Berberine Hydrochloride Reduces the Intracellular Survival of Salmonella Typhimurium by Enhancing Host Autophagic Flux Through the Inhibition of the Type III Secretion System
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Strains, Cells and Mice
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4. Growth Curve
2.5. Observation of Biofilm Formation
2.6. Swimming Motility Assay
2.7. Observation of Bacteria and Biofilms by Field Emission Scanning Electron Microscopy (FESEM)
2.8. Determination of Cell Membrane Integrity
2.9. Reverse Transcription Quantitative Real-Time PCR (RT-qPCR)
2.10. Determination of Cytotoxicity and Intracellular Bacterial Load
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Determination of Reactive Oxygen Species (ROS)-Related Indicators
2.13. CLSM for Autophagy Detection
2.14. Transmission Electron Microscopy (TEM) for Autophagosome Detection
2.15. Mouse Experiments
2.16. Identification of Targets for the Interaction Between 14028 Strain and BH
2.17. Bioinformatics Combined with Molecular Docking
2.18. Statistical Analysis
3. Results
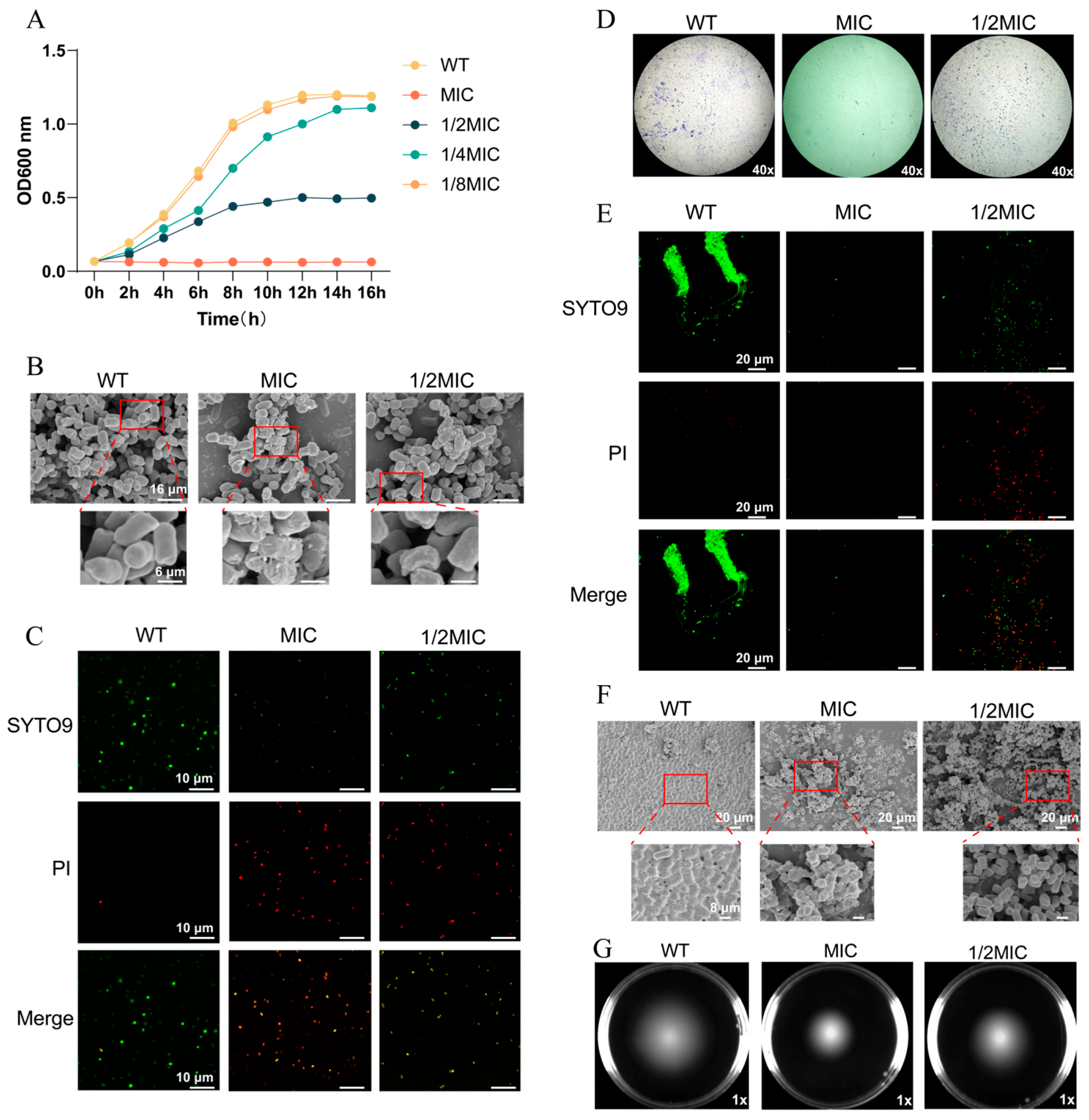
3.1. BH Inhibits the Survival of Salmonella Typhimurium
3.2. BH Inhibits the Expression of T3SS-Related Genes in 14028 Strain
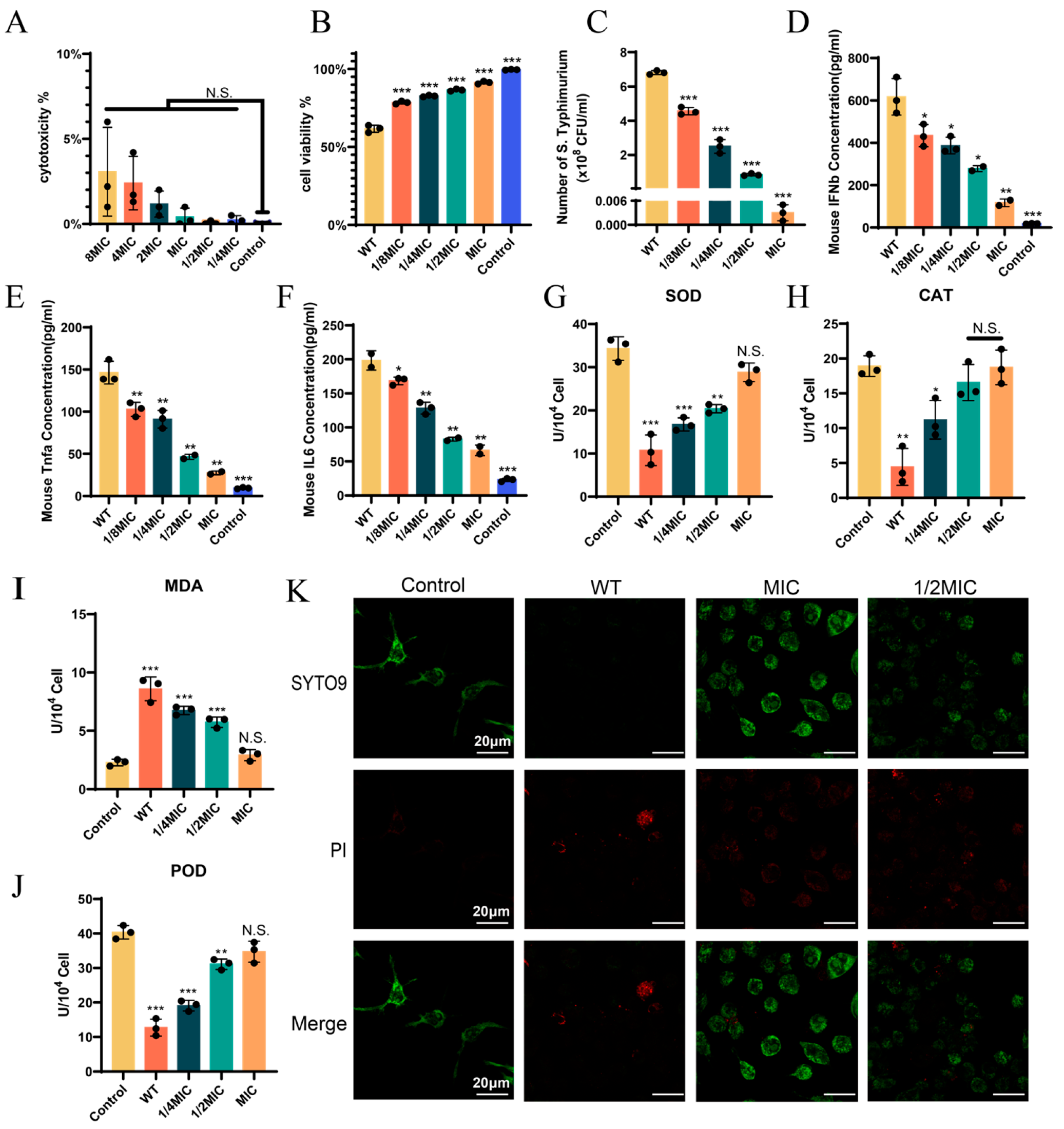
3.3. BH Treatment Can Enhance the Viability of 14028 Strain-Infected Cells
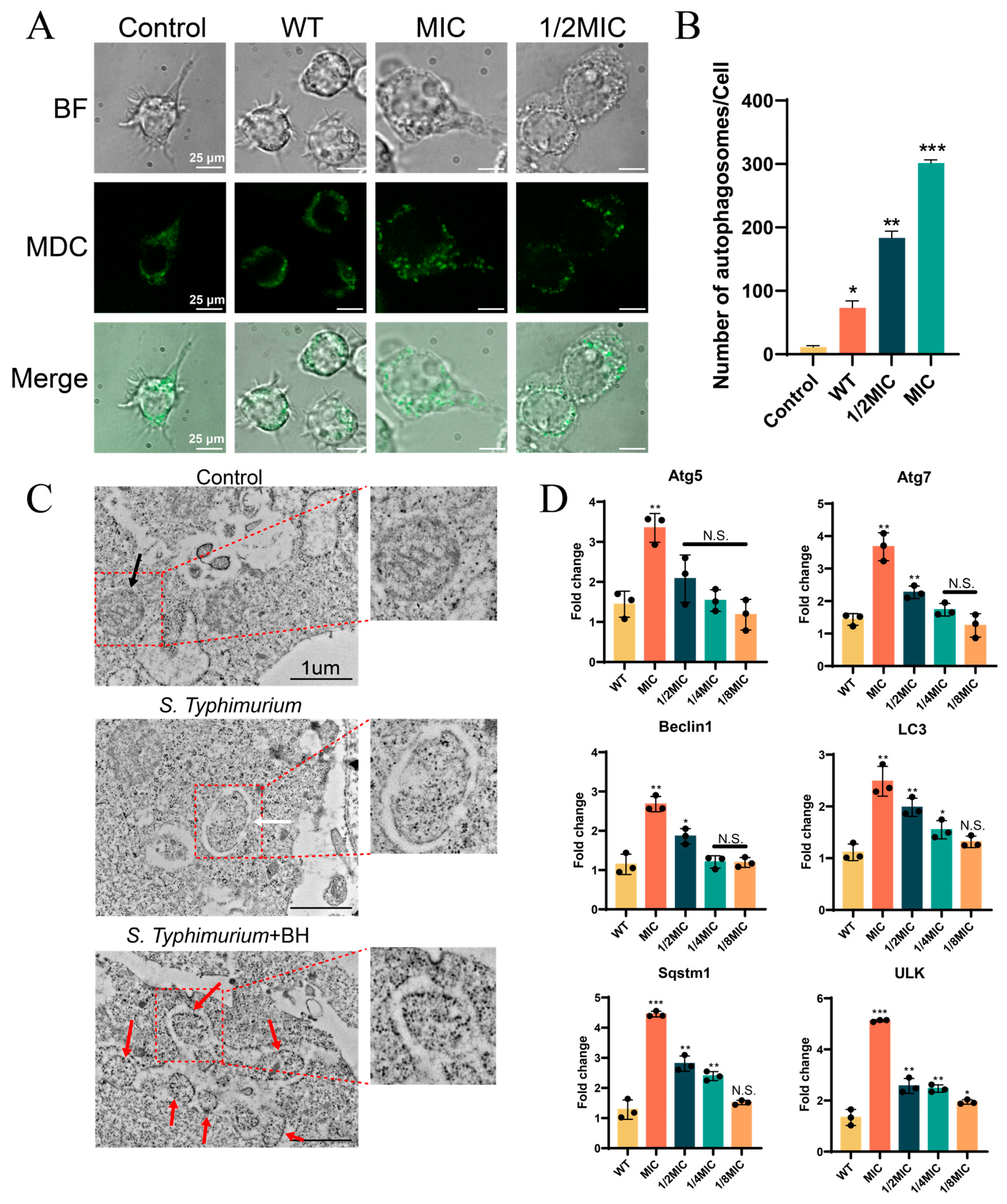
3.4. BH Enhances Autophagy in Macrophages via Inhibition of T3SS
3.5. BH Reduced S. Typhimurium-Induced Gastroenteritis Symptoms in Mice
3.6. Explore the Interacting Proteins of BH Using the GraphBAN Model
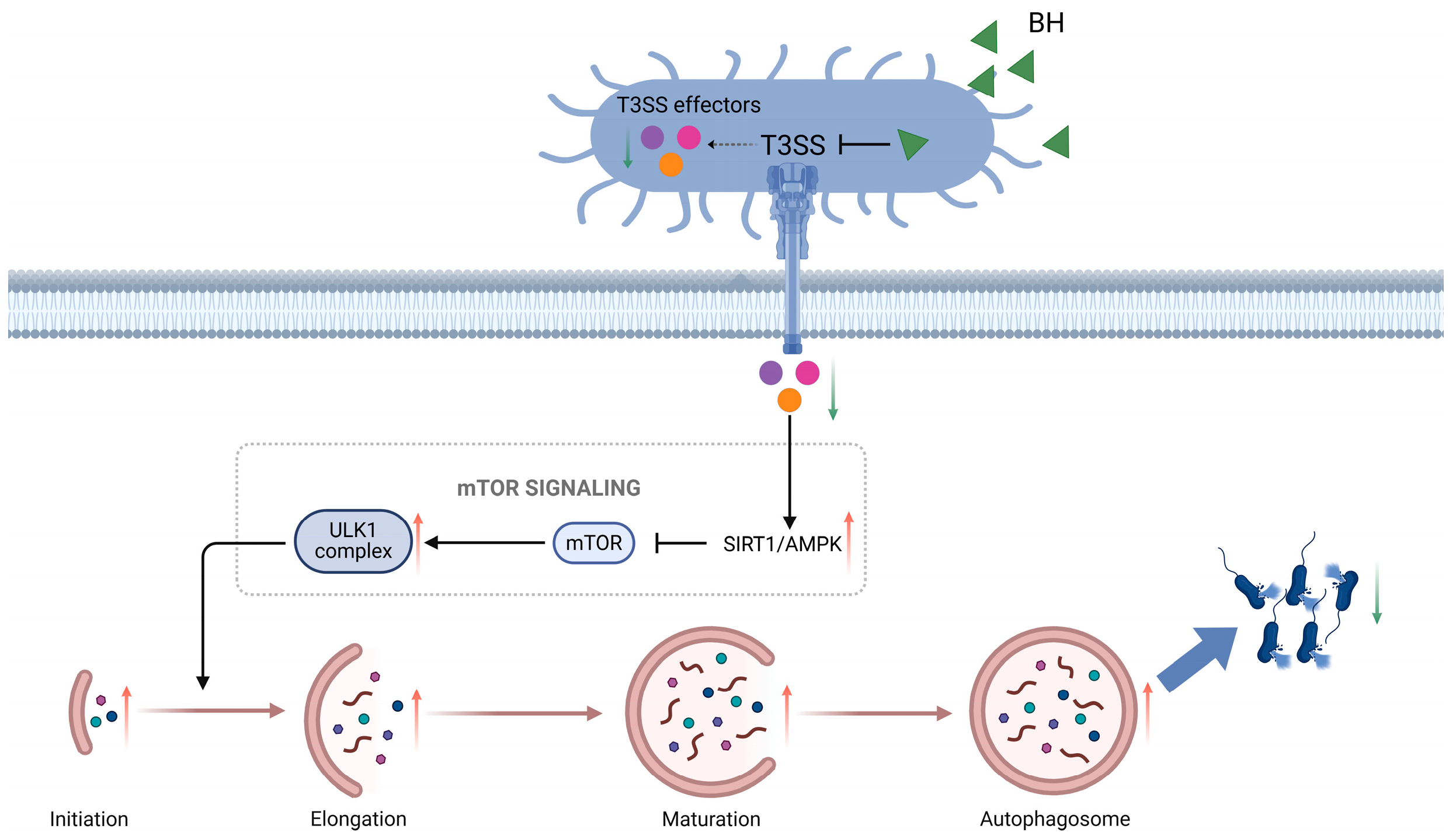
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BH | Berberine hydrochloride |
| T3SS | Type III secretion system |
| PI | Propidium iodide |
| FBS | Fetal bovine serum |
| SPF | Specific pathogen-free |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| CLSI | Clinical and Laboratory Standards Institute |
| CFU | Colony-forming unit |
| WT | Wild-type |
| FESEM | Field emission scanning electron microscopy |
| PBS | Phosphate-buffered saline |
| CLSM | Confocal laser scanning microscopy |
| RT-qPCR | Reverse transcription quantitative real-time PCR |
| ELISA | Enzyme-linked immunosorbent assay |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| MDC | Monodansylcadaverine |
| TEM | Transmission electron microscopy |
| PPI | Protein–protein interaction |
| T6SS | Type VI secretion system |
| SPI-1 | Salmonella Pathogenicity Island 1 |
| SPI-2 | Salmonella Pathogenicity Island 2 |
| IFN-β | Interferon-β |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| GO | Gene Ontology |
References
- Wotzka, S.Y.; Nguyen, B.D.; Hardt, W.-D. Salmonella Typhimurium Diarrhea Reveals Basic Principles of Enteropathogen Infection and Disease-Promoted DNA Exchange. Cell Host Microbe 2017, 21, 443–454. [Google Scholar] [CrossRef]
