Plasma Membrane Lipid Composition and Turnover in Human Midbrain Neurons Investigated by Time-of-Flight Mass Spectrometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Preparation
2.2. Immunocytochemistry
2.3. ToF-SIMS Imaging
2.4. Data Analysis
3. Results and Discussion
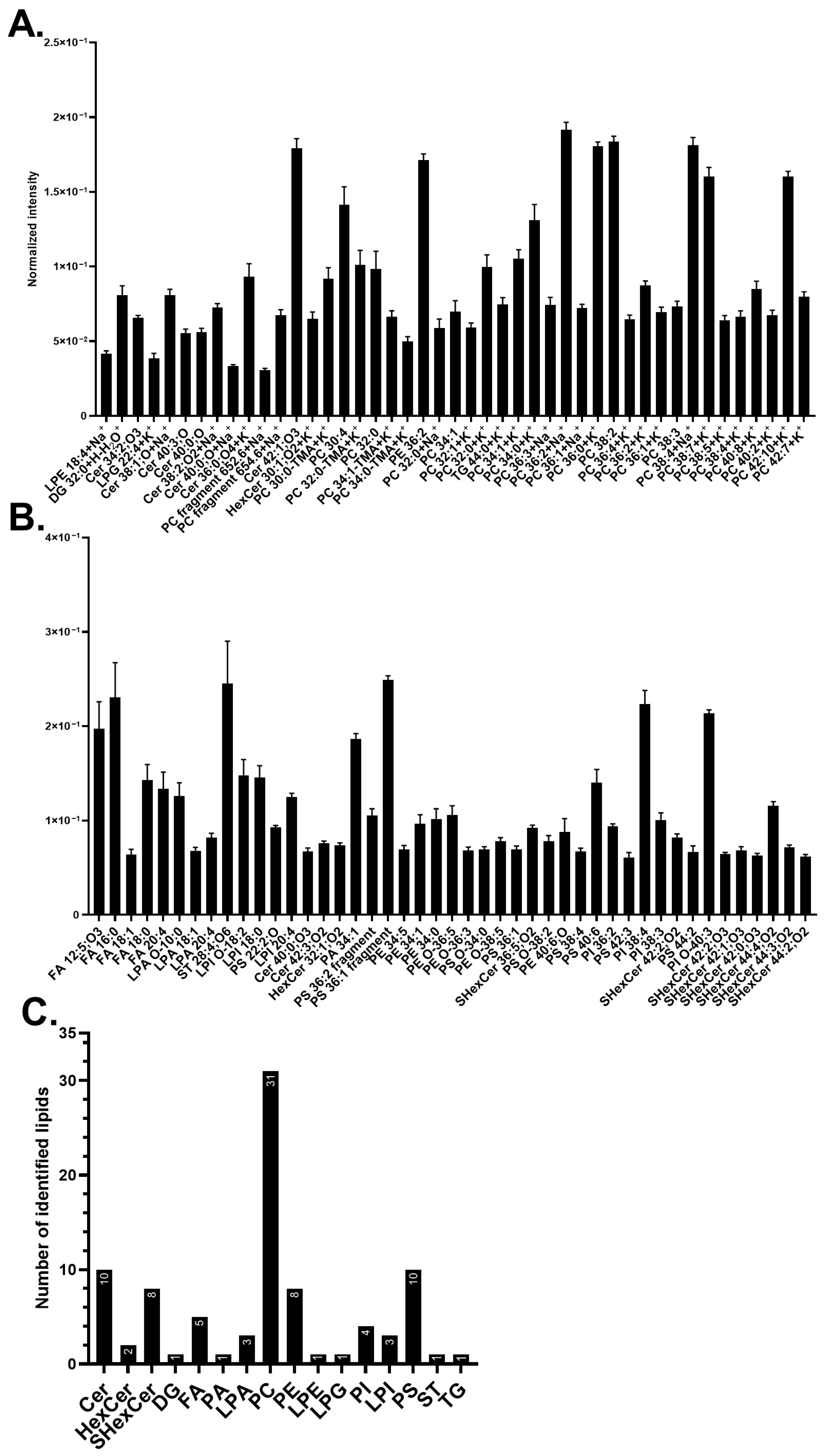
3.1. Lipid Abundancy in Neuronal Plasma Membrane
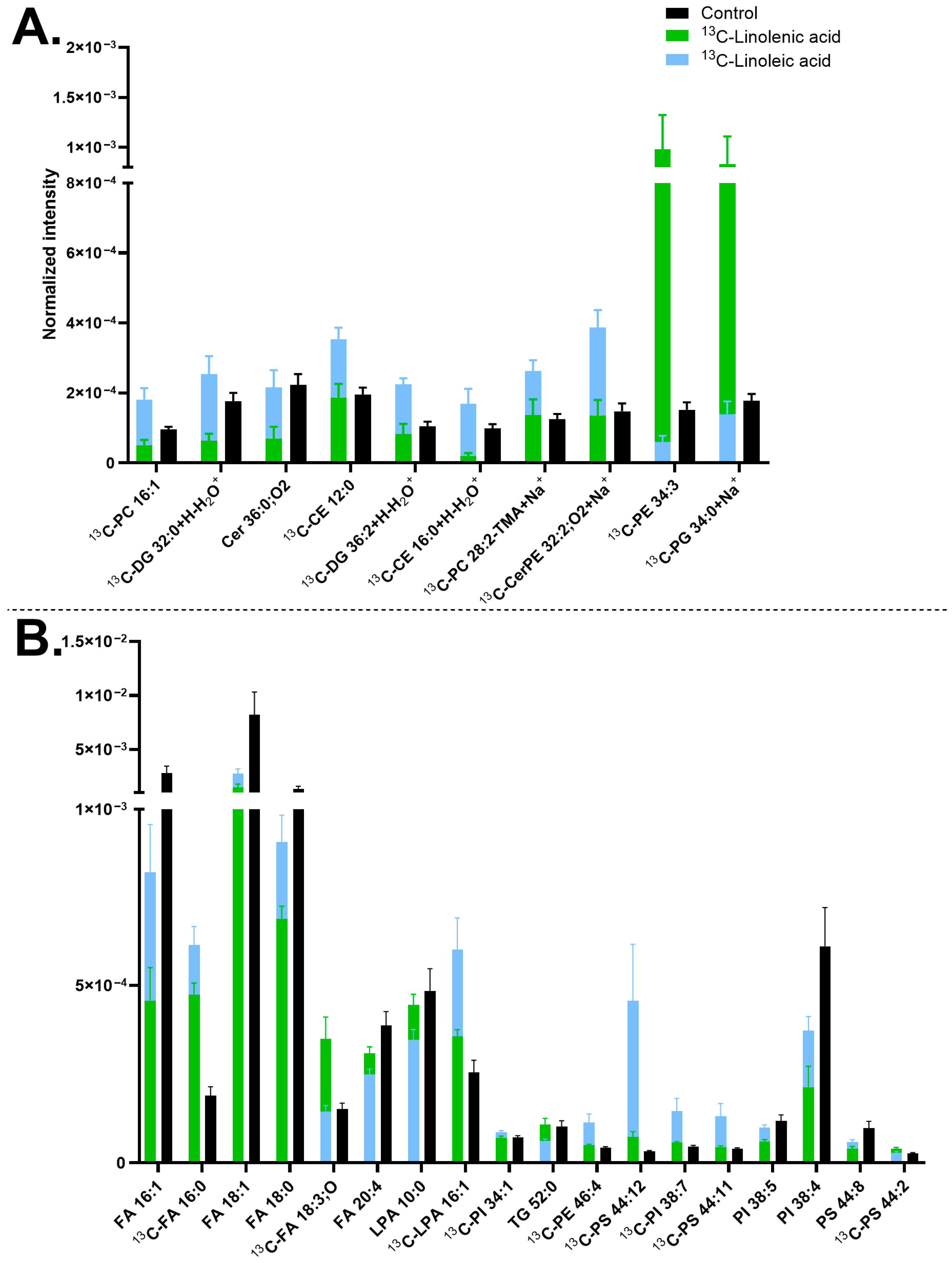
3.2. Effects of Lipid Head Groups on Neuronal Membrane Lipid Turnover—Ethanolamine Versus Choline
3.3. Effects of the Unsaturation Level on Neuronal Membrane Lipid Turnover—Linolenic Acid Versus Linoleic Acid
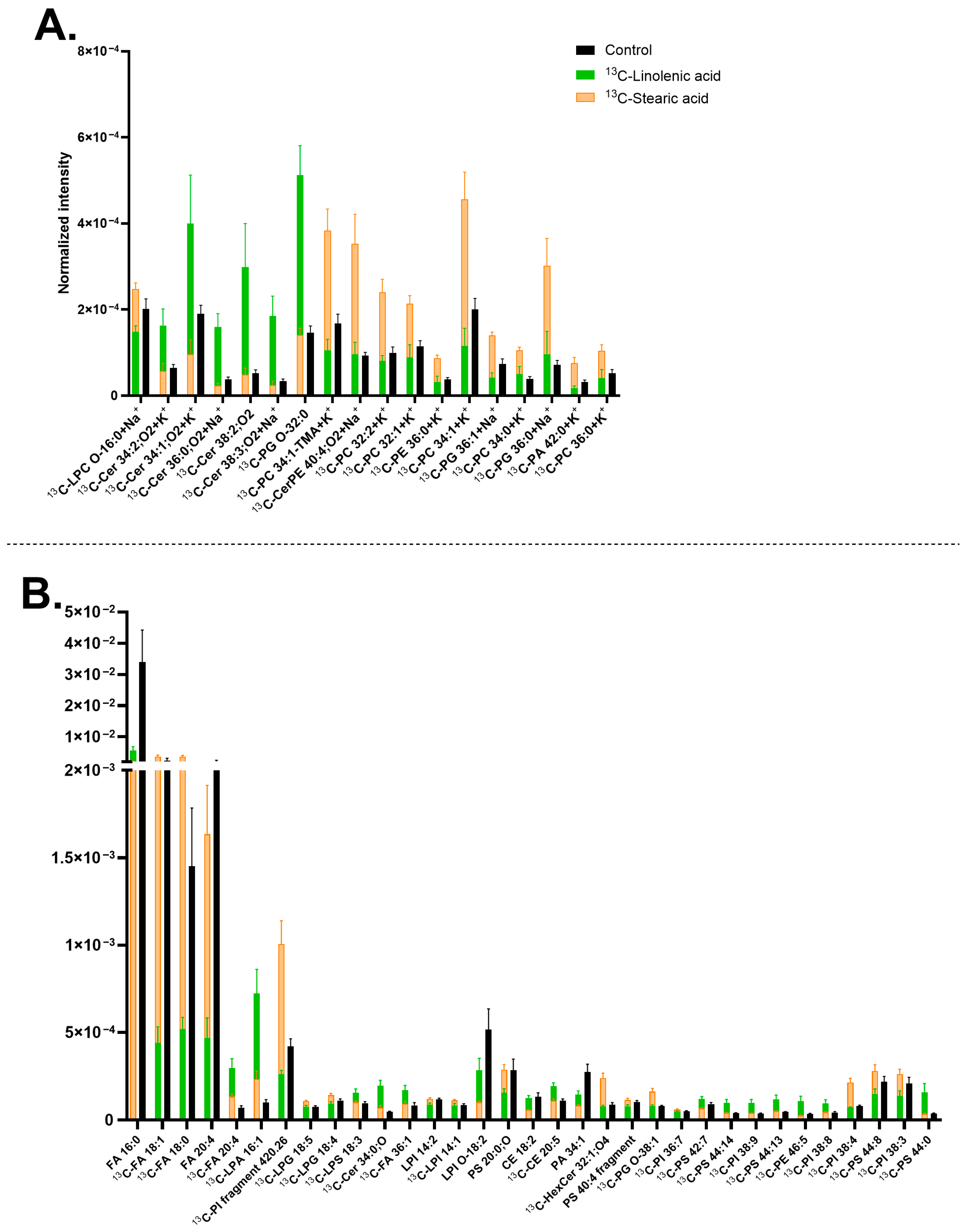
3.4. Effects of Carbon Chain Saturation of Lipid Precursor on Neuronal Membrane Lipid Turnover—Linoleic Acid and Linolenic Acid Versus Stearic Acid
3.5. Effect of Carbon Chain Length in Neuronal Membrane Lipid Turnover—Stearic Acid Versus Lauric Acid

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruchalski, K.; Hathout, G.M. A Medley of Midbrain Maladies: A Brief Review of Midbrain Anatomy and Syndromology for Radiologists. Radiol. Res. Pract. 2012, 2012, 258524. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, S.V.; Sullivan, A.M.; O’Keeffe, G.W. Midbrain Dopaminergic Neurons: A Review of the Molecular Circuitry That Regulates Their Development. Dev. Biol. 2013, 379, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Root, D.H. Glutamate Neurons within the Midbrain Dopamine Regions. Neuroscience 2014, 282, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Ingólfsson, H.I.; Carpenter, T.S.; Bhatia, H.; Bremer, P.T.; Marrink, S.J.; Lightstone, F.C. Computational Lipidomics of the Neuronal Plasma Membrane. Biophys. J. 2017, 113, 2271–2280. [Google Scholar] [CrossRef]
- Samhan-Arias, A.K.; Poejo, J.; Marques-da-Silva, D.; Martínez-Costa, O.H.; Gutierrez-Merino, C. Are There Lipid Membrane-Domain Subtypes in Neurons with Different Roles in Calcium Signaling? Molecules 2023, 28, 7909. [Google Scholar] [CrossRef]
- Duménieu, M.; Oulé, M.; Kreutz, M.R.; Lopez-Rojas, J. The Segregated Expression of Voltage-Gated Potassium and Sodium Channels in Neuronal Membranes: Functional Implications and Regulatory Mechanisms. Front. Cell. Neurosci. 2017, 11, 115. [Google Scholar] [CrossRef]
- Antelmi, E.; Rocchi, L.; Latorre, A.; Belvisi, D.; Magrinelli, F.; Bhatia, K.P.; Tinazzi, M. Restless Legs Syndrome: Known Knowns and Known Unknowns. Brain Sci. 2022, 12, 118. [Google Scholar] [CrossRef]
- Sonnenschein, S.F.; Gomes, F.V.; Grace, A.A. Dysregulation of Midbrain Dopamine System and the Pathophysiology of Schizophrenia. Front. Psychiatry 2020, 11, 613. [Google Scholar] [CrossRef]
- Triarhou, L.C. Dopamine and Parkinson’s Disease. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Ernst, M.; Zametkin, A.J.; Matochik, J.A.; Pascualvaca, D.; Jons, P.H.; Cohen, R.M. High Midbrain [18F]DOPA Accumulation in Children with Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry 1999, 156, 1209–1215. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Agüi-Gonzalez, P.; Guobin, B.; Gomes De Castro, M.A.; Rizzoli, S.O.; Phan, N.T.N. Secondary Ion Mass Spectrometry Imaging Reveals Changes in the Lipid Structure of the Plasma Membranes of Hippocampal Neurons Following Drugs Affecting Neuronal Activity. ACS Chem. Neurosci. 2021, 12, 1542–1551. [Google Scholar] [CrossRef]
- Egawa, J.; Pearn, M.L.; Lemkuil, B.P.; Patel, P.M.; Head, B.P. Membrane Lipid Rafts and Neurobiology: Age—Related Changes in Membrane Lipids and Loss of Neuronal Function. J. Physiol. 2016, 594, 4565–4579. [Google Scholar] [CrossRef]
