Overexpression of TpGSDMT in Rice Seedlings Promotes High Levels of Glycine Betaine and Enhances Tolerance to Salt and Low Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Vector Construction of TpGSDMT and Transformation of Rice
2.3. GB Extraction and Quantification
2.4. Analysis of Tolerance to Salt and Low-Temperature Stresses of Transgenic Rice
2.5. Statistical Analysis
3. Results
3.1. Identification of Transgenic Rice
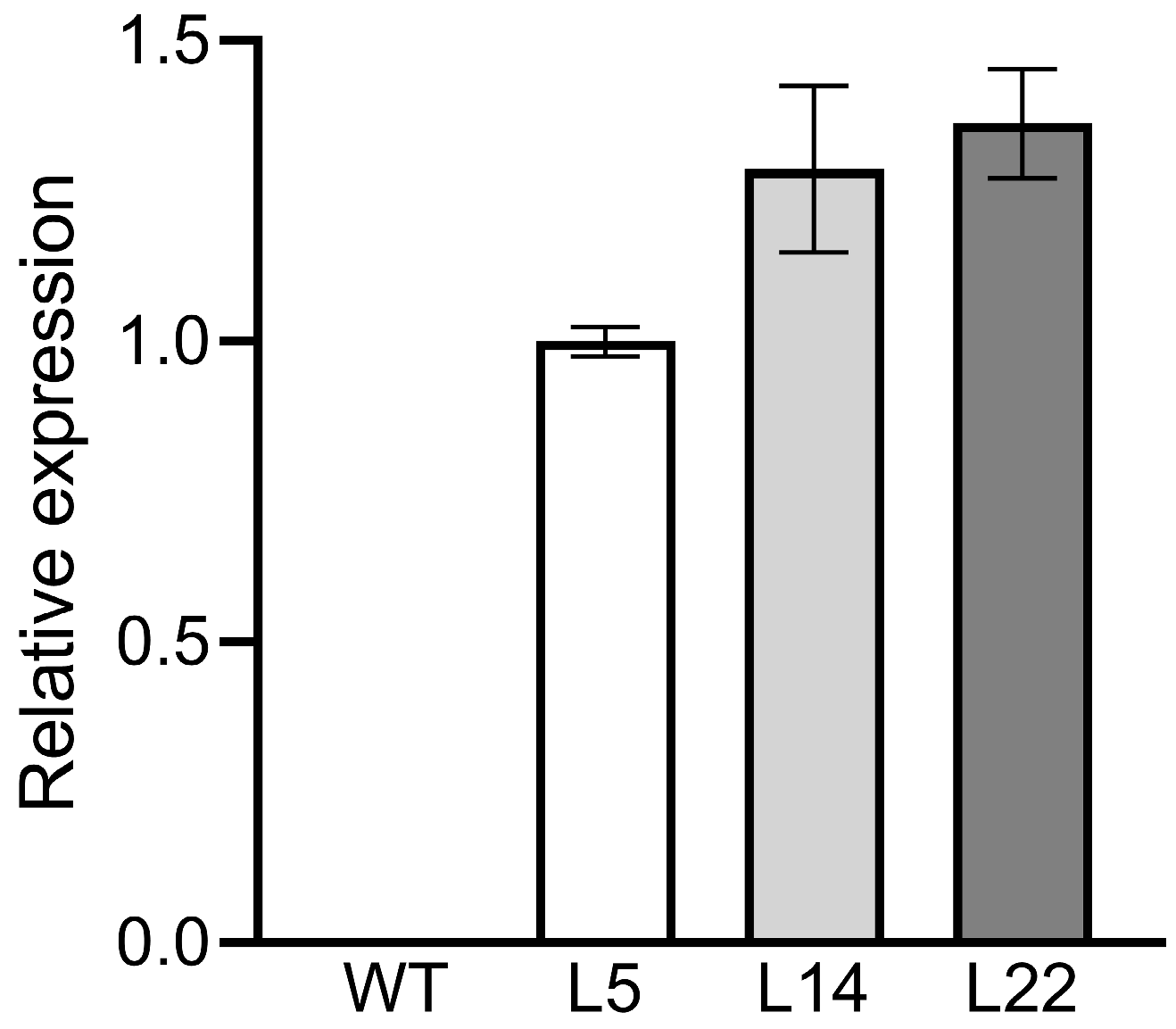
3.2. Expression Analysis of TpGSDMT in Transgenic Rice
3.3. Determination of GB Content in Transgenic Rice
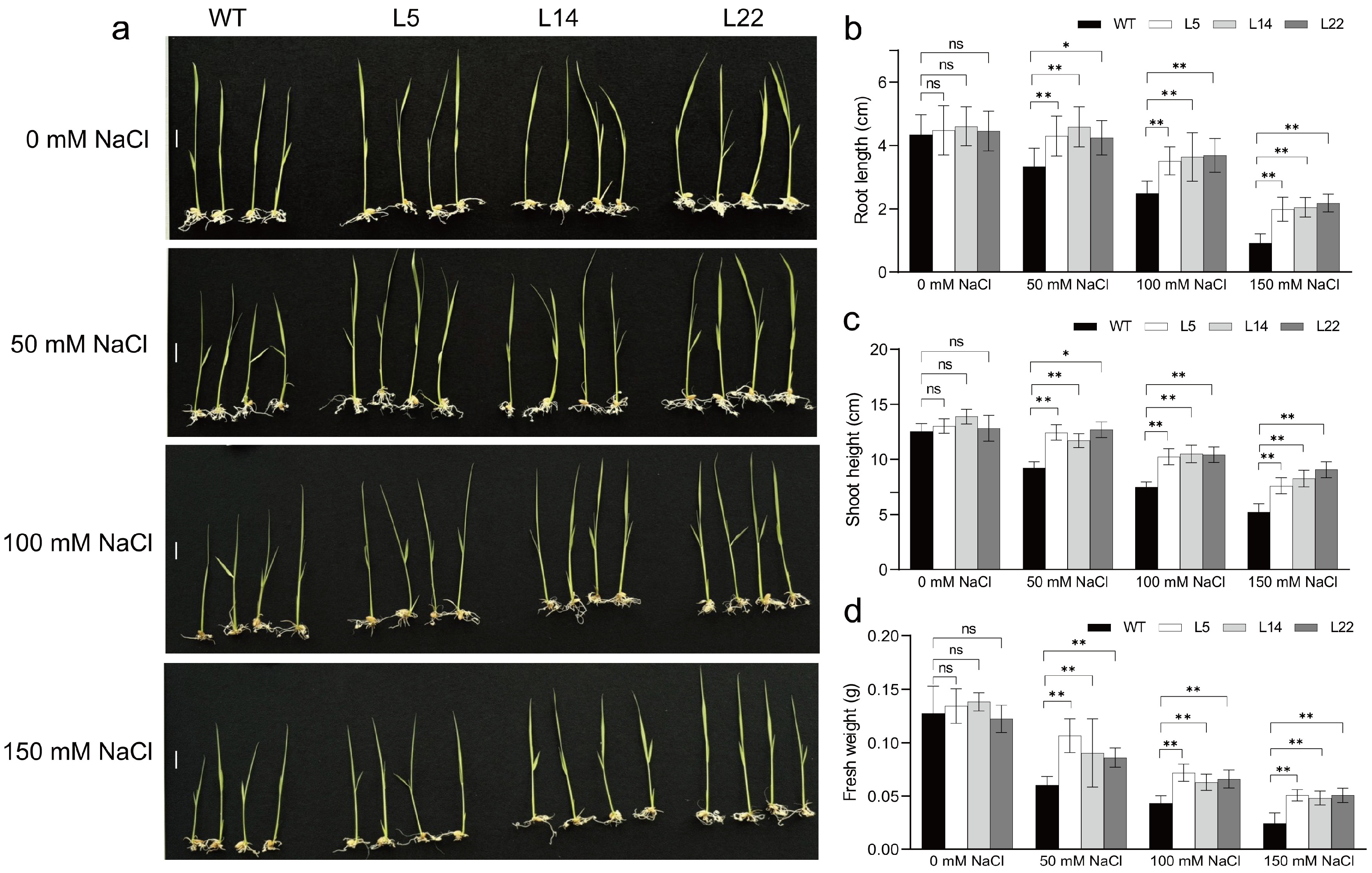
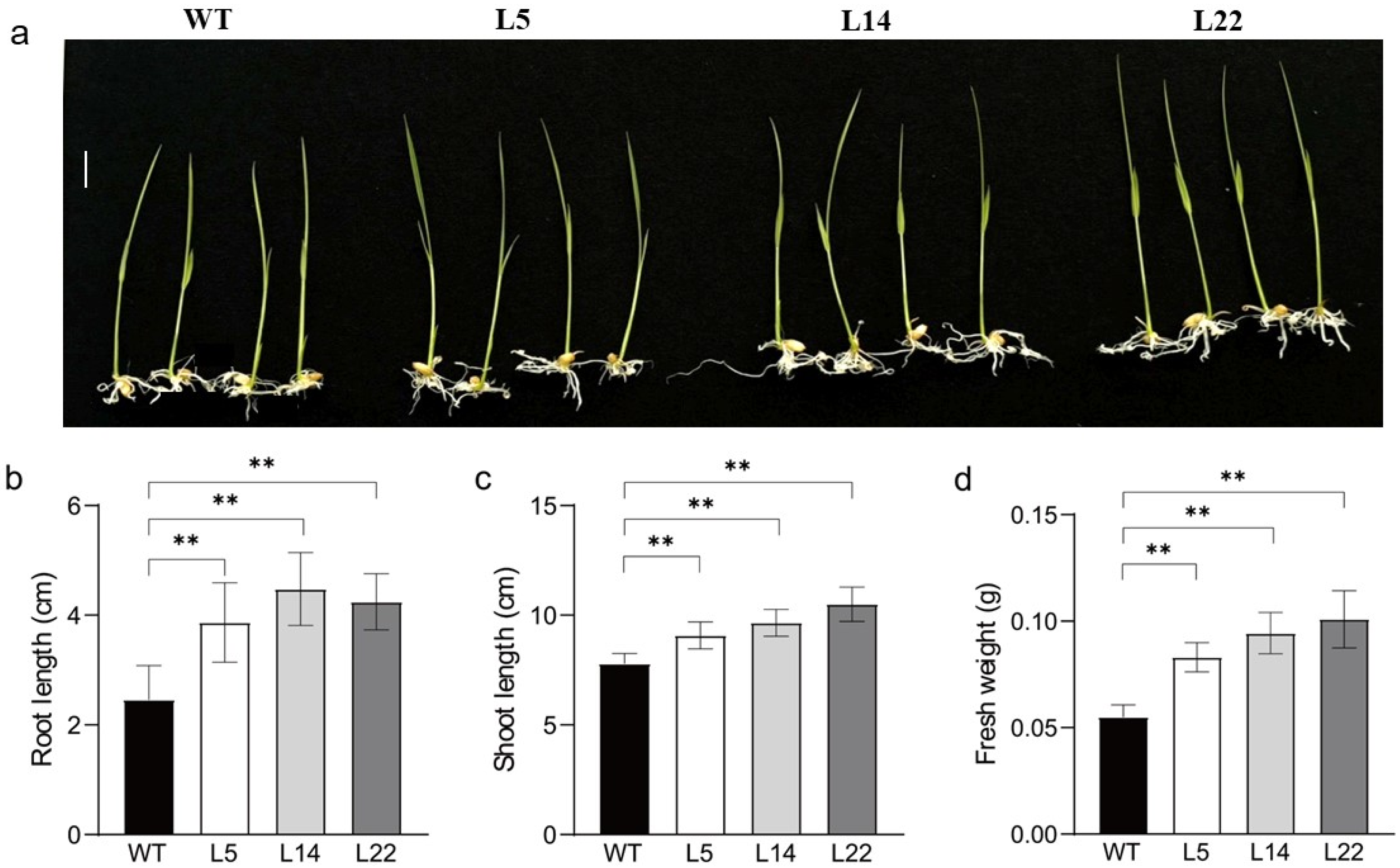
3.4. Salt and Low-Temperature Tolerance of Transgenic Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | One-way analysis of variance |
| BADH | Betaine aldehyde dehydrogenase |
| CAT | Catalase |
| CDH | Choline dehydrogenase |
| CDS | Coding sequences |
| CMO | Choline monooxygenase |
| COX | Choline oxidase |
| DMT | Sarcosine and dimethylglycine methyltransferase |
| FW | Fresh weight |
| GB | Glycine betaine |
| GSDMT | Glycine, sarcosine and dimethylglycine methyltransferase |
| GSMT | Glycine and sarcosine methyltransferase |
| HPLC | High-performance liquid chromatography |
| Hyg | Hygromycin phosphotransferase |
| LB | Left T-DNA border |
| MDA | Malondialdehyde |
| RB Right | Right T-DNA border |
| ROS | Reactive oxygen species |
| RT-qPCR | Real-time fluorescence quantitative PCR |
| SAM | S-adenosylmethionine |
| SDMT | Sarcosine and dimethylglycine methyltransferase |
| SE | Standard error |
| SOD | Superoxide dismutase |
| Tnos | Nopaline synthase polyadenlylation terminator |
| WT | Wild-type |
| 35SP | Cauliflower mosaic virus 35S promoter |
References
- Seleiman, M.F. Use of Plant Nutrients in Improving Abiotic Stress Tolerance in Wheat. In Wheat Production in Changing Environments: Responses, Adaptation and Tolerance; Springer: Berlin/Heidelberg, Germany, 2019; pp. 481–495. [Google Scholar]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Shuaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Rafey, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Badawy, A.A.; Alamri, A.A.; Hussein, H.A.A.; Salem, N.F.; Mashlawi, A.M.; Kenawy, S.K.; El-Shabasy, A. Glycine betaine mitigates heavy metal toxicity in beta vulgaris (L.): An antioxidant-driven approach. Agronomy 2024, 14, 797. