia, S.; Castellani, V.; Badagliacca, R.; et al. Implications of Oxidative Stress in the Pathophysiological Pathways of Heart Failure. Int. J. Mol. Sci. 2025, 26, 5165. [Google Scholar] [CrossRef]
- Yndigegn, T.; Lindahl, B.; Alfredsson, J.; Benatar, J.; Brandin, L.; Erlinge, D.; Haaga, U.; Held, C.; Johansson, P.; Karlström, P.; et al. Design and Rationale of Randomized Evaluation of Decreased Usage of Beta-Blockers after Acute Myocardial Infarction (REDUCE-AMI). Eur. Heart J. Cardiovasc. Pharmacother. 2023, 9, 192–197. [Google Scholar] [CrossRef]
- Yndigegn, T.; Lindahl, B.; Mars, K.; Alfredsson, J.; Benatar, J.; Brandin, L.; Erlinge, D.; Hallen, O.; Held, C.; Hjalmarsson, P.; et al. Beta-Blockers after Myocardial Infarction and Preserved Ejection Fraction. N. Engl. J. Med. 2024, 390, 1372–1381. [Google Scholar] [CrossRef]
- Cruickshank, J.M. Beta-Blockers and Heart Failure. Indian Heart J. 2010, 62, 101–110. [Google Scholar] [PubMed]
- Anzai, A.; Ko, S.; Fukuda, K. Immune and Inflammatory Networks in Myocardial Infarction: Current Research and Its Potential Implications for the Clinic. Int. J. Mol. Sci. 2022, 23, 5214. [Google Scholar] [CrossRef]
- Tirdea, C.; Hostiuc, S.; Moldovan, H.; Scafa-Udriste, A. Identification of Risk Genes Associated with Myocardial Infarction—Big Data Analysis and Literature Review. Int. J. Mol. Sci. 2022, 23, 15008. [Google Scholar] [CrossRef] [PubMed]
- Nohria, R.; Viera, A.J. Acute Coronary Syndrome: Diagnosis and Initial Management. Am. Fam. Physician 2024, 109, 34–42. [Google Scholar] [PubMed]
- Chi, K.-Y.; Nanna, M.G. Stabilizing Stable Coronary Artery Disease. JACC Adv. 2025, 4, 101569. [Google Scholar] [CrossRef]
- Dai, Y.; Mei, Z.; Zhang, S.; Shali, S.; Ren, D.; Xu, L.; Gao, W.; Chang, S.; Zheng, Y.; Qian, J.; et al. Sexual Dysfunction and the Impact of Beta-Blockers in Young Males with Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 708200. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Guo, J.; Yang, Y.; Huang, M.; Li, L.; Wang, Y.; Qin, Y.; Zhang, M. Serum Cytokines Predict the Severity of Coronary Artery Disease Without Acute Myocardial Infarction. Front. Cardiovasc. Med. 2022, 9, 896810. [Google Scholar] [CrossRef]
- Schröder, L.C.; Holkeri, A.; Eranti, A.; Haukilahti, M.A.E.; Kerola, T.; Kenttä, T.V.; Noponen, K.; Seppänen, T.; Rissanen, H.; Heliövaara, M.; et al. Poor R-Wave Progression as a Predictor of Sudden Cardiac Death in the General Population and Subjects with Coronary Artery Disease. Heart Rhythm. 2022, 19, 952–959. [Google Scholar] [CrossRef]
- Oba, Y.; Kabutoya, T.; Kohro, T.; Imai, Y.; Kario, K.; Sato, H.; Nochioka, K.; Nakayama, M.; Fujita, H.; Mizuno, Y.; et al. Relationships Among Heart Rate, β-Blocker Dosage, and Prognosis in Patients with Coronary Artery Disease in a Real-World Database Using a Multimodal Data Acquisition System. Circ. J. 2023, 87, CJ-22-0314. [Google Scholar] [CrossRef]
- Malinowski, D.; Bochniak, O.; Luterek-Puszyńska, K.; Puszyński, M.; Pawlik, A. Genetic Risk Factors Related to Coronary Artery Disease and Role of Transforming Growth Factor Beta 1 Polymorphisms. Genes 2023, 14, 1425. [Google Scholar] [CrossRef]
- Sharma, V.; Maheshwari, V.; Thayumanavan, T.; Sahai, A.; Singh, S.; Kar, B. Heart on Fire: Unmasking RyR2 Mutation in Stress-Induced Ventricular Arrhythmias. Methodist. Debakey Cardiovasc. J. 2025, 21, 25–29. [Google Scholar] [CrossRef]
- Nattel, S. Comparative Mechanisms of Action of Antiarrhythmic Drugs. Am. J. Cardiol. 1993, 72, F13–F17. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Takekita, Y.; Aoki, N.; Suwa, T.; Yasuda, K.; Uchinuma, N.; Tominaga, H.; Kojima, Y.; Kawashima, H.; Kato, M.; et al. Beta-Blockers for Electroconvulsive Therapy: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 183, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A.; McCrink, K.A.; Brill, A. Impact of CYP2D6 Genetic Variation on the Response of the Cardiovascular Patient to Carvedilol and Metoprolol. Curr. Drug Metab. 2015, 17, 30–36. [Google Scholar] [CrossRef]
- Hall, M.E.; Rocco, M.V.; Morgan, T.M.; Hamilton, C.A.; Jordan, J.H.; Edwards, M.S.; Hall, J.E.; Hundley, W.G. Beta-Blocker Use Is Associated with Higher Renal Tissue Oxygenation in Hypertensive Patients Suspected of Renal Artery Stenosis. Cardiorenal Med. 2016, 6, 261–268. [Google Scholar] [CrossRef]
