High-Intensity Pulse Magnetic Fields Affect Redox Homeostasis and Survival Rate of Escherichia coli According to Initial Level of Intracellular Glucose
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Sample Preparation
2.2. HI-PMF Treatment
2.3. Starvation Stimulation and Glucose Stimulation
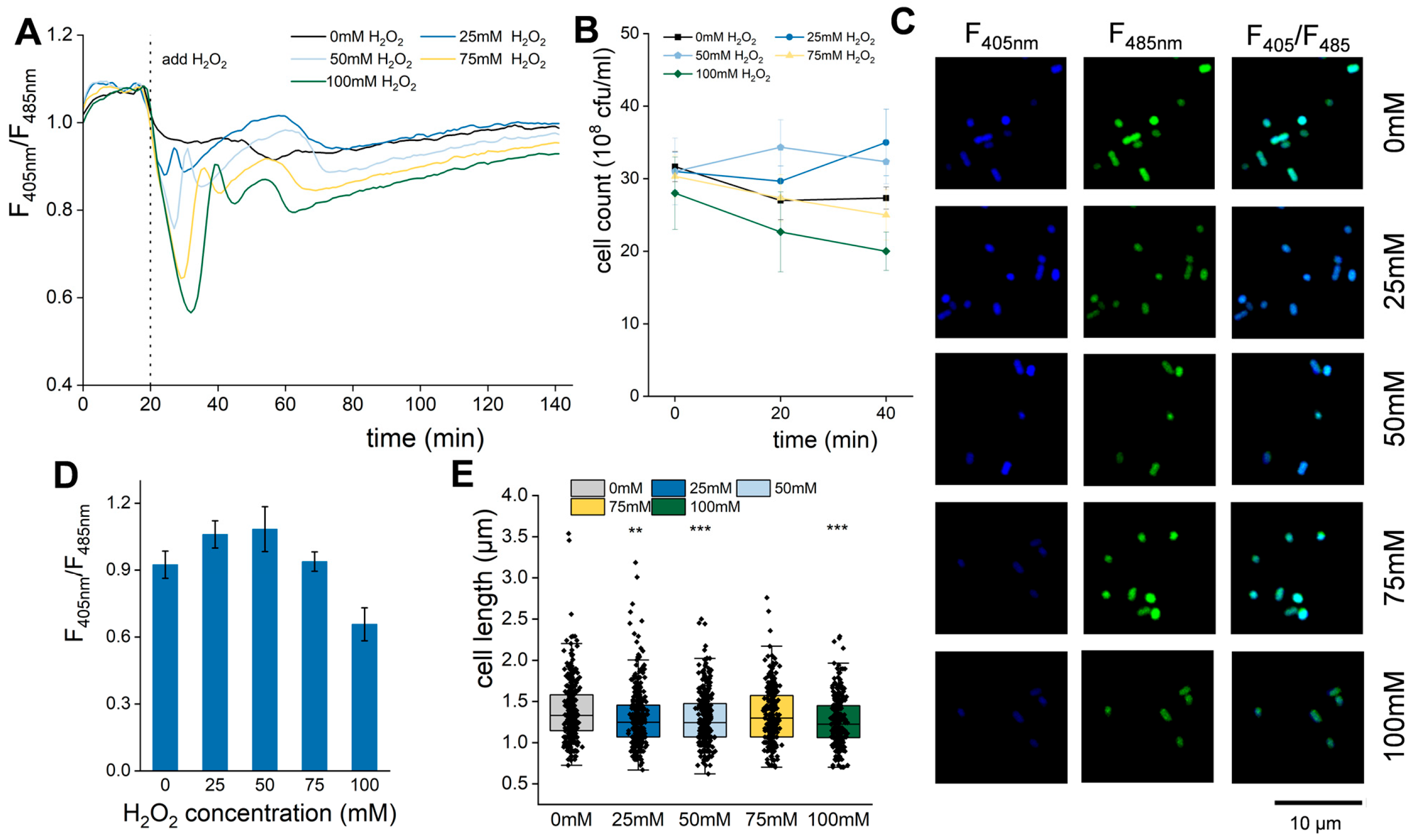
2.4. H2O2 Stimulation
2.5. Detection of Growth
2.6. Cell Counting
2.7. Image Collection and Cell Length Measurement
2.8. Fluorescence Intensity Detection
2.9. RNA Extraction and Sequencing
2.10. Transcriptome Sequencing, Analysis and qRT-PCR
2.11. Glycogen and Glucose Concentration Measurement
2.12. Statistical Analysis
3. Results
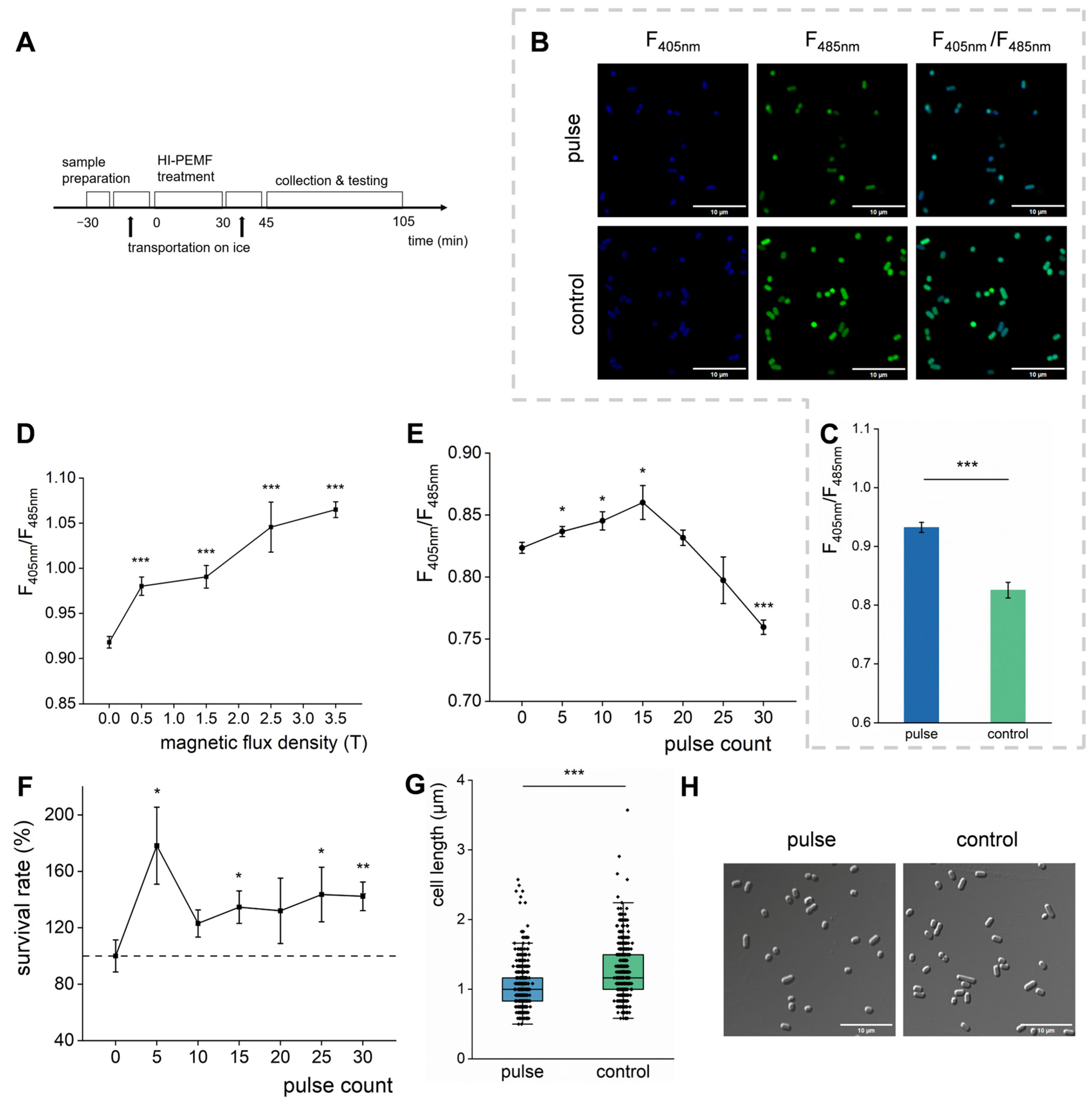
3.1. HI-PMF Affects the Ratio of NADH and NAD+ in E. coli
3.2. HI-PMF Increases the Survival Rate of E. coli Cells by Promoting Their Division
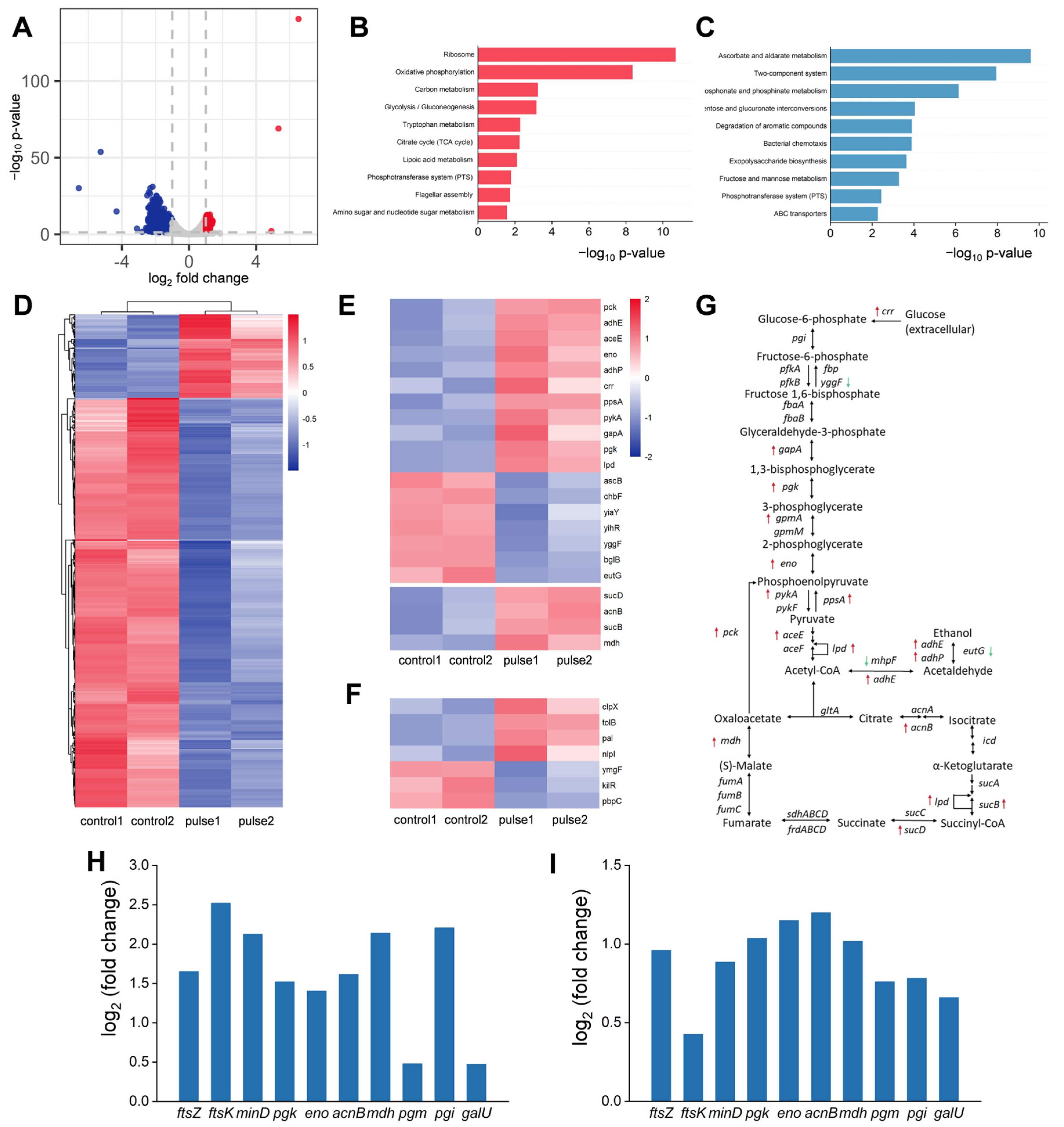
3.3. Differentially Expressed Genes in E. coli After Being Treated by HI-PMF and Their Expression Profiles
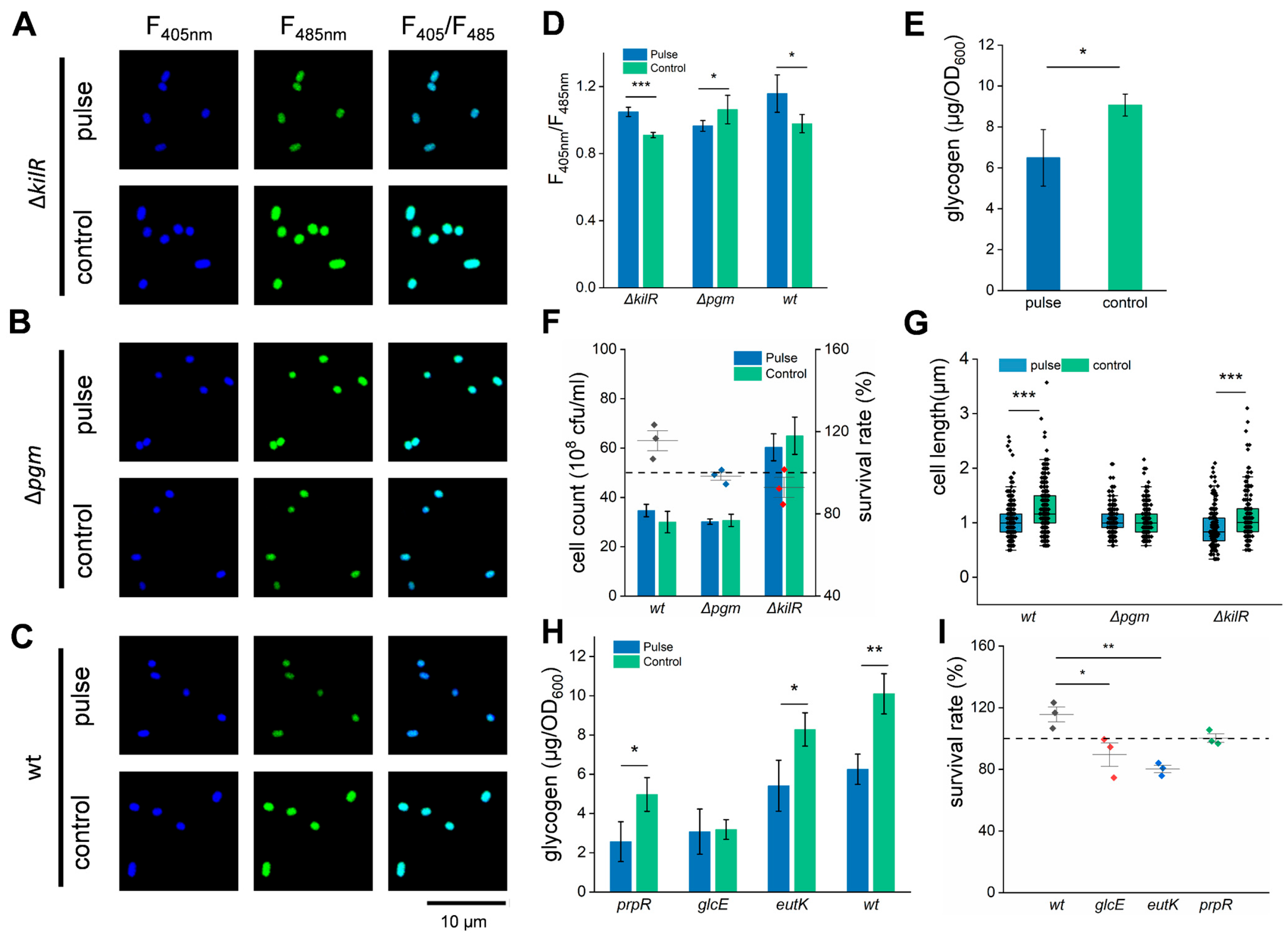
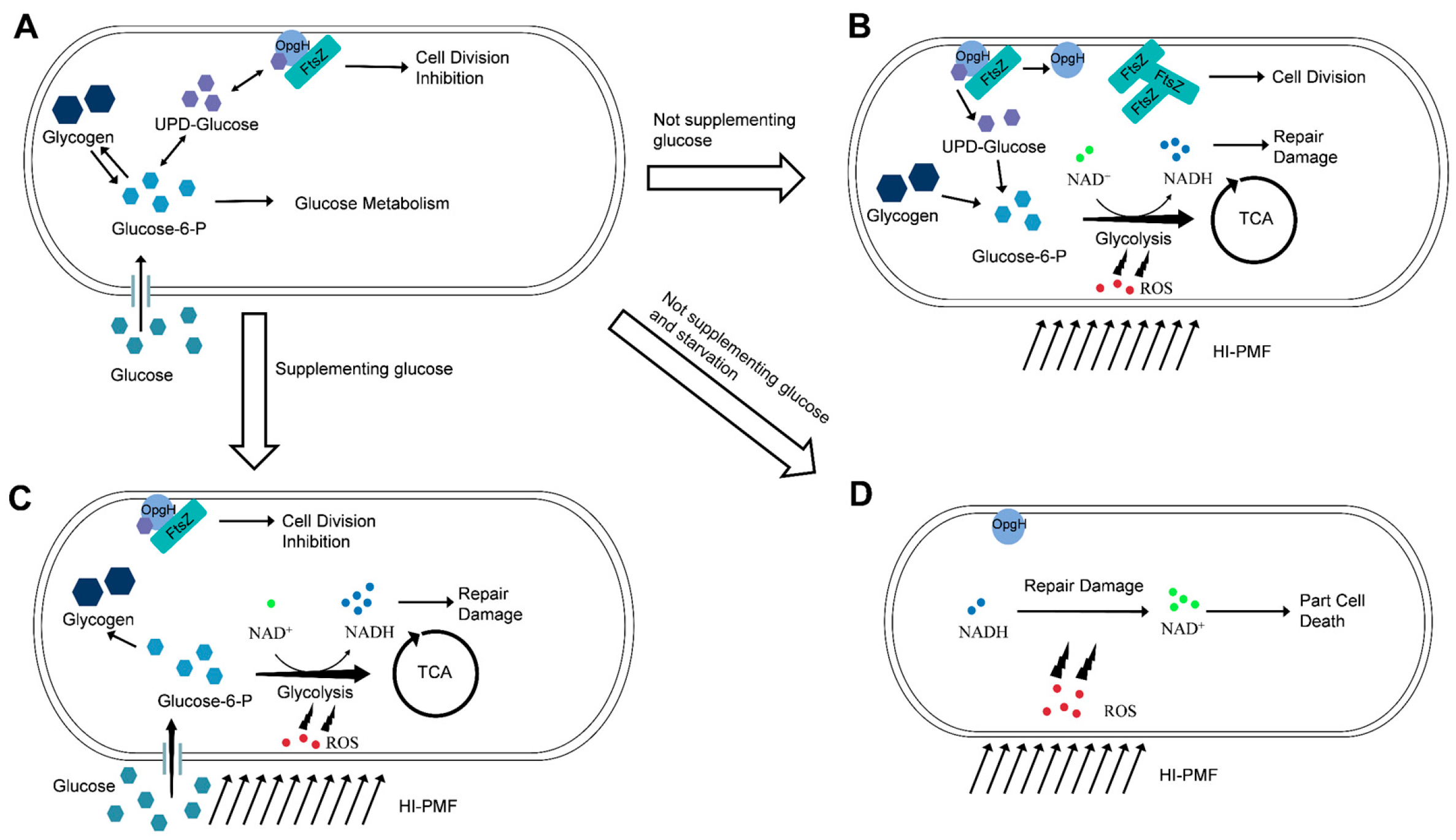
3.4. HI-PMF Treatment Relieves Nutritional Inhibition on Cell Division of E. coli
3.5. Starvation Incubation Decreases the Survival of E. coli Under HI-PMF Treatment
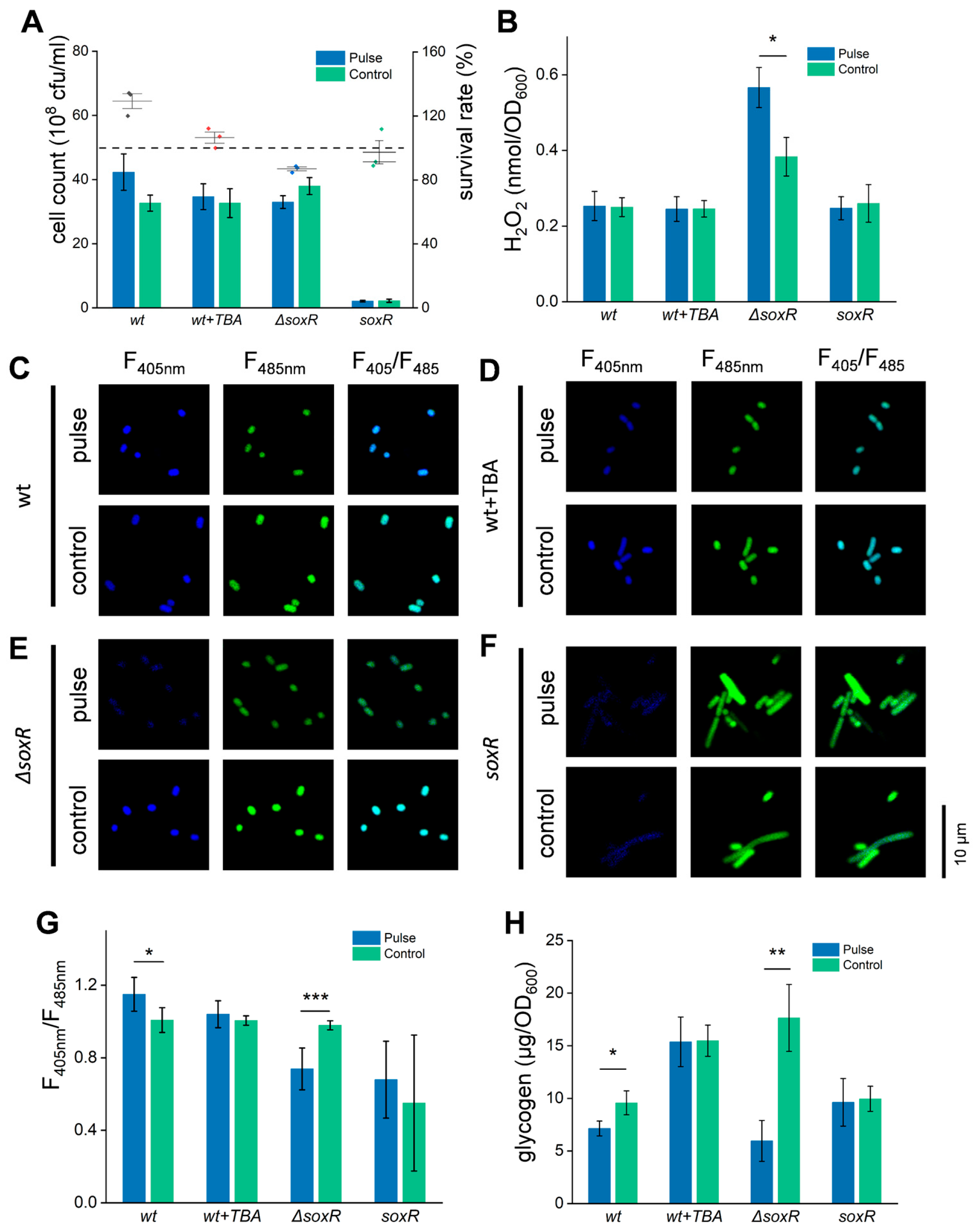
3.6. ROS Participates in the Response of E. coli Cells to HI-PMF Treatment
3.7. Appropriate ROS Stimulation Can Promote E. coli Cell Division
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MF | Magnetic field |
| HI-PMF | High-intensity pulse magnetic field |
| ROS | Reactive oxygen species |
| GMF | Geomagnetic field |
| EMF | Electromagnetic field |
| WI-MFs | Weak-intensity magnetic fields |
| MRI | Magnetic resonance imaging |
| RPM | Radical pair mechanism |
| FDR | False discovery rate |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FC | Fold change |
| DEGs | Differentially expressed genes |
| TBA | Tert-butylalcohol |
References
- Ozturk, S.F.; Bhowmick, D.K.; Kapon, Y.; Sang, Y.; Kumar, A.; Paltiel, Y.; Naaman, R.; Sasselov, D.D. Chirality-Induced Avalanche Magnetization of Magnetite by an RNA Precursor. Nat. Commun. 2023, 14, 6351. [Google Scholar] [CrossRef]
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J.C.; et al. Magnetic Sensitivity of Cryptochrome 4 from a Migratory Songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef]