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, J.; Wang, M.; Li, D.; Chen, T.H.; Liu, Y. Genetic engineering of the biosynthesis of glycinebetaine enhances the fruit development and size of tomato. Plant Sci. 2019, 280, 355–366. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; Kalderis, D. Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zeng, W.; Chen, F.; Ji, X.; Zhuang, Z.; Jin, B.; Wang, J.; Jia, L.; Peng, Y. Transcriptome expression profiles reveal response mechanisms to drought and drought-stress mitigation mechanisms by exogenous glycine betaine in maize. Biotechnol. Lett. 2022, 44, 367–386. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Ebrahim, N.E.; Mohamed, G.Z. Effect of water stress and foliar application of chitosan and glycine betaine on lettuce. Sci. Rep. 2023, 13, 17274. [Google Scholar] [CrossRef]
- Niu, T.; Zhang, J.; Li, J.; Gao, X.; Ma, H.; Gao, Y.; Chang, Y.; Xie, J. Effects of exogenous glycine betaine and cycloleucine on photosynthetic capacity, amino acid composition, and hormone metabolism in Solanum melongena L. Sci. Rep. 2023, 13, 7626. [Google Scholar] [CrossRef]
- Basit, F.; Alyafei, M.; Hayat, F.; Al-Zayadneh, W.; El-Keblawy, A.; Sulieman, S.; Sheteiwy, M.S. Deciphering the role of glycine betaine in enhancing plant performance and defense mechanisms against environmental stresses. Front. Plant Sci. 2025, 16, 1582332. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 1, 701596. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Singh, H.P.; Cha-Um, S. Foliar application of glycinebetaine regulates soluble sugars and modulates physiological adaptations in sweet potato (Ipomoea batatas) under water deficit. Protoplasma 2020, 257, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Ban, S.; Han, L.; Li, L.; Zhang, Y.; Zhang, Y.; Zhu, W. Effects of exogenous glycine betaine on growth and development of tomato seedlings under cold stress. Front. Plant Sci. 2024, 15, 1332583. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H. Role of glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Xing, W.; Rajashekar, C.B. Alleviation of water stress in beans by exogenous glycine betaine. Plant Sci. 1999, 148, 185–192. [Google Scholar] [CrossRef]