- Garai, P.; Gnanadhas, D.P.; Chakravortty, D. Salmonella Enterica Serovars Typhimurium and Typhi as Model Organisms: Revealing Paradigm of Host-Pathogen Interactions. Virulence 2012, 3, 377–388. [Google Scholar] [CrossRef]
- Galán, J.E. Salmonella Typhimurium and Inflammation: A Pathogen-Centric Affair. Nat. Rev. Microbiol. 2021, 19, 716–725. [Google Scholar] [CrossRef]
- Patel, S.; McCormick, B.A. Mucosal Inflammatory Response to Salmonella Typhimurium Infection. Front. Immunol. 2014, 5, 311. [Google Scholar] [CrossRef]
- Diepold, A.; Armitage, J.P. Type III Secretion Systems: The Bacterial Flagellum and the Injectisome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20150020. [Google Scholar] [CrossRef]
- Finn, C.E.; Chong, A.; Cooper, K.G.; Starr, T.; Steele-Mortimer, O. A Second Wave of Salmonella T3SS1 Activity Prolongs the Lifespan of Infected Epithelial Cells. PLoS Pathog. 2017, 13, e1006354. [Google Scholar] [CrossRef]
- Ganesan, R.; Hos, N.J.; Gutierrez, S.; Fischer, J.; Stepek, J.M.; Daglidu, E.; Krönke, M.; Robinson, N. Salmonella Typhimurium Disrupts Sirt1/AMPK Checkpoint Control of mTOR to Impair Autophagy. PLoS Pathog. 2017, 13, e1006227. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Z.; Yang, F.; Wang, Y.; Jiang, H.; Huang, X.; Zhang, Y. Salmonella Enterica Serovar Typhi Influences Inflammation and Autophagy in Macrophages. Braz. J. Microbiol. 2022, 53, 525–534. [Google Scholar] [CrossRef]
- Threlfall, E.J. Antimicrobial Drug Resistance in Salmonella: Problems and Perspectives in Food- and Water-Borne Infections. FEMS Microbiol. Rev. 2002, 26, 141–148. [Google Scholar] [CrossRef]
- Bhan, M.K.; Bahl, R.; Bhatnagar, S. Typhoid and Paratyphoid Fever. Lancet 2005, 366, 749–762. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Y.; Jiang, S.; Yu, X.; Zhu, H.; Xie, X.; Ning, X. Mechanisms of Action of Berberine Hydrochloride in Planktonic Cells and Biofilms of Pseudomonas Aeruginosa. Microb. Pathog. 2024, 193, 106774. [Google Scholar] [CrossRef]
- Shang, X.-F.; Yang, C.-J.; Morris-Natschke, S.L.; Li, J.-C.; Yin, X.-D.; Liu, Y.-Q.; Guo, X.; Peng, J.-W.; Goto, M.; Zhang, J.-Y.; et al. Biologically Active Isoquinoline Alkaloids Covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef]
- Xiao, Y.; Cui, Y.; Zhang, Y.; Fu, W.; Liu, Y.; Liu, F. Berberine Hydrochloride Enhances Innate Immunity to Protect against Pathogen Infection via P38 MAPK Pathway. Front. Immunol. 2025, 16, 1536143. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, M.; Yi, Y.; Patel, A.; Song, Z.; Li, Y. Inhibition of Berberine Hydrochloride on Candida Albicans Biofilm Formation. Biotechnol. Lett. 2020, 42, 2263–2269. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Gao, Z.; Zhang, Q.; Gu, C. The Mechanism of Berberine Alleviating Metabolic Disorder Based on Gut Microbiome. Front. Cell. Infect. Microbiol. 2022, 12, 854885. [Google Scholar] [CrossRef]