- Miranda, A.M.; Bravo, F.V.; Chan, R.B.; Sousa, N.; Di Paolo, G.; Oliveira, T.G. Differential Lipid Composition and Regulation along the Hippocampal Longitudinal Axis. Transl. Psychiatry 2019, 9, 144. [Google Scholar] [CrossRef]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Philipsen, M.H.; Phan, N.T.N.; Fletcher, J.S.; Ewing, A.G. Interplay between Cocaine, Drug Removal, and Methylphenidate Reversal on Phospholipid Alterations in Drosophila Brain Determined by Imaging Mass Spectrometry. ACS Chem. Neurosci. 2020, 11, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, B.G.; Mandad, S.; Truckenbrodt, S.; Kröhnert, K.; Schäfer, C.; Rammner, B.; Koo, S.J.; Claßen, G.A.; Krauss, M.; Haucke, V.; et al. Composition of Isolated Synaptic Boutons Reveals the Amounts of Vesicle Trafficking Proteins. Science 2014, 344, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.G.; Park, S.S.; Park, J.H.; Lee, S.B. Dysregulated Plasma Membrane Turnover Underlying Dendritic Pathology in Neurodegenerative Diseases. Front. Cell. Neurosci. 2020, 14, 556461. [Google Scholar] [CrossRef] [PubMed]
- Porcellati, G.; Goracci, G.; Arienti, G. Lipid Turnover. In Handbook of Neurochemistry; Springer: Boston, MA, USA, 1983; pp. 277–294. [Google Scholar] [CrossRef]
- Schmitt, A.; Maras, A.; Petroianu, G.; Braus, D.F.; Scheuer, L.; Gattaz, W.F. Effects of Antipsychotic Treatment on Membrane Phospholipid Metabolism in Schizophrenia. J. Neural Transm. 2001, 108, 1081–1091. [Google Scholar] [CrossRef]
- Jensen, J.E.; Miller, J.; Williamson, P.C.; Neufeld, R.W.J.; Menon, R.S.; Malla, A.; Manchanda, R.; Schaefer, B.; Densmore, M.; Drost, D.J. Grey and White Matter Differences in Brain Energy Metabolism in First Episode Schizophrenia: 31P-MRS Chemical Shift Imaging at 4 Tesla. Psychiatry Res. Neuroimaging 2006, 146, 127–135. [Google Scholar] [CrossRef]
- Tessier, C.; Sweers, K.; Frajerman, A.; Bergaoui, H.; Ferreri, F.; Delva, C.; Lapidus, N.; Lamaziere, A.; Roiser, J.P.; De Hert, M.; et al. Membrane Lipidomics in Schizophrenia Patients: A Correlational Study with Clinical and Cognitive Manifestations. Transl. Psychiatry 2016, 6, e906. [Google Scholar] [CrossRef]
- Nuss, P.; Tessier, C.; Ferreri, F.; De Hert, M.; Peuskens, J.; Trugnan, G.; Masliah, J.; Wolf, C. Abnormal Transbilayer Distribution of Phospholipids in Red Blood Cell Membranes in Schizophrenia. Psychiatry Res. 2009, 169, 91–96. [Google Scholar] [CrossRef]
- Horrobin, D.F.; Glen, A.I.M.; Vaddadi, K. The Membrane Hypothesis of Schizophrenia. Schizophr. Res. 1994, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F.; Bennett, C.N. The Membrane Phospholipid Concept of Schizophrenia. In Search for the Causes of Schizophrenia; Springer: Darmstadt, Germany, 1999; pp. 261–277. [Google Scholar] [CrossRef]
- Hannestad, J.K.; Rocha, S.; Agnarsson, B.; Zhdanov, V.P.; Wittung-Stafshede, P.; Höök, F. Single-Vesicle Imaging Reveals Lipid-Selective and Stepwise Membrane Disruption by Monomeric α-Synuclein. Proc. Natl. Acad. Sci. USA 2020, 117, 14178–14186. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Lee, J.C. Unroofing Site-Specific α-Synuclein Lipid Interactions at the Plasma Membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 18977–18983. [Google Scholar] [CrossRef] [PubMed]
- Plotegher, N.; Bubacco, L.; Greggio, E.; Civiero, L. Ceramides in Parkinson’s Disease: From Recent Evidence to New Hypotheses. Front. Neurosci. 2019, 13, 330. [Google Scholar] [CrossRef]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef]
- Schulte, E.C.; Altmaier, E.; Berger, H.S.; Do, K.T.; Kastenmüller, G.; Wahl, S.; Adamski, J.; Peters, A.; Krumsiek, J.; Suhre, K.; et al. Alterations in Lipid and Inositol Metabolisms in Two Dopaminergic Disorders. PLoS ONE 2016, 11, e0147129. [Google Scholar] [CrossRef]
- Haynes, P.R.; Pyfrom, E.S.; Li, Y.; Stein, C.; Cuddapah, V.A.; Jacobs, J.A.; Yue, Z.; Sehgal, A. A Neuron–Glia Lipid Metabolic Cycle Couples Daily Sleep to Mitochondrial Homeostasis. Nat. Neurosci. 2024, 27, 666–678. [Google Scholar] [CrossRef]
- Ugur, C.; Uneri, O.S.; Goker, Z.; Sekmen, E.; Aydemir, H.; Solmaz, E. The Assessment of Serum Lipid Profiles of Children with Attention Deficit Hyperactivity Disorder. Psychiatry Res. 2018, 264, 231–235. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Wucher, A.; Winograd, N. Molecular Depth Profiling with Argon Gas Cluster Ion Beams. J. Phys. Chem. C 2015, 119, 15316–15324. [Google Scholar] [CrossRef]