- Ashraf, M.F.M.R.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Jarin, A.; Ghosh, U.K.; Hossain, M.S.; Mahmud, A.; Khan, M.A.R. Glycine betaine in plant responses and tolerance to abiotic stresses. Discov. Agric. 2024, 2, 127. [Google Scholar] [CrossRef]
- Li, N.; Gao, Y.; Zhang, M.; Wang, T.; Li, J.; Xie, J. Glycine betaine enhances tolerance of low temperature combined with low light in pepper (Capsicum annuum L.) by improving the antioxidant capacity and regulating GB metabolism. Plant Physiol. Biol. Chem. 2025, 222, 109705. [Google Scholar] [CrossRef]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving Photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef]
- Huang, S.; Zuo, T.; Ni, W. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 2020, 251, 36. [Google Scholar] [CrossRef]
- Hernandez-Leon, S.G.; Valenzuela-Soto, E.M. Glycine betaine is a phytohormone-like plant growth and development regulator under stress conditions. J. Plant Growth Regul. 2023, 42, 5029–5040. [Google Scholar] [CrossRef]
- He, C.; Zhang, W.; Gao, Q.; Yang, A.; Hu, X.; Zhang, J. Enhancement of drought resistance and biomass by increasing the amount of glycine betaine in wheat seedlings. Euphytica 2011, 177, 151–167. [Google Scholar] [CrossRef]
- Zhao, F.; Luo, J.; Ibrahim, E.; Chen, L.; Shen, Y.; Ibrahim, M.; Alonazi, W.B.; Lu, J.; Luo, Y.; Wu, H. Engineering stress resistance: Advances in glycine betaine production for sustainable agriculture. Crop Health 2025, 3, 5. [Google Scholar] [CrossRef]
- Rontein, D.; Basset, G.; Hanson, A.D. Metabolic Engineering of Osmoprotectant Accumulation in Plants. Metab. Eng. 2002, 4, 49–56. [Google Scholar] [CrossRef]
- Chen, T.H.; Murata, N. Glycinebetaine protects plants against abiotic stress: Mechanisms and biotechnological applications. Plant Cell Environ. 2011, 34, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Tanaka, Y.; Takabe, T. Biosynthetic pathways of glycinebetaine in Thalassiosira pseudonana; functional characterization of enzyme catalyzing three-step methylation of glycine. Plant Physiol. Biochem. 2018, 127, 248–255. [Google Scholar] [CrossRef]
- Rathinasabapathi, B.; Burnet, M.; Russell, B.L.; Gage, D.A.; Liao, P.-C.; Nye, G.J.; Scott, P.; Golbeck, J.H.; Hanson, A.D. Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plants: Prosthetic group characterization and cDNA cloning. Proc. Natl. Acad. Sci. USA 1997, 94, 3454–3458. [Google Scholar] [CrossRef]