- Kong, Y.; Li, L.; Zhao, L.-G.; Yu, P.; Li, D.-D. A Patent Review of Berberine and Its Derivatives with Various Pharmacological Activities (2016-2020). Expert Opin. Ther. Pat. 2022, 32, 211–223. [Google Scholar] [CrossRef]
- Xi, R.; Abdulla, R.; Zhang, M.; Sherzod, Z.; Ivanovna, V.V.; Habasi, M.; Liu, Y. Pharmacokinetic Study and Metabolite Identification of 1-(3′-Bromophenyl)-Heliamine in Rats. Pharmaceuticals 2022, 15, 1483. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Pallathadka, H.; Gupta, J.; Ma, H.; Al-Shukri, H.H.K.; Kareem, A.K.; Zwamel, A.H.; Mustafa, Y.F. Berberine and Berberine Nanoformulations in Cancer Therapy: Focusing on Lung Cancer. Phytother. Res. 2024, 38, 4336–4350. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Inhibition of Biofilm Formation and Quorum Sensing Mediated Phenotypes by Berberine in Pseudomonas Aeruginosa and Salmonella Typhimurium. RSC Adv. 2018, 8, 36133–36141. [Google Scholar] [CrossRef]
- Xu, C.; Wang, F.; Huang, F.; Yang, M.; He, D.; Deng, L. Targeting Effect of Berberine on Type I Fimbriae of Salmonella Typhimurium and Its Effective Inhibition of Biofilm. Appl. Microbiol. Biotechnol. 2021, 105, 1563–1573. [Google Scholar] [CrossRef]
- Meng, J.; Wang, W.; Ding, J.; Gu, B.; Zhou, F.; Wu, D.; Fu, X.; Qiao, M.; Liu, J. The Synergy Effect of Matrine and Berberine Hydrochloride on Treating Colibacillosis Caused by an Avian Highly Pathogenic Multidrug-Resistant Escherichia coli. Poult. Sci. 2024, 103, 104151. [Google Scholar] [CrossRef]
- Tong, J.; Hou, X.; Cui, D.; Chen, W.; Yao, H.; Xiong, B.; Cai, L.; Zhang, H.; Jiang, L. A Berberine Hydrochloride-Carboxymethyl Chitosan Hydrogel Protects against Staphylococcus Aureus Infection in a Rat Mastitis Model. Carbohydr. Polym. 2022, 278, 118910. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Zhao, Y.; Xu, B.; Qin, W.; Yan, Y.; Yin, B.; Xi, C.; Ma, L. Anti-inflammatory Mechanism of Berberine on Lipopolysaccharide-induced IEC-18 Models Based on Comparative Transcriptomics. Mol. Med. Rep. 2020, 22, 5163–5180. [Google Scholar] [CrossRef]
- Galluzzi, L.; Pietrocola, F.; Levine, B.; Kroemer, G. Metabolic Control of Autophagy. Cell 2014, 159, 1263–1276. [Google Scholar] [CrossRef]
- Russell, R.C.; Yuan, H.-X.; Guan, K.-L. Autophagy Regulation by Nutrient Signaling. Cell Res. 2014, 24, 42–57. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A Nutrient and Energy Sensor That Maintains Energy Homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Price, N.L.; Gomes, A.P.; Ling, A.J.Y.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Zheng, Q.; Duan, L.; Zhang, Y.; Li, J.; Zhang, S.; Wang, H. A Dynamically Evolving War between Autophagy and Pathogenic Microorganisms. J. Zhejiang Univ. Sci. B 2022, 23, 19–41. [Google Scholar] [CrossRef]
