Molecular Basis of Simalikalactone D Sensitivity in Triple-Negative Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of SKD and General Experimental Procedures
2.2. Cell Lines and Cell Culture Maintenance
2.3. Cell Viability Assay
2.4. Cell Growth and Proliferation Studies
2.5. Wound Healing Assay for Cell Migration
2.6. Caspase-3 Activity Assay
2.7. Western Blot Analysis
2.8. Explorer and JAK/STAT Phospho-Antibody Arrays
2.9. Proteomics & Ingenuity Pathway Analysis (IPA)
2.10. Clustering and Network Analysis
2.11. Computational Analyses and Molecular Docking of SKD
2.12. Statistical Analysis
3. Results
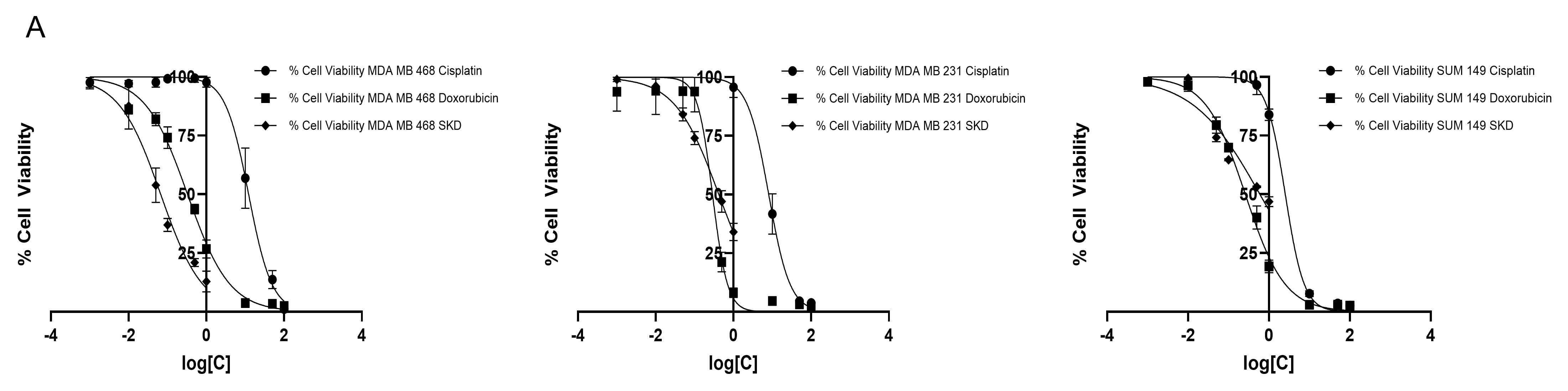
3.1. MDA-MB-468, MDA-MB-231 and SUM-149 Exhibited Different Sensitivity to SKD Treatment
3.2. Low Concentration of SKD Inhibits Cell Migration in MDA-MB-468, MDA-MB-231 and SUM-149 Cells
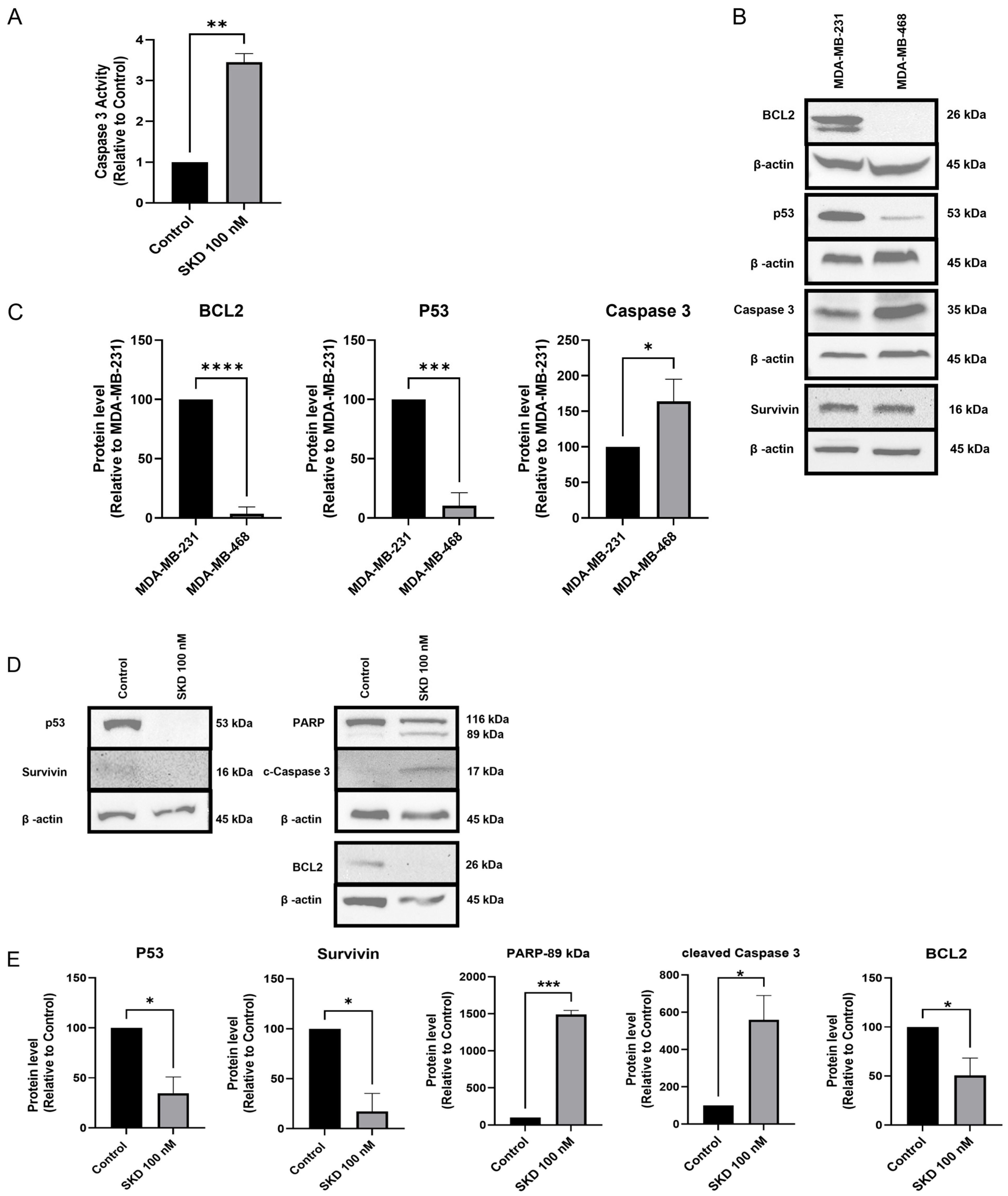
3.3. SKD Induces Apoptosis in MDA-MB-468 Cells
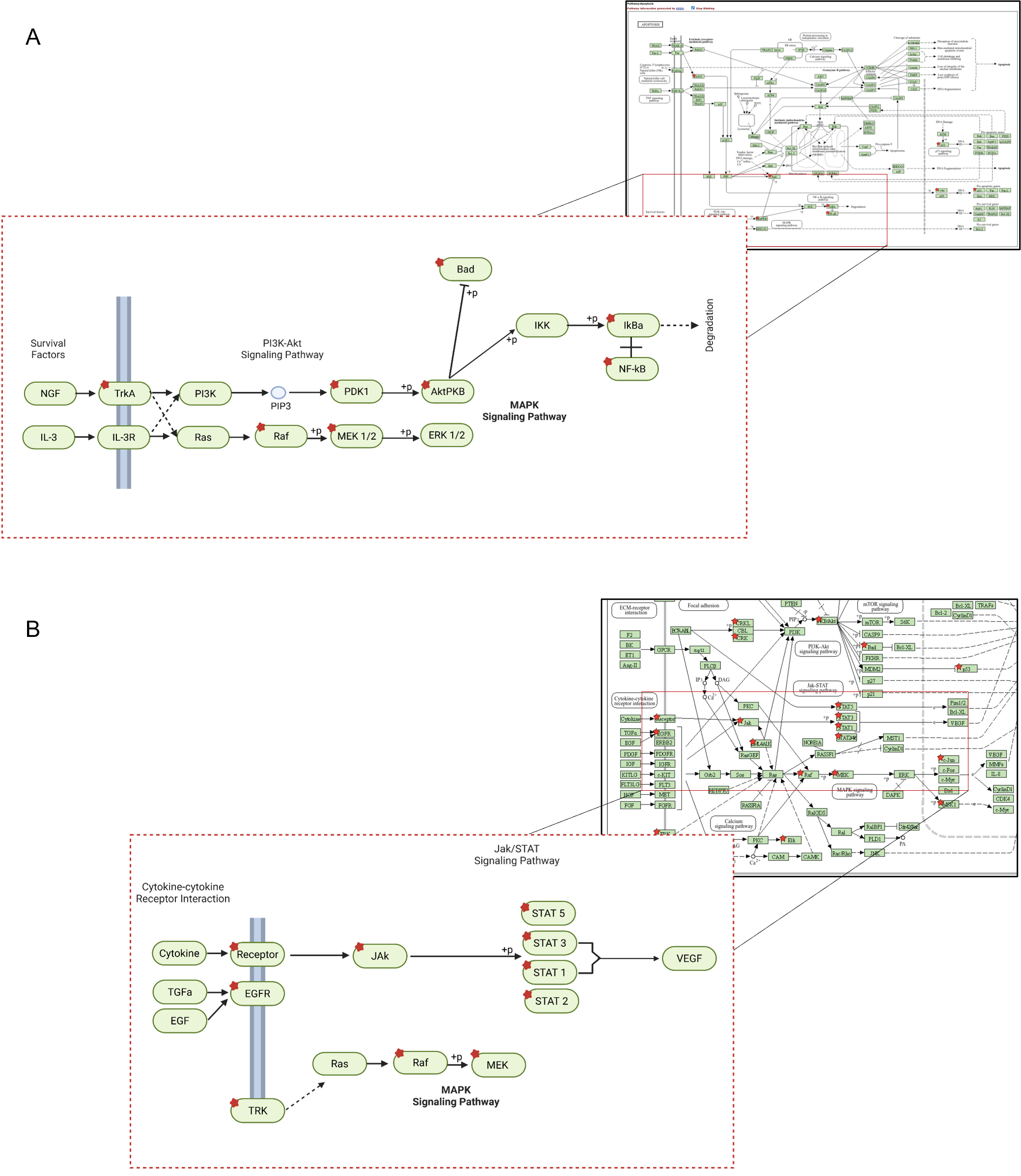
3.4. SKD Modulates EGFR and JAK–STAT Signaling
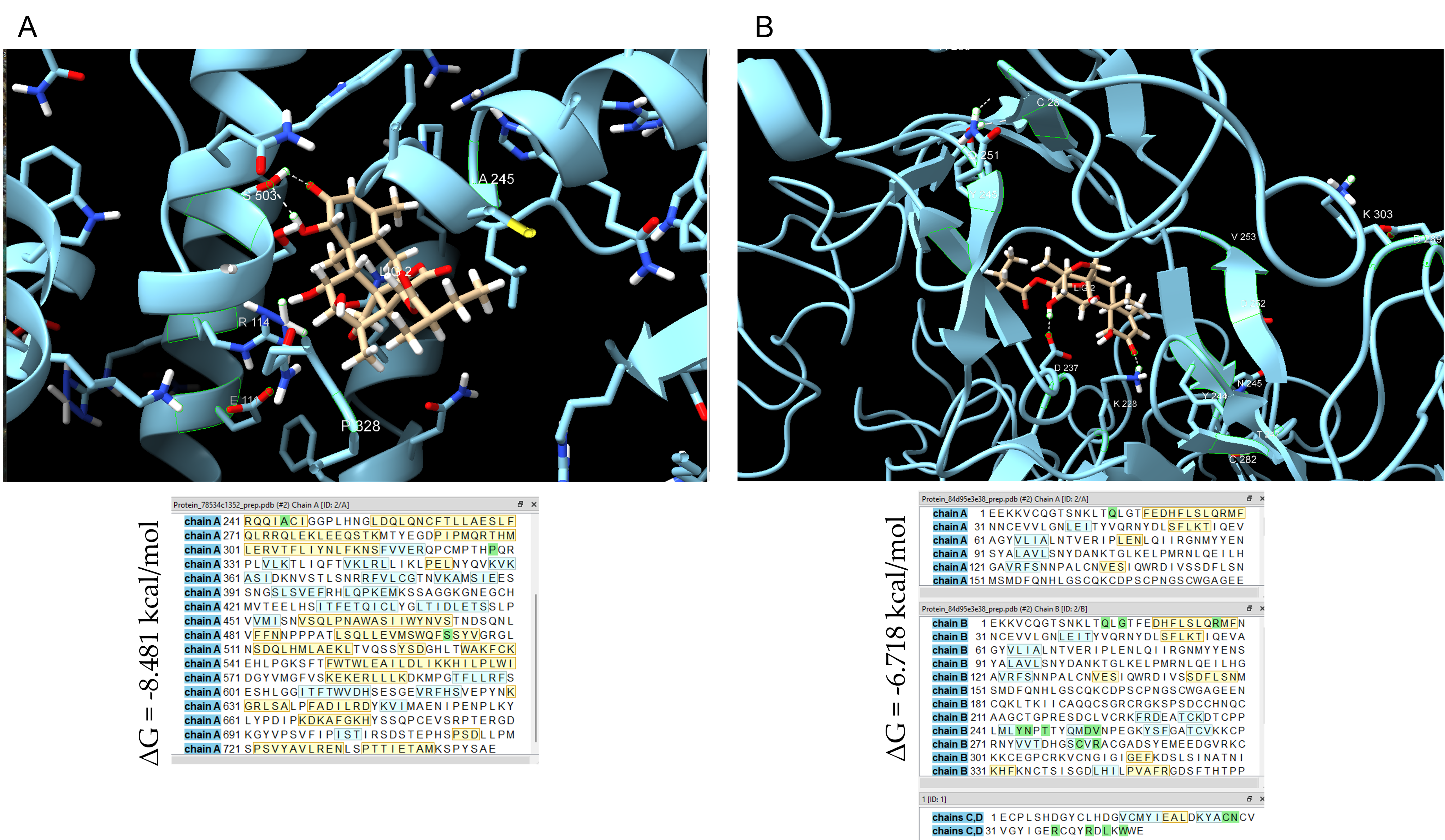
3.5. Computational Docking Supports SKD Interaction with STAT4 and EGFR
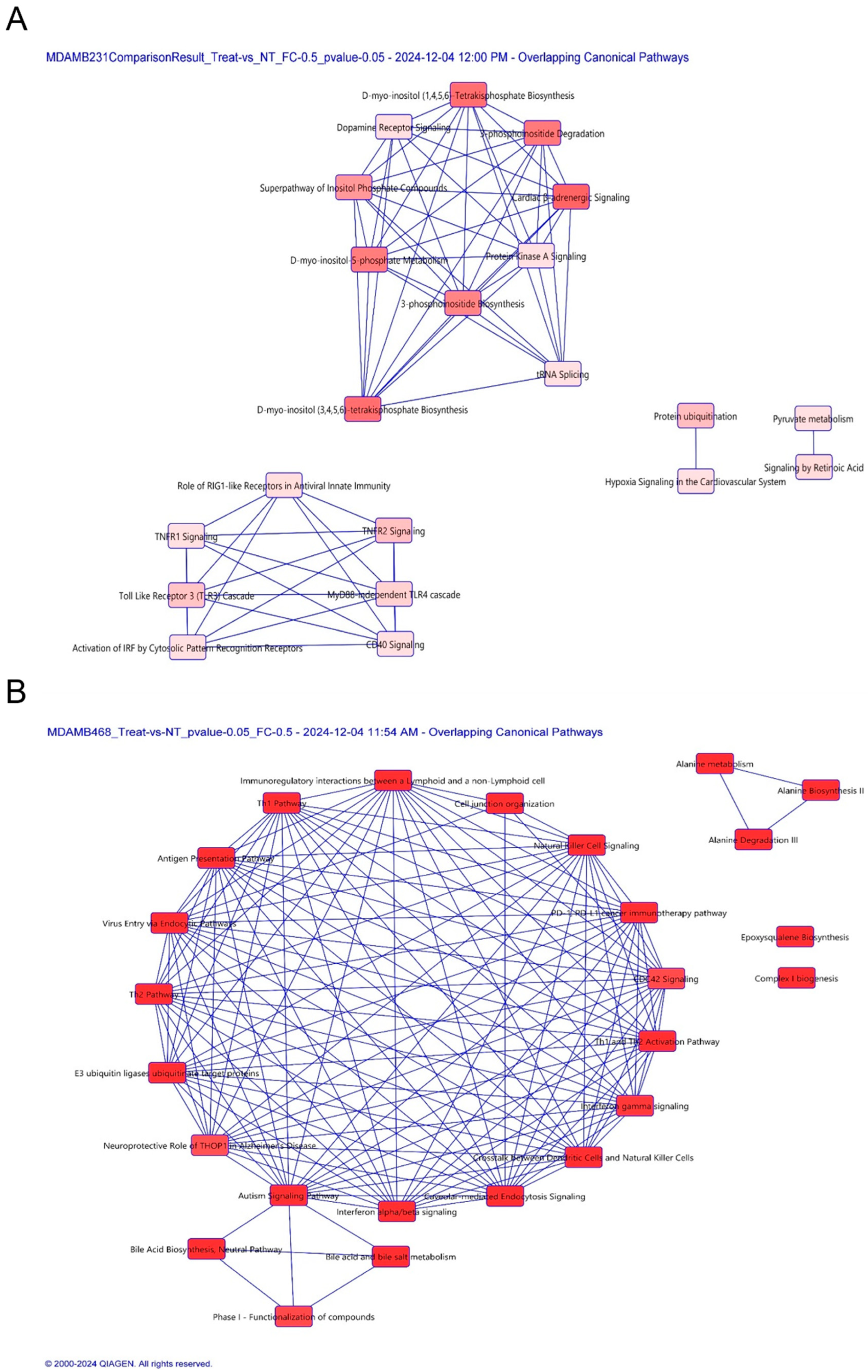
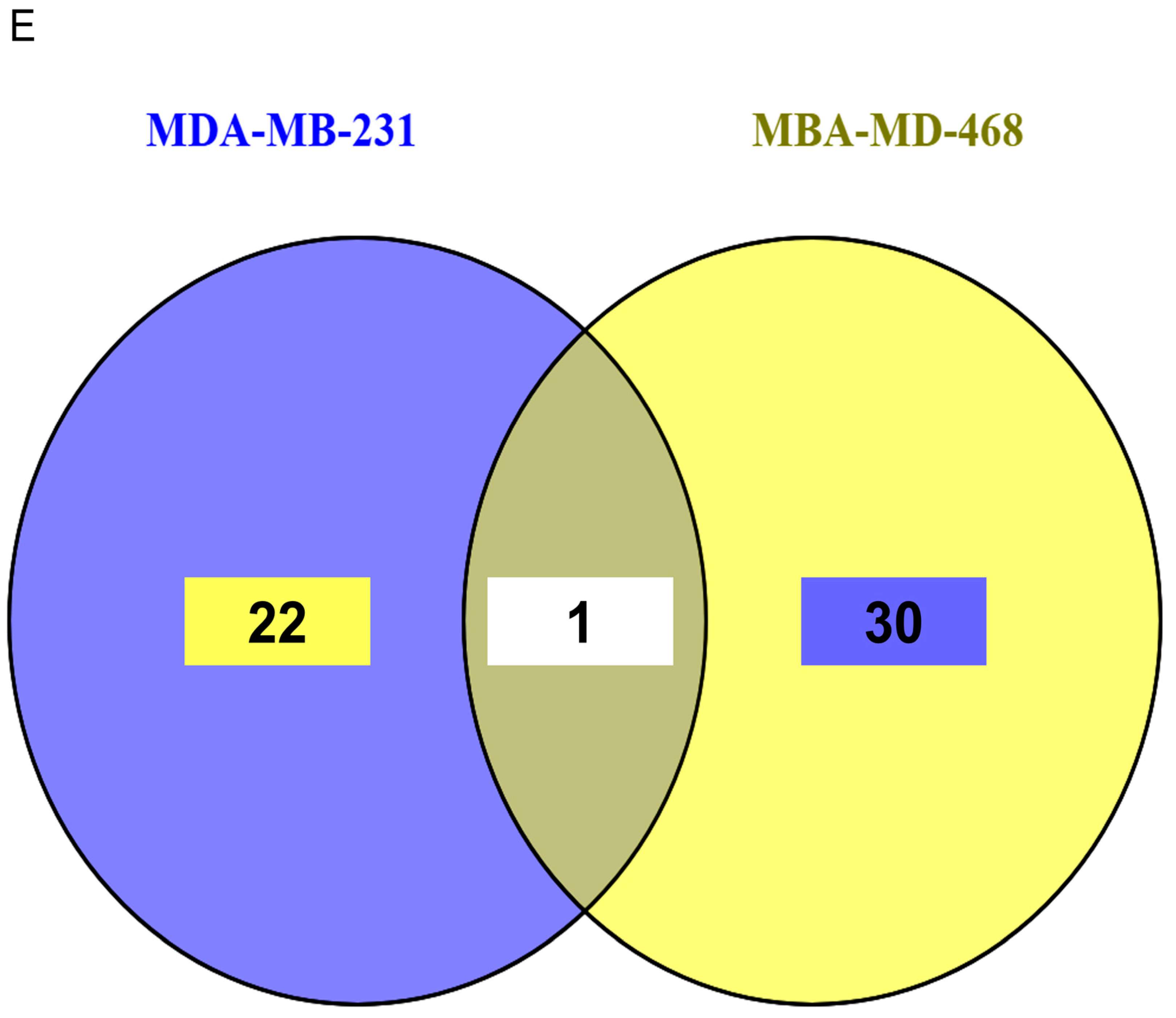
3.6. Proteomic Profiling Reveals Cell-Specific Disruption of Apoptosis and Migration Pathways
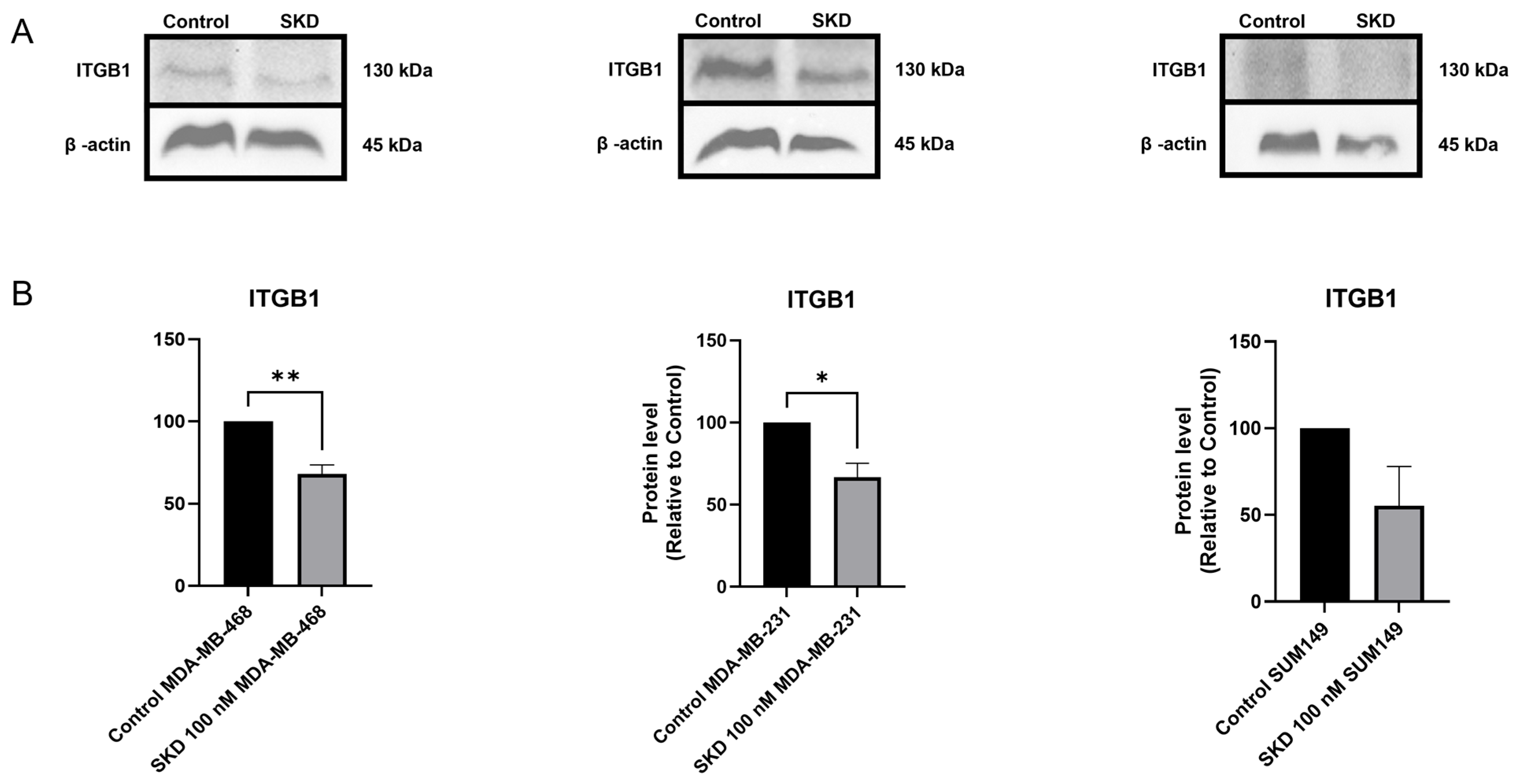
3.7. SKD Consistently Reduces ITGB1 Protein Expression in TNBC Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13 Pt 1, 4429–4434. [Google Scholar] [CrossRef]
- Hercules, S.M.; Hercules, J.C.; Ansari, A.; Date, S.A.J.; Skeete, D.H.A.; Smith Connell, S.P.; Pond, G.R.; Daniel, J.M. High triple-negative breast cancer prevalence and aggressive prognostic factors in Barbadian women with breast cancer. Cancer 2020, 126, 2217–2224. [Google Scholar] [CrossRef]
- Ventura, A.M.; Taiwo, E.; Leuva, H.; Chiu, E. Triple negative breast cancer presentation in Afro-Caribbean women in New York City. J. Clin. Oncol. 2022, 40 (Suppl. S16), e18726. [Google Scholar] [CrossRef]
- Mendez, B.; Reyes, J.; Conde, I.; Ramos, Z.; Lozada, E.; Cruz, A.M.; Asencio, G.; Carvajal, A.; Dharmawardhane, S.; Piñero-Cruz, D.M.; et al. Simalikalactone D, a Potential Anticancer Compound from Simarouba tulae, an Endemic Plant of Puerto Rico. Plants 2020, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Hooker, S.E., Jr.; Woods-Burnham, L.; Bathina, M.; Lloyd, S.; Gorjala, P.; Mitra, R.; Nonn, L.; Kimbro, K.S.; Kittles, R.A. Genetic Ancestry Analysis Reveals Misclassification of Commonly Used Cancer Cell Lines. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1003–1009. [Google Scholar] [CrossRef]