- Holmström, K.O.; Somersalo, S.; Mandal, A.; Palva, T.E.; Welin, B. Improved tolerance to salinity and low temperature in transgenic tobacco producing glycine betaine. J. Exp. Bot. 2000, 51, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Salvi, F.; Gadda, G. Human choline dehydrogenase: Medical promises and biochemical challenges. Arch. Biochem. Biophys. 2013, 537, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, S.; Imamura, S.; Misaki, H.; Horiuti, Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J. Biochem. 1977, 82, 1741–1749. [Google Scholar] [CrossRef]
- Waditee, R.; Tanaka, Y.; Aoki, K.; Hibino, T.; Jikuya, H.; Takano, J.; Takabe, T.; Takabe, T. Isolation and Functional Characterization of N-Methyltransferases That Catalyze Betaine Synthesis from Glycine in a Halotolerant Photosynthetic Organism Aphanothece halophytica. J. Biol. Chem. 2003, 278, 4932–4942. [Google Scholar] [CrossRef]
- Nyyssölä, A.; Kerovuo, J.; Kaukinen, P.; von Weymarn, N.; Reinikainen, T. Extreme Halophiles Synthesize Betaine from Glycine by Methylation. J. Biol. Chem. 2000, 275, 22196–22201. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Wang, C.C.; Chuang, M.J.; Wu, Y.C.; Lee, Y.C. Effects of substrate and potassium on the betaine-synthesizing enzyme glycine sarcosine dimethylglycine N-methyltransferase from a halophilic methanoarchaeon Methanohalophilus portucalensis. Res. Microbiol. 2006, 157, 948–955. [Google Scholar] [CrossRef]
- Lai, S.J.; Lai, M.C. Characterization and regulation of the osmolyte betaine synthesizing enzyme GSMT and SDMT from halophilic methanogen Methanohalophilus portucalensis. PLoS ONE 2011, 6, e25090. [Google Scholar] [CrossRef] [PubMed]
- Sulpice, R.; Tsukaya, H.; Nonaka, H.; Mustardy, L.; Murata, N. Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the synthesis of glycine betaine. Plant J. 2003, 36, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Hirji, R.; Zhang, L.; He, C.; Selvaraj, G.; Wu, R. Evaluation of the stress-inducible production of choline oxidase in transgenic rice as a strategy for producing the stress-protectant glycine betaine. J. Exp. Bot. 2006, 57, 1129–1135. [Google Scholar] [CrossRef]
- Park, E.J.; Jeknić, Z.; Chen, T.H.H.; Murata, N. The codA transgene for glycinebetaine synthesis increases the size of flowers and fruits in tomato. Plant Biotechnol. J. 2007, 5, 422–430. [Google Scholar] [CrossRef]
