From Lab to Field: Context-Dependent Impacts of Pseudomonas-Produced 2,4-Diacetylphloroglucinol on Soil Microbial Ecology
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sample Preparation and Experimental Design
- Control: Soil amended with a 0.01% (v/v) aqueous solution of dimethyl sulfoxide (DMSO).
- D-1: Soil amended with 2,4-DAPG at a concentration of 1 mg kg−1 soil.
- D-10: Soil amended with 2,4-DAPG at a concentration of 10 mg kg−1 soil.
2.2. Chemical Analysis
2.3. Determination of Soil Microbiological Properties
2.4. Soil Enzyme Activities
2.5. Metabarcoding Sample Preparation and Sequencing
2.6. Sequencing Data Processing and Analysis
2.7. Statistical Processing
3. Results
3.1. Soil Organic Carbon
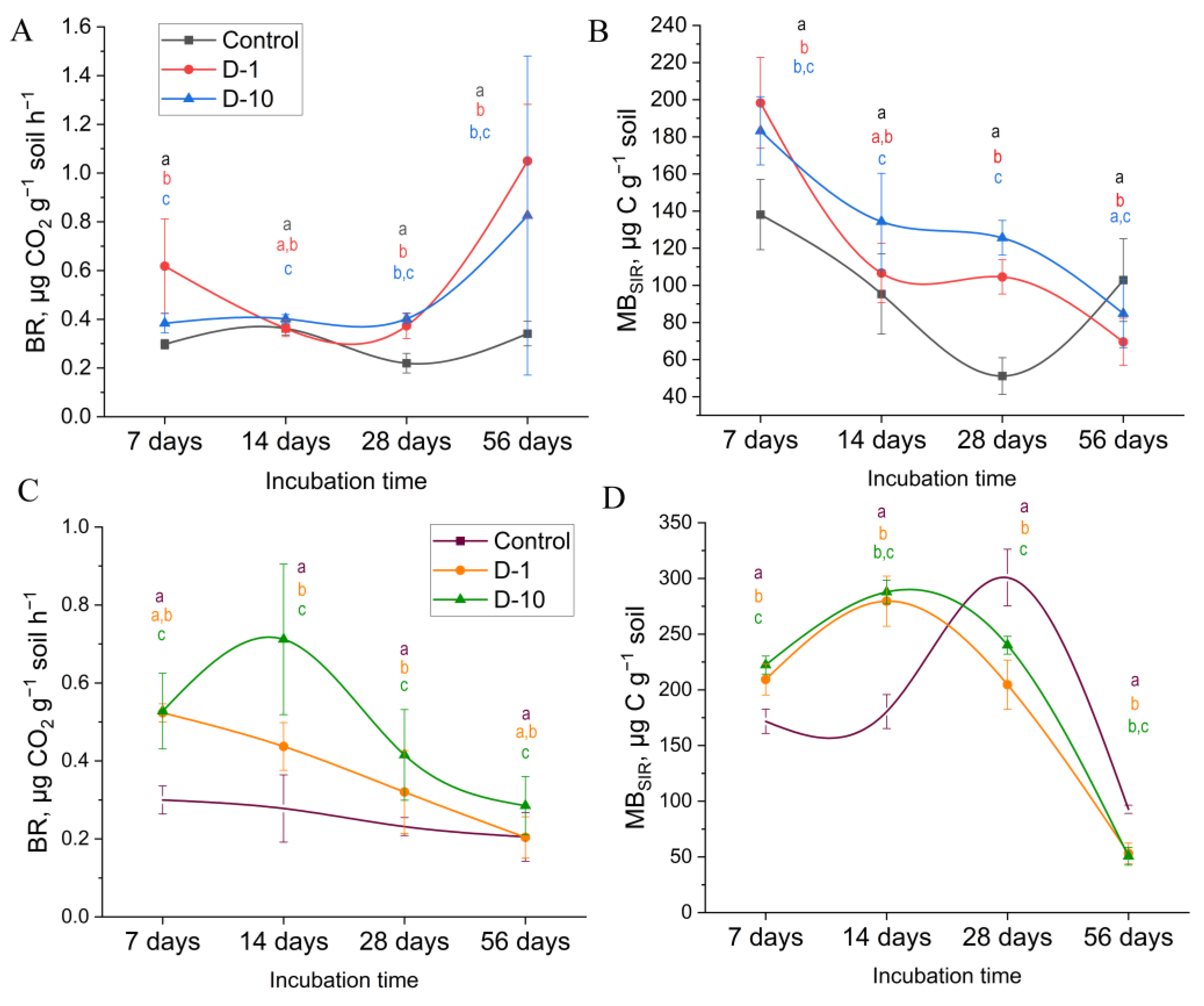
3.2. Microbiological Activity
3.2.1. Controlled Laboratory Experiments
3.2.2. Field Experiment
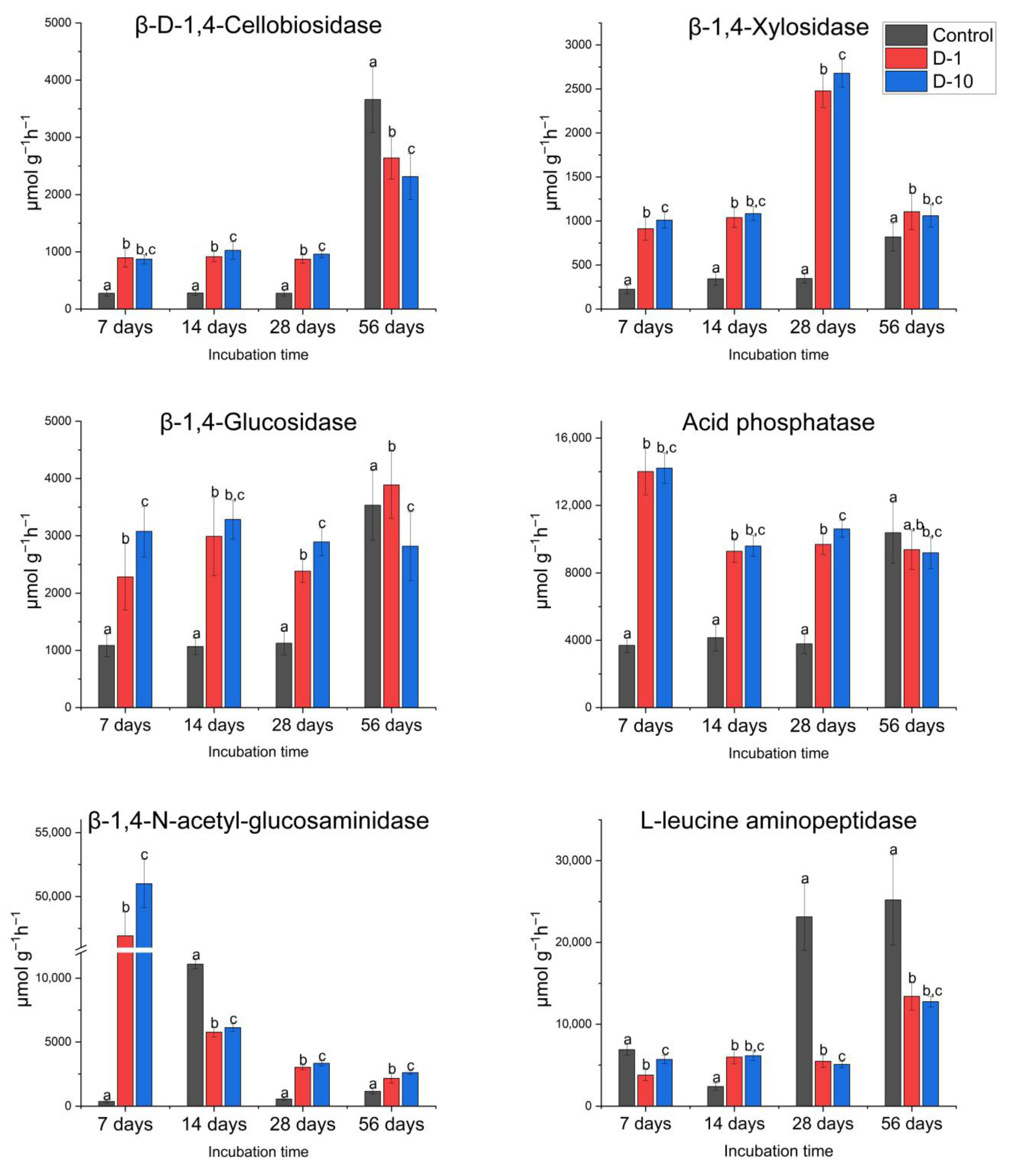
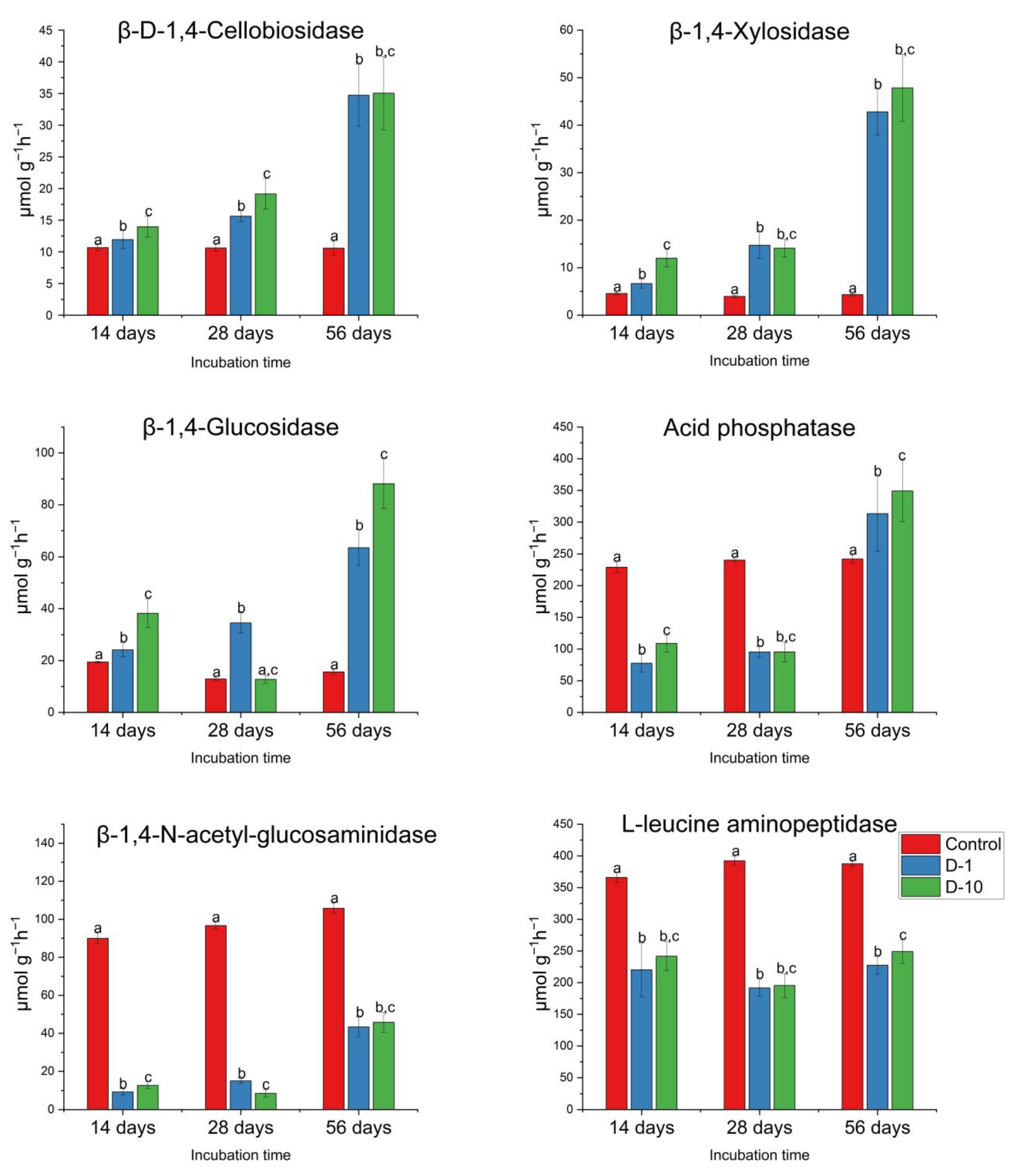
3.3. The Effects of 2,4-DAPG on Soil Hydrolytic Enzyme Activities
3.3.1. Controlled Laboratory Experiments
3.3.2. Field Experiment
3.4. Environmental Modulation of Microbial Community Function
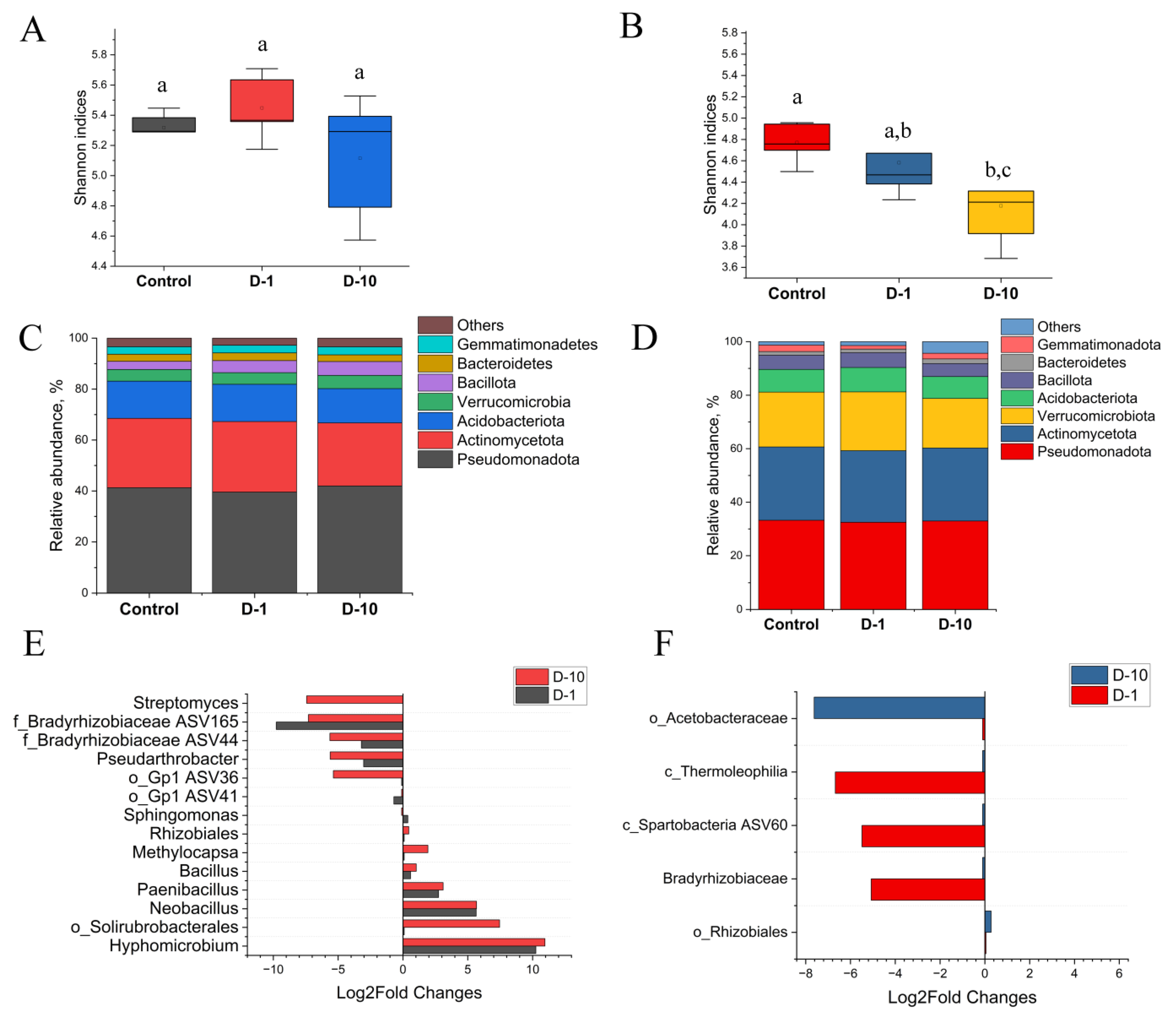
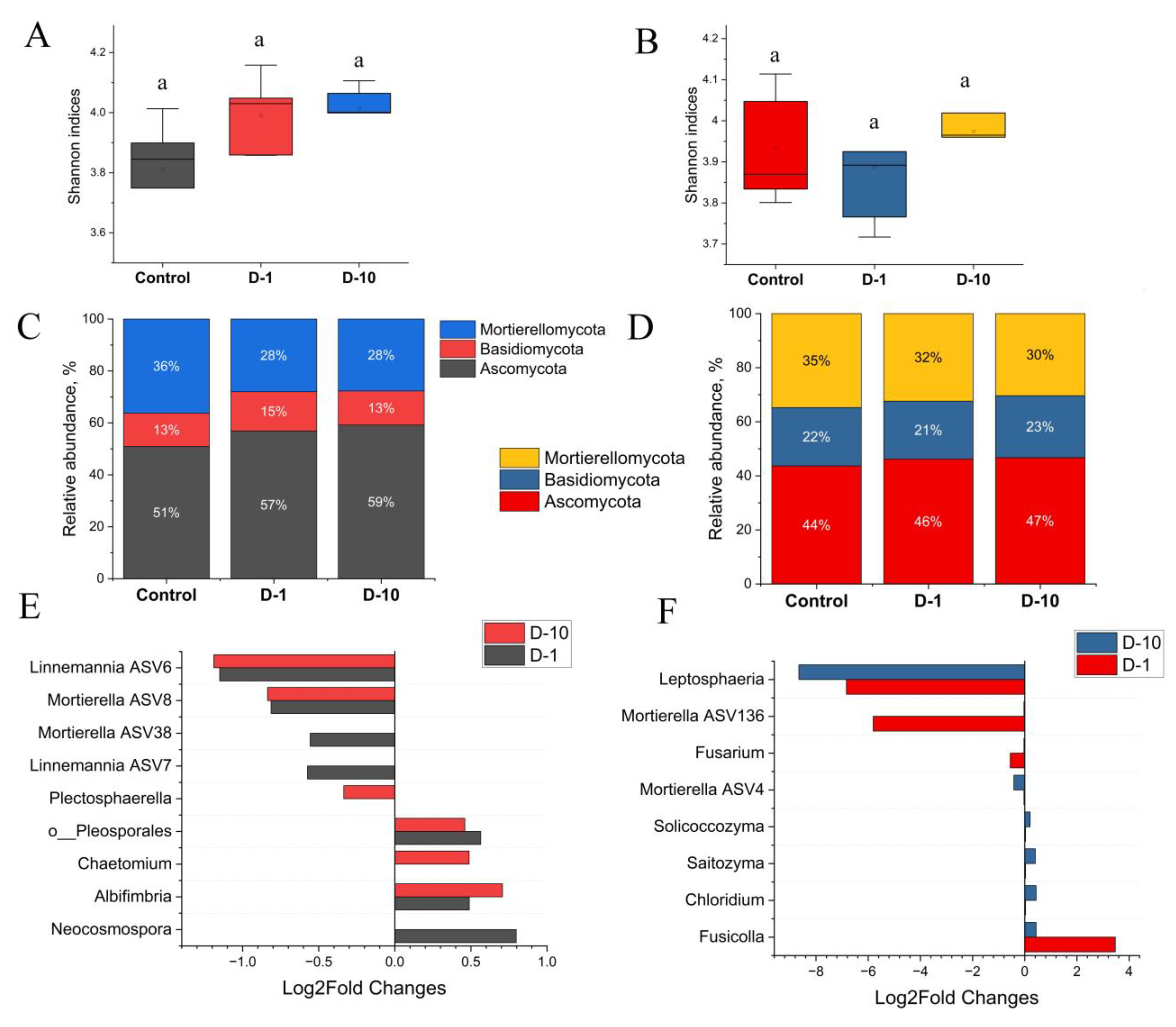
3.5. Diversity and Composition of Bacterial Soil Community
3.6. Diversity and Composition of Fungal Soil Community
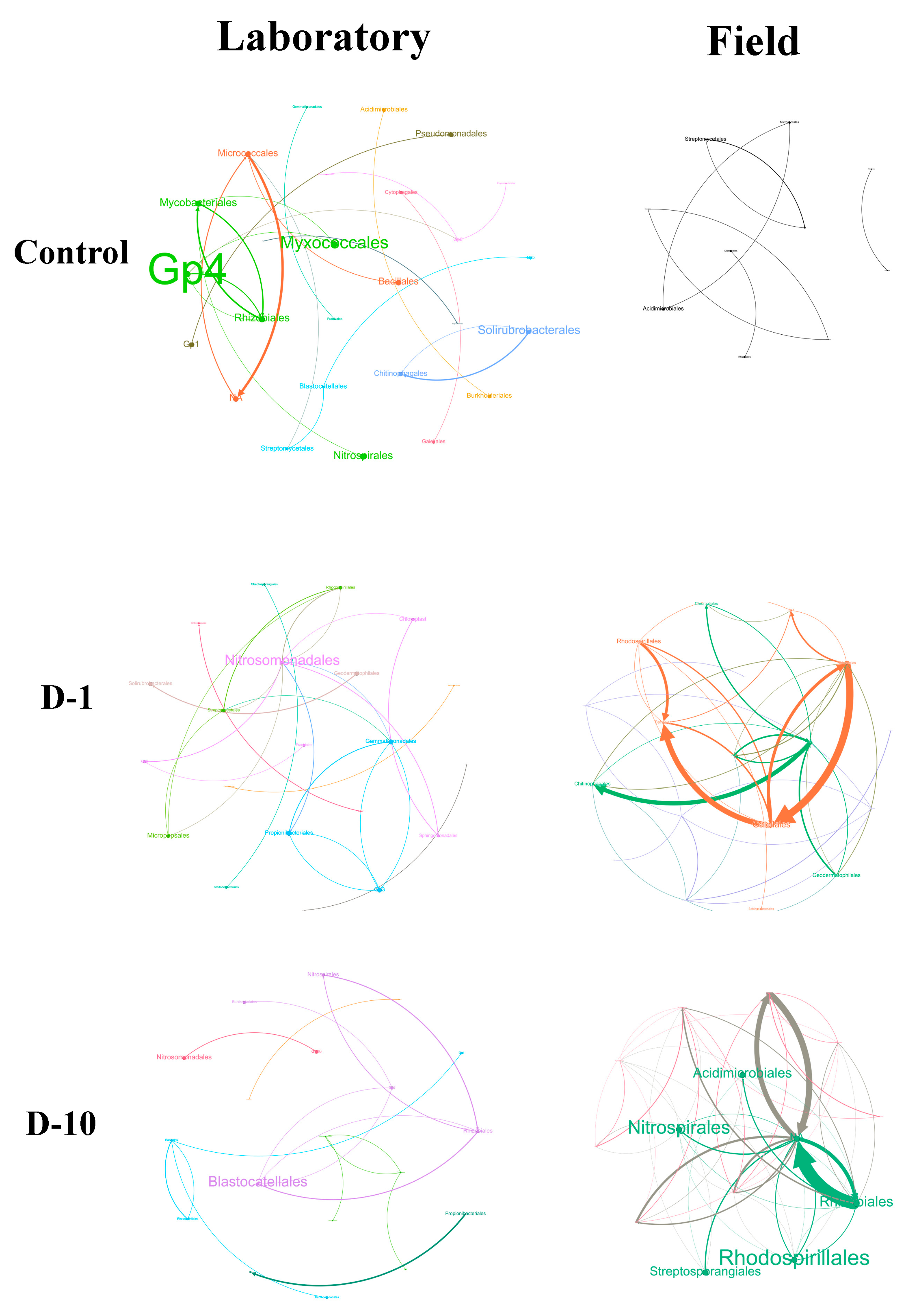
3.7. Analysis of the Ecological Network Structure of Bacterial and Fungal Communities
4. Discussion
4.1. Biochemical and Ecological Drivers of Microbial Community Responses to 2,4-DAPG
4.2. Dose-Dependent Effects on Microbial Activity
4.3. Functional Shifts in Nutrient Cycling: Enzyme Responses
4.4. Structural Reorganization of Bacterial and Fungal Communities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASV | Amplicon Sequence Variant |
| SOC | soil organic carbon |
| TN | total nitrogen |
| TC | total organic carbon |
| BR | basal respiration |
| SIR | substrate-induced respiration |
| MBSIR | microbial biomass carbon (SIR method) |
| QR | the coefficient of the microbial respiration |
| qCO2 | the metabolic coefficient |
| MB-SIR/SOC | the share of microbial biomass carbon in organic carbon |
| qCO2/SOC | the relationship between C-use efficiency and of the available organic carbon in soil |
Appendix A
| Topological Properties | Explanation |
|---|---|
| Node | An individual taxon (ASV) in the network. Node size often reflects its degree of connectivity (number of links) |
