Hypoxia-Induced Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization and Enhance Angiogenesis to Promote Diabetic Wound Healing
Abstract
1. Introduction
2. Method Materials
2.1. Cell Culture
2.2. Collection and Characterization of hy-EVs Derived from HUCMSCs
2.3. Investigation of hy-EVs Uptake by HUVECs
2.4. Cell Counting Kit-8 (CCK-8)
2.5. Live–Dead Cell Staining
2.6. Transwell Assay
2.7. Scratch Wound Assay
2.8. Tube Formation Assay
2.9. Cell Immunofluorescence
2.10. ROS Measurement
2.11. Protein Extraction and Western Blot
2.12. Animal Experiments
2.13. Histological Analysis
2.14. Immunohistochemical Analysis and Immunofluorescence Analysis
2.15. Statistical Analysis
3. Results
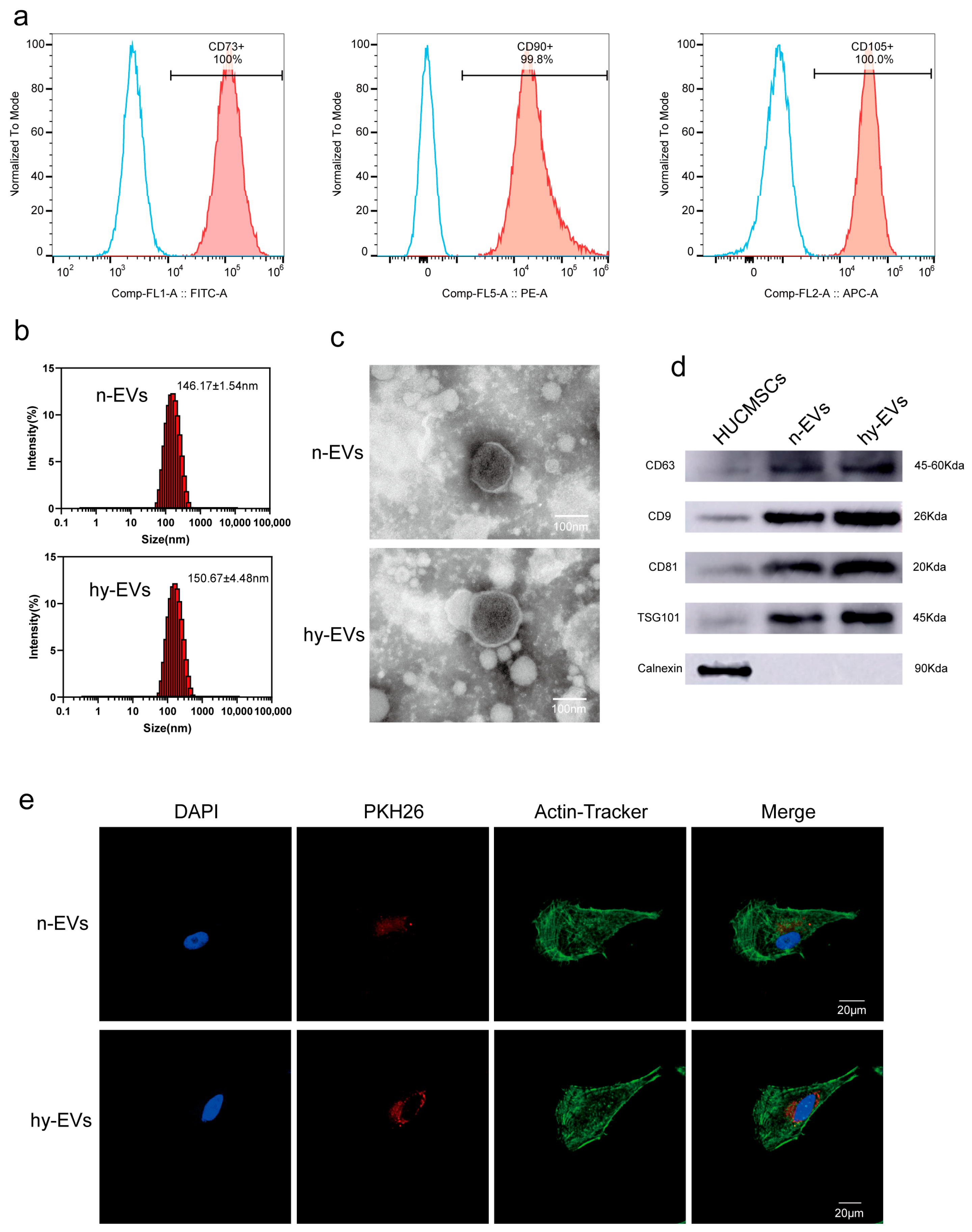
3.1. Isolation and Identification of hy-EVs Derived from HUMSCs
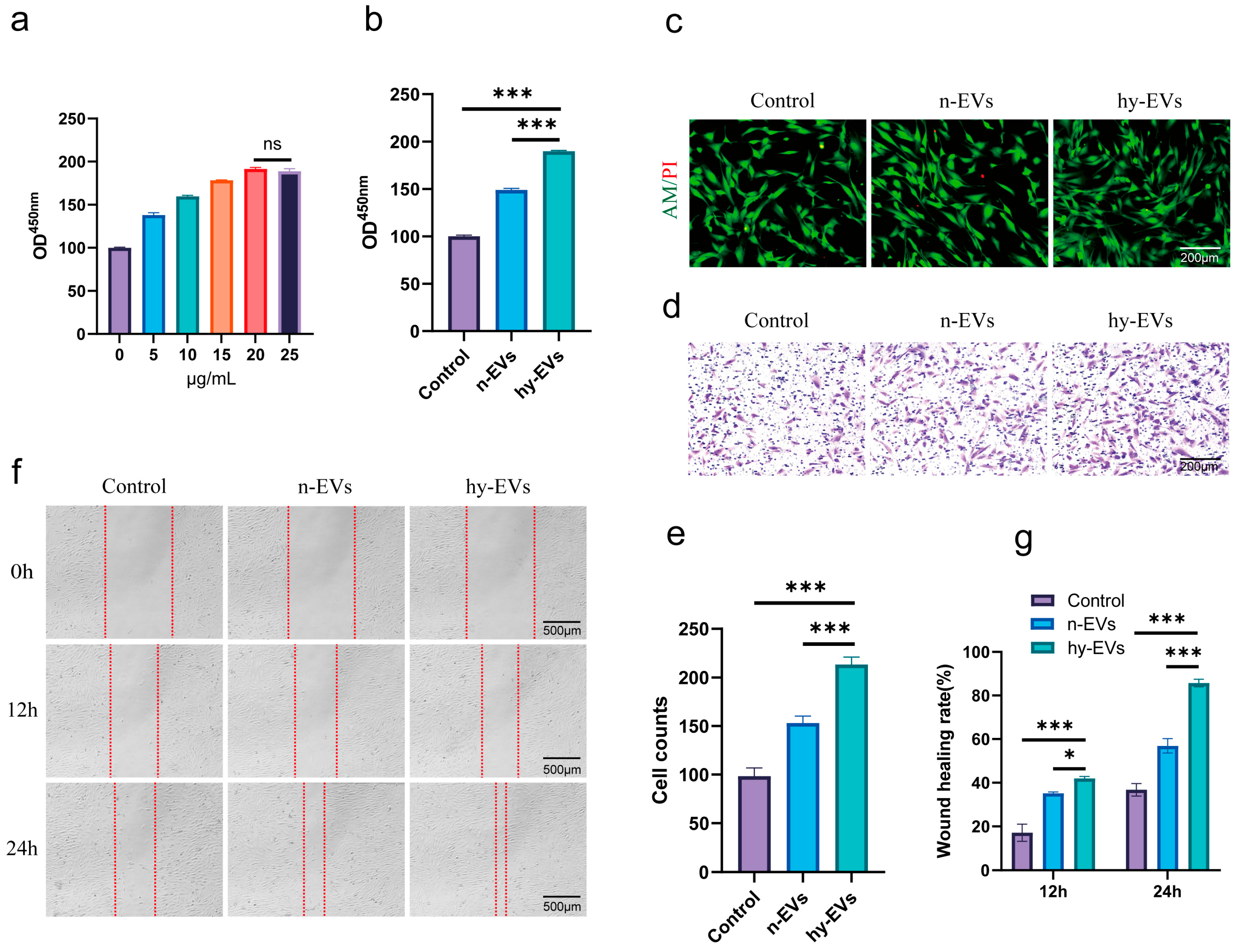
3.2. hy-EVs Derived from HUMSCs Enhanced the Functions of HSFs
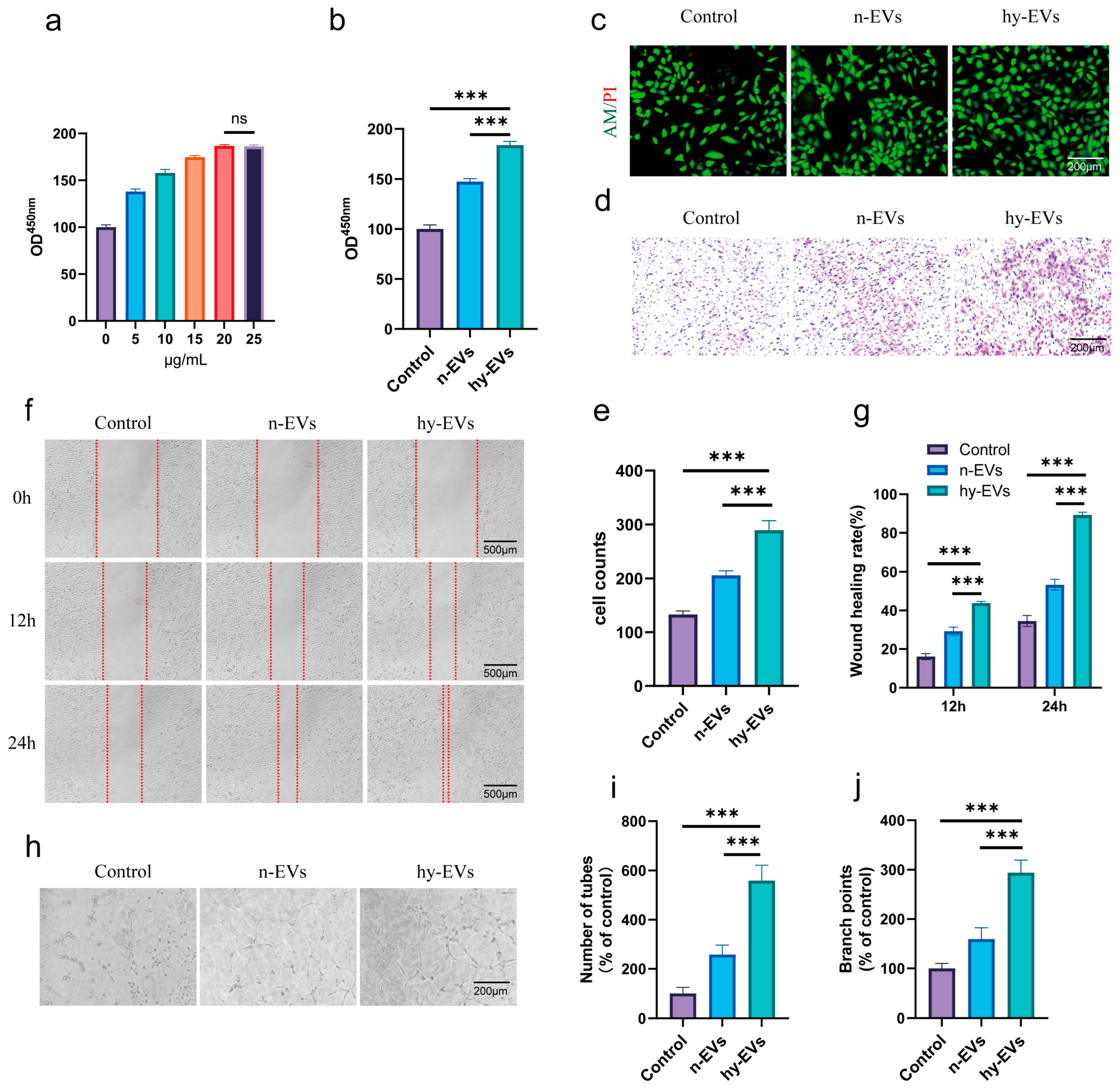
3.3. hy-EVs Derived from HUMSCs Enhanced the Functions of HUVECs
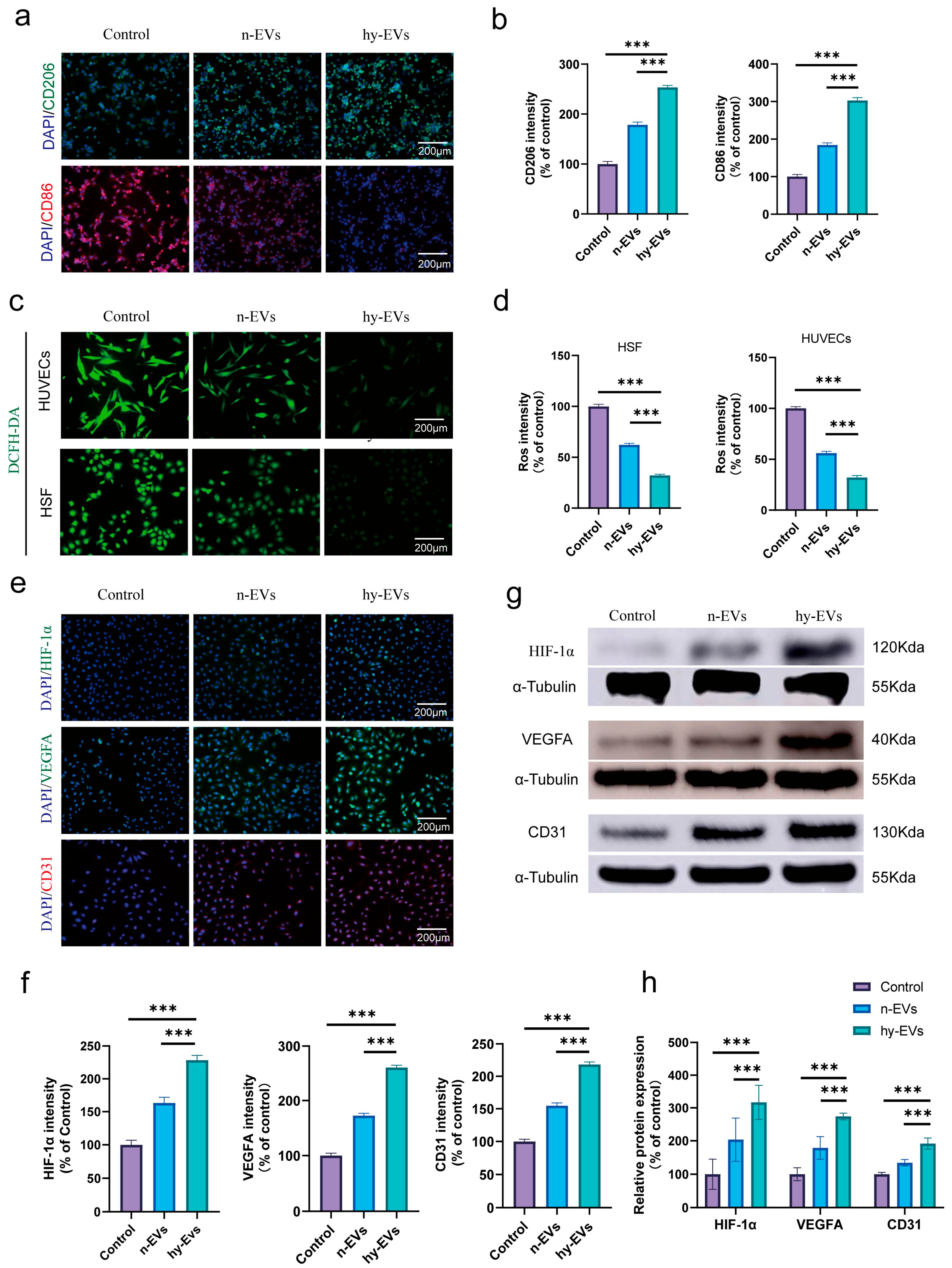
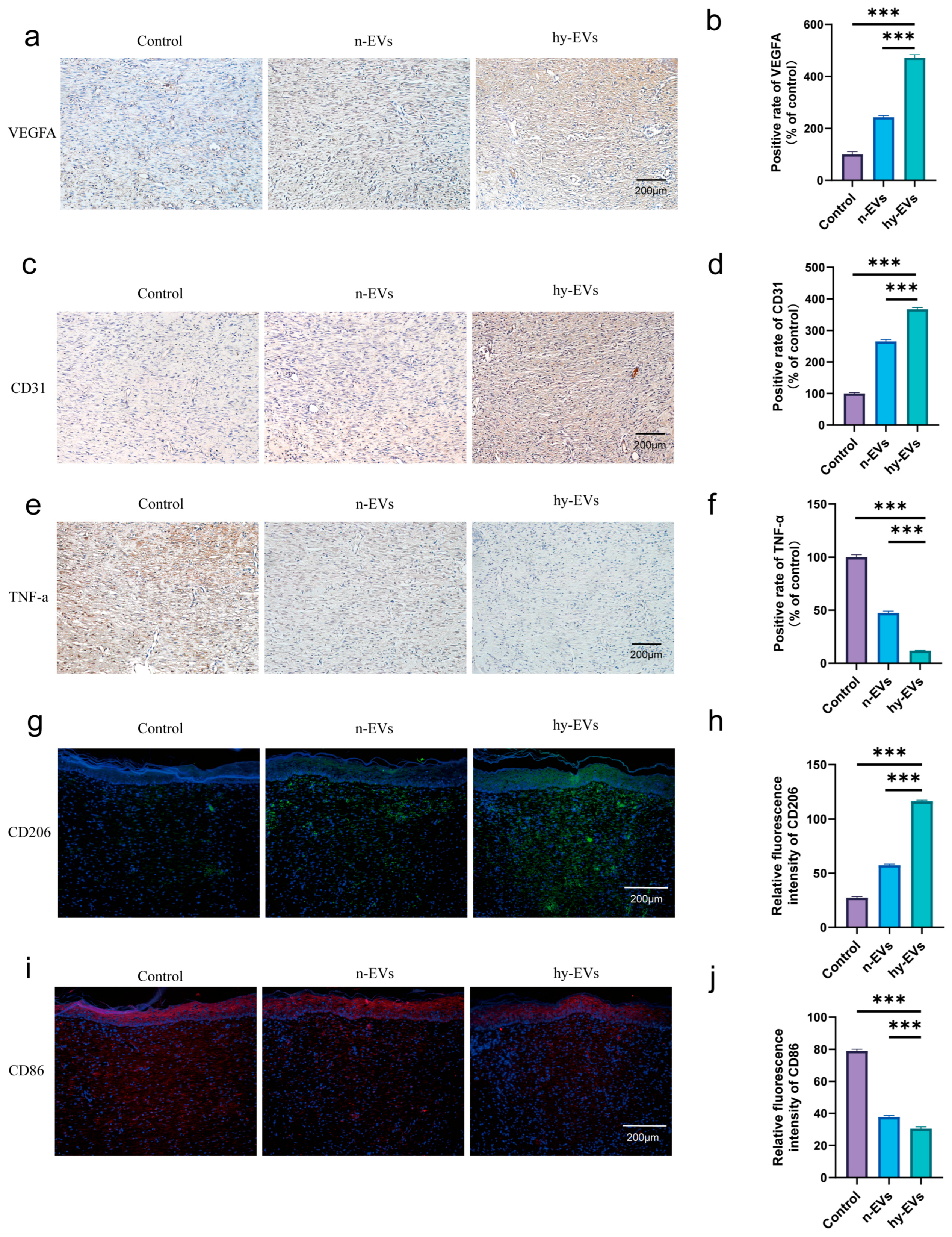
3.4. hy-EVs Exhibited Anti-Inflammatory Effect and Mediate Angiogenesis Through the HIF-1α Pathway
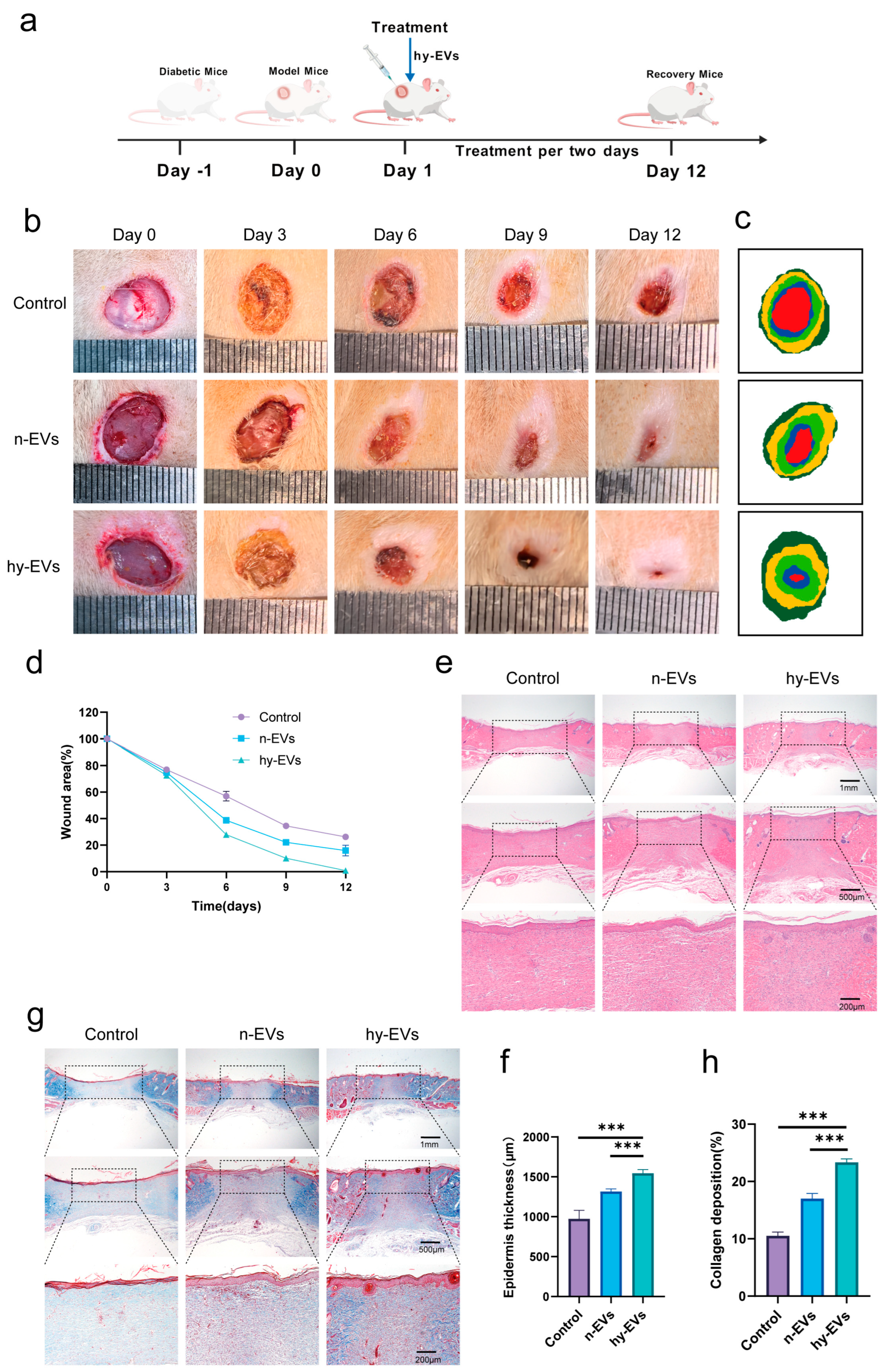
3.5. hy-EVs Promoted the Healing of Diabetic Wounds In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Liang, F.; Huang, N.; Tian, Y.; Fang, Y.; Huang, C.; Qiu, B.; Lu, T.; Zheng, L.; Cheng, J.; Zhu, B. Extracellular