- Ahmad, R.; Kim, M.D.; Back, K.H.; Kim, H.S.; Lee, H.S.; Kwon, S.Y.; Murata, N.; Chung, W.I.; Kwak, S.S. Stress-induced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. Plant Cell Rep. 2008, 27, 687–698. [Google Scholar] [CrossRef]
- Luo, D.; Niu, X.; Yu, J.; Yan, J.; Gou, X.; Lu, B.R.; Liu, Y. Rice choline monooxygenase (OsCMO) protein functions in enhancing glycine betaine biosynthesis in transgenic tobacco but does not accumulate in rice (Oryza sativa L. ssp. japonica). Plant Cell Rep. 2012, 31, 1625–1635. [Google Scholar] [CrossRef]
- Yu, J.; Li, Y.; Tang, W.; Liu, J.; Lu, B.R.; Liu, Y. The Accumulation of Glycine Betaine Is Dependent on Choline Monooxygenase (OsCMO), Not on Phosphoethanolamine N-Methyltransferase (OsPEAMT1), in Rice (Oryza sativa L. ssp. japonica). Plant Mol. Biol. Rep. 2014, 32, 916–922. [Google Scholar] [CrossRef]
- Waditee, R.; Bhuiyan, M.N.H.; Rai, V.; Aoki, K.; Tanaka, Y.; Hibino, T.; Suzuki, S.; Takano, J.; Jagendorf, A.T.; Takabe, T.; et al. Genes for direct methylation of glycine provide high levels of glycinebetaine and abiotic-stress tolerance in Synechococcus and Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 1318–1323. [Google Scholar] [CrossRef]
- Niu, X.; Xiong, F.; Liu, J.; Sui, Y.; Zeng, Z.; Lu, B.R.; Liu, Y. Co-expression of ApGSMT and ApDMT promotes biosynthesis of glycine betaine in rice (Oryza sativa L.) and enhances salt and cold tolerance. Environ. Exp. Bot. 2014, 104, 16–25. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef]
- Raldugina, G.N.; Bogoutdinova, L.R.; Shelepova, O.V.; Kondrateva, V.V.; Platonova, E.V.; Nechaeva, T.L.; Kazantseva, V.V.; Lapshin, P.V.; Rostovtseva, H.I.; Aniskina, T.S.; et al. Heterologous codA gene expression leads to mitigation of salt stress effects and modulates developmental processes. Int. J. Mol. Sci. 2023, 24, 13998. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhu, M.; Li, Q.; Wang, X.; Wan, J.; Zhang, Y. Glycine betaine-mediated root priming improves water stress tolerance in wheat (Triticum aestivum L.). Agriculture 2021, 11, 1127. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; González-Orenga, S.; Tofei, A.M.; Boscaiu, M.; Moreno-Ramón, H.; Ibáñez-Asensio, S.; Vicente, O. Effect of a biostimulant based on polyphenols and glycine betaine on tomato plants’ responses to salt stress. Agronomy 2022, 12, 2142. [Google Scholar] [CrossRef]