| Link | A connection (co-occurrence or correlation) between two taxa (nodes) in the network |
| Number of nodes | The total number of taxa (ASVs) included in the network analysis. Reflects taxonomic richness |
| Number of links | The total number of significant connections between all nodes in the network. Indicates overall network complexity |
| avgCC | Average Clustering Coefficient. Measures the tendency of nodes to form clusters (how interconnected a node’s neighbors are). High values indicate high local clustering and network stability. |
| CD | Degree Centralization. Measures how centralized the network is around a few highly connected nodes (hubs). High values indicate network vulnerability. |
| Power-law (R2) | Coefficient of determination for the power-law distribution. Indicates how well the network fits a scale-free model (presence of few highly connected hubs). High R2 (close to 1) suggests a stable and robust structure. |
| max degree | Maximum degree. The highest number of connections held by a single node in the network. Identifies keystone hub taxa. |
| avgK | Average degree/Mean connectivity. The average number of connections per node in the network. Reflects overall community connectedness. |
| Density | Network density. The ratio of observed links to the maximum possible number of links. Indicates the overall connectedness of the network. |
| CB | Betweenness Centrality. Measures how often a node acts as a “bridge” on the shortest path between other nodes. High values indicate a taxon’s key role in maintaining network integrity. |
| E | Global Efficiency. A measure of how efficiently information or resources are exchanged across the network. A decrease indicates loss of integrity and stability. |
| Eigenvector centrality | Measures a node’s influence in the network, considering both its own connections and the connections of its neighbors. High values indicate connections to other influential nodes. |
| Module | A group of nodes that are more densely connected to each other than to nodes in other groups. Reflects ecological guilds or functional groups. |
| Fragmentation | The breakdown of a single network into isolated or poorly connected modules/components. A sign of strong disturbance. |
| Trophic plasticity | The ability of an organism or community to switch between different food sources or resource consumption strategies |
References