- Dean, L.; Kane, M. Metoprolol Therapy and CYP2D6 Genotype. In Medical Genetics Summaries; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Kopańko, M.; Zabłudowska, M.; Pawlak, D.; Sieklucka, B.; Krupa, A.; Sokołowska, K.; Ziemińska, M.; Pawlak, K. The Possible Effect of β-Blocker Use on the Circulating MMP-2/TIMP-2 System in Patients with Chronic Kidney Disease on Conservative Treatment. J. Clin. Med. 2024, 13, 1847. [Google Scholar] [CrossRef]
- Rizvi, S. Association of Genetic Variants with Diabetic Nephropathy. World J. Diabetes 2014, 5, 809. [Google Scholar] [CrossRef] [PubMed]
- Galis, P.; Bartosova, L.; Farkasova, V.; Bartekova, M.; Ferenczyova, K.; Rajtik, T. Update on Clinical and Experimental Management of Diabetic Cardiomyopathy: Addressing Current and Future Therapy. Front. Endocrinol. 2024, 15, 1451100. [Google Scholar] [CrossRef]
- DeMorrow, S. Role of the Hypothalamic–Pituitary–Adrenal Axis in Health and Disease. Int. J. Mol. Sci. 2018, 19, 986. [Google Scholar] [CrossRef]
- Gullestad, L.; Dolva, L.O.; Kjeldsen, S.E.; Eide, I.; Kjekshus, J. Effect of Beta-Adrenergic Blockade on Hormonal Responses during Continuous and Intermittent Exercise. Cardiovasc. Drugs Ther. 1989, 3, 63–71. [Google Scholar] [CrossRef]
- Burford, N.; Webster, N.; Cruz-Topete, D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef] [PubMed]
- Bugajski, J.; Gadek-Michalska, A.; Ołowska, A.; Borycz, J.; Głód, R.; Bugajski, A.J. Adrenergic Regulation of the Hypothalamic-Pituitary-Adrenal Axis under Basal and Social Stress Conditions. J. Physiol. Pharmacol. 1995, 46, 297–312. [Google Scholar] [PubMed]
- Oki, K.; Yamane, K.; Nakanishi, S.; Nakashima, R.; Jitsuiki, K.; Kohno, N. Improvement of Hypercortisolism by β-Blocker Therapy in Subclinical Cushing’s Syndrome Associated with ACTH-Independent Macronodular Adrenocortical Hyperplasia. Endocrine 2009, 36, 372–376. [Google Scholar] [CrossRef]
- McGill, J.B. Reexamining Misconceptions About β-Blockers in Patients with Diabetes. Clin. Diabetes 2009, 27, 36–46. [Google Scholar] [CrossRef]
- Kveiborg, B.; Hermann, T.S.; Major-Pedersen, A.; Christiansen, B.; Rask-Madsen, C.; Raunsø, J.; Køber, L.; Torp-Pedersen, C.; Dominguez, H. Metoprolol Compared to Carvedilol Deteriorates Insulin-Stimulated Endothelial Function in Patients with Type 2 Diabetes—A Randomized Study. Cardiovasc. Diabetol. 2010, 9, 21. [Google Scholar] [CrossRef]
- Zanchetti, A.; Leonetti, G.; Terzoli, L.; Sala, C. β-Blockers and Renin. Drugs 1983, 25, 58–63. [Google Scholar] [CrossRef]
- Knox, F.G.; Burnett, J.C.; Kohan, D.E.; Spielman, W.S.; Strand, J.C. Escape from the Sodium-Retaining Effects of Mineralocorticoids. Kidney Int. 1980, 17, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Parichatikanond, W.; Duangrat, R.; Kurose, H.; Mangmool, S. Regulation of β-Adrenergic Receptors in the Heart: A Review on Emerging Therapeutic Strategies for Heart Failure. Cells 2024, 13, 1674. [Google Scholar] [CrossRef]
- Khan, O.; Patel, M.; Tomdio, A.N.; Beall, J.; Jovin, I.S. Beta-Blockers in the Prevention and Treatment of Ischemic Heart Disease. Heart Views 2023, 24, 41–49. [Google Scholar] [CrossRef]
- Prijic, S.; Buchhorn, R. Mechanisms of Beta-Blockers Action in Patients with Heart Failure. Rev. Recent Clin. Trials 2014, 9, 58–60. [Google Scholar] [CrossRef]
- Han, L.; Liu, F.; Li, Q.; Qing, T.; Zhai, Z.; Xia, Z.; Li, J. The Efficacy of Beta-Blockers in Patients with Long QT Syndrome 1–3 According to Individuals’ Gender, Age, and QTc Intervals: A Network Meta-Analysis. Front. Pharmacol. 2020, 11, 579525. [Google Scholar] [CrossRef]
- Kurtz, A. Control of Renin Synthesis and Secretion. Am. J. Hypertens. 2012, 25, 839–847. [Google Scholar] [CrossRef]