- Casanova, J.E. Bacterial Autophagy: Offense and Defense at the Host-Pathogen Interface. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 237–243. [Google Scholar] [CrossRef]
- Khater, S.I.; Almanaa, T.N.; Fattah, D.M.A.; Khamis, T.; Seif, M.M.; Dahran, N.; Alqahtani, L.S.; Metwally, M.M.M.; Mostafa, M.; Albedair, R.A.; et al. Liposome-Encapsulated Berberine Alleviates Liver Injury in Type 2 Diabetes via Promoting AMPK/mTOR-Mediated Autophagy and Reducing ER Stress: Morphometric and Immunohistochemical Scoring. Antioxidants 2023, 12, 1220. [Google Scholar] [CrossRef]
- Park, G.-S.; Park, B.; Lee, M.-Y. Berberine Induces Autophagic Cell Death by Inactivating the Akt/mTOR Signaling Pathway. Planta Med. 2022, 88, 1116–1122. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A.; Barchiesi, F.; Cuenca-Estrella, M.; Pfaller, M.A.; Rinaldi, M.; Rodriguez-Tudela, J.L.; Verweij, P.E. International and Multicenter Comparison of EUCAST and CLSI M27-A2 Broth Microdilution Methods for Testing Susceptibilities of Candida Spp. to Fluconazole, Itraconazole, Posaconazole, and Voriconazole. J. Clin. Microbiol. 2005, 43, 3884–3889. [Google Scholar] [CrossRef]
- Pelletier, L.L.; Baker, C.B. Oxacillin, Cephalothin, and Vancomycin Tube Macrodilution MBC Result Reproducibility and Equivalence to MIC Results for Methicillin-Susceptible and Reputedly Tolerant Staphylococcus Aureus Isolates. Antimicrob. Agents Chemother. 1988, 32, 374–377. [Google Scholar] [CrossRef]
- Qiu, J.; Feng, H.; Xiang, H.; Wang, D.; Xia, L.; Jiang, Y.; Song, K.; Lu, J.; Yu, L.; Deng, X. Influence of Subinhibitory Concentrations of Licochalcone A on the Secretion of Enterotoxins A and B by Staphylococcus Aureus. FEMS Microbiol. Lett. 2010, 307, 135–141. [Google Scholar] [CrossRef]
- Gomes, L.C.; Moreira, J.M.R.; Simões, M.; Melo, L.F.; Mergulhão, F.J. Biofilm Localization in the Vertical Wall of Shaking 96-Well Plates. Scientifica 2014, 2014, 231083. [Google Scholar] [CrossRef]
- Djordjevic, D.; Wiedmann, M.; McLandsborough, L.A. Microtiter Plate Assay for Assessment of Listeria Monocytogenes Biofilm Formation. Appl. Environ. Microbiol. 2002, 68, 2950–2958. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin Inhibits Salmonella Virulence Factors and Has Anti-Quorum-Sensing Potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- Lim, S.; Choi, J.; Kim, M.; Yoon, H. Temporal Regulation of Salmonella Pathogenicity Island 1 (SPI-1) hilA by Hfq in Salmonella Enterica Serovar Typhimurium. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 169–172. [Google Scholar] [CrossRef]
- Kreitlow, A.; Becker, A.; Schotte, U.; Malorny, B.; Plötz, M.; Abdulmawjood, A. Evaluation of Different Target Genes for the Detection of Salmonella Sp. by Loop-Mediated Isothermal Amplification. Lett. Appl. Microbiol. 2021, 72, 420–426. [Google Scholar] [CrossRef]