- Craney, A.; Ahmed, S.; Nodwell, J. Towards a new science of secondary metabolism. J. Antibiot. 2013, 66, 387–400. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, L.; Mavrodi, D.V.; Thomashow, L.S.; Weller, D.M. Utilization of trehalose, benzoate, valerate, and seed and root exudates by genotypes of 2,4-diacetylphloroglucinol-producing Pseudomonas fluorescens. Soil Biol. Biochem. 2007, 39, 2712–2722. [Google Scholar] [CrossRef]
- Almario, J.; Bruto, M.; Vacheron, J.; Prigent-Combaret, C.; Moënne-Loccoz, Y.; Muller, D. Distribution of 2,4-Diacetylphloroglucinol Biosynthetic Genes among the Pseudomonas spp. Reveals Unexpected Polyphyletism. Front. Microbiol. 2017, 8, 1218. [Google Scholar] [CrossRef]
- Reddi, T.K.; Borovkov, A.V. Antibioticheskie svoistva 2,4-diatsetilflorogliutsina, produtsiruemogo Pseudo-monas fluorescens shtamm 26-o [Antibiotic prop-erties of 2,4-diacetylphloroglucinol produced by Pseudomonas fluo-rescens strain 26-o]. Antibiotiki 1970, 15, 19–21. (In Russian) [Google Scholar]
- Müller, T.; Ruppel, S.; Behrendt, U.; Lentzsch, P.; Müller, M.E.H. Antagonistic Potential of Fluorescent Pseudomonads Colonizing Wheat Heads Against Mycotoxin Producing Alternaria and Fusaria. Front. Microbiol. 2018, 9, 2124. [Google Scholar] [CrossRef]
- Suresh, P.; Varathraju, G.; Shanmugaiah, V.; Almaary, K.S.; Elbadawi, Y.B.; Mubarak, A. Partial purification and characterization of 2,4-diacetylphloroglucinol producing Pseudomonas fluorescens VSMKU3054 against bacterial wilt disease of tomato. Saudi J. Biol. Sci. 2021, 28, 2155–2167. [Google Scholar] [CrossRef]
- Isnansetyo, A.; Cui, L.; Hiramatsu, K.; Kamei, Y. Antibacterial activity of 2,4-diacetylphloroglucinol produced by Pseudomonas sp. AMSN isolated from a marine alga, against vancomycin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2003, 22, 545–547. [Google Scholar] [CrossRef]
- Zhong, Y.; He, X.; Tao, W.; Feng, J.; Zhang, R.; Gong, H.; Tang, Z.; Huang, C.; He, Y. 2,4-Diacetylphloroglucinol (DAPG) derivatives rapidly eradicate methicillin-resistant Staphylococcus aureus without resistance development by disrupting membrane. Eur. J. Med. Chem. 2023, 261, 115823. [Google Scholar] [CrossRef]
- Meyer, S.L.F.; Halbrendt, J.M.; Carta, L.K.; Skantar, A.M.; Liu, T.; E Abdelnabby, H.M.; Vinyard, B.T. Toxicity of 2,4-diacetylphloroglucinol (DAPG) to plant-parasitic and bacterial-feeding nematodes. J. Nematol. 2009, 41, 274–280. [Google Scholar]