- Angerer, T.B.; Blenkinsopp, P.; Fletcher, J.S. High Energy Gas Cluster Ions for Organic and Biological Analysis by Time-of-Flight Secondary Ion Mass Spectrometry. Int. J. Mass Spectrom. 2015, 377, 591–598. [Google Scholar] [CrossRef]
- Tian, H.; Maciążek, D.; Postawa, Z.; Garrison, B.J.; Winograd, N. CO2 Cluster Ion Beam, an Alternative Projectile for Secondary Ion Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2016, 27, 1476–1482. [Google Scholar] [CrossRef]
- Tian, H.; Sheraz Née Rabbani, S.; Vickerman, J.C.; Winograd, N. Multiomics Imaging Using High-Energy Water Gas Cluster Ion Beam Secondary Ion Mass Spectrometry [(H2O)n-GCIB-SIMS] of Frozen-Hydrated Cells and Tissue. Anal. Chem. 2021, 93, 7808–7814. [Google Scholar] [CrossRef]
- Ogiso, H.; Taniguchi, M.; Okazaki, T. Analysis of Lipid-Composition Changes in Plasma Membrane Microdomains. J. Lipid Res. 2015, 56, 1594–1605. [Google Scholar] [CrossRef]
- Agüi-Gonzalez, P.; Jähne, S.; Phan, N.T.N. SIMS Imaging in Neurobiology and Cell Biology. J. Anal. At. Spectrom. 2019, 34, 1355–1368. [Google Scholar] [CrossRef]
- Philipsen, M.H.; Sämfors, S.; Malmberg, P.; Ewing, A.G. Relative Quantification of Deuterated Omega-3 and -6 Fatty Acids and Their Lipid Turnover in PC12 Cell Membranes Using TOF-SIMS. J. Lipid Res. 2018, 59, 2098–2107. [Google Scholar] [CrossRef]
- Troiano, J.M.; Olenick, L.L.; Kuech, T.R.; Melby, E.S.; Hu, D.; Lohse, S.E.; Mensch, A.C.; Dogangun, M.; Vartanian, A.M.; Torelli, M.D.; et al. Direct probes of 4 nm diameter gold nanoparticles interacting with supported lipid bilayers. J. Phys. Chem. C. 2015, 119, 534–546. [Google Scholar] [CrossRef]
- Vaezian, B.; Anderton, C.R.; Kraft, M.L. Discriminating and Imaging Different Phosphatidylcholine Species within Phase-Separated Model Membranes by Principal Component Analysis of TOF-Secondary Ion Mass Spectrometry Images. Anal. Chem. 2010, 82, 10006–10014. [Google Scholar] [CrossRef] [PubMed]
- Prinz, C.; Höök, F.; Malm, J.; Sjövall, P. Structural Effects in the Analysis of Supported Lipid Bilayers by Time-of-Flight Secondary Ion Mass Spectrometry. Langmuir 2007, 23, 8035–8041. [Google Scholar] [CrossRef] [PubMed]
- Kunze, A.; Sjövall, P.; Kasemo, B.; Svedhem, S. In Situ Preparation and Modification of Supported Lipid Layers by Lipid Transfer from Vesicles Studied by QCM-D and TOF-SIMS. J. Am. Chem. Soc. 2009, 131, 2450–2451. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, M.K.; Winograd, N. Lipid Imaging with Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS). Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2011, 1811, 976–990. [Google Scholar] [CrossRef]
- Munem, M.; Zaar, O.; Dimovska Nilsson, K.; Neittaanmäki, N.; Paoli, J.; Fletcher, J.S. Chemical Imaging of Aggressive Basal Cell Carcinoma Using Time-of-Flight Secondary Ion Mass Spectrometry. Biointerphases 2018, 13, 03B402. [Google Scholar] [CrossRef]
- Dimovska Nilsson, K.; Neittaanmäki, N.; Zaar, O.; Angerer, T.B.; Paoli, J.; Fletcher, J.S. TOF-SIMS Imaging Reveals Tumor Heterogeneity and Inflammatory Response Markers in the Microenvironment of Basal Cell Carcinoma. Biointerphases 2020, 15, 041012. [Google Scholar] [CrossRef]
- Siljeström, S.; Parenteau, M.N.; Jahnke, L.L.; Cady, S.L. A Comparative ToF-SIMS and GC–MS Analysis of Phototrophic Communities Collected from an Alkaline Silica-Depositing Hot Spring. Org. Geochem. 2017, 109, 14–30. [Google Scholar] [CrossRef]
- Yang, K.; Han, X. Lipidomics: Techniques, Applications, and Outcomes Related to Biomedical Sciences. Trends Biochem. Sci. 2016, 41, 954–969. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Rabbani, S.; Henderson, A.; Blenkinsopp, P.; Thompson, S.P.; Lockyer, N.P.; Vickerman, J.C. A New Dynamic in Mass Spectral Imaging of Single Biological Cells. Anal. Chem. 2008, 80, 9058–9064. [Google Scholar] [CrossRef]