vesicle-derived lncRNA VIM-AS1 promotes diabetic wound healing by promoting glycolysis and alleviating cellular senescence. Stem Cell Res. Ther. 2025, 16, 341. [Google Scholar] [CrossRef]
- Zhou, P.; Feng, H.; Qin, W.; Li, Q. KRT17 from skin cells with high glucose stimulation promotes keratinocytes proliferation and migration. Front. Endocrinol. 2023, 14, 1237048. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Wolde, S.; Hulskes, R.; Weenink, R.; Hollmann, M.; Hulst, R. The effects of hyperbaric oxygenation on oxidative stress. Inflamm. Angiogenesis Biomol. 2021, 11, 1210. [Google Scholar]
- Jeffcoate, W.; Boyko, E.J.; Game, F.; Cowled, P.; Senneville, E.; Fitridge, R. Causes, prevention, and management of diabetes-related foot ulcers. Lancet Diabetes Endocrinol. 2024, 12, 472–482. [Google Scholar] [CrossRef]
- Wong, A.Y.W.; Hooi, N.M.F.; Yeo, B.S.Y.; Sultana, R.; Bee, Y.M.; Lee, A.R.Y.B.; Tay, S.M. Improving diabetic wound-healing outcomes with topical growth factor therapies. J. Clin. Endocrinol. Metab. 2024, 109, e1642–e1651. [Google Scholar] [CrossRef]
- Yang, L.; Rong, G.-C.; Wu, Q.-N. Diabetic foot ulcer: Challenges and future. World J. Diabetes 2022, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, Z.; Li, Y.; Wu, R.; Jiang, J. Pretreatment of Human Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes with Quercetin Enhances the Healing of Diabetic Skin Wounds by Modulating Host-Microbiota Interactions. Int. J. Nanomed. 