- Cronin, D.; Moenne-Loccoz, Y.; Fenton, A.; Dunne, C.; Dowling, D.N.; O’GAra, F. Role of 2,4-Diacetylphloroglucinol in the Interactions of the Biocontrol Pseudomonad Strain F113 with the Potato Cyst Nematode Globodera rostochiensis. Appl. Environ. Microbiol. 1997, 63, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, P.; Immanuel, J.E.; Gnanamanickam, S.S.; Thomashow, L. Biological control of rice bacterial blight by plant-associated bacteria producing 2,4-diacetylphloroglucinol. Can. J. Microbiol. 2006, 52, 56–65. [Google Scholar] [CrossRef]
- Julian, W.T.; Vasilchenko, A.V.; Shpindyuk, D.D.; Poshvina, D.V.; Vasilchenko, A.S. Bacterial-Derived Plant Protection Metabolite 2,4-Diacetylphloroglucinol: Effects on Bacterial Cells at Inhibitory and Subinhibitory Concentrations. Biomolecules 2021, 11, 13. [Google Scholar] [CrossRef]
- Fenton, A.M.; Stephens, P.M.; Crowley, J.; O’Callaghan, M.; O’Gara, F. Exploitation of gene(s) involved in 2,4-diacetylphloroglucinol biosynthesis to confer a new biocontrol capability to a Pseudomonas strain. Appl. Environ. Microbiol. 1992, 58, 3873–3878. [Google Scholar] [CrossRef]
- Appel, H.M. Phenolics in ecological interactions: The importance of oxidation. J. Chem. Ecol. 1993, 19, 1521–1552. [Google Scholar] [CrossRef]
- Hebatpuria, V.M.; Arafat, H.A.; Rho, H.S.; Bishop, P.L.; Pinto, N.G.; Buchanan, R.C. Immobilization of phenol in cement-based solidified/stabilized hazardous wastes using regenerated activated carbon: Leaching studies. J. Hazard. Mater. 1999, 70, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.; Hempel, M.; Philipp, B.; Gross, E.M. Degradation of gallic acid and hydrolysable polyphenols is constitutively activated in the freshwater plant-associated bacterium Matsuebacter sp. FB25. Aquat. Microb. Ecol. 2007, 47, 83–90. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of Triazole Fungicides on Soil Microbiota and on the Activities of Enzymes Found in Soil: A Review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Vasilchenko, A.V.; Poshvina, D.V.; Semenov, M.V.; Timofeev, V.N.; Iashnikov, A.V.; Stepanov, A.A.; Pervushina, A.N.; Vasilchenko, A.S. Triazoles and Strobilurin Mixture Affects Soil Microbial Community and Incidences of Wheat Diseases. Plants 2023, 12, 660. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; An, Y.; Shu, L.; Li, S.; Sun, K.; Zhang, W. Effect of fungicides on soil respiration, microbial community, and enzyme activity: A global meta-analysis (1975–2024). Ecotoxicol. Environ. Saf. 2025, 289, 117433. [Google Scholar] [CrossRef]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of Soil Microbial Diversity through Sustainable Agricultural Practices and Its Evaluation by Omics Approaches: A Perspective for the Environment, Food Quality and Human Safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef]