- Eskra, L.; Mathison, A.; Splitter, G. Microarray Analysis of mRNA Levels from RAW264.7 Macrophages Infected with Brucella Abortus. Infect. Immun. 2003, 71, 1125–1133. [Google Scholar] [CrossRef]
- Peng, H.; Endo, Y.; Wu, W.J. Define Critical Parameters of Trastuzumab-Mediated ADCC Assays via Assay Optimization Processes, Focusing on the Impact of Cryopreserved Effector Cells on Assay Performance. Cancers 2024, 16, 2367. [Google Scholar] [CrossRef]
- Li, Z.; Wu, Q.; Liu, L.; Sun, S.; Sun, S.; Wang, Z.; Li, J. Determination of Mitophagy by Electron Microscope. Methods Cell Biol. 2021, 165, 103–110. [Google Scholar] [CrossRef]
- Wang, X.; Feng, S.; Ding, N.; He, Y.; Li, C.; Li, M.; Ding, X.; Ding, H.; Li, J.; Wu, J.; et al. Anti-Inflammatory Effects of Berberine Hydrochloride in an LPS-Induced Murine Model of Mastitis. Evid. Based Complement. Altern. Med. 2018, 2018, 5164314. [Google Scholar] [CrossRef]
- Xu, L.; Li, M.; Yang, Y.; Zhang, C.; Xie, Z.; Tang, J.; Shi, Z.; Chen, S.; Li, G.; Gu, Y.; et al. Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release. mBio 2022, 13, e0363221. [Google Scholar] [CrossRef]
- Hadipour, H.; Li, Y.Y.; Sun, Y.; Deng, C.; Lac, L.; Davis, R.; Cardona, S.T.; Hu, P. GraphBAN: An Inductive Graph-Based Approach for Enhanced Prediction of Compound-Protein Interactions. Nat. Commun. 2025, 16, 2541. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhou, Y.; Wang, T.; Deng, X.; Chu, X.; Zhou, T. Cinnamaldehyde Inhibits Type Three Secretion System in Salmonella Enterica Serovar Typhimurium by Affecting the Expression of Key Effector Proteins. Vet. Microbiol. 2019, 239, 108463. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chu, X.-L.; Su, J.-Q.; Cui, Z.-Q.; Zhang, L.-Y.; Yu, Z.-J.; Wu, Z.-M.; Cai, M.-L.; Li, H.-X.; Zhang, Z.-J. Baicalin Protects Mice against Salmonella Typhimurium Infection via the Modulation of Both Bacterial Virulence and Host Response. Phytomedicine 2018, 48, 21–31. [Google Scholar] [CrossRef]
- Ibarra, J.A.; Steele-Mortimer, O. Salmonella—the Ultimate Insider. Salmonella Virulence Factors That Modulate Intracellular Survival. Cell Microbiol. 2009, 11, 1579–1586. [Google Scholar] [CrossRef]
- Sana, T.G.; Flaugnatti, N.; Lugo, K.A.; Lam, L.H.; Jacobson, A.; Baylot, V.; Durand, E.; Journet, L.; Cascales, E.; Monack, D.M. Salmonella Typhimurium Utilizes a T6SS-Mediated Antibacterial Weapon to Establish in the Host Gut. Proc. Natl. Acad. Sci. USA 2016, 113, E5044–E5051. [Google Scholar] [CrossRef]
- Troxell, B. A Type 6 Secretion System (T6SS) Encoded Gene within Salmonella Enterica Serovar Enteritidis Contributes to Virulence. Virulence 2018, 9, 585–587. [Google Scholar] [CrossRef]
- Hensel, M. Salmonella Pathogenicity Island 2. Mol. Microbiol. 2000, 36, 1015–1023. [Google Scholar] [CrossRef]