- Manis, A.D.; Palygin, O.; Khedr, S.; Levchenko, V.; Hodges, M.R.; Staruschenko, A. Relationship between the Renin–Angiotensin–Aldosterone System and Renal Kir5.1 Channels. Clin. Sci. 2019, 133, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dev, S.; Khalid, M.U.; Siddenthi, S.M.; Noman, M.; John, C.; Akubuiro, C.; Haider, A.; Rani, R.; Kashif, M.; et al. The Bidirectional Link Between Diabetes and Kidney Disease: Mechanisms and Management. Cureus 2023, 15, e45615. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.H.; Hall, A.S.; Narkiewicz, K. The Combination of Beta-Blockers and ACE Inhibitors Across the Spectrum of Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2023, 37, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Abosamak, N.R.; Shahin, M.H. Beta2 Receptor Agonists and Antagonists. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Loth, D.W.; Brusselle, G.G.; Lahousse, L.; Hofman, A.; Leufkens, H.G.M.; Stricker, B.H. Β-adrenoceptor Blockers and Pulmonary Function in the General Population: The Rotterdam Study. Br. J. Clin. Pharmacol. 2014, 77, 190–200. [Google Scholar] [CrossRef]
- Mangmool, S.; Denkaew, T.; Parichatikanond, W.; Kurose, H. β-Adrenergic Receptor and Insulin Resistance in the Heart. Biomol. Ther. 2017, 25, 44–56. [Google Scholar] [CrossRef]
- Marketou, M.; Gupta, Y.; Jain, S.; Vardas, P. Differential Metabolic Effects of Beta-Blockers: An Updated Systematic Review of Nebivolol. Curr. Hypertens. Rep. 2017, 19, 22. [Google Scholar] [CrossRef]
- Senn, J.R.; Löliger, R.C.; Fischer, J.G.W.; Bur, F.; Maushart, C.I.; Betz, M.J. Acute Effect of Propranolol on Resting Energy Expenditure in Hyperthyroid Patients. Front. Endocrinol. 2023, 13, 1026998. [Google Scholar] [CrossRef]
- Samanta, S.; Bagchi, D.; Bagchi, M. Physiological and Metabolic Functions of the Β3-Adrenergic Receptor and an Approach to Therapeutic Achievements. J. Physiol. Biochem. 2024, 80, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Morioka, T.; Emoto, M.; Yamazaki, Y.; Kawano, N.; Imamura, S.; Numaguchi, R.; Urata, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; et al. Leptin Is Associated with Vascular Endothelial Function in Overweight Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2014, 13, 10. [Google Scholar] [CrossRef]
- Cardoso, V.G.; Gonçalves, G.L.; Costa-Pessoa, J.M.; Thieme, K.; Lins, B.B.; Casare, F.A.M.; de Ponte, M.C.; Camara, N.O.S.; Oliveira-Souza, M. Angiotensin II-Induced Podocyte Apoptosis Is Mediated by Endoplasmic Reticulum Stress/PKC-δ/P38 MAPK Pathway Activation and Trough Increased Na+/H+ Exchanger Isoform 1 Activity. BMC Nephrol. 2018, 19, 179. [Google Scholar] [CrossRef]
- Shanmugam, N.; Gaw Gonzalo, I.T.; Natarajan, R. Molecular Mechanisms of High Glucose-Induced Cyclooxygenase-2 Expression in Monocytes. Diabetes 2004, 53, 795–802. [Google Scholar] [CrossRef]
- Fatima, N.; Patel, S.N.; Hussain, T. Angiotensin II Type 2 Receptor: A Target for Protection Against Hypertension, Metabolic Dysfunction, and Organ Remodeling. Hypertension 2021, 77, 1845–1856. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible Nitric Oxide Synthase and Inflammatory Diseases. Mol. Med. 2000, 6, 347–373. [Google Scholar] [CrossRef]
- Leppänen, T.; Korhonen, R.; Laavola, M.; Nieminen, R.; Tuominen, R.K.; Moilanen, E. Down-Regulation of Protein Kinase Cδ Inhibits Inducible Nitric Oxide Synthase Expression through IRF1. PLoS ONE 2013, 8, e52741. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The Role of COX-2 and PGE2 in the Regulation of Immunomodulation and Other Functions of Mesenchymal Stromal Cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- Louden, J.D. Regulation of Fluid and Electrolyte Balance. Anaesth. Intensive Care Med. 2015, 16, 278–285. [Google Scholar] [CrossRef]
- Siragy, H.M. Interaction of the Renin Angiotensin and Cox Systems in the Kidney. Front. Biosci. 2016, 8, 459. [Google Scholar] [CrossRef]
- Hyman, D.A.; Siebert, V.R.; Birnbaum, G.D.; Alam, M.; Birnbaum, Y. A Modern History RAAS Inhibition and Beta Blockade for Heart Failure to Underscore the Non-Equivalency of ACEIs and ARBs. Cardiovasc. Drugs Ther. 2020, 34, 215–221. [Google Scholar] [CrossRef]
- Frank, G.D.; Saito, S.; Motley, E.D.; Sasaki, T.; Ohba, M.; Kuroki, T.; Inagami, T.; Eguchi, S. Requirement of Ca2+ and PKCδ for Janus Kinase 2 Activation by Angiotensin II: Involvement of PYK2. Mol. Endocrinol. 2002, 16, 367–377. [Google Scholar] [CrossRef]
- McWhinney, C.D.; Hunt, R.A.; Conrad, K.M.; Dostal, D.E.; Baker, K.M. The Type I Angiotensin II Receptor Couples to Stat1 and Stat3 Activation Through Jak2 Kinase in Neonatal Rat Cardiac Myocytes. J. Mol. Cell Cardiol. 1997, 29, 2513–2524. [Google Scholar] [CrossRef]
- Patel, S.N.; Fatima, N.; Ali, R.; Hussain, T. Emerging Role of Angiotensin AT2 Receptor in Anti-Inflammation: An Update. Curr. Pharm. Des. 2020, 26, 492–500. [Google Scholar] [CrossRef]