- Karipidis, K.; Baaken, D.; Loney, T.; Blettner, M.; Brzozek, C.; Elwood, M.; Narh, C.; Orsini, N.; Röösli, M.; Paulo, M.S.; et al. The Effect of Exposure to Radiofrequency Fields on Cancer Risk in the General and Working Population: A Systematic Review of Human Observational Studies—Part I: Most Researched Outcomes. Environ. Int. 2024, 191, 108983. [Google Scholar] [CrossRef]
- Moon, J.-H. Health Effects of Electromagnetic Fields on Children. Clin. Exp. Pediatr. 2020, 63, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, B.; Lu, Y.; Zhao, Y.; Tang, X.; Shi, Y. Interactions between Electromagnetic Radiation and Biological Systems. iScience 2024, 27, 109201. [Google Scholar] [CrossRef] [PubMed]
- Ikeya, N.; Woodward, J.R. Cellular Autofluorescence Is Magnetic Field Sensitive. Proc. Natl. Acad. Sci. USA 2021, 118, e2018043118. [Google Scholar] [CrossRef] [PubMed]
- Del Sol-Fernández, S.; Martínez-Vicente, P.; Gomollón-Zueco, P.; Castro-Hinojosa, C.; Gutiérrez, L.; Fratila, R.M.; Moros, M. Magnetogenetics: Remote Activation of Cellular Functions Triggered by Magnetic Switches. Nanoscale 2022, 14, 2091–2118. [Google Scholar] [CrossRef]
- Nimpf, S.; Keays, D.A. Is Magnetogenetics the New Optogenetics? EMBO J. 2017, 36, 1643–1646. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Gu, J.; Xia, L.; Cao, Y.; Zhu, X.; Wen, H.; Ouyang, S.; Liu, R.; Li, J.; et al. Magnetically Driven Capsules with Multimodal Response and Multifunctionality for Biomedical Applications. Nat. Commun. 2024, 15, 1839. [Google Scholar] [CrossRef]
- Dufor, T.; Grehl, S.; Tang, A.D.; Doulazmi, M.; Traoré, M.; Debray, N.; Dubacq, C.; Deng, Z.-D.; Mariani, J.; Lohof, A.M.; et al. Neural Circuit Repair by Low-Intensity Magnetic Stimulation Requires Cellular Magnetoreceptors and Specific Stimulation Patterns. Sci. Adv. 2019, 5, eaav9847. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, J.; Wang, X.; Xu, Y.; Liang, Z.; Gu, X.; He, C. Coupling Induction of Osteogenesis and Type H Vessels by Pulsed Electromagnetic Fields in Ovariectomy-Induced Osteoporosis in Mice. Bone 2022, 154, 116211. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, C.M.; Cogo, A.J.D.; Perez, V.H.; Dos Santos, N.F.; Okorokova-Façanha, A.L.; Justo, O.R.; Façanha, A.R. Increases of Bioethanol Productivity by S. cerevisiae in Unconventional Bioreactor under ELF-Magnetic Field: New Advances in the Biophysical Mechanism Elucidation on Yeasts. Renew. Energy 2021, 169, 836–842. [Google Scholar] [CrossRef]
- Li, W.-W.; Sheng, G.-P.; Liu, X.-W.; Cai, P.-J.; Sun, M.; Xiao, X.; Wang, Y.-K.; Tong, Z.-H.; Dong, F.; Yu, H.-Q. Impact of a Static Magnetic Field on the Electricity Production of Shewanella-Inoculated Microbial Fuel Cells. Biosens. Bioelectron. 2011, 26, 3987–3992. [Google Scholar] [CrossRef]