- Lee, C.A.; Jones, B.D.; Falkow, S. Identification of a Salmonella Typhimurium Invasion Locus by Selection for Hyperinvasive Mutants. Proc. Natl. Acad. Sci. USA 1992, 89, 1847–1851. [Google Scholar] [CrossRef]
- Sheng, L.; Sun, X.; Mo, C.; Hao, M.; Wei, X.; Ma, A. Relationship between Antioxidant Enzymes and Sclerotial Formation of Pleurotus Tuber-Regium under Abiotic Stress. Appl. Microbiol. Biotechnol. 2023, 107, 1391–1404. [Google Scholar] [CrossRef]
- Brkljača Bottegaro, N.; Gotić, J.; Šuran, J.; Brozić, D.; Klobučar, K.; Bojanić, K.; Vrbanac, Z. Effect of Prolonged Submaximal Exercise on Serum Oxidative Stress Biomarkers (d-ROMs, MDA, BAP) and Oxidative Stress Index in Endurance Horses. BMC Vet. Res. 2018, 14, 216. [Google Scholar] [CrossRef]
- Magariños, M.; Pulido, S.; Aburto, M.R.; de Iriarte Rodríguez, R.; Varela-Nieto, I. Autophagy in the Vertebrate Inner Ear. Front. Cell Dev. Biol. 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Yoshimori, T. How to Interpret LC3 Immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Castro-Gonzalez, S.; Shi, Y.; Colomer-Lluch, M.; Song, Y.; Mowery, K.; Almodovar, S.; Bansal, A.; Kirchhoff, F.; Sparrer, K.; Liang, C.; et al. HIV-1 Nef Counteracts Autophagy Restriction by Enhancing the Association between BECN1 and Its Inhibitor BCL2 in a PRKN-Dependent Manner. Autophagy 2021, 17, 553–577. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, L.; Xu, J.; Liu, S.; Song, Z.; Chen, T.; Deng, X.; Wang, J.; Lv, Q. Quercitrin Is a Novel Inhibitor of Salmonella Enterica Serovar Typhimurium Type III Secretion System. Molecules 2023, 28, 5455. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, G.; Li, C.; Li, Z.; Lu, C.; Shen, Y. Structural Optimization of Natural Product Fusaric Acid to Discover Novel T3SS Inhibitors of Salmonella. Biochem. Biophys. Res. Commun. 2021, 582, 72–76. [Google Scholar] [CrossRef]
- Zhao, Z.; Fux, B.; Goodwin, M.; Dunay, I.R.; Strong, D.; Miller, B.C.; Cadwell, K.; Delgado, M.A.; Ponpuak, M.; Green, K.G.; et al. Autophagosome-Independent Essential Function for the Autophagy Protein Atg5 in Cellular Immunity to Intracellular Pathogens. Cell Host Microbe 2008, 4, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lu, Y.; Zhao, L.; Hu, L.; Qiu, Q.; Zhang, Z.; Li, M.; Hong, G.; Wu, B.; Zhao, G.; et al. Curcumin Attenuates Sepsis-Induced Acute Organ Dysfunction by Preventing Inflammation and Enhancing the Suppressive Function of Tregs. Int. Immunopharmacol. 2018, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hussein, Y.; Loutfy, S.A.; Kamoun, E.A.; El-Moslamy, S.H.; Radwan, E.M.; Elbehairi, S.E.I. Enhanced Anti-Cancer Activity by Localized Delivery of Curcumin Form PVA/CNCs Hydrogel Membranes: Preparation and in Vitro Bioevaluation. Int. J. Biol. Macromol. 2021, 170, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Dereli Can, G.; Taner, G.; İnci Aydemir, Ç. Evaluation of the Cytotoxic and Genotoxic/Antigenotoxic Effects of Resveratrol in Human Limbal Explant Cultures. Int. Ophthalmol. 2023, 43, 1977–1985. [Google Scholar] [CrossRef]