- Cailleau, R.; Olivé, M.; Cruciger, Q.V. Long-term human breast carcinoma cell lines of metastatic origin: Preliminary characterization. In Vitro 1978, 14, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Walerych, D.; Napoli, M.; Collavin, L.; Del Sal, G. The rebel angel: Mutant p53 as the driving oncogene in breast cancer. Carcinogenesis 2012, 33, 2007–2017. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.S.; Tiong, K.H.; Choo, H.L.; Fei-Lei Chung, F.; Hii, L.W.; Tan, S.H.; Yap, I.K.; Pani, S.; Khor, N.T.; Wong, S.F.; et al. Mutant p53-R273H mediates cancer cell survival and anoikis resistance through AKT-dependent suppression of BCL2-modifying factor (BMF). Cell Death Dis. 2015, 6, e1826. [Google Scholar] [CrossRef]
- Filmus, J.; Pollak, M.N.; Cailleau, R.; Buick, R.N. MDA-468, a human breast cancer cell line with a high number of epidermal growth factor (EGF) receptors, has an amplified EGF receptor gene and is growth inhibited by EGF. Biochem. Biophys. Res. Commun. 1985, 128, 898–905. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef]
- Su, Y.; Pogash, T.J.; Nguyen, T.D.; Russo, J. Development and characterization of two human triple-negative breast cancer cell lines with highly tumorigenic and metastatic capabilities. Cancer Med. 2016, 5, 558–573. [Google Scholar] [CrossRef]
- Hollestelle, A.; Elstrodt, F.; Nagel, J.H.A.; Kallemeijn, W.W.; Schutte, M. Phosphatidylinositol-3-OH Kinase or RAS Pathway Mutations in Human Breast Cancer Cell Lines. Mol. Cancer Res. 2007, 5, 195–201. [Google Scholar] [CrossRef]
- Kim, R.K.; Suh, Y.; Yoo, K.C.; Cui, Y.H.; Kim, H.; Kim, M.J.; Gyu Kim, I.; Lee, S.J. Activation of KRAS promotes the mesenchymal features of basal-type breast cancer. Exp. Mol. Med. 2015, 47, e137. [Google Scholar] [CrossRef]
- Hu, X.; Stern, H.M.; Ge, L.; O’Brien, C.; Haydu, L.; Honchell, C.D.; Haverty, P.M.; Peters, B.A.; Wu, T.D.; Amler, L.C.; et al. Genetic Alterations and Oncogenic Pathways Associated with Breast Cancer Subtypes. Mol. Cancer Res. 2009, 7, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Isert, L.; Mehta, A.; Loiudice, G.; Oliva, A.; Roidl, A.; Merkel, O.M. An In Vitro Approach to Model EMT in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 7757. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.J.; Husmann, A.; Hume, R.D.; Watson, C.J.; Cameron, R.E. Development of three-dimensional collagen scaffolds with controlled architecture for cell migration studies using breast cancer cell lines. Biomaterials 2017, 114, 34–43. [Google Scholar] [CrossRef]