- Asgari, G.; Seid-Mohammadi, A.; Shokoohi, R.; Samarghandi, M.R.; Daigger, G.T.; Malekolkalami, B.; Khoshniyat, R. Exposure of the Static Magnetic Fields on the Microbial Growth Rate and the Sludge Properties in the Complete-Mix Activated Sludge Process (a Lab-Scale Study). Microb. Cell Fact. 2023, 22, 195. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Q.; Zhang, Y. Applying Dynamic Magnetic Field to Promote Anaerobic Digestion via Enhancing the Electron Transfer of a Microbial Respiration Chain. Environ. Sci. Technol. 2023, 57, 2138–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X. Magnetic Fields and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 2175. [Google Scholar] [CrossRef]
- Krylov, V.V.; Osipova, E.A. Molecular Biological Effects of Weak Low-Frequency Magnetic Fields: Frequency–Amplitude Efficiency Windows and Possible Mechanisms. Int. J. Mol. Sci. 2023, 24, 10989. [Google Scholar] [CrossRef]
- Van Huizen, A.V.; Morton, J.M.; Kinsey, L.J.; Von Kannon, D.G.; Saad, M.A.; Birkholz, T.R.; Czajka, J.M.; Cyrus, J.; Barnes, F.S.; Beane, W.S. Weak Magnetic Fields Alter Stem Cell–Mediated Growth. Sci. Adv. 2019, 5, eaau7201. [Google Scholar] [CrossRef]
- Inhan-Garip, A.; Aksu, B.; Akan, Z.; Akakin, D.; Ozaydin, A.N.; San, T. Effect of Extremely Low Frequency Electromagnetic Fields on Growth Rate and Morphology of Bacteria. Int. J. Radiat. Biol. 2011, 87, 1155–1161. [Google Scholar] [CrossRef]
- Neuman, K.; Zhang, X.; Lejeune, B.T.; Pizzarella, D.; Vázquez, M.; Lewis, L.H.; Koppes, A.N.; Koppes, R.A. Static Magnetic Stimulation and Magnetic Microwires Synergistically Enhance and Guide Neurite Outgrowth. Adv. Healthc. Mater. 2025, 14, 2403956. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, J.; Dubrowska, K.; Augustyniak, A.; Kordas, M.; Rakoczy, R. Application of Magnetically Assisted Reactors for Modulation of Growth and Pyocyanin Production by Pseudomonas aeruginosa. Front. Bioeng. Biotechnol. 2022, 10, 795871. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhan, A.; Wang, M.; Tian, L.; Guo, W.; Pan, Y. Long-Term Exposure to a Hypomagnetic Field Attenuates Adult Hippocampal Neurogenesis and Cognition. Nat. Commun. 2021, 12, 1174. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jin, Y.; Yang, N.; Wei, L.; Xu, D.; Xu, X. Improving Microbial Production of Value-Added Products through the Intervention of Magnetic Fields. Bioresour. Technol. 2024, 393, 130087. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Gessi, S.; Merighi, S.; Iannotta, V.; Cattabriga, E.; Spisani, S.; Cadossi, R.; Borea, P.A. Effect of Low Frequency Electromagnetic Fields on A2A Adenosine Receptors in Human Neutrophils. Br. J. Pharmacol. 2002, 136, 57–66. [Google Scholar] [CrossRef]