- Roque-Borda, C.A.; Chávez-Morán, M.R.; Primo, L.M.D.G.; Márquez Montesinos, J.C.E.; Borges Cardoso, V.M.; Saraiva, M.M.S.; Marcos, C.M.; Chorilli, M.; Albericio, F.; de la Torre, B.G.; et al. Alginate-Pectin Microparticles Embedding Self-Assembling Antimicrobial Peptides and Resveratrol for Antimicrobial and Anti-Inflammatory Applications. Food Hydrocoll. 2025, 167, 111454. [Google Scholar] [CrossRef]
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J.; et al. Anti-SARS-CoV-2 Activity of Andrographis Paniculata Extract and Its Major Component Andrographolide in Human Lung Epithelial Cells and Cytotoxicity Evaluation in Major Organ Cell Representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Wen, L.; Xia, N.; Chen, X.; Li, Y.; Hong, Y.; Liu, Y.; Wang, Z.; Liu, Y. Activity of Antibacterial, Antiviral, Anti-Inflammatory in Compounds Andrographolide Salt. Eur. J. Pharmacol. 2014, 740, 421–427. [Google Scholar] [CrossRef]
- Hurley, J.C. Antibiotic-Induced Release of Endotoxin: A Reappraisal. Clin. Infect. Dis. 1992, 15, 840–854. [Google Scholar] [CrossRef]
- Mulder, D.T.; Cooper, C.A.; Coombes, B.K. Type VI Secretion System-Associated Gene Clusters Contribute to Pathogenesis of Salmonella Enterica Serovar Typhimurium. Infect. Immun. 2012, 80, 1996–2007. [Google Scholar] [CrossRef]
- Howard, S.; Furniss, C.; Bonini, D.; Amin, H.; Paracuellos-Torrecilla, P.; Costa, T.; Mavridou, D.; Filloux, A. The Type VI Secretion System of Pseudomonas Aeruginosa: A Gun Loaded with Antimicrobial Bullets. Access Microbiol. 2020, 2, 725. [Google Scholar] [CrossRef]
- Mori, N.; Oda, K.; Takakura, H.; Tanaka, Y.; Yokooji, T.; Murakami, T. Comparison of Berberine Bioavailability between Oral and Rectal Administrations in Rats. Biol. Pharm. Bull. 2023, 46, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.; Le, K.N.M.; Nguyen, M.C.N. Spray-Dried Solid Lipid Nanoparticles for Enhancing Berberine Bioavailability via Oral Administration. Curr. Pharm. Des. 2023, 29, 3050–3059. [Google Scholar] [CrossRef]
- Gries, R.; Sala, C.; Rybniker, J. Host-Directed Therapies and Anti-Virulence Compounds to Address Anti-Microbial Resistant Tuberculosis Infection. Appl. Sci. 2020, 10, 2688. [Google Scholar] [CrossRef]



| Primers (For qPCR Only) | Sequence (5′-3′) | Size (bp) |
|---|---|---|
| RpoD-F | GTGAAATGGGCACTGTTGAACTG | 23 |
| RpoD-R | TTCCAGCAGATAGGTAATGGCTTC | 24 |
| SipA-F | TGCAAGCCATCAACGGTAGT | 20 |
| SipA-R | ATTGCACTGCAGTTTGCCAG | 20 |
| SipB-F | GCCGTTTTCTTATCGACGCC | 20 |
| SipB-R | CGTTGTGGCCGCTGTTTTTA | 20 |
| SipD-F | GCCAGGCTTGATATTTGGCG | 20 |
| SipD-R | ACCGTTGATCTGACGCCATT | 20 |
| SseA-F | ACCAAATCCGGGCTAAGGTG | 20 |
| SseA-R | CCGGGGCTTGAGCATTAAGT | 20 |
| SseBa-F | CAGCAAAATCCGTTTGCCGA | 20 |