- Nagaria, T.S.; Shi, C.; Leduc, C.; Hoskin, V.; Sikdar, S.; Sangrar, W.; Greer, P.A. Combined targeting of Raf and Mek synergistically inhibits tumorigenesis in triple negative breast cancer model systems. Oncotarget 2017, 8, 80804. [Google Scholar] [CrossRef]
- She, Q.-B.; Solit, D.; Basso, A.; Moasser, M.M. Resistance to Gefitinib in PTEN-Null HER-Overexpressing Tumor Cells Can Be Overcome through Restoration of PTEN Function or Pharmacologic Modulation of Constitutive Phosphatidylinositol 3′-Kinase/Akt Pathway Signaling. Clin. Cancer Res. 2003, 9, 4340–4346. [Google Scholar] [PubMed]
- Jonkman, J.E.; Cathcart, J.A.; Xu, F.; Bartolini, M.E.; Amon, J.E.; Stevens, K.M.; Colarusso, P. An introduction to the wound healing assay using live-cell microscopy. Cell Adhes. Migr. 2014, 8, 440–451. [Google Scholar] [CrossRef]
- Suzuki-Hatano, S.; Tsai, A.C.; Daugherty, A.; Pacak, C.A. TMT Sample Preparation for Proteomics Facility Submission and Subsequent Data Analysis. J. Vis. Exp. 2020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rabelo-Fernández, R.J.; Santiago-Sánchez, G.S.; Sharma, R.K.; Roche-Lima, A.; Carrion, K.C.; Rivera, R.A.N.; Quiñones-Díaz, B.I.; Rajasekaran, S.; Siddiqui, J.; Miles, W.; et al. Reduced RBPMS Levels Promote Cell Proliferation and Decrease Cisplatin Sensitivity in Ovarian Cancer Cells. Int. J. Mol. Sci. 2022, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Borges-Vélez, G.; Rosado-Philippi, J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrion, K.; Roche-Lima, A.; Pérez-Vargas, J.; González-Martínez, A.; Correa-Rivas, M.S.; Meléndez, L.M. Zika virus infection of the placenta alters extracellular matrix proteome. J. Mol. Histol. 2022, 53, 199–214. [Google Scholar] [CrossRef]
- Rosario-Rodríguez, L.J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrión, K.; Rosa-Díaz, A.; Rodríguez-De Jesús, A.E.; Rivera-Nieves, V.; Tosado-Rodríguez, E.L.; Méndez, L.B.; Roche-Lima, A.; Bertrán, J.; et al. Plasma Proteins Associated with COVID-19 Severity in Puerto Rico. Int. J. Mol. Sci. 2024, 25, 5426. [Google Scholar] [CrossRef] [PubMed]
- Zenón-Meléndez, C.N.; Carrasquillo Carrión, K.; Cantres Rosario, Y.; Roche Lima, A.; Meléndez, L.M. Inhibition of Cathepsin B and SAPC Secreted by HIV-Infected Macrophages Reverses Common and Unique Apoptosis Pathways. J. Proteome Res. 2022, 21, 301–312. [Google Scholar] [CrossRef]