- Barki-Harrington, L.; Luttrell, L.M.; Rockman, H.A. Dual Inhibition of β-Adrenergic and Angiotensin II Receptors by a Single Antagonist. Circulation 2003, 108, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Zenke, K.; Muroi, M.; Tanamoto, K. IRF1 Supports DNA Binding of STAT1 by Promoting Its Phosphorylation. Immunol. Cell Biol. 2018, 96, 1095–1103. [Google Scholar] [CrossRef]
- Uddin, S.; Sassano, A.; Deb, D.K.; Verma, A.; Majchrzak, B.; Rahman, A.; Malik, A.B.; Fish, E.N.; Platanias, L.C. Protein Kinase C-δ (PKC-δ) Is Activated by Type I Interferons and Mediates Phosphorylation of Stat1 on Serine 727. J. Biol. Chem. 2002, 277, 14408–14416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.-S.; Li, L.; Huang, L.; Gong, J.; Xia, H.; Liu, X.; Wan, N.; Wei, X.; Zhu, X.; Chen, Y.; et al. Interferon Regulatory Factor 1 Is Required for Cardiac Remodeling in Response to Pressure Overload. Hypertension 2014, 64, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Ollerstam, A.; Persson, A. Macula Densa Neuronal Nitric Oxide Synthase. Cardiovasc. Res. 2002, 56, 189–196. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Gomez, R.A.; Sequeira-Lopez, M.L.S. Renin Cells, From Vascular Development to Blood Pressure Sensing. Hypertension 2023, 80, 1580–1589. [Google Scholar] [CrossRef]
- Reid, I.A. Role of Nitric Oxide in the Regulation of Renin and Vasopressin Secretion. Front. Neuroendocrinol. 1994, 15, 351–383. [Google Scholar] [CrossRef]
- Riedel, W. Role of Nitric Oxide in the Control of the Hypothalamic-Pituitary-Adrenocortical Axis. Z. Rheumatol. 2000, 59, II36–II42. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Szlachcic, A.; Dembinski, A.; Warzecha, Z.; Jaworek, J.; Stachura, J. Nitric Oxide in Pancreatic Secretion and Hormone-Induced Pancreatitis in Rats. Int. J. Pancreatol. 1994, 15, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.W. Nitric Oxide in Immunity and Inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K. The Role of Nitric Oxide in Cardiovascular Diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Blazquez-Navarro, A.; Mittmann, L.; Thieme, C.J.; Anft, M.; Paniskaki, K.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Konik, M.J.; Berger, M.M.; et al. Impact of Low EGFR on the Immune Response against COVID-19. J. Nephrol. 2022, 36, 199–202. [Google Scholar] [CrossRef]
- Jufar, A.H.; Lankadeva, Y.R.; May, C.N.; Cochrane, A.D.; Bellomo, R.; Evans, R.G. Renal Functional Reserve: From Physiological Phenomenon to Clinical Biomarker and Beyond. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 319, R690–R702. [Google Scholar] [CrossRef]
- Arif, E.; Nihalani, D. Beta2-adrenergic Receptor in Kidney Biology: A Current Prospective. Nephrology 2019, 24, 497–503. [Google Scholar] [CrossRef]
- Maroz, N.; Simman, R. Wound Healing in Patients with Impaired Kidney Function. J. Am. Coll. Clin. Wound Spec. 2013, 5, 2–7. [Google Scholar] [CrossRef]
- Gorin, Y.; Ricono, J.M.; Wagner, B.; Kim, N.-H.; Bhandari, B.; Choudhury, G.G.; Abboud, H.E. Angiotensin II-Induced ERK1/ERK2 Activation and Protein Synthesis Are Redox-Dependent in Glomerular Mesangial Cells. Biochem. J. 2004, 381, 231–239. [Google Scholar] [CrossRef]
- Lira, V.A.; Soltow, Q.A.; Long, J.H.D.; Betters, J.L.; Sellman, J.E.; Criswell, D.S. Nitric Oxide Increases GLUT4 Expression and Regulates AMPK Signaling in Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1062–E1068. [Google Scholar] [CrossRef]
- Peifer-Weiß, L.; Al-Hasani, H.; Chadt, A. AMPK and Beyond: The Signaling Network Controlling RabGAPs and Contraction-Mediated Glucose Uptake in Skeletal Muscle. Int. J. Mol. Sci. 2024, 25, 1910. [Google Scholar] [CrossRef]
- Griendling, K.K.; Berk, B.C.; Socorro, L.; Tsuda, T.; Delafontaine, P.; Alexander, R.W. Secondary Signaling Mechanisms in Angiotensin II-Stimulated Vascular Smooth Muscle Cells. Clin. Exp. Pharmacol. Physiol. 1988, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ruiz-Ortega, M.; Lorenzo, O.; Ruperez, M.; Esteban, V.; Egido, J. Inflammation and Angiotensin II. Int. J. Biochem. Cell Biol. 2003, 35, 881–900. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and Pathogenic Functions of Macrophage Subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Fares, H.; Niazi, A.K.; Chatterjee, S.; D’Ascenzo, F.; Cerrato, E.; Biondi-Zoccai, G.; Lavie, C.J.; Bell, D.S.; O’Keefe, J.H. β-Blockers in Hypertension, Diabetes, Heart Failure and Acute Myocardial Infarction: A Review of the Literature. Open Heart 2015, 2, e000230. [Google Scholar] [CrossRef] [PubMed]