- Spagnolo, F.; Trujillo, M.; Dennehy, J.J. Why Do Antibiotics Exist? mBio 2021, 12, e0196621. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Application of eco-physiological quotients (qCO2 and qD) on microbial biomasses from soils of different cropping histories. Soil Biol. Biochem. 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Domsch, K.H.A. Physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Vasilchenko, A.V.; Galaktionova, L.V.; Tretyakov, N.Y.; Dyachkov, S.M.; Vasilchenko, A.S. Impact of agricultural land use on distribution of microbial biomass and activity within soil aggregates. Soil Use Manag. 2023, 39, 618–633. [Google Scholar] [CrossRef]
- Teslya, A.V.; Gurina, E.V.; Poshvina, D.V.; Stepanov, A.A.; Iashnikov, A.V.; Vasilchenko, A.S. Fungal secondary metabolite gliotoxin enhances enzymatic activity in soils by reshaping their microbiome. Rhizosphere 2024, 32, 100960. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Xiao, N.; Zhou, A.; Kempher, M.L.; Zhou, B.Y.; Shi, Z.J.; Yuan, M.; Guo, X.; Wu, L.; Ning, D.; Van Nostrand, J.; et al. Disentangling direct from indirect relationships in association networks. Proc. Natl. Acad. Sci. USA 2022, 119, e2109995119. [Google Scholar] [CrossRef]
- Hansen, M.L.; He, Z.; Wibowo, M.; Jelsbak, L. A whole-cell biosensor for detection of 2,4-diacetylphloroglucinol (DAPG)-producing bacteria from grassland soil. Appl. Environ. Microbiol. 2021, 87, e01400-20. [Google Scholar] [CrossRef]
- Poshvina, D.V.; Balkin, A.S.; Dilbaryan, D.S.; Vasilchenko, A.S. Unravelling the response of the soil microbiome to macrolactin A: A metagenomic study. Chemosphere 2025, 387, 144645. [Google Scholar] [CrossRef]
- Jousset, A.; Becker, J.; Chatterjee, S.; Karlovsky, P.; Scheu, S.; Eisenhauer, N. Biodiversity and species identity shape the antifungal activity of bacterial communities. Ecology 2014, 95, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Z.; Xu, F.; Yang, Z.; Li, Z.; Shen, D.; Wang, L.; Wu, H.; Li, T.; Yan, Q.; et al. Soil bacterium manipulates antifungal weapons by sensing intracellular type IVA secretion system effectors of a competitor. ISME J. 2023, 17, 2232–2246. [Google Scholar] [CrossRef]