- Huang, T.; Choi, M.; Tzouros, M.; Golling, S.; Pandya, N.J.; Banfai, B.; Dunkley, T.; Vitek, O. MSstatsTMT: Statistical Detection of Differentially Abundant Proteins in Experiments with Isobaric Labeling and Multiple Mixtures. Mol. Cell Proteom. 2020, 19, 1706–1723. [Google Scholar] [CrossRef]
- Guedes, I.A.; Pereira da Silva, M.M.; Galheigo, M.; Krempser, E.; de Magalhães, C.S.; Correa Barbosa, H.J.; Dardenne, L.E. DockThor-VS: A Free Platform for Receptor-Ligand Virtual Screening. J. Mol. Biol. 2024, 436, 168548. [Google Scholar] [CrossRef]
- Guedes, I.A.; Costa, L.S.C.; dos Santos, K.B.; Karl, A.L.M.; Rocha, G.K.; Teixeira, I.M.; Galheigo, M.M.; Medeiros, V.; Krempser, E.; Custódio, F.L.; et al. Drug design and repurposing with DockThor-VS web server focusing on SARS-CoV-2 therapeutic targets and their non-synonym variants. Sci. Rep. 2021, 11, 5543. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- National Library of Medicine (US). N.C. f. B. I. PubChem Compound Summary for CID 441808, Simalikilactone D. PubChem. 2004. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Simalikilactone-D (accessed on 14 February 2025).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Wang, Y.; Hu, P.; Wang, J.; Wang, R.Y.L. Pristimerin exerts antitumor activity against MDA-MB-231 triple-negative breast cancer cells by reversing of epithelial-mesenchymal transition via downregulation of integrin β3. Biomed. J. 2021, 44 (Suppl. S1), S84–S92. [Google Scholar] [CrossRef] [PubMed]
- Oren, M.; Rotter, V. Mutant p53 Gain-of-Function in Cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a001107. [Google Scholar] [CrossRef] [PubMed]
- Willmarth, N.E.; Baillo, A.; Dziubinski, M.L.; Wilson, K.; Riese, D.J., 2nd; Ethier, S.P. Altered EGFR localization and degradation in human breast cancer cells with an amphiregulin/EGFR autocrine loop. Cell Signal 2009, 21, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Quesnelle, K.M.; Boehm, A.L.; Grandis, J.R. STAT-mediated EGFR signaling in cancer. J. Cell Biochem. 2007, 102, 311–319. [Google Scholar] [CrossRef]
- Müller-Dethlefs, K.; Hobza, P. Noncovalent Interactions: A Challenge for Experiment and Theory. Chem. Rev. 2000, 100, 143–168. [Google Scholar] [CrossRef]
- Matsuhashi, S.; Manirujjaman, M.; Hamajima, H.; Ozaki, I. Control Mechanisms of the Tumor Suppressor PDCD4: Expression and Functions. Int. J. Mol. Sci. 2019, 20, 2304. [Google Scholar] [CrossRef]
- Su, C.; Mo, J.; Dong, S.; Liao, Z.; Zhang, B.; Zhu, P. Integrinβ-1 in disorders and cancers: Molecular mechanisms and therapeutic targets. Cell Commun. Signal 2024, 22, 71. [Google Scholar] [CrossRef]