- Ghorbani, A.; Ghasemi-Omran, V.O.; Chen, M. The effect of glycine betaine on nitrogen and polyamine metabolisms, expression of glycoside-related biosynthetic enzymes, and K/Na balance of stevia under salt stress. Plants 2023, 12, 1628. [Google Scholar] [CrossRef]
- Ruan, B.; Shang, L.; Zhang, B.; Hu, J.; Wang, Y.; Lin, H.; Zhang, A.; Liu, C.; Peng, Y.; Zhu, L. Natural variation in the promoter of TGW2 determines grain width and weight in rice. New Phytol. 2020, 227, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Ali, M.M.; Mahadi, M.R.; Abdullah, A.F.; Wayayok, A.; Kassim, M.S.M.; Jamaluddin, A. Smart farming for sustainable rice production: An insight into application, challenge, and future prospect. Rice Sci. 2024, 31, 47–61. [Google Scholar] [CrossRef]
- Fu, D.; Wu, W.; Mustafa, G.; Yang, Y.; Yang, P. Molecular mechanisms of rice seed germination. New Crops 2025, 2, 100051. [Google Scholar] [CrossRef]
- Wei, S.; Li, X.; Lu, Z.; Zhang, H.; Ye, X.; Zhou, Y.; Li, J.; Yan, Y.; Pei, H.; Duan, F.; et al. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science 2022, 377, eabi8455. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using exogenous melatonin, glutathione, proline, and glycine betaine treatments to combat abiotic stresses in crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Engler, C.; Kandzia, R.; Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef]
- Shao, J.; Tang, W.; Huang, K.; Ding, C.; Wang, H.; Zhang, W.; Li, R.; Aamer, M.; Hassan, M.U.; Elnour, R.O.; et al. How does zinc improve salinity tolerance? Mechanisms and future prospects. Plants 2023, 12, 3207. [Google Scholar] [CrossRef]
- Ghaderi, N.; Hatami, M.R.; Mozafari, A.; Siosehmardeh, A. Change in antioxidant enzymes activity and some morpho-physiological characteristics of strawberry under long-term salt stress. Physiol. Mol. Biol. Plants 2018, 24, 833–843. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Hoefsloot, H.C.; Mol, S.; Feron, R.; de Boer, G.J.; Haring, M.A.; Testerink, C. Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 2014, 166, 1387–1402. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Lu, B.; Liu, L.; Duan, W.; Meng, Y.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Dong, H.; et al. Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biol. 2021, 21, 331. [Google Scholar] [CrossRef]