- Jousset, A.; Bonkowski, M. The model predator Acanthamoeba castellanii induces the production of 2,4, DAPG by the biocontrol strain Pseudomonas fluorescens Q2-87. Soil Biol. Biochem. 2010, 42, 1647–1649. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Poshvina, D.V.; Vasilchenko, A.S. 2,4-Diacetylphloroglucinol Modulates Candida albicans Virulence. J. Fungi 2022, 8, 1018. [Google Scholar] [CrossRef]
- Stepanov, A.A.; Shulaev, N.A.; Vasilchenko, A.S. The Ecological Strategy Determines the Response of Fungi to Stress: A Study of the 2,4-diacetylphloroglucinol Activity Against Aspergillus and Fusarium Species. J Basic Microbiol. 2024, 65, e2400334. [Google Scholar] [CrossRef]
- Yan, X.; Yang, R.; Zhao, R.-X.; Han, J.-T.; Jia, W.-J.; Li, D.-Y.; Wang, Y.; Zhang, N.; Wu, Y.; Zhang, L.-Q.; et al. Transcriptional Regulator PhlH Modulates 2,4-Diacetylphloroglucinol Biosynthesis in Response to the Biosynthetic Intermediate and End Product. Appl. Environ. Microbiol. 2017, 83, e01419-17. [Google Scholar] [CrossRef]
- Chander, K.; Dyckmans, J.; Joergensen, R.; Meyer, B.; Raubuch, M. Different sources of heavy metals and their long-term effects on soil microbial properties. Biol. Fertil. Soils 2001, 34, 241–247. [Google Scholar] [CrossRef]
- Anderson, T.H. Microbial eco-physiological indicators to assess soil quality. Agric. Ecosyst. Environ. 2003, 98, 285–293. [Google Scholar] [CrossRef]
- Frische, T.; Höper, H. Soil microbial parameters and luminescent bacteria assays as indicators for in situ bioremediation of TNT-contaminated soils. Chemosphere 2003, 50, 415–427. [Google Scholar] [CrossRef]
- Lucas, J.M.; Sone, B.M.; Whitmore, D.; Strickland, M.S. Antibiotics and temperature interact to disrupt soil communities and nutrient cycling. Soil Biol. Biochem. 2021, 163, 108437. [Google Scholar] [CrossRef]
- Maurhofer, M.; Baehler, E.; Notz, R.; Martinez, V.; Keel, C. Cross talk between 2,4-diacetylphloroglucinol-producing biocontrol pseudomonads on wheat roots. Appl. Environ. Microbiol. 2004, 70, 1990–1998. [Google Scholar] [CrossRef][Green Version]
- Powers, M.J.; Sanabria-Valentín, E.; Bowers, A.A.; Shank, E.A. Inhibition of Cell Differentiation in Bacillus subtilis by Pseudomonas protegens. J. Bacteriol. 2015, 197, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.D.; Owusu-Ansah, C.; Ellenbogen, J.B.; Malone, Z.D.; Ricketts, M.P.; Frolking, S.E.; Ernakovich, J.G.; Ibba, M.; Bagby, S.C.; Weissman, J. What is microbial dormancy? Trends Microbiol. 2024, 32, 142–150. [Google Scholar] [CrossRef]
- Bloor, J.M.G.; Si-Moussi, S.; Taberlet, P.; Carrère, P.; Hedde, M. Analysis of complex trophic networks reveals the signature of land-use intensification on soil communities in agroecosystems. Sci. Rep. 2021, 11, 18260. [Google Scholar] [CrossRef] [PubMed]