- Xu, L.; Shao, Y.; Ren, L.; Liu, X.; Li, Y.; Xu, J.; Ye, Y. IQGAP2 Inhibits Migration and Invasion of Gastric Cancer Cells via Elevating SHIP2 Phosphatase Activity. Int. J. Mol. Sci. 2020, 21, 1968. [Google Scholar] [CrossRef]
- Oliveros, J.C. Venny: An Interactive Tool for Comparing Lists with Venn’s Diagrams, Version 2.1.0; BioinfoGP Service: Madrid, Spain, 2024; Available online: https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 14 February 2025).
- Yin, H.L.; Wu, C.C.; Lin, C.H.; Chai, C.Y.; Hou, M.F.; Chang, S.J.; Tsai, H.P.; Hung, W.C.; Pan, M.R.; Luo, C.W. β1 Integrin as a Prognostic and Predictive Marker in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1432. [Google Scholar] [CrossRef]
- Conway, J.R.W.; Joshi, O.; Kaivola, J.; Follain, G.; Gounis, M.; Kühl, D.; Ivaska, J. Dynamic regulation of integrin β1 phosphorylation supports invasion of breast cancer cells. Nat. Cell Biol. 2025, 27, 1021–1034. [Google Scholar] [CrossRef]
- Wang, D.-Y.; Hong, Y.; Chen, Y.-G.; Dong, P.-Z.; Liu, S.-Y.; Gao, Y.-R.; Lu, D.; Li, H.-M.; Li, T.; Guo, J.-C.; et al. PEST-containing nuclear protein regulates cell proliferation, migration, and invasion in lung adenocarcinoma. Oncogenesis 2019, 8, 22. [Google Scholar] [CrossRef]
- Chen, S.; Li, Y.; Wu, M.; Xue, L.; Zhu, J.; Wu, M.; Zhang, Q.; He, G.; Li, G.; Fu, S.; et al. Nucleolar and Coiled-Body Phosphoprotein 1 Is Associated With Stemness and Represents a Potential Therapeutic Target in Triple-Negative Breast Cancer. Front. Oncol. 2022, 12, 731528. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Hu, H.; Gao, N.; Yan, X.; Zhou, Q.; Liu, H. TANK shapes an immunosuppressive microenvironment and predicts prognosis and therapeutic response in glioma. Front. Immunol. 2023, 14, 1138203. [Google Scholar] [CrossRef]
- Li, P.; Chi, W.-R.; Xiu, B.; Zhang, Q.; Zhang, L.; Chen, M.; Xue, J.; Huang, X.; Chi, Y.; Wu, J. Abstract P5-02-41: UBE2E3 promotes the progression of HER2-positive breast cancer and influences the efficacy of targeted therapy via EGFR stabilization. Cancer Res. 2023, 83 (Suppl. 5), P5-02-41. [Google Scholar] [CrossRef]
- Attalla, S.S.; Boucher, J.; Proud, H.; Taifour, T.; Zuo, D.; Sanguin-Gendreau, V.; Ling, C.; Johnson, G.; Li, V.; Luo, R.B.; et al. HER2Δ16 Engages ENPP1 to Promote an Immune-Cold Microenvironment in Breast Cancer. Cancer Immunol. Res. 2023, 11, 1184–1202. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. 2024. Available online: https://www.proteinatlas.org/ENSG00000128609-NDUFA5/cancer (accessed on 6 December 2024).