- Kehat, I.; Molkentin, J.D. Molecular Pathways Underlying Cardiac Remodeling During Pathophysiological Stimulation. Circulation 2010, 122, 2727–2735. [Google Scholar] [CrossRef]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Arós, F.; Loma-Osorio, Á.; Vila, J.; López-Bescós, L.; Cuñat, J.; Rodríguez, E.; San José, J.M.; Heras, M.; Marrugat, J.; Investigadores del PRIAMHO II. Effect of Combined Beta-Blocker and Angiotensin-Converting Enzyme Inhibitor Treatment on 1-Year Survival After Acute Myocardial Infarction: Findings of the PRIAMHO-II Registry. Rev. Esp. Cardiol. 2006, 59, 313–320. [Google Scholar] [CrossRef]
- Kim, J.A.; Berliner, J.A.; Nadler, J.L. Angiotensin II Increases Monocyte Binding to Endothelial Cells. Biochem. Biophys. Res. Commun. 1996, 226, 862–868. [Google Scholar] [CrossRef]
- Pueyo, M.E.; Gonzalez, W.; Nicoletti, A.; Savoie, F.; Arnal, J.-F.; Michel, J.-B. Angiotensin II Stimulates Endothelial Vascular Cell Adhesion Molecule-1 via Nuclear Factor-ΚB Activation Induced by Intracellular Oxidative Stress. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 645–651. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Matrix Metalloproteinase Inhibition Therapy for Vascular Diseases. Vasc. Pharmacol. 2012, 56, 232–244. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Marchesi, C.; Paradis, P.; Schiffrin, E.L. Role of the Renin–Angiotensin System in Vascular Inflammation. Trends Pharmacol. Sci. 2008, 29, 367–374. [Google Scholar] [CrossRef]
- Beltrami, M.; Milli, M.; Dei, L.L.; Palazzuoli, A. The Treatment of Heart Failure in Patients with Chronic Kidney Disease: Doubts and New Developments from the Last ESC Guidelines. J. Clin. Med. 2022, 11, 2243. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; Pecoraro, M.; Rusciano, M.R.; Ciccarelli, M.; Popolo, A. Cross-Talk between Neurohormonal Pathways and the Immune System in Heart Failure: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 1698. [Google Scholar] [CrossRef]
- Fung, J.W.H.; Yu, C.M.; Yip, G.; Chan, S.; Yandle, T.G.; Richards, A.M.; Nicholls, M.G.; Sanderson, J.E. Effect of Beta Blockade (Carvedilol or Metoprolol) on Activation of the Renin-Angiotensin-Aldosterone System and Natriuretic Peptides in Chronic Heart Failure. Am. J. Cardiol. 2003, 92, 406–410. [Google Scholar] [CrossRef]
- van der Horst, I.C.C.; Voors, A.A.; van Veldhuisen, D.J. Treatment of Heart Failure with ACE Inhibitors and Beta-Blockers. Clin. Res. Cardiol. 2007, 96, 193. [Google Scholar] [CrossRef]
- Camafort, M.; Park, S.-M.; Kang, S.-M. Lifestyle Modification in Heart Failure Management: Are We Using Evidence-Based Recommendations in Real World Practice? Int. J. Heart Fail. 2023, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Murray, P.J. Cytokine Signaling Modules in Inflammatory Responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Vieceli Dalla Sega, F.; Fortini, F.; Aquila, G.; Campo, G.; Vaccarezza, M.; Rizzo, P. Notch Signaling Regulates Immune Responses in Atherosclerosis. Front. Immunol. 2019, 10, 1130. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Shuai, W.; Meng, J.; Feng, J.; Han, Z. Macrophage Polarization and Its Role in the Pathogenesis of Acute Lung Injury/Acute Respiratory Distress Syndrome. Inflamm. Res. 2020, 69, 883–895. [Google Scholar] [CrossRef]
- Ban, J.-Q.; Ao, L.-H.; He, X.; Zhao, H.; Li, J. Advances in Macrophage-Myofibroblast Transformation in Fibrotic Diseases. Front. Immunol. 2024, 15, 1461919. [Google Scholar] [CrossRef]
- Ahmadi, M.; Bekeschus, S.; Weltmann, K.-D.; von Woedtke, T.; Wende, K. Non-Steroidal Anti-Inflammatory Drugs: Recent Advances in the Use of Synthetic COX-2 Inhibitors. RSC Med. Chem. 2022, 13, 471–496. [Google Scholar] [CrossRef]
- Tison, G.H. Using Machine Learning to Uncover Heterogeneity of Beta Blocker Response in Heart Failure. Cell Rep. Med. 2022, 3, 100504. [Google Scholar] [CrossRef]
- DeGroat, W.; Mendhe, D.; Bhusari, A.; Abdelhalim, H.; Zeeshan, S.; Ahmed, Z. IntelliGenes: A Novel Machine Learning Pipeline for Biomarker Discovery and Predictive Analysis Using Multi-Genomic Profiles. Bioinformatics 2023, 39, btad755. [Google Scholar] [CrossRef]