- Feofilova, E.P.; Ivashechkin, A.A.; Alekhin, A.I.; Sergeeva, Y.E. Fungal spores: Dormancy, germination, chemical composition, and role in biotechnology (review). Appl. Biochem. Microbiol. 2012, 48, 5–17. [Google Scholar] [CrossRef]
- Ponizovskaya, V.B.; Dyakov, M.Y.; Antropova, A.B.; Bilanenko, E.N.; Mokeeva, V.L.; Ilyin, V.K. The survival of micromycetes exposed to space conditions. Mosc. Univ. Biol. Sci. Bull. 2017, 72, 6–12. [Google Scholar] [CrossRef][Green Version]
- Seekles, S.J. The breaking of fungal spore dormancy: A coordinated transition. PLoS Biol. 2023, 21, e3002077. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, B.; Zhang, Y. A meta-analysis on the responses of soil microbial biomass and community structure to antibiotics. Appl. Soil Ecol. 2023, 184, 104786. [Google Scholar] [CrossRef]
- Selyanoinov, G.T. About agricultural estimate of climate. Proc. Agric. Meteorol. 1928, 20, 165–177. [Google Scholar]
- Devyatova, E.; Kochugova, E.; Cydenzapov, M. Comparison of Selyaninov’s Hydrothermal Coefficient (Aridity Criterion) over Buryatia, Russia, in the Summer Period from 1979 to 2019 according to Meteorological Stations and ECMWF ERA5. Environ. Sci. Proc. 2022, 19, 55. [Google Scholar] [CrossRef]

| Microcosms | Laboratory | Field | ||||||
|---|---|---|---|---|---|---|---|---|
| Incubation Time, Days | ||||||||
| 7 | 14 | 28 | 56 | 7 | 14 | 28 | 56 | |
| Control | 17.4 ± 0.69 a | 17.5 ± 1.09 a | 17.4 ± 0.53 a | 17.3 ± 1.13 a | 15.5 ± 0.58 a | 15.5 ± 0.43 a | 15.7 ± 1.36 a | 15.0 ± 0.69 a |
| D-1 | 18.9 ± 1.07 b | 19.1 ± 0.69 b | 18.7 ± 0.78 b | 18.4 ± 0.76 b | 13.4 ± 0.43 b | 16.5 ± 1.07 b | 15.8 ± 0.60 a | 15.6 ± 0.82 b |
| D-10 | 18.9 ± 0.93 b,c | 19.1 ± 0.59 b,c | 19.8 ± 0.63 c | 18.3 ± 0.75 b,c | 14.7 ± 1.01 c | 14.7 ± 1.03 c | 16.2 ± 0.69 a | 16.0 ± 0.50 b,c |
| Microcosms | Laboratory Conditions | Field Conditions | ||||||
|---|---|---|---|---|---|---|---|---|
| Incubation Time, Days | ||||||||
| 7 | 14 | 28 | 56 | 7 | 14 | 28 | 56 | |
| QR | ||||||||
| Control | 0.1 ± 0.01 a | 0.16 ± 0.05 a | 0.19 ± 0.08 a | 0.14 ± 0.04 a | 0.07 ± 0.01 a | 0.06 ± 0.02 a | 0.03 ± 0.01 a | 0.09 ± 0.04 a |
| D-1 | 0.13 ± 0.05 b | 0.14 ± 0.04 a,b | 0.15 ± 0.02 a,b | 0.67 ± 0.37 b | 0.10 ± 0.02 b | 0.06 ± 0.01 a | 0.06 ± 0.02 b | 0.16 ± 0.05 b |
| D-10 | 0.1 ± 0.01 a | 0.13 ± 0.03 b | 0.13 ± 0.02 b | 0.39 ± 0.31 b | 0.10 ± 0.02 b | 0.10 ± 0.03 b | 0.07 ± 0.02 b | 0.24 ± 0.09 c |