- Johnston, S.R.D.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef]
- Chen, Z.; Yuan, Y.C.; Wang, Y.; Liu, Z.; Chan, H.J.; Chen, S. Down-regulation of programmed cell death 4 (PDCD4) is associated with aromatase inhibitor resistance and a poor prognosis in estrogen receptor-positive breast cancer. Breast Cancer Res. Treat. 2015, 152, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=CCNB2 (accessed on 7 December 2024).
- Huang, C.; Park, C.C.; Hilsenbeck, S.G.; Ward, R.; Rimawi, M.F.; Wang, Y.-C.; Shou, J.; Bissell, M.J.; Osborne, C.K.; Schiff, R. β1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res. 2011, 13, R84. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ni, Z.; Li, B.-s.; Yong, X.; Yang, X.; Zhang, J.-w.; Zhang, D.; Qin, Y.; Jie, M.-m.; Dong, H.; et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2017, 66, 31. [Google Scholar] [CrossRef]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006, 66, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- HLA-A. 2024. Available online: https://www.proteinatlas.org/ENSG00000206503-HLA-A/cancer (accessed on 7 December 2024).
- Li, J.; Zhao, L.; Pan, Y.; Ma, X.; Liu, L.; Wang, W.; You, W. SMYD3 overexpression indicates poor prognosis and promotes cell proliferation, migration and invasion in non-small cell lung cancer. Int. J. Oncol. 2020, 57, 756–766. [Google Scholar] [CrossRef]
- Yue, F.R.; Wei, Z.B.; Yan, R.Z.; Guo, Q.H.; Liu, B.; Zhang, J.H.; Li, Z. SMYD3 promotes colon adenocarcinoma (COAD) progression by mediating cell proliferation and apoptosis. Exp. Ther. Med. 2020, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Brasacchio, D.; Alsop, A.E.; Noori, T.; Lufti, M.; Iyer, S.; Simpson, K.J.; Bird, P.I.; Kluck, R.M.; Johnstone, R.W.; Trapani, J.A. Epigenetic control of mitochondrial cell death through PACS1-mediated regulation of BAX/BAK oligomerization. Cell Death Differ. 2017, 24, 961–970. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, L.; He, Q.; Chang, H.; Wang, Z.; Cao, H.; Zhou, Y.; Pan, R.; Chen, Y. Targeting TRMT5 suppresses hepatocellular carcinoma progression via inhibiting the HIF-1α pathways. J. Zhejiang Univ. Sci. B 2023, 24, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Peng, L.; Li, J.; Liu, X.; Xia, X.; Li, G. PPP1R14B Is a Prognostic and Immunological Biomarker in Pan-Cancer. Front. Genet. 2021, 12, 763561. [Google Scholar] [CrossRef]
- Patel, S.A.; Hassan, M.K.; Dixit, M. Oncogenic activation of EEF1A2 expression: A journey from a putative to an established oncogene. Cell. Mol. Biol. Lett. 2024, 29, 6. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, J.; Yu, S.; Sun, B.; Zhang, W. Anticancer Small-Molecule Agents Targeting Eukaryotic Elongation Factor 1A: State of the Art. Int. J. Mol. Sci. 2023, 24, 5184. [Google Scholar] [CrossRef]
- Giudici, F.; Petracci, E.; Nanni, O.; Bottin, C.; Pinamonti, M.; Zanconati, F.; Scaggiante, B. Correction: Elevated levels of eEF1A2 protein expression in triple negative breast cancer relate with poor prognosis. PLoS ONE 2019, 14, e0227068. [Google Scholar] [CrossRef]
- Mattijssen, S.; Kozlov, G.; Fonseca, B.D.; Gehring, K.; Maraia, R.J. LARP1 and LARP4: Up close with PABP for mRNA 3′ poly(A) protection and stabilization. RNA Biol. 2021, 18, 259–274. [Google Scholar] [CrossRef]
- Ranjan, A.; Mattijssen, S.; Charlly, N.; Gallardo, I.C.; Pitman, L.F.; Coleman, J.C.; Conte, M.R.; Maraia, R.J. The short conserved region-2 of LARP4 interacts with ribosome-associated RACK1 and promotes translation. Nucleic Acids Res. 2025, 53, gkaf053. [Google Scholar] [CrossRef]
- Coleman, J.C.; Hallett, S.R.; Grigoriadis, A.E.; Conte, M.R. LARP4A and LARP4B in cancer: The new kids on the block. Int. J. Biochem. Cell Biol. 2023, 161, 106441. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiu, Y.; Ching, W.-K.; Zhao, P.; Zou, Q. PCB: A pseudotemporal causality-based Bayesian approach to identify EMT-associated regulatory relationships of AS events and RBPs during breast cancer progression. PLoS Comput. Biol. 2023, 19, e1010939. [Google Scholar] [CrossRef]
- You, D.; Du, D.; Li, X.; Hu, X. Expression of Malic Enzyme 3 in Breast Cancer and Precancerous Lesions: A Promising Novel Biomarker for Carcinogenesis and Prognosis. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Kumar, D.; Hassan, M.K.; Pattnaik, N.; Mohapatra, N.; Dixit, M. Reduced expression of IQGAP2 and higher expression of IQGAP3 correlates with poor prognosis in cancers. PLoS ONE 2017, 12, e0186977. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Allan, A.L. Anti-proliferative and anti-migratory effects of EGFR and c-Met tyrosine kinase inhibitors in triple negative breast cancer cells. Precis. Cancer Med. 2021, 4. [Google Scholar] [CrossRef]
- Liao, Y.; Xia, X.; Liu, N.; Cai, J.; Guo, Z.; Li, Y.; Jiang, L.; Dou, Q.P.; Tang, D.; Huang, H.; et al. Growth arrest and apoptosis induction in androgen receptor-positive human breast cancer cells by inhibition of USP14-mediated androgen receptor deubiquitination. Oncogene 2018, 37, 1896–1910. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Imani, S.; Jomhori, M.; Ling, K.H. Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs. the current chemotherapy. Am. J. Cancer Res. 2021, 11, 5155–5183. [Google Scholar]
- Guo, Y.; Pei, X. Tetrandrine-Induced Autophagy in MDA-MB-231 Triple-Negative Breast Cancer Cell through the Inhibition of PI3K/AKT/mTOR Signaling. Evid. Based Complement. Alternat Med. 2019, 2019, 7517431. [Google Scholar] [CrossRef]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef]
- Gautam, P.; Karhinen, L.; Szwajda, A.; Jha, S.K.; Yadav, B.; Aittokallio, T.; Wennerberg, K. Identification of selective cytotoxic and synthetic lethal drug responses in triple negative breast cancer cells. Mol. Cancer 2016, 15, 34. [Google Scholar] [CrossRef]
- Sorice, A.; Guerriero, E.; Volpe, M.G.; Capone, F.; La Cara, F.; Ciliberto, G.; Colonna, G.; Costantini, S. Differential Response of Two Human Breast Cancer Cell Lines to the Phenolic Extract from Flaxseed Oil. Molecules 2016, 21, 319. [Google Scholar] [CrossRef]