- Angerer, T.B.; Dowlatshahi Pour, M.; Malmberg, P.; Fletcher, J.S. Improved Molecular Imaging in Rodent Brain with Time-of-Flight-Secondary Ion Mass Spectrometry Using Gas Cluster Ion Beams and Reactive Vapor Exposure. Anal. Chem. 2015, 87, 4305–4313. [Google Scholar] [CrossRef]
- Fransson, A.; Dimovska Nilsson, K.; Henderson, A.; Farewell, A.; Fletcher, J.S. PCA, PC-CVA, and Random Forest of GCIB-SIMS Data for the Elucidation of Bacterial Envelope Differences in Antibiotic Resistance Research. Anal. Chem. 2024, 96, 14168–14177. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A. ChiToolbox. 2024. Available online: https://zenodo.org/records/10932517 (accessed on 19 September 2024).
- Sjövall, P.; Gregoire, S.; Wargniez, W.; Skedung, L.; Luengo, G.S. 3D Molecular Imaging of Stratum Corneum by Mass Spectrometry Suggests Distinct Distribution of Cholesteryl Esters Compared to Other Skin Lipids. Int. J. Mol. Sci. 2022, 23, 13799. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Sjövall, P.; Lausmaa, J.; Leefmann, T.; Thiel, V. Spectral Characterisation of Eight Glycerolipids and Their Detection in Natural Samples Using Time-of-Flight Secondary Ion Mass Spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 2741–2753. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.J.; DeBord, J.D.; Fernandez-Lima, F. Lipid Specific Molecular Ion Emission as a Function of the Primary Ion Characteristics in TOF-SIMS. J. Vac. Sci. Technol. B 2016, 34, 051084. [Google Scholar] [CrossRef]
- Keating, E.; Waring, A.J.; Walther, F.J.; Possmayer, F.; Veldhuizen, R.A.W.; Petersen, N.O. A ToF-SIMS Study of the Lateral Organization of Lipids and Proteins in Pulmonary Surfactant Systems. Biochim. Biophys. Acta (BBA)—Biomembr. 2011, 1808, 614–621. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.-W.; Chen, L.; Zhang, M.; Liu, Y.-X.; Zhang, B.-W.; Xu, R.; Miao, Y.-Y.; Xu, X.-M.; Hua, X.; et al. Mass Spectrometry Imaging-Based Single-Cell Lipidomics Profiles Metabolic Signatures of Heart Failure. Research 2023, 6, 0019. [Google Scholar] [CrossRef]
- Cappuccio, G.; Khalil, S.M.; Osenberg, S.; Li, F.; Maletic-Savatic, M. Mass Spectrometry Imaging as an Emerging Tool for Studying Metabolism in Human Brain Organoids. Front. Mol. Biosci. 2023, 10, 1181965. [Google Scholar] [CrossRef]
- Sjövall, P.; Skedung, L.; Gregoire, S.; Biganska, O.; Clément, F.; Luengo, G.S. Imaging the Distribution of Skin Lipids and Topically Applied Compounds in Human Skin Using Mass Spectrometry. Sci. Rep. 2018, 8, 16683. [Google Scholar] [CrossRef]
- Starr, N.J.; Khan, M.H.; Edney, M.K.; Trindade, G.F.; Kern, S.; Pirkl, A.; Kleine-Boymann, M.; Elms, C.; O’Mahony, M.M.; Bell, M.; et al. Elucidating the Molecular Landscape of the Stratum Corneum. Proc. Natl. Acad. Sci. USA 2022, 119, e2114380119. [Google Scholar] [CrossRef]
- Slijkhuis, N.; Towers, M.; Mirzaian, M.; Korteland, S.A.; Heijs, B.; van Gaalen, K.; Nieuwenhuizen, I.; Nigg, A.; van der Heiden, K.; de Rijke, Y.B.; et al. Identifying Lipid Traces of Atherogenic Mechanisms in Human Carotid Plaque. Atherosclerosis 2023, 385, 117340. [Google Scholar] [CrossRef] [PubMed]
- Benabdellah, F.; Seyer, A.; Quinton, L.; Touboul, D.; Brunelle, A.; Laprévote, O. Mass Spectrometry Imaging of Rat Brain Sections: Nanomolar Sensitivity with MALDI versus Nanometer Resolution by TOF-SIMS. Anal. Bioanal. Chem. 2010, 396, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.B.; Lu, J.G.; Jiang, Z.H.; Zhang, W.; Li, W.J.; Qian, Z.M.; Bai, L.P. In Situ Chemical Profiling and Imaging of Cultured and Natural Cordyceps Sinensis by TOF-SIMS. Front. Chem. 2022, 10, 862007. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zou, L.; Lin, Q.; Ong, C.N. Use of Liquid Chromatography/Tandem Mass Spectrometry and Online Databases for Identification of Phosphocholines and Lysophosphatidylcholines in Human Red Blood Cells. Rapid Commun. Mass Spectrom. 2009, 23, 3243–3254. [Google Scholar] [CrossRef]
- CHEBI:131441—Phosphatidylcholine 16:2. Available online: https://www.ebi.ac.uk/chebi/CHEBI:131441 (accessed on 29 October 2025).