- Pell, G.S.; Roth, Y.; Zangen, A. Modulation of Cortical Excitability Induced by Repetitive Transcranial Magnetic Stimulation: Influence of Timing and Geometrical Parameters and Underlying Mechanisms. Prog. Neurobiol. 2011, 93, 59–98. [Google Scholar] [CrossRef]
- Jabłońska, J.; Augustyniak, A.; Kordas, M.; Dubrowska, K.; Sołoducha, D.; Borowski, T.; Konopacki, M.; Grygorcewicz, B.; Roszak, M.; Dołęgowska, B.; et al. Evaluation of Ferrofluid-Coated Rotating Magnetic Field-Assisted Bioreactor for Biomass Production. Chem. Eng. J. 2022, 431, 133913. [Google Scholar] [CrossRef]
- Tessaro, L.W.E.; Murugan, N.J.; Persinger, M.A. Bacterial Growth Rates Are Influenced by Cellular Characteristics of Individual Species When Immersed in Electromagnetic Fields. Microbiol. Res. 2015, 172, 26–33. [Google Scholar] [CrossRef]
- Tang, L.; Qiu, C.; Zhang, H.; Ren, D. Effects of 0.4 T, 3.0 T and 9.4 T Static Magnetic Fields on Development, Behaviour and Immune Response in Zebrafish (Danio Rerio). NeuroImage 2023, 282, 120398. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, Y.; Tian, X.; Yu, B.; Song, C.; Feng, C.; Zhang, L.; Ji, X.; Zablotskii, V.; Zhang, X. The Anti-Depressive Effects of Ultra-High Static Magnetic Field. Magn. Reson. Imaging 2022, 56, 354–365. [Google Scholar] [CrossRef]
- Khan, M.H.; Huang, X.; Tian, X.; Ouyang, C.; Wang, D.; Feng, S.; Chen, J.; Xue, T.; Bao, J.; Zhang, X. Short- and Long-Term Effects of 3.5–23.0 Tesla Ultra-High Magnetic Fields on Mice Behaviour. Eur. Radiol. 2022, 32, 5596–5605. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yu, X.; Yu, B.; Ji, X.; Tian, X.; Song, C.; Zhang, X. Life on Magnet: Long-Term Exposure of Moderate Static Magnetic Fields on the Lifespan and Healthspan of Mice. Antioxidants 2022, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, C.; Yu, B.; Fan, Y.; Zhang, L.; Zhang, X. 9.4 T Static Magnetic Field Ameliorates Imatinib Mesylate-Induced Toxicity and Depression in Mice. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ji, X.; Fan, Y.; Yu, B.; Wang, X.; Feng, C.; Zhang, L.; Song, C.; Zhang, X. Static Magnetic Fields Protect against Cisplatin-Induced Kidney Toxicity. Antioxidants 2022, 12, 73. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, L.; Li, Z.; Tian, X.; Fang, J.; Lu, Q.; Zhang, X. Cellular ATP Levels Are Affected by Moderate and Strong Static Magnetic Fields. Bioelectromagnetics 2018, 39, 352–360. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, Y.; Li, Z.; Ji, X.; Wang, Z.; Wang, H.; Tian, X.; Yu, F.; Yang, Z.; Pi, L.; et al. 27 T Ultra-High Static Magnetic Field Changes Orientation and Morphology of Mitotic Spindles in Human Cells. eLife 2017, 6, e22911. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.; Zhou, J.; Pan, H. Effects of a Strong Static Magnetic Field on bacterium Shewanella oneidensis: An Assessment by Using Whole Genome Microarray. Bioelectromagnetics 2005, 26, 558–563. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; He, R.; Cui, H. Action Mechanism of Pulsed Magnetic Field against E. coli O157:H7 and Its Application in Vegetable Juice. Food Control 2019, 95, 150–156. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, M.; Dai, C.; Huo, S.; Ma, H. Transcriptomic Analysis of Listeria monocytogenes under Pulsed Magnetic Field Treatment. Food Res. Int. 2020, 133, 109195. [Google Scholar] [CrossRef]
- San Martín, M.F.; Harte, F.M.; Lelieveld, H.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation Effect of an 18-T Pulsed Magnetic Field Combined with Other Technologies on Escherichia coli. Innov. Food Sci. Emerg. Technol. 2001, 2, 273–277. [Google Scholar] [CrossRef]
- Kranjc, M.; Polajžer, T.; Novickij, V.; Miklavčič, D. Determination of the Impact of High-Intensity Pulsed Electromagnetic Fields on the Release of Damage-Associated Molecular Pattern Molecules. Int. J. Mol. Sci. 2023, 24, 14607. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef]
- Cartee, L.A.; Plonsey, R. The Transient Subthreshold Response of Spherical and Cylindrical Cell Models to Extracellular Stimulation. IEEE Trans. Biomed. Eng. 1992, 39, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Cotic, M.; Carlen, P.L. Transmembrane Potential Induced in a Spherical Cell Model under Low-Frequency Magnetic Stimulation. J. Neural Eng. 2007, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Denton, M.C.J.; Smith, L.D.; Xu, W.; Pugsley, J.; Toghill, A.; Kattnig, D.R. Magnetosensitivity of Tightly Bound Radical Pairs in Cryptochrome Is Enabled by the Quantum Zeno Effect. Nat. Commun. 2024, 15, 10823. [Google Scholar] [CrossRef]
- Yang, Z.-S.; Gao, S.; Zhang, J.-L. Magnetic Manipulation of the Reactivity of Singlet Oxygen: From Test Tubes to Living Cells. Natl. Sci. Rev. 2024, 11, nwae069. [Google Scholar] [CrossRef]