- Wang, X.; Reyes, M.E.; Zhang, D.; Funakoshi, Y.; Trape, A.P.; Gong, Y.; Kogawa, T.; Eckhardt, B.L.; Masuda, H.; Pirman Jr, D.A.; et al. EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer. Oncotarget 2017, 8, 67904. [Google Scholar] [CrossRef]
- Alvarado-Ortiz, E.; de la Cruz-López, K.G.; Becerril-Rico, J.; Sarabia-Sánchez, M.A.; Ortiz-Sánchez, E.; García-Carrancá, A. Mutant p53 Gain-of-Function: Role in Cancer Development, Progression, and Therapeutic Approaches. Front. Cell Dev. Biol. 2020, 8, 607670. [Google Scholar] [CrossRef]
- Fan, Q.W.; Cheng, C.K.; Gustafson, W.C.; Charron, E.; Zipper, P.; Wong, R.A.; Chen, J.; Lau, J.; Knobbe-Thomsen, C.; Weller, M.; et al. EGFR phosphorylates tumor-derived EGFRvIII driving STAT3/5 and progression in glioblastoma. Cancer Cell 2013, 24, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.A.; Chu, P.Y.; Tan, Z.L.; Hsu, F.T.; Lee, Y.C.; Wu, H.J. STAT3 Inactivation and Induction of Apoptosis Associate With Fluoxetine-inhibited Epithelial-mesenchymal Transition and Growth of Triple-negative Breast Cancer In Vivo. Anticancer Res. 2022, 42, 3807–3814. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lei, Y.; Zhang, H.; Zhang, M.; Dayton, A. Interleukin-12 treatment down-regulates STAT4 and induces apoptosis with increasing ROS production in human natural killer cells. J. Leukoc. Biol. 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Dumon, S.; Santos, S.C.R.; Debierre-Grockiego, F.; Gouilleux-Gruart, V.; Cocault, L.; Boucheron, C.; Mollat, P.; Gisselbrecht, S.; Gouilleux, F. IL-3 dependent regulation of Bcl-xL gene expression by STAT5 in a bone marrow derived cell line. Oncogene 1999, 18, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Kieslinger, M.; Woldman, I.; Moriggl, R.; Hofmann, J.; Marine, J.C.; Ihle, J.N.; Beug, H.; Decker, T. Antiapoptotic activity of Stat5 required during terminal stages of myeloid differentiation. Genes Dev. 2000, 14, 232–244. [Google Scholar] [CrossRef]
- Hassel, J.C.; Winnemöller, D.; Schartl, M.; Wellbrock, C. STAT5 contributes to antiapoptosis in melanoma. Melanoma Res. 2008, 18, 378–385. [Google Scholar] [CrossRef]
- El Ahanidi, H.; El Azzouzi, M.; Addoum, B.; Mohammed; Hassan, I.; Al Bouzidi, A.; Oukabli, M.; Hafidi Alaoui, C.; Chaoui, I.; Benbacer, L.; et al. STAT1 and STAT4 expression as prognostic biomarkers in patients with bladder cancer. Mol. Clin. Oncol. 2025, 22, 33. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Shi, W.; Li, X.; Yu, L. Prognostic roles of signal transducers and activators of transcription family in human breast cancer. Biosci. Rep. 2018, 38, BSR20171175. [Google Scholar] [CrossRef] [PubMed]
- Klahan, S.; Huang, W.C.; Chang, C.M.; Wong, H.S.; Huang, C.C.; Wu, M.S.; Lin, Y.C.; Lu, H.F.; Hou, M.F.; Chang, W.C. Gene expression profiling combined with functional analysis identify integrin beta1 (ITGB1) as a potential prognosis biomarker in triple negative breast cancer. Pharmacol. Res. 2016, 104, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guan, Y.; Zhang, Y.; Jia, Y.; Li, W.; Guo, C.; Li, Y.; Wang, X.; Shi, Y.; Wang, Q.; et al. Programmed cell death 4 modulates lysosomal function by inhibiting TFEB translation. Cell Death Differ. 2021, 28, 1237–1250. [Google Scholar] [CrossRef]
- Li, R.; Jiang, X.; Zhang, Y.; Wang, S.; Chen, X.; Yu, X.; Ma, J.; Huang, X. Cyclin B2 Overexpression in Human Hepatocellular Carcinoma is Associated with Poor Prognosis. Arch. Med. Res. 2019, 50, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, A.D.; Juric, I.; Singh, S.; Rayman, P.A.; Pavicic, P.G.; Powers, J.; Parthasarathy, P.B.; Al-Hilli, Z.; Ko, J.S.; Chan, T.; et al. Responses to checkpoint inhibition in metastatic triple negative breast cancer driven by divergent myeloid phenotypes. Commun. Med. 2025, 5, 180. [Google Scholar] [CrossRef]


| Name | Relative Levels |
|---|---|
| VE-Cadherin (Phospho-Tyr731) | 0.3925 |
| MAP3K8/COT (Phospho-Thr290) | 0.4743 |
| BTK (Phospho-Tyr223) | 0.5395 |
| CrkII (Phospho-Tyr221) | 0.5563 |
| IkB-alpha (Phospho-Ser32/36) | 0.5578 |
| Cell Line | Name | Relative Levels |
|---|---|---|
| MDA-MB-231 | STAT4(Phospho-Tyr-693) | 0.563 |
| JAK2(Phospho-Tyr1007) | 0.785 | |
| Mek1(Phospho-Ser221) | 0.939 | |
| MDA-MB-468 | STAT4(Phospho-Tyr-693) | 0.719 |
| STAT6(Phospho-Thr-645) | 0.800 | |
| TYK2(Phospho-Tyr1054) | 0.817 |
| Protein of Interest | Binding Affinity (kcal/mol) | Vdw Energy (kcal/mol) | Elec. Energy (kcal/mol) |
|---|---|---|---|
| STAT4 | −8.481 | −24.299 | −9.018 |
| EGFR | −6.718 | −7.117 | −23.382 |
| Pathway in MDA-MB-231 | p-value | Number of Proteins | Proteins |
| Protein ubiquitination | 2.39 × 10−2 | 1 | UBE2E3 |
| TNFR2 | 2.46 × 10−2 | 1 | TANK |
| D-myo-inositol (1,4,5,6)-tetrakisphosphate biosynthesis | 9.95 × 10−3 | 2 | ENPP1, PPP1R14B |
| Protein Kinase A Signaling | 3.99 × 10−2 | 2 | ENPP1, PPP1R14B |
| Pathway in MDA-MB-468 | p-value | Number of Proteins | Proteins |
| E3 ubiquitin ligases ubiquitinate target proteins | 1.80 × 10−3 | 2 | HLA-A, HLA-B |
| PD-1, PDL-1 immunotherapy pathway | 2.24 × 10−4 | 3 | HLA-A, HLA-B, PDCD4 |
| Natural Killer Cell Signaling | 1.42 × 10−3 | 3 | HLA-A, HLA-B, ITGB1 |
| Cell Junction Organization | 4.94 × 10−3 | 2 | CLDN7, ITGB1 |
| Protein | Protein Symbol | log2 Fold Change | p-Value | Biological Role |
|---|---|---|---|---|
| Decreased Protein Levels in MDA-MB-231 | ||||
| Q8WW12 | PCNP | −0.80167 | 0.002767 | PEST-containing nuclear protein (PCNP) promotes proliferation, migration, and invasion in lung adenocarcinoma cells while inhibiting apoptosis through p-STAT3 and p-STAT5 activation [45]. |
| Q14978 | NOLC1 | −0.83644 | 0.025729 | Nucleolar and coiled-body phosphoprotein 1 (NOLC1) supports cancer stem cell properties, promoting tumor growth, therapy resistance, and relapse. Its silence in TNBC cells reduces stemness markers (MYC, ALDH) and sphere formation. High NOLC1 levels are linked to poor prognosis, highlighting its potential as a therapeutic target [46]. |
| Q92844 | TANK | −0.63767 | 0.007082 | TRAF family member-associated NF-κB activator (TANK) contributes to an immunosuppressive tumor microenvironment and regulates genes involved in cell survival and inflammation. Its overexpression is linked to poor glioma prognosis [47]. |