2024, 19, 12557–12581. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Yang, J.; Zheng, W.; Chu, Z.; Wang, W.; Qian, H.; Xu, L. Comprehensive management of diabetic ulceration: Strategies and perspectives. J. Control. Release 2025, 385, 114058. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yan, G.; Huang, H.; Zheng, M.; Ma, K.; Cui, X.; Lu, D.; Zheng, L.; Zhu, B.; Cheng, J. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J. Nanobiotechnol. 2022, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, S.; Wang, J.; Lan, Y.; Feng, L.; Zhu, M.; Xiao, Y.; Cheng, B.; Xue, W.; Guo, R. Enhanced cutaneous wound healing by functional injectable thermo-sensitive chitosan-based hydrogel encapsulated human umbilical cord-mesenchymal stem cells. Int. J. Biol. Macromol. 2019, 137, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.M.; Cancemi, P.; Geraci, F. Mesenchymal and induced pluripotent stem cells-derived extracellular vesicles: The new frontier for regenerative medicine? Cells 2020, 9, 1163. [Google Scholar] [CrossRef]
- Tang, S.; Chen, P.; Zhang, H.; Weng, H.; Fang, Z.; Chen, C.; Peng, G.; Gao, H.; Hu, K.; Chen, J. Comparison of curative effect of human umbilical cord-derived mesenchymal stem cells and their small extracellular vesicles in treating osteoarthritis. Int. J. Nanomed. 2021, 16, 8185–8202. [Google Scholar] [CrossRef]
- Kumari, S.; Lausted, C.; Scherler, K.; Ng, A.H.; Lu, Y.; Lee, I.; Hood, L.; Wang, K. Approaches and challenges in characterizing the molecular content of extracellular vesicles for biomarker discovery. Biomolecules 2024, 14, 1599. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Wang, S.; Shi, C. MSC-Derived Extracellular Vesicles: Roles and Molecular Mechanisms for Tissue Repair. Int. J. Nanomed. 2025, 20, 7953–7974. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, B.; Yang, Y.; Jiang, Q.; Li, T.; Gong, J.; Tang, H.; Zhang, Q. Stem cell-derived exosomes: Emerging therapeutic opportunities for wound healing. Stem Cell Res. Ther. 2023, 14, 107. [Google Scholar] [CrossRef]
- Liu, J.-L.; Kang, D.-L.; Mi