- Drygała, S.; Radzikowski, M.; Maciejczyk, M. β-Blockers and Metabolic Modulation: Unraveling the Complex Interplay with Glucose Metabolism, Inflammation and Oxidative Stress. Front. Pharmacol. 2024, 15, 1489657. [Google Scholar] [CrossRef]
- Schenone, M.; Dančík, V.; Wagner, B.K.; Clemons, P.A. Target Identification and Mechanism of Action in Chemical Biology and Drug Discovery. Nat. Chem. Biol. 2013, 9, 232–240. [Google Scholar] [CrossRef]
- Duncavage, E.J.; Bagg, A.; Hasserjian, R.P.; DiNardo, C.D.; Godley, L.A.; Iacobucci, I.; Jaiswal, S.; Malcovati, L.; Vannucchi, A.M.; Patel, K.P.; et al. Genomic Profiling for Clinical Decision Making in Myeloid Neoplasms and Acute Leukemia. Blood 2022, 140, 2228–2247. [Google Scholar] [CrossRef] [PubMed]
- Devereux, G.; Cotton, S.; Nath, M.; McMeekin, N.; Campbell, K.; Chaudhuri, R.; Choudhury, G.; De Soyza, A.; Fielding, S.; Gompertz, S.; et al. Bisoprolol in Patients with Chronic Obstructive Pulmonary Disease at High Risk of Exacerbation. JAMA 2024, 332, 462. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, H.S.; Sheer, R.; Honarpour, N.; Casebeer, A.W.; Simmons, J.D.; Kurtz, C.E.; Pasquale, M.K.; Globe, G. Real-World Analysis of Guideline-Based Therapy After Hospitalization for Heart Failure. J. Am. Heart Assoc. 2020, 9, 16. [Google Scholar] [CrossRef]
- Fuchs, B.; Heesen, P. From Data Integration to Precision Medicine: A Value-Based Healthcare Approach for Sarcoma Care. J. Clin. Med. 2024, 13, 6500. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M.; Nebert, D.W.; Lauschke, V.M. Emerging Trends in Pharmacogenomics: From Common Variant Associations toward Comprehensive Genomic Profiling. Hum. Genom. 2023, 17, 105. [Google Scholar] [CrossRef]
- Subramanian, M.; Wojtusciszyn, A.; Favre, L.; Boughorbel, S.; Shan, J.; Letaief, K.B.; Pitteloud, N.; Chouchane, L. Precision Medicine in the Era of Artificial Intelligence: Implications in Chronic Disease Management. J. Transl. Med. 2020, 18, 472. [Google Scholar] [CrossRef]
| Mechanistic Domain | Molecular Targets | β-Blocker Type | Effect or Outcome | References |
|---|---|---|---|---|
| Genetic Variants | ADRB1 Arg389Gly, GRK5, CYP2D6 | Any BB (dose-adjusted) | Pharmacogenomic modifiers of BB response and metabolism | [9,10,11,12] |
| Sympathetic Nervous System | β1/β2-adrenergic receptors | BBs with ISA (e.g., Carteolol) | ↓ HR and contractility; preserved resting tone; ↓ exercise tachycardia | [13,14] |
| RAAS Inhibition | Renin, Ang II, AT1R | β1-selective (e.g., metoprolol) | ↓ Renin secretion; ↓ Ang II activity; ↓ vascular remodeling | [15,16,17] |
| NO Signaling Preservation | eNOS, iNOS, PGE2 | Vasodilatory BBs (e.g., nebivolol) | ↑ NO bioavailability; ↑ endothelial function | [18,19,20,21] |
| Fibrosis Pathway Modulation | TGF-β, Smad3, PI3K-AKT, MMT | Cardioselective BBs (e.g., bisoprolol) | ↓ Cardiac and renal fibrosis; improved M1/M2 macrophage balance | [22,23] |
| Cytokine Modulation | TNF-α, IL-6, MCP-1 | Non-selective (e.g., propranolol) | ↓ Pro-inflammatory cytokine production; ↓ macrophage activation | [24,25] |
| Disease Context | Primary Mechanism Involved | β-Blocker Type | Clinical Outcomes and Benefits | References |
|---|---|---|---|---|
| Arrhythmias (AF, VT) | AV node conduction suppression | β1-selective or non-selective BBs | ↓ Rhythm and rate disturbances; ↓ sudden cardiac death | [1,26] |
| Heart Failure with Reduced Ejection Fraction (HFrEF) | RAAS inhibition, SNS blockade | β1-selective (e.g., bisoprolol, metoprolol) | ↓ Mortality; ↑ LVEF; ↓ hospitalizations | [27,28] |
| Hypertension | RAAS inhibition, reduced CO | β1-selective (e.g., atenolol); BBs with ISA (e.g., pindolol) | ↓ Blood pressure; ↑ vascular compliance | [29,30,31] |
| Myocardial Infarction | Sympathetic modulation, anti-arrhythmic | Non-selective or β1-selective (e.g., atenolol, propranolol) | ↓ Reinfarction; ↓ mortality; ↓ arrhythmias | [32,33,34] |
| Chronic Kidney Disease | RAAS inhibition, cytokine modulation | β1-selective (e.g., bisoprolol); vasodilatory (e.g., nebivolol) | ↓ Proteinuria; ↓ CKD progression; ↑ renal protection | [31,35,36] |