- Sherrard, R.M.; Morellini, N.; Jourdan, N.; El-Esawi, M.; Arthaut, L.-D.; Niessner, C.; Rouyer, F.; Klarsfeld, A.; Doulazmi, M.; Witczak, J.; et al. Low-Intensity Electromagnetic Fields Induce Human Cryptochrome to Modulate Intracellular Reactive Oxygen Species. PLoS Biol. 2018, 16, e2006229. [Google Scholar] [CrossRef]
- Mumtaz, S.; Javed, R.; Rana, J.N.; Iqbal, M.; Choi, E.H. Pulsed High Power Microwave Seeds Priming Modulates Germination, Growth, Redox Homeostasis, and Hormonal Shifts in Barley for Improved Seedling Growth: Unleashing the Molecular Dynamics. Free Radic. Biol. Med. 2024, 222, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.S.; Buske, P.J.; Shi, Y.; Levin, P.A. A Moonlighting Enzyme Links Escherichia coli Cell Size with Central Metabolism. PLoS Genet. 2013, 9, e1003663. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene Editing in the Escherichia coli Genome via the CRISPR-Cas9 System. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef]
- Anton-Leberre, V.; Haanappel, E.; Marsaud, N.; Trouilh, L.; Benbadis, L.; Boucherie, H.; Massou, S.; François, J.M. Exposure to High Static or Pulsed Magnetic Fields Does Not Affect Cellular Processes in the Yeast Saccharomyces cerevisiae. Bioelectromagnetics 2009, 31, 28–38. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, Q.; Cheng, F.; Su, N.; Wang, A.; Zou, Y.; Hu, H.; Chen, X.; Zhou, H.-M.; Huang, X.; et al. SoNar, a Highly Responsive NAD+/NADH Sensor, Allows High-Throughput Metabolic Screening of Anti-Tumor Agents. Cell Metab. 2015, 21, 777–789. [Google Scholar] [CrossRef]
- Nascimento, L.F.C.; Botura, G., Jr.; Mota, R.P. Glucose Consume and Growth of E. coli under Electromagnetic Field. Rev. Inst. Med. Trop. S. Paulo 2003, 45, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, P.; Fijałkowski, K.; Struk, M.; Kordas, M.; Rakoczy, R. Effects of 50 Hz Rotating Magnetic Field on the Viability of Escherichia coli and Staphylococcus aureus. Electromagn. Biol. Med. 2014, 33, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, E.; Bes, M.T.; González, A.; Peleato, M.L.; Fillat, M.F. Redox-Based Transcriptional Regulation in Prokaryotes: Revisiting Model Mechanisms. Antioxid. Redox Signal. 2019, 30, 1651–1696. [Google Scholar] [CrossRef]
- Willis, L.; Huang, K.C. Sizing up the Bacterial Cell Cycle. Nat. Rev. Microbiol. 2017, 15, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Q.; Tan, X.; Wang, Z.; Wang, M.; Wise, M.J.; Li, C.; Ma, C.; Li, E.; Deng, B.; et al. Molecular Structure of Glycogen in Escherichia coli. Biomacromolecules 2019, 20, 2821–2829. [Google Scholar] [CrossRef]