- Konjevod, M.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Sáiz, J.; Tudor, L.; Uzun, S.; Kozumplik, O.; Svob Strac, D.; Zarkovic, N.; Pivac, N. Metabolomics in Posttraumatic Stress Disorder: Untargeted Metabolomic Analysis of Plasma Samples from Croatian War Veterans. Free Radic. Biol. Med. 2021, 162, 636–641. [Google Scholar] [CrossRef]
- Philipsen, M.H.; Phan, N.T.N.; Fletcher, J.S.; Malmberg, P.; Ewing, A.G. Mass Spectrometry Imaging Shows Cocaine and Methylphenidate Have Opposite Effects on Major Lipids in Drosophila Brain. ACS Chem. Neurosci. 2018, 9, 1462–1468. [Google Scholar] [CrossRef]
- Hsu, F.F.; Turk, J. Characterization of Phosphatidylinositol, Phosphatidylinositol-4-Phosphate, and Phosphatidylinositol-4,5-Bisphosphate by Electrospray Ionization Tandem Mass Spectrometry: A Mechanistic Study. J. Am. Soc. Mass Spectrom. 2000, 11, 986–999. [Google Scholar] [CrossRef]
- Saud, Z.; Tyrrell, V.J.; Zaragkoulias, A.; Protty, M.B.; Statkute, E.; Rubina, A.; Bentley, K.; White, D.A.; Dos Santos Rodrigues, P.; Murphy, R.C.; et al. The SARS-CoV2 Envelope Differs from Host Cells, Exposes Procoagulant Lipids, and Is Disrupted in Vivo by Oral Rinses. J. Lipid Res. 2022, 63, 100208. [Google Scholar] [CrossRef]
- Jackson, S.N.; Wang, H.Y.J.; Woods, A.S. In Situ Structural Characterization of Glycerophospholipids and Sulfatides in Brain Tissue Using MALDI-MS/MS. J. Am. Soc. Mass Spectrom. 2007, 18, 17–26. [Google Scholar] [CrossRef]
- Bakker, B.; Eijkel, G.B.; Heeren, R.M.A.; Karperien, M.; Post, J.N.; Cillero-Pastor, B. Oxygen-Dependent Lipid Profiles of Three-Dimensional Cultured Human Chondrocytes Revealed by MALDI-MSI. Anal. Chem. 2017, 89, 9438–9444. [Google Scholar] [CrossRef]
- Gopalan, A.B.; van Uden, L.; Sprenger, R.R.; Marx, N.F.N.; Bogetofte, H.; Neveu, P.A.; Meyer, M.; Noh, K.M.; Diz-Muñoz, A.; Ejsing, C.S. Lipotype Acquisition during Neural Development Is Not Recapitulated in Stem Cell–Derived Neurons. Life Sci. Alliance 2024, 7, e202402622. [Google Scholar] [CrossRef] [PubMed]
- Marlet, F.R.; Muñoz, S.S.; Sotiraki, N.; Eliasen, J.N.; Woessmann, J.; Weicher, J.; Dreier, J.E.; Schoof, E.M.; Kohlmeier, K.A.; Maeda, K.; et al. Lipid Levels Correlate with Neuronal and Dopaminergic Markers during the Differentiation of SH-SY5Y Cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2024, 1870, 167212. [Google Scholar] [CrossRef] [PubMed]
- Osetrova, M.; Tkachev, A.; Mair, W.; Guijarro Larraz, P.; Efimova, O.; Kurochkin, I.; Stekolshchikova, E.; Anikanov, N.; Foo, J.C.; Cazenave-Gassiot, A.; et al. Lipidome Atlas of the Adult Human Brain. Nat. Commun. 2024, 15, 4455. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.L.; Cho, K.J.; Chang, J.T.; Du, G.; Waxham, M.N.; Hancock, J.F.; Levental, I.; Levental, K.R. Lipidomic Atlas of Mammalian Cell Membranes Reveals Hierarchical Variation Induced by Culture Conditions, Subcellular Membranes, and Cell Lineages. Soft Matter 2021, 17, 288–297. [Google Scholar] [CrossRef]
- Fitzner, D.; Bader, J.M.; Penkert, H.; Bergner, C.G.; Su, M.; Weil, M.T.; Surma, M.A.; Mann, M.; Klose, C.; Simons, M. Cell-Type- and Brain-Region-Resolved Mouse Brain Lipidome. Cell Rep. 2020, 32, 108132. [Google Scholar] [CrossRef]
- Bhaduri, A.; Neumann, E.K.; Kriegstein, A.R.; Sweedler, J.V. Identification of Lipid Heterogeneity and Diversity in the Developing Human Brain. JACS Au 2021, 1, 2261–2270. [Google Scholar] [CrossRef]
- Guschina, I.; Millership, S.; O’Donnell, V.; Ninkina, N.; Harwood, J.; Buchman, V. Lipid Classes and Fatty Acid Patterns Are Altered in the Brain of γ-Synuclein Null Mutant Mice. Lipids 2010, 46, 121–130. [Google Scholar] [CrossRef]
- Berlin, E.; Lork, A.A.; Bornecrantz, M.; Ernst, C.; Phan, N.T.N. Lipid Organization and Turnover in the Plasma Membrane of Human Differentiating Neural Progenitor Cells Revealed by Time-of-Flight Secondary Ion Mass Spectrometry Imaging. Talanta 2024, 272, 125762. [Google Scholar] [CrossRef]
- Choi, J.; Yin, T.; Shinozaki, K.; Lampe, J.W.; Stevens, J.F.; Becker, L.B.; Kim, J. Comprehensive Analysis of Phospholipids in the Brain, Heart, Kidney, and Liver: Brain Phospholipids Are Least Enriched with Polyunsaturated Fatty Acids. Mol. Cell. Biochem. 2017, 442, 187–201. [Google Scholar] [CrossRef]
- Setou, M.; Kurabe, N. Mass Microscopy: High-Resolution Imaging Mass Spectrometry. J. Electron Microsc. 2011, 60, 47–56. [Google Scholar] [CrossRef]
- Kuge, H.; Akahori, K.; Yagyu, K.I.; Honke, K. Functional Compartmentalization of the Plasma Membrane of Neurons by a Unique Acyl Chain Composition of Phospholipids. J. Biol. Chem. 2014, 289, 26783–26793. [Google Scholar] [CrossRef] [PubMed]
- Guschina, I.A.; Ninkina, N.; Roman, A.; Pokrovskiy, M.V.; Buchman, V.L. Triple-Knockout, Synuclein-Free Mice Display Compromised Lipid Pattern. Molecules 2021, 26, 3078. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y. Novel Metabolism of Docosahexaenoic Acid in Neural Cells. J. Biol. Chem. 2007, 282, 18661–18665. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, I.; Chaudhary, M.N.; Ali, K.; Mushtaq, A.; Jiang, B.; Zheng, L.; Pan, Y.; Hu, J.; Zou, X. Phosphatidylserine: A Comprehensive Overview of Synthesis, Metabolism, and Nutrition. Chem. Phys. Lipids 2024, 264, 105422. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Wang, W.; Zhang, M.; Yang, B.; Miao, Z. Phosphatidylserine, Inflammation, and Central Nervous System Diseases. Front. Aging Neurosci. 2022, 14, 975176. [Google Scholar] [CrossRef]