| SseBa-R | CTCAGGCACCTCCTCTTTGG | 20 |
| SseD-F | TGTTGTCGGGTGTACTGACG | 20 |
| SseD-R | ATTGGGCCCCATTTTGTTGC | 20 |
| IcmF-F | GCTGGCGTAAAATCTTCGAG | 20 |
| IcmF-R | GGTAAACCACCAGTCGCAGT | 20 |
| VgrG-F | TGGCGGTAAACGACATATC | 19 |
| VgrG-R | TATTCCGCCAGAACCTCATC | 20 |
| ClpV-F | CCAGCGCCATTAGTGATTTTTC | 22 |
| ClpV-R | CGATCAACGAGGGCAGTATTTC | 22 |
| HliA-F | GGGCAGATGATACCCGATGG | 20 |
| HliA-R | AAGAGAGAAGCGGGTTGGTG | 20 |
| HilC-F | GGACTTGTTGCCAGGGATGA | 20 |
| HilC-R | GCGGGTGAGATCGCTGATAA | 20 |
| SlyA-F | AAGCCTCTGGAATTGACGCA | 20 |
| SlyA-R | GCAGGTTTGCCGCGAAATTA | 20 |
| FliZ-F | ACGCCTTGGCAATTACCTCA | 20 |
| FliZ-R | CTGGCGGTAAAGGGGGATTT | 20 |
| SsrB-F | ACGCTGACACGACCAATCAT | 20 |
| SsrB-R | CCTCATTCTTCGGGCACAGT | 20 |
| SsrA-F | TCCGATGAATGGCGTACTCG | 20 |
| SsrA-R | ATTGCCTGGTCCAGTAACGG | 20 |
| Atg5-F | AGCCAGGTGATGATTCACGG | 20 |
| Atg5-R | CTGGGTAGCTCAGATGCTCG | 20 |
| Lc3-F | TGACCCAGCTTAAGCGACTG | 20 |
| Lc3-R | AACCACATCCTAAGGCCAGC | 20 |
| Atg7-F | AGTGTTCAAGTGGCACACCA | 20 |
| Atg7-R | GCTTCTCCCATCCCTGGAAC | 20 |
| Beclin1-F | ACCCCATCCCTCTAGGTCAC | 20 |
| Beclin1-R | CTCCCCTCCCTAAGCTCCAT | 20 |
| P62-F | GACTGGCATTGAGGGACACA | 20 |
| P62-R | CCTTGCAACTGCACAACCTC | 20 |
| Ulk1-F | GAGACCGTTGCTGACTCCAA | 20 |
| Ulk1-R | TCCTAGAGAGAACAGGGGGC | 20 |
| Atg14-F | GCTGGAGTCTGTTCTGTGCT | 20 |
| Atg14-R | TTGCTGTAGGCGGTAGTTGG | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Lu, J.; Wu, C.; Chen, S.; Chang, T.; Xu, L.; Shen, X.; Bakhsh, Q.; Qin, B.; Qian, W.; et al. Berberine Hydrochloride Reduces the Intracellular Survival of Salmonella Typhimurium by Enhancing Host Autophagic Flux Through the Inhibition of the Type III Secretion System. Biomolecules 2025, 15, 1589. https://doi.org/10.3390/biom15111589
Huang J, Lu J, Wu C, Chen S, Chang T, Xu L, Shen X, Bakhsh Q, Qin B, Qian W, et al. Berberine Hydrochloride Reduces the Intracellular Survival of Salmonella Typhimurium by Enhancing Host Autophagic Flux Through the Inhibition of the Type III Secretion System. Biomolecules. 2025; 15(11):1589. https://doi.org/10.3390/biom15111589
Chicago/Turabian StyleHuang, Jianan, Jiaxing Lu, Conghui Wu, Sidi Chen, Tianyuan Chang, Lei Xu, Xihui Shen, Qadir Bakhsh, Baofu Qin, Weidong Qian, and et al. 2025. "Berberine Hydrochloride Reduces the Intracellular Survival of Salmonella Typhimurium by Enhancing Host Autophagic Flux Through the Inhibition of the Type III Secretion System" Biomolecules 15, no. 11: 1589. https://doi.org/10.3390/biom15111589
APA StyleHuang, J., Lu, J., Wu, C., Chen, S., Chang, T., Xu, L., Shen, X., Bakhsh, Q., Qin, B., Qian, W., & Wang, Y. (2025). Berberine Hydrochloride Reduces the Intracellular Survival of Salmonella Typhimurium by Enhancing Host Autophagic Flux Through the Inhibition of the Type III Secretion System. Biomolecules, 15(11), 1589. https://doi.org/10.3390/biom15111589