- Barshishat, S.; Elgrably-Weiss, M.; Edelstein, J.; Georg, J.; Govindarajan, S.; Haviv, M.; Wright, P.R.; Hess, W.R.; Altuvia, S. OxyS Small RNA Induces Cell Cycle Arrest to Allow DNA Damage Repair. EMBO J. 2018, 37, 413–426. [Google Scholar] [CrossRef]
- Pomposiello, P.J.; Bennik, M.H.J.; Demple, B. Genome-Wide Transcriptional Profiling of the Escherichia coli Responses to Superoxide Stress and Sodium Salicylate. J. Bacteriol. 2001, 183, 3890–3902. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, J.; Ma, J.; Yang, Y.; Luo, C.; Huangfu, X.; Guo, Z. Role of the Propagation Reactions on the Hydroxyl Radical Formation in Ozonation and Peroxone (Ozone/Hydrogen Peroxide) Processes. Water Res. 2015, 68, 750–758. [Google Scholar] [CrossRef]
- Steffan, R.J.; McClay, K.; Vainberg, S.; Condee, C.W.; Zhang, D. Biodegradation of the Gasoline Oxygenates Methyl Tert-Butyl Ether, Ethyl Tert-Butyl Ether, and Tert-Amyl Methyl Ether by Propane-Oxidizing Bacteria. Appl. Environ. Microbiol. 1997, 63, 4216–4222. [Google Scholar] [CrossRef]
- Knoshaug, E.P.; Zhang, M. Butanol Tolerance in a Selection of Microorganisms. Appl. Biochem. Biotechnol. 2009, 153, 13–20. [Google Scholar] [CrossRef]
- Imlay, J.A.; Linn, S. Mutagenesis and Stress Responses Induced in Escherichia coli by Hydrogen Peroxide. J. Bacteriol. 1987, 169, 2967–2976. [Google Scholar] [CrossRef] [PubMed]
- Stratford, J.P.; Edwards, C.L.A.; Ghanshyam, M.J.; Malyshev, D.; Delise, M.A.; Hayashi, Y.; Asally, M. Electrically Induced Bacterial Membrane-Potential Dynamics Correspond to Cellular Proliferation Capacity. Proc. Natl. Acad. Sci. USA 2019, 116, 9552–9557. [Google Scholar] [CrossRef]
- Nyström, T. Stationary-Phase Physiology. Annu. Rev. Microbiol. 2004, 58, 161–181. [Google Scholar] [CrossRef]
- Akerlund, T.; Nordström, K.; Bernander, R. Analysis of Cell Size and DNA Content in Exponentially Growing and Stationary-Phase Batch Cultures of Escherichia coli. J. Bacteriol. 1995, 177, 6791–6797. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic Field Effects in Biology from the Perspective of the Radical Pair Mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N. Nonspecific Magnetic Biological Effects: A Model Assuming the Spin-Orbit Coupling. J. Chem. Phys. 2019, 151, 204101. [Google Scholar] [CrossRef] [PubMed]
- Madjid Ansari, A.; Majidzadeh-A, K.; Darvishi, B.; Sanati, H.; Farahmand, L.; Norouzian, D. Extremely Low Frequency Magnetic Field Enhances Glucose Oxidase Expression in Pichia Pastoris GS115. Enzym. Microb. Technol. 2017, 98, 67–75. [Google Scholar] [CrossRef]