- Dou, T.; Kurouski, D. Phosphatidylcholine and Phosphatidylserine Uniquely Modify the Secondary Structure of α-Synuclein Oligomers Formed in Their Presence at the Early Stages of Protein Aggregation. ACS Chem. Neurosci. 2022, 13, 2380–2385. [Google Scholar] [CrossRef]
- Ali, A.; Zhaliazka, K.; Dou, T.; Holman, A.P.; Kurouski, D. The Toxicities of A30P and A53T α-Synuclein Fibrils Can Be Uniquely Altered by the Length and Saturation of Fatty Acids in Phosphatidylserine. J. Biol. Chem. 2023, 299, 105383. [Google Scholar] [CrossRef]
- Custodia, A.; Aramburu-Núñez, M.; Correa-Paz, C.; Posado-Fernández, A.; Gómez-Larrauri, A.; Castillo, J.; Gómez-Muñoz, A.; Sobrino, T.; Ouro, A. Ceramide Metabolism and Parkinson’s Disease—Therapeutic Targets. Biomolecules 2021, 11, 945. [Google Scholar] [CrossRef]
- Abbott, S.K.; Li, H.; Muñoz, S.S.; Knoch, B.; Batterham, M.; Murphy, K.E.; Halliday, G.M.; Garner, B. Altered Ceramide Acyl Chain Length and Ceramide Synthase Gene Expression in Parkinson’s Disease. Mov. Disord. 2014, 29, 518–526. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Luo, X.; Gong, H.B.; Gao, H.Y.; Wu, Y.P.; Sun, W.Y.; Li, Z.Q.; Wang, G.; Liu, B.; Liang, L.; Kurihara, H.; et al. Oxygenated Phosphatidylethanolamine Navigates Phagocytosis of Ferroptotic Cells by Interacting with TLR2. Cell Death Differ. 2021, 28, 1971–1989. [Google Scholar] [CrossRef] [PubMed]
- St Germain, M.; Iraji, R.; Bakovic, M. Phosphatidylethanolamine Homeostasis under Conditions of Impaired CDP-Ethanolamine Pathway or Phosphatidylserine Decarboxylation. Front. Nutr. 2023, 9, 1094273. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, M.; Zetterberg, H.; Blennow, K.; Månsson, J.E. Sulfatide in Health and Disease. The Evaluation of Sulfatide in Cerebrospinal Fluid as a Possible Biomarker for Neurodegeneration. Mol. Cell. Neurosci. 2021, 116, 103670. [Google Scholar] [CrossRef]
- Takahashi, T.; Suzuki, T. Role of Sulfatide in Normal and Pathological Cells and Tissues. J. Lipid Res. 2012, 53, 1437–1450. [Google Scholar] [CrossRef]
- Berntson, Z.; Hansson, E.; Rönnbäck, L.; Fredman, P. Intracellular Sulfatide Expression in a Subpopulation of Astrocytes in Primary Cultures. J. Neurosci. Res. 1998, 52, 559–568. [Google Scholar] [CrossRef]
- Pernber, Z.; Molander-Melin, M.; Berthold, C.H.; Hansson, E.; Fredman, P. Expression of the Myelin and Oligodendrocyte Progenitor Marker Sulfatide in Neurons and Astrocytes of Adult Rat Brain. J. Neurosci. Res. 2002, 69, 86–93. [Google Scholar] [CrossRef]
- Cockcroft, S. Mammalian Lipids: Structure, Synthesis and Function. Essays Biochem. 2021, 65, 813–845. [Google Scholar] [CrossRef]
- Uchiyama, Y.; Maxson, M.M.; Sawada, T.; Nakano, A.; Ewing, A.G. Phospholipid Mediated Plasticity in Exocytosis Observed in PC12 Cells. Brain Res. 2007, 1151, 46–54. [Google Scholar] [CrossRef]
- Rosenberger, T.A.; Oki, J.; Purdon, A.D.; Rapoport, S.I.; Murphy, E.J. Rapid Synthesis and Turnover of Brain Microsomal Ether Phospholipids in the Adult Rat. J. Lipid Res. 2002, 43, 59–68. [Google Scholar] [CrossRef]
- Xu, Z.; Byers, D.M.; Palmer, F.B.S.C.; Spence, M.W.; Cook, H.W. Limited Metabolic Interaction of Serine with Ethanolamine and Choline in the Turnover of Phosphatidylserine, Phosphatidylethanolamine and Plasmalogens in Cultured Glioma Cells. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1993, 1168, 167–174. [Google Scholar] [CrossRef]
- van Echten-Deckert, G.; Herget, T. Sphingolipid Metabolism in Neural Cells. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 1978–1994. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Koshibu, K.; Rytz, A.; Giuffrida, F.; Sultan, S.; Patin, A.; Gaudin, M.; Tomezyk, A.; Steiner, P.; Schneider, N. Early Life to Adult Brain Lipidome Dynamic: A Temporospatial Study Investigating Dietary Polar Lipid Supplementation Efficacy. Front. Nutr. 2022, 9, 898655. [Google Scholar] [CrossRef]
- Malgat, M.; Maurice, A.; Baraud, J. Sphingomyelin and Ceramide-Phosphoethanolamine Synthesis by Microsomes and Plasma Membranes from Rat Liver and Brain. J. Lipid Res. 1987, 27, 251–260. [Google Scholar] [CrossRef]
- Michno, W.; Bowman, A.; Jha, D.; Minta, K.; Ge, J.; Koutarapu, S.; Zetterberg, H.; Blennow, K.; Lashley, T.; Heeren, R.M.A.; et al. Spatial Neurolipidomics at the Single Amyloid-β Plaque Level in Postmortem Human Alzheimer’s Disease Brain. ACS Chem. Neurosci. 2024, 15, 877–888. [Google Scholar] [CrossRef]
- Beger, A.W.; Dudzik, B.; Woltjer, R.L.; Wood, P.L. Human Brain Lipidomics: Pilot Analysis of the Basal Ganglia Sphingolipidome in Parkinson’s Disease and Lewy Body Disease. Metabolites 2022, 12, 187. [Google Scholar] [CrossRef]
- Térová, B.; Heczko, R.; Slotte, J.P. On the Importance of the Phosphocholine Methyl Groups for Sphingomyelin/Cholesterol Interactions in Membranes: A Study with Ceramide Phosphoethanolamine. Biophys. J. 2005, 88, 2661–2669. [Google Scholar] [CrossRef]
- Chen, T.C.; Hsu, W.L.; Wu, C.Y.; Lai, Y.R.; Chao, H.R.; Chen, C.H.; Tsai, M.H. Effect of Omega-6 Linoleic Acid on Neurobehavioral Development in Caenorhabditis Elegans. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102557. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M. Role of Docosahexaenoic Acid in the Modulation of Glial Cells in Alzheimer’s Disease. J. Neuroinflammation 2016, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; He, J.; Wang, L.; Tian, Z.; Wang, C. Protective Effects of Omega-3 Fatty Acids against Alzheimer’s Disease in Rat Brain Endothelial Cells. Brain Behav. 2018, 8, e01037. [Google Scholar] [CrossRef] [PubMed]