| Thyrotoxicosis | β1-, β2-receptor blockade | Non-selective (e.g., propranolol) | ↓ Tremor and palpitations; ↓ T4→T3 conversion | [37,38] |
| Authors (Year) | Effect of BB Therapy | Clinical Insight/Implication |
|---|---|---|
| Gullestad et al. (1989) [65] | Modulates neurohormonal responses (cortisol, epinephrine, prolactin) during physical stress | BBs blunt stress hormone surges during exertion; may limit physiologic adaptation |
| DeMorrow (2018) [64] | Describes HPA axis physiology and BBs influence on stress adaptation | Chronic HPA modulation by BBs may alter endocrine resilience and stress response |
| Burford et al. (2017) [66] | Alters glucocorticoid signaling via HPA axis and cardiovascular effects | Prolonged BBs use may increase CV risk by disrupting glucocorticoid homeostasis |
| Bugajski et al. (1995) [67] | Shows β-adrenergic stimulation of HPA axis under stress | BBs reduce stress-induced cortisol secretion; consider stress-sensitive disorders |
| Macdonald et al. (1984) [30] | Inhibits lactate and free fatty acid mobilization during exercise | BBs alter exercise metabolism; caution in cardiac patients requiring energy reserve |
| Oki et al. (2009) [68] | Propranolol normalizes cortisol in AIMAH (adrenal incidentaloma) | May be therapeutic in subclinical Cushing’s syndrome via β-receptor modulation |
| Wiersinga (1991) [37] | Inhibits type 1 deiodinase, lowering T3 levels | Effective in hyperthyroidism; monitor for hypothyroid risk with long-term use |
| Geffner & Hershman (1992) [38] | Reduces adrenergic symptoms in thyrotoxicosis | BBs alleviate hyperthyroid symptoms; recommend thyroid function monitoring |
| McGill (2009) [69] | Non-selective BBs impair insulin sensitivity in diabetes | Avoid non-selective BBs in diabetics; consider vasodilatory agents |
| Kveiborg et al. (2010) [70] | Carvedilol preserves, metoprolol impairs insulin-stimulated endothelial function | Choose carvedilol over metoprolol for improved metabolic safety in diabetics |
| Zanchetti et al. (1983) [71] | Suppresses plasma renin activity | Monitor RAAS suppression; adjust antihypertensive regimen accordingly |
| Knox et al. (1980) [72] | Alters renal sodium balance under mineralocorticoid influence | Monitor fluid/electrolyte status in long-term BBs therapy for volume-sensitive patients |
| Citation | Study Type | BB Type | Mechanism of Action | Target System | Physiologic/Clinical Effect | Example Drugs | Model |
|---|---|---|---|---|---|---|---|
| Wang et al. (2006) [7] | Experimental | β1-stimulation (untreated) | Preserves renal afferent arterioles from NE-induced oxidative stress via cAMP | Renal | Protective against oxidative injury | N/A | Animal (Rabbit) |
| Bruck et al. (2004) [13] | Clinical (in vivo) | BBs with ISA | Partial β2 agonism → mild vasodilation & ↑ HR | Cardiac & vascular | ↓ HR/bradycardia risk; preserved exercise tolerance | Carteolol, Alprenolol | Human |
| Nyberg et al. (1979) [14] | Clinical (pharmacology) | BBs with ISA | Partial β2 agonism → mild vasodilation & ↑ HR | Cardiac & vascular | ↓ HR/bradycardia risk; preserved exercise tolerance | Penbutolol | Human |
| Cruickshank et al. (2010) [45] | Review (HF/ISA) | BBs with ISA | Partial β2 agonism → mild vasodilation & ↑ HR | Cardiac & vascular | ↓ HR/bradycardia risk; preserved exercise tolerance | Carteolol, Alprenolol | Human |
| Khan et al. (2023) [74] | Clinical review | β1-selective BBs | Inhibit β1-adrenergic stimulation → ↓ HR, ↓ contractility | Cardiac | ↓ Myocardial O2 demand; ↑ diastolic filling; anti-ischemic | Metoprolol, Bisoprolol | Human |
| Khan et al. (2023) [74] | Clinical review | Vasodilatory BBs | α1-blockade → ↓ PVR & vasodilation | Vascular | ↓ Afterload; ↑ CO | Carvedilol, Labetalol | Human |
| Khan et al. (2023) [74] | Clinical review | Vasodilatory BBs | ↑ NO bioactivity → ↓ PVR | Vascular | ↓ BP; ↑ CO | Nebivolol | Human |
| Prijic et al. (2014) [75] | Clinical review | BBs (general) | ↓ SNS overdrive → ↓ afterload; reverse remodeling; ↓ arrhythmias | Cardiac | ↓ Angina; ↓ HF symptoms; ↑ survival | Carvedilol, Metoprolol | Human |
| Wołowiec et al. (2023) [1] | Review (clinical) | Non-selective BBs | Block β1 & β2 → ↓ HR & ↓ renin (possible ↑ vasoconstriction) | Cardiorenal | Mixed BP effects; caution in asthma | Propranolol, Nadolol | Human |