- Da Costa Menestrino, B.; Sala, L.; Costa, J.A.V.; Buffon, J.G.; Santos, L.O. Magnetic Fields Exhibit a Positive Impact on Lipid and Biomass Yield during Phototrophic Cultivation of Spirulina sp. Bioprocess Biosyst. Eng. 2021, 44, 2087–2097. [Google Scholar] [CrossRef]
- Emamdadi, N.; Gholizadeh, M.; Housaindokht, M.R. Investigation of Static Magnetic Field Effect on Horseradish Peroxidase Enzyme Activity and Stability in Enzymatic Oxidation Process. Int. J. Biol. Macromol. 2021, 170, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Tang, X.; Zhao, Y.; Jiang, T.; Zhou, J.; Wang, X.; Huang, B.; Liu, L.; Deng, H.; Huang, Y.; et al. Analysis of Electromagnetic Response of Cells and Lipid Membranes Using a Model-Free Method. Bioelectrochemistry 2023, 152, 108444. [Google Scholar] [CrossRef] [PubMed]
- Prindle, A.; Liu, J.; Asally, M.; Ly, S.; Garcia-Ojalvo, J.; Süel, G.M. Ion Channels Enable Electrical Communication in Bacterial Communities. Nature 2015, 527, 59–63. [Google Scholar] [CrossRef]
- Lee, D.D.; Galera-Laporta, L.; Bialecka-Fornal, M.; Moon, E.C.; Shen, Z.; Briggs, S.P.; Garcia-Ojalvo, J.; Süel, G.M. Magnesium Flux Modulates Ribosomes to Increase Bacterial Survival. Cell 2019, 177, 352–360.e13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Du, L.; Li, Y.; Xu, Z.; Ye, L.; Dai, S.; Xu, L.; Yan, J.; Xie, X.; Cao, Q.; et al. High-Intensity Pulse Magnetic Fields Affect Redox Homeostasis and Survival Rate of Escherichia coli According to Initial Level of Intracellular Glucose. Biomolecules 2025, 15, 1550. https://doi.org/10.3390/biom15111550
Wang P, Du L, Li Y, Xu Z, Ye L, Dai S, Xu L, Yan J, Xie X, Cao Q, et al. High-Intensity Pulse Magnetic Fields Affect Redox Homeostasis and Survival Rate of Escherichia coli According to Initial Level of Intracellular Glucose. Biomolecules. 2025; 15(11):1550. https://doi.org/10.3390/biom15111550
Chicago/Turabian StyleWang, Pengbo, Limeng Du, Yunchong Li, Zitang Xu, Luona Ye, Shuhan Dai, Li Xu, Jinyong Yan, Xiaoman Xie, Quanliang Cao, and et al. 2025. "High-Intensity Pulse Magnetic Fields Affect Redox Homeostasis and Survival Rate of Escherichia coli According to Initial Level of Intracellular Glucose" Biomolecules 15, no. 11: 1550. https://doi.org/10.3390/biom15111550
APA StyleWang, P., Du, L., Li, Y., Xu, Z., Ye, L., Dai, S., Xu, L., Yan, J., Xie, X., Cao, Q., Yang, M., Han, X., & Yan, Y. (2025). High-Intensity Pulse Magnetic Fields Affect Redox Homeostasis and Survival Rate of Escherichia coli According to Initial Level of Intracellular Glucose. Biomolecules, 15(11), 1550. https://doi.org/10.3390/biom15111550