- Belkind-Gerson, J.; Carreón-Rodríguez, A.; Contreras-Ochoa, C.O.; Estrada-Mondaca, S.; Parra-Cabrera, M.S. Fatty Acids and Neurodevelopment. J. Pediatr. Gastroenterol. Nutr. 2008, 47, S7–S9. [Google Scholar] [CrossRef] [PubMed]
- Dec, K.; Alsaqati, M.; Morgan, J.; Deshpande, S.; Wood, J.; Hall, J.; Harwood, A.J. A High Ratio of Linoleic Acid (n-6 PUFA) to Alpha-Linolenic Acid (n-3 PUFA) Adversely Affects Early Stage of Human Neuronal Differentiation and Electrophysiological Activity of Glutamatergic Neurons in Vitro. Front. Cell Dev. Biol. 2023, 11, 1166808. [Google Scholar] [CrossRef]
- Sheppard, K.W.; Cheatham, C.L. Omega-6 to Omega-3 Fatty Acid Ratio and Higher-Order Cognitive Functions in 7- to 9-y-Olds: A Cross-Sectional Study. Am. J. Clin. Nutr. 2013, 98, 659–667. [Google Scholar] [CrossRef]
- Gu, C.; Philipsen, M.H.; Ewing, A.G. Omega-3 and -6 Fatty Acids Alter the Membrane Lipid Composition and Vesicle Size to Regulate Exocytosis and Storage of Catecholamines. ACS Chem. Neurosci. 2024, 15, 816–826. [Google Scholar] [CrossRef]
- Ordóñez-Gutiérrez, L.; Fábrias, G.; Casas, J.; Wandosell, F. Diets with Higher ω-6/ω-3 Ratios Show Differences in Ceramides and Fatty Acid Levels Accompanied by Increased Amyloid-Beta in the Brains of Male APP/PS1 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 10907. [Google Scholar] [CrossRef]
- Rapoport, S.I.; Chang, M.C.J.; Spector, A.A. Delivery and Turnover of Plasma-Derived Essential PUFAs in Mammalian Brain. J. Lipid Res. 2001, 42, 678–685. [Google Scholar] [CrossRef]
- Novak, E.M.; Dyer, R.A.; Innis, S.M. High Dietary ω-6 Fatty Acids Contribute to Reduced Docosahexaenoic Acid in the Developing Brain and Inhibit Secondary Neurite Growth. Brain Res. 2008, 1237, 136–145. [Google Scholar] [CrossRef]
- Levental, K.R.; Malmberg, E.; Symons, J.L.; Fan, Y.Y.; Chapkin, R.S.; Ernst, R.; Levental, I. Lipidomic and Biophysical Homeostasis of Mammalian Membranes Counteracts Dietary Lipid Perturbations to Maintain Cellular Fitness. Nat. Commun. 2020, 11, 1339. [Google Scholar] [CrossRef]
- Carson, R.H.; Lewis, C.R.; Erickson, M.N.; Zagieboylo, A.P.; Naylor, B.C.; Li, K.W.; Farnsworth, P.B.; Price, J.C. Imaging Regiospecific Lipid Turnover in Mouse Brain with Desorption Electrospray Ionization Mass Spectrometry. J. Lipid Res. 2017, 58, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, P.H.; Murphy, R.C. Quantitative Analysis of Phospholipids Containing Arachidonate and Docosahexaenoate Chains in Microdissected Regions of Mouse Brain. J. Lipid Res. 2010, 51, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Little, S.J.; Lynch, M.A.; Manku, M.; Nicolaou, A. Docosahexaenoic Acid-Induced Changes in Phospholipids in Cortex of Young and Aged Rats: A Lipidomic Analysis. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sakayori, N.; Katakura, M.; Hamazaki, K.; Higuchi, O.; Fujii, K.; Fukabori, R.; Iguchi, Y.; Setogawa, S.; Takao, K.; Miyazawa, T.; et al. Maternal Dietary Imbalance between Omega-6 and Omega-3 Fatty Acids Triggers the Offspring’s Overeating in Mice. Commun. Biol. 2020, 3, 473. [Google Scholar] [CrossRef]
- Yavin, E.; Menkes, J.H. Effect of Temperature on Fatty Acid Metabolism in Dissociated Cell Cultures of Developing Brain. Pediatr. Res. 1974, 8, 263–269. [Google Scholar] [CrossRef]
- Bosch-Bouju, C.; Layé, S. Dietary Omega-6/Omega-3 and Endocannabinoids: Implications for Brain Health and Diseases. In Cannabinoids Health Disease; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Borasio, F.; De Cosmi, V.; D’Oria, V.; Scaglioni, S.; Syren, M.L.E.; Turolo, S.; Agostoni, C.; Coniglio, M.; Molteni, M.; Antonietti, A.; et al. Associations between Dietary Intake, Blood Levels of Omega-3 and Omega-6 Fatty Acids and Reading Abilities in Children. Biomolecules 2023, 13, 368. [Google Scholar] [CrossRef]
- Zock, P.L.; Mensink, R.P.; Harryvan, J.; De Vries, J.H.M.; Katan, M.B. Fatty Acids in Serum Cholesteryl Esters as Quantitative Biomarkers of Dietary Intake in Humans. Am. J. Epidemiol. 1997, 145, 1114–1122. [Google Scholar] [CrossRef]
- Corrigan, F.M.; Horrobin, D.F.; Skinner, E.R.; Besson, J.A.O.; Cooper, M.B. Abnormal Content of N−6 and N−3 Long-Chain Unsaturated Fatty Acids in the Phosphoglycerides and Cholesterol Esters of Parahippocampal Cortex from Alzheimer’s Disease Patients and Its Relationship to Acetyl CoA Content. Int. J. Biochem. Cell Biol. 1998, 30, 197–207. [Google Scholar] [CrossRef]
- Wood, P.L.; Medicherla, S.; Sheikh, N.; Terry, B.; Phillipps, A.; Kaye, J.A.; Quinn, J.F.; Woltjer, R.L. Targeted Lipidomics of Fontal Cortex and Plasma Diacylglycerols (DAG) in Mild Cognitive Impairment (MCI) and Alzheimer’s Disease: Validation of DAG Accumulation Early in the Pathophysiology of Alzheimer’s Disease. J. Alzheimers’s Dis. 2015, 48, 537–546. [Google Scholar] [CrossRef]
- Strosznajder, J. Incorporation of Linoleic Acid into Membrane Glycerophospholipids from Rat Brain Submitted to Ischemia and Hypoxia. Neurochem. Res. 1980, 5, 1265–1277. [Google Scholar] [CrossRef]