- Baranova, E.N.; Gulevich, A.A. Asymmetry of plant cell divisions under salt stress. Symmetry 2021, 13, 1811. [Google Scholar] [CrossRef]
- Bogoutdinova, L.R.; Lazareva, E.M.; Chaban, I.A.; Kononenko, N.V.; Dilovarova, T.; Khaliluev, M.R.; Kurenina, L.V.; Gulevich, A.A.; Smirnova, E.A.; Baranova, E.N. Salt stress-induced structural changes are mitigated in transgenic tomato plants over-expressing superoxide dismutase. Biology 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Nassar, R.M.; Kamel, H.A.; Ghoniem, A.E.; Alarcón, J.J.; Sekara, A.; Ulrichs, C.; Abdelhamid, M.T. Physiological and anatomical mechanisms in wheat to cope with salt stress induced by seawater. Plants 2020, 9, 237. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Wang, M.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Genetic Engineering of the Biosynthesis of Glycine Betaine Modulates Phosphate Homeostasis by Regulating Phosphate Acquisition in Tomato. Front. Plant Sci. 2019, 9, 1995. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotechnol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.P.; Chao, D.Y.; Lin, H.X. Understanding abiotic stress tolerance mechanisms: Recent studies on stress response in rice. J. Integr. Plant Biol. 2007, 49, 742–750. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Zhang, Z.; Zhao, N.; Feng, X.; Zong, D.; Zhao, L. Overexpression of TpGSDMT in Rice Seedlings Promotes High Levels of Glycine Betaine and Enhances Tolerance to Salt and Low Temperature. Biomolecules 2025, 15, 1576. https://doi.org/10.3390/biom15111576
Yu J, Zhang Z, Zhao N, Feng X, Zong D, Zhao L. Overexpression of TpGSDMT in Rice Seedlings Promotes High Levels of Glycine Betaine and Enhances Tolerance to Salt and Low Temperature. Biomolecules. 2025; 15(11):1576. https://doi.org/10.3390/biom15111576
Chicago/Turabian StyleYu, Jinde, Zihan Zhang, Ning Zhao, Xiaofei Feng, Dan Zong, and Lihua Zhao. 2025. "Overexpression of TpGSDMT in Rice Seedlings Promotes High Levels of Glycine Betaine and Enhances Tolerance to Salt and Low Temperature" Biomolecules 15, no. 11: 1576. https://doi.org/10.3390/biom15111576
APA StyleYu, J., Zhang, Z., Zhao, N., Feng, X., Zong, D., & Zhao, L. (2025). Overexpression of TpGSDMT in Rice Seedlings Promotes High Levels of Glycine Betaine and Enhances Tolerance to Salt and Low Temperature. Biomolecules, 15(11), 1576. https://doi.org/10.3390/biom15111576