| Q969T4 | UBE2E3 | −0.60915 | 0.034159 | Ubiquitin-conjugating enzyme E2 E3 (UBE2E3) interacts with c-Cbl to upregulate EGFR, activating the MAPK pathway and driving tumor growth and progression [48]. |
| P22413 | ENPP1 | −0.51164 | 0.035984 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1) suppression in HER2Δ16 tumors slows growth and increases immune infiltration, highlighting its role in limiting immune response in these tumors [49]. |
| Decreased Protein Levels in MDA-MB-468 | ||||
| Q16718 | NDUFA5 | −0.88316 | 0.032715 | NADH:Ubiquinone Oxidoreductase Subunit A5 (NDUFA5) mutations or downregulation of this gene impairs mitochondrial activity, reducing ATP production and increasing oxidative stress [50]. |
| Q53EL6 | PDCD4 | −0.94785 | 0.002245 | Programmed Cell Death Protein 4 (PDCD4) is a well-known tumor suppressor gene. Its low expression is associated with Aromatase inhibitor resistance—a drug primarily used in the treatment of hormone receptor-positive breast cancer, especially in postmenopausal women [51,52]. Its low expression is also associated with poor prognosis and reduced disease-free survival in ER-positive, high-grade tumors [52]. |
| O95067 | CCNB2 | −0.82997 | 0.025429 | Cyclin B2 (CCNB2) is associated with breast cancer and anauxetic dysplasia 1, playing roles in mitotic cell cycle regulation and microtubule organization [53]. |
| P05556 | ITGB1 | −0.93412 | 0.028627 | β1 integrin (ITGB1) drives breast tumor progression, supporting proliferation, survival, and resistance to lapatinib. Inhibiting β1 integrin in early-stage breast cancer cells reverses malignant behavior in laminin-rich extracellular matrix cultures [54,55,56]. |
| P04439 | HLA-A | −0.87185 | 0.026263 | Human Leukocyte Antigen A (HLA-A) plays a key role in the immune system by encoding proteins that present peptides to T cells, crucial for pathogen defense and identifying abnormal or cancerous cells [57]. |
| Increased Protein Levels in MDA-MB-231 | ||||
| Q9H7B4 | SMYD3 | 0.536685 | 0.038703 | SET and MYND Domain Containing 3 (SMYD3) upregulation is linked to poor prognosis in cancers by activating oncogenes and promoting cell survival. Its inhibition could suppress tumor growth and enhance chemotherapy sensitivity [58,59]. |
| Q6VY07 | PACS1 | 0.508768 | 0.046877 | Phosphofurin Acidic Cluster Sorting Protein 1 (PACS1) regulates the intrinsic apoptotic pathway by controlling BAX/BAK oligomerization and mitochondrial outer membrane permeabilization. Cells with reduced PACS1 expression resist apoptosis from various stimuli but remain sensitive to TRAIL receptor ligation [60]. |
| Q32P41 | TRMT5 | 0.610423 | 0.015878 | tRNA Methyltransferase 5 (TRMT5) is linked to cancer progression and targeting it may inhibit hepatocellular carcinoma progression and enhance chemotherapy sensitivity [61]. |
| Q96C90 | PPP1R14B | 0.750876 | 0.007414 | Upregulation of Protein Phosphatase 1 Regulatory Inhibitor Subunit 14B (PPP1R14B) is linked to poor prognosis in cancers, correlating with increased immune cell infiltration, particularly myeloid-derived suppressor cells. This may contribute to an immunosuppressive tumor microenvironment, promoting tumor progression in pancreatic cancer [62]. |
| Q8N6R0 | eEF1A-KNMT | 0.92339 | 0.051941 | Eukaryotic Translation Elongation Factor 1A Lysine N-Methyltransferase (eEF1A-KNMT) regulates cancer progression by affecting protein synthesis and tumor cell survival. Its overexpression is linked to increased tumor proliferation and metastasis, including triple-negative breast cancer [63,64,65]. |
| Increased Protein Levels in MDA-MB-468 | ||||
| Q71RC2 | LARP4 | 0.511559 | 0.008935 | La Ribonucleoprotein 4 (LARP4) interacts with mRNAs to regulate cell proliferation by stabilizing transcripts and enhancing their translation. It binds to 3′ UTRs or competes with microRNA machinery [66], and through interaction with PABPC1, promotes synthesis of proteins involved in proliferation and survival [67]. LARP4 is also implicated in survival and metastasis, and its expression decreases during epithelial-to-mesenchymal transition in breast cancer [68,69]. |
| Q16798 | ME3 | 0.679279 | 0.039963 | Malic Enzyme 3, NADP(+)-Dependent, Mitochondrial (ME3), expression is linked to negative lymph node metastasis, and patients with positive ME3 expression have a better prognosis in breast cancer [70]. |
| Q13576 | IQGAP2 | 0.689599 | 0.053686 | IQ Motif Containing GTPase Activating Protein 2 (IQGAP2) may act as a tumor suppressor, with its downregulation linked to poor prognosis in cancers like breast, lung, and gastric. Reduced IQGAP2 expression promotes cell migration, invasion, and EMT, key processes in tumor progression and metastasis [71]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Álvarez, A.O.; Nieves-Reyes, J.; Borges-Vélez, G.; Pérez-Santiago, J.; Rivera-García, M.; Alicea-Ayala, S.; Ospina-Millan, C.; Valiyeva, F.; Vivas-Mejia, P.E. Molecular Basis of Simalikalactone D Sensitivity in Triple-Negative Breast Cancer Cells. Biomolecules 2025, 15, 1561. https://doi.org/10.3390/biom15111561
Sánchez-Álvarez AO, Nieves-Reyes J, Borges-Vélez G, Pérez-Santiago J, Rivera-García M, Alicea-Ayala S, Ospina-Millan C, Valiyeva F, Vivas-Mejia PE. Molecular Basis of Simalikalactone D Sensitivity in Triple-Negative Breast Cancer Cells. Biomolecules. 2025; 15(11):1561. https://doi.org/10.3390/biom15111561
Chicago/Turabian StyleSánchez-Álvarez, Annelis O., Joshua Nieves-Reyes, Gabriel Borges-Vélez, Josué Pérez-Santiago, Misael Rivera-García, Stella Alicea-Ayala, Claudia Ospina-Millan, Fatima Valiyeva, and Pablo E. Vivas-Mejia. 2025. "Molecular Basis of Simalikalactone D Sensitivity in Triple-Negative Breast Cancer Cells" Biomolecules 15, no. 11: 1561. https://doi.org/10.3390/biom15111561
APA StyleSánchez-Álvarez, A. O., Nieves-Reyes, J., Borges-Vélez, G., Pérez-Santiago, J., Rivera-García, M., Alicea-Ayala, S., Ospina-Millan, C., Valiyeva, F., & Vivas-Mejia, P. E. (2025). Molecular Basis of Simalikalactone D Sensitivity in Triple-Negative Breast Cancer Cells. Biomolecules, 15(11), 1561. https://doi.org/10.3390/biom15111561