- Almena, M.; Mérida, I. Shaping up the Membrane: Diacylglycerol Coordinates Spatial Orientation of Signaling. Trends Biochem. Sci. 2011, 36, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Hennessey, T.; Ahmed, Z. Manipulation of Plasma Membrane Fatty Acid Composition of Fetal Rat Brain Cells Grown in a Serum-Free Denned Medium. J. Neurochem. 1990, 55, 1537–1545. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio: Health Implications. Ol. Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Kang, J.X.; Wan, J.B.; He, C. Concise Review: Regulation of Stem Cell Proliferation and Differentiation by Essential Fatty Acids and Their Metabolites. Stem Cells 2014, 32, 1092–1098. [Google Scholar] [CrossRef]
- Connor, W.E.; Neuringer, M.; Lin, D.S. Dietary Effects on Brain Fatty Acid Composition: The Reversibility of n-3 Fatty Acid Deficiency and Turnover of Docosahexaenoic Acid in the Brain, Erythrocytes, and Plasma of Rhesus Monkeys. J. Lipid Res. 1990, 31, 237–247. [Google Scholar] [CrossRef]
- Kim, H.Y.; Akbar, M.; Kim, Y.S. Phosphatidylserine-Dependent Neuroprotective Signaling Promoted by Docosahexaenoic Acid. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 165–172. [Google Scholar] [CrossRef]
- Hamilton, J.; Greiner, R.; Salem, N.J.; Kim, H.Y. N-3 Fatty Acid Deficiency Decreases Phosphatidylserine Accumulation Selectively in Neuronal Tissues. Lipids 2000, 35, 863–869. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the Brain: Metabolism and Function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-Chain Omega-3 Fatty Acids and the Brain: A Review of the Independent and Shared Effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- DeMar, J.C.; Lee, H.J.; Ma, K.; Chang, L.; Bell, J.M.; Rapoport, S.I.; Bazinet, R.P. Brain Elongation of Linoleic Acid Is a Negligible Source of the Arachidonate in Brain Phospholipids of Adult Rats. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2006, 1761, 1050–1059. [Google Scholar] [CrossRef]
- Gimenez da Silva-Santi, L.; Masetto Antunes, M.; Mori, M.A.; Biesdorf de Almeida-Souza, C.; Vergílio Visentainer, J.; Carbonera, F.; Rabello Crisma, A.; Nunes Masi, L.; Massao Hirabara, S.; Curi, R.; et al. Brain Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2018, 10, 1277. [Google Scholar] [CrossRef]
- Taha, A.Y.; Cheon, Y.; Ma, K.; Rapoport, S.I.; Rao, J.S. Altered Fatty Acid Concentrations in Prefrontal Cortex of Schizophrenic Patients. J. Psychiatr. Res. 2013, 47, 636–643. [Google Scholar] [CrossRef]
- Taha, A.Y.; Basselin, M.; Ramadan, E.; Modi, H.R.; Rapoport, S.I.; Cheon, Y. Altered Lipid Concentrations of Liver, Heart and Plasma but Not Brain in HIV-1 Transgenic Rats. Prostaglandins Leukot. Essent. Fat. Acids 2012, 87, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gozlan-Devillierre, N.; Baumann, N.; Bourre, J.M. Incorporation of Stearic Acid Into Brain Lipids in the Developing Brain: Blood-Brain Relationships during Development. Developmental Neuroscience. Dev. Neurosci. 1978, 1, 153–158. [Google Scholar] [CrossRef]
- Gozlan-Devillierre, N.; Baumann, N.A.; Bourre, J.M. Mouse Brain Uptake and Metabolism of Stearic Acid. Biochimie 1976, 58, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Morand, O.; Baumann, N.; Bourre, J.M. In Vivo Incorporation of Exogenous [1-14C]Stearic Acid into Neurons and Astrocytes. Neurosci. Lett. 1979, 13, 177–181. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Camdzic, M.; Aga, D.S.; Atilla-Gokcumen, G.E. Cellular Lipidome Changes during Retinoic Acid (RA)-Induced Differentiation in SH-SY5Y Cells: A Comprehensive In Vitro Model for Assessing Neurotoxicity of Contaminants. Environ. Health 2023, 1, 110–120. [Google Scholar] [CrossRef]
- Martínez, M.; Mougan, I. Fatty Acid Composition of Human Brain Phospholipids during Normal Development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef]
- Knobloch, M.; Pilz, G.-A.; Ghesquière, B.; Kovacs, W.J.; Wegleiter, T.; Moore, D.L.; Hruzova, M.; Zamboni, N.; Carmeliet, P.; Jessberger, S. A Fatty Acid Oxidation-Dependent Metabolic Shift Regulates Adult Neural Stem Cell Activity. Cell Rep. 2017, 20, 2144–2155. [Google Scholar] [CrossRef]
- Wallis, T.P.; Venkatesh, B.G.; Narayana, V.K.; Kvaskoff, D.; Ho, A.; Sullivan, R.K.; Windels, F.; Sah, P.; Meunier, F.A. Saturated Free Fatty Acids and Association with Memory Formation. Nat. Commun. 2021, 12, 3443. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlin, E.; Lork, A.A.; Ernst, C.; Fletcher, J.S.; Phan, N.T.N. Plasma Membrane Lipid Composition and Turnover in Human Midbrain Neurons Investigated by Time-of-Flight Mass Spectrometry. Biomolecules 2025, 15, 1650. https://doi.org/10.3390/biom15121650
Berlin E, Lork AA, Ernst C, Fletcher JS, Phan NTN. Plasma Membrane Lipid Composition and Turnover in Human Midbrain Neurons Investigated by Time-of-Flight Mass Spectrometry. Biomolecules. 2025; 15(12):1650. https://doi.org/10.3390/biom15121650
Chicago/Turabian StyleBerlin, Emmanuel, Alicia A. Lork, Carl Ernst, John S. Fletcher, and Nhu T. N. Phan. 2025. "Plasma Membrane Lipid Composition and Turnover in Human Midbrain Neurons Investigated by Time-of-Flight Mass Spectrometry" Biomolecules 15, no. 12: 1650. https://doi.org/10.3390/biom15121650
APA StyleBerlin, E., Lork, A. A., Ernst, C., Fletcher, J. S., & Phan, N. T. N. (2025). Plasma Membrane Lipid Composition and Turnover in Human Midbrain Neurons Investigated by Time-of-Flight Mass Spectrometry. Biomolecules, 15(12), 1650. https://doi.org/10.3390/biom15121650