| Baltogiannis et al. (2019) [26] | Brief review | Non-selective BBs | Block β1 & β2 → ↓ HR & ↓ renin (possible ↑ vasoconstriction) | Cardiorenal | Mixed BP effects; caution in asthma | Propranolol, Nadolol | Human |
| Han et al. (2020) [76] | Network meta-analysis | Non-selective BBs | Block β1 & β2 → ↓ HR & ↓ renin (possible ↑ vasoconstriction) | Cardiorenal | Mixed BP effects; caution in asthma | Propranolol, Nadolol | Human |
| Han et al. (2020) [76] | Network meta-analysis | Nadolol (non-selective) | ↓ SNS effect on heart → ↓ QT dispersion | Cardiac electrical | ↓ Risk of sudden death in LQTS | Nadolol | Human |
| Schweda et al. (2007) [15] | Review (renal physiology) | β1-selective BBs | Inhibit β1 on JG cells → ↓cAMP → ↓ renin | Renal | ↓ RAAS activation; ↓ BP | Atenolol, Metoprolol | Animal & Human |
| Kurtz et al. (2012) [77] | Review (renal physiology) | β1-selective BBs | Inhibit β1 on JG cells → ↓ cAMP → ↓ renin | Renal | ↓ RAAS activation; ↓ BP | Atenolol, Metoprolol | Animal & Human |
| Kurtz et al. (2011) [16] | Review (renal physiology) | β1-selective BBs | Inhibit β1 on JG cells → ↓ cAMP → ↓ renin | Renal | ↓ RAAS activation; ↓ BP | Atenolol, Metoprolol | Animal & Human |
| Manis et al. (2019) [78] | Experimental physiology | BBs (general) | ↓ Renin, ↓ Na+ reabsorption, ↑ renal O2 balance | Renal | Stabilizes BP; ↑ renal tissue oxygenation | Atenolol, Metoprolol, Propranolol | Animal & Human |
| Kumar et al. (2023) [79] | Review (diabetes/renal) | BBs (general) | ↓ Renin, ↓ Na+ reabsorption, ↑ renal O2 balance | Renal | Stabilizes BP; ↑ renal tissue oxygenation | Carvedilol, Nebivolol, Bisoprolol | Human |
| Hall et al. (2016) [59] | Observational study | BBs (general) | ↓ Renin, ↓ Na+ reabsorption, ↑ renal O2 balance | Renal | Stabilizes BP; ↑ renal tissue oxygenation | Metoprolol, Bisoprolol, Carvedilol | Human |
| Strauss et al. (2023) [80] | Clinical review | BBs (general) | ↓ Renin, ↓ Na+ reabsorption, ↑ renal O2 balance | Renal | Stabilizes BP; ↑ renal tissue oxygenation | Atenolol, Metoprolol, Nebivolol | Human |
| BBs | RAAS | COX-2 | PGE2 and iNOS/NO Pathway | NO | Aldosterone |
|---|---|---|---|---|---|
| Block β1-adrenergic receptors on juxtaglomerular cells, reducing renin release and suppressing RAAS activity. | Under normal conditions, RAAS activation leads to: ANG II–mediated COX-2 upregulation. Aldosterone secretion via adrenal cortex stimulation. Vasopressin (ADH) release via hypothalamic–pituitary axis. β-blockers attenuate these downstream effects. | ANG II stimulates COX-2 expression in vascular and renal cells. COX-2 catalyzes arachidonic acid conversion into prostaglandins, including PGE2, which regulate inflammation and vascular tone. | COX-2–derived PGE2 may inhibit iNOS expression via EP2/EP4 receptors, leading to decreased NO synthesis. NO acts as a vasodilator and modulates immune cell differentiation. | NO mediates: Vasodilation, lowering blood pressure. Anti-inflammatory effects. Maintenance of renal blood flow and function. Reduced NO contributes to hypertension, inflammation, and renal dysfunction. | Aldosterone binds mineralocorticoid receptors, promoting sodium and water retention, affecting renal function through volume status and elevating blood pressure. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Hamanaka, A.; Park, I.; Abdelhady, H.G. Chronic β-Blockade and Systemic Homeostasis: Molecular Integration of Cardiorenal and Immune Pathways, a Narrative Review. Biomolecules 2025, 15, 1653. https://doi.org/10.3390/biom15121653
Park J, Hamanaka A, Park I, Abdelhady HG. Chronic β-Blockade and Systemic Homeostasis: Molecular Integration of Cardiorenal and Immune Pathways, a Narrative Review. Biomolecules. 2025; 15(12):1653. https://doi.org/10.3390/biom15121653
Chicago/Turabian StylePark, Jason, Amethyst Hamanaka, Issac Park, and Hosam Gharib Abdelhady. 2025. "Chronic β-Blockade and Systemic Homeostasis: Molecular Integration of Cardiorenal and Immune Pathways, a Narrative Review" Biomolecules 15, no. 12: 1653. https://doi.org/10.3390/biom15121653
APA StylePark, J., Hamanaka, A., Park, I., & Abdelhady, H. G. (2025). Chronic β-Blockade and Systemic Homeostasis: Molecular Integration of Cardiorenal and Immune Pathways, a Narrative Review. Biomolecules, 15(12), 1653. https://doi.org/10.3390/biom15121653