| qCO2 | ||||||||
| Control | 2.18 ± 0.24 a | 4.06 ± 1.13 a | 4.73 ± 1.87 a | 3.53 ± 1.02 a | 1.76 ± 0.26 a | 1.55 ± 0.48 a | 0.79 ± 0.22 a | 2.25 ± 0.86 a |
| D-1 | 3.21 ± 1.15 b | 3.48 ± 0.98 a,b | 3.60 ± 0.59 a,b | 16.6 ± 9.04 b | 2.52 ± 0.45 b | 1.56 ± 0.14 a | 1.57 ± 0.52 b | 3.95 ± 1.23 b |
| D-10 | 2.09 ± 0.18 a | 3.13 ± 0.73 b | 3.24 ± 0.38 b | 9.61 ± 3.82 b | 2.36 ± 0.41 b | 2.50 ± 0.71 b | 1.74 ± 0.51 b | 5.84 ± 2.12 c |
| MBSIR/SOC | ||||||||
| Control | 0.79 ± 0.13 a | 0.55 ± 0.15 a | 0.30 ± 0.09 a | 0.60 ± 0.17 a | 1.11 ± 0.09 a | 1.16 ± 0.11 a | 1.93 ± 0.345 a | 0.62 ± 0.05 a |
| D-1 | 1.05 ± 0.14 b | 0.56 ± 0.09 a | 0.57 ± 0.0 b | 0.38 ± 0.08 b | 1.57 ± 0.12 b | 1.72 ± 0.18 b | 1.29 ± 0.16 b | 0.34 ± 0.06 b |
| D-10 | 0.98 ± 0.14 b | 0.70 ± 0.16 b | 0.63 ± 0.08 c | 0.47 ± 0.11 c | 1.52 ± 0.16 b | 1.97 ± 0.18 c | 1.48 ± 0.13 c | 0.32 ± 0.05 b |
| qCO2/SOC | ||||||||
| Control | 0.13 ± 0.02 a | 0.23 ± 0.06 a | 0.27 ± 0.11 a | 0.21 ± 0.06 a | 0.11 ± 0.02 a | 0.10 ± 0.03 a | 0.05 ± 0.02 a | 0.15 ± 0.06 a |
| D-1 | 0.17 ± 0.07 b | 0.18 ± 0.05 b | 0.19 ± 0.03 b | 0.90 ± 0.49 b | 0.19 ± 0.04 b | 0.10 ± 0.01 a | 0.09 ± 0.03 b | 0.25 ± 0.07 b |
| D-10 | 0.11 ± 0.01 c | 0.16 ± 0.04 b | 0.16 ± 0.02 c | 0.52 ± 0.41 b | 0.16 ± 0.03 b | 0.17 ± 0.05 b | 0.11 ± 0.03 b | 0.36 ± 0.12 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teslya, A.V.; Stepanov, A.A.; Poshvina, D.V.; Petrushin, I.S.; Vasilchenko, A.S. From Lab to Field: Context-Dependent Impacts of Pseudomonas-Produced 2,4-Diacetylphloroglucinol on Soil Microbial Ecology. Biomolecules 2025, 15, 1578. https://doi.org/10.3390/biom15111578
Teslya AV, Stepanov AA, Poshvina DV, Petrushin IS, Vasilchenko AS. From Lab to Field: Context-Dependent Impacts of Pseudomonas-Produced 2,4-Diacetylphloroglucinol on Soil Microbial Ecology. Biomolecules. 2025; 15(11):1578. https://doi.org/10.3390/biom15111578
Chicago/Turabian StyleTeslya, Anastasia V., Artyom A. Stepanov, Darya V. Poshvina, Ivan S. Petrushin, and Alexey S. Vasilchenko. 2025. "From Lab to Field: Context-Dependent Impacts of Pseudomonas-Produced 2,4-Diacetylphloroglucinol on Soil Microbial Ecology" Biomolecules 15, no. 11: 1578. https://doi.org/10.3390/biom15111578
APA StyleTeslya, A. V., Stepanov, A. A., Poshvina, D. V., Petrushin, I. S., & Vasilchenko, A. S. (2025). From Lab to Field: Context-Dependent Impacts of Pseudomonas-Produced 2,4-Diacetylphloroglucinol on Soil Microbial Ecology. Biomolecules, 15(11), 1578. https://doi.org/10.3390/biom15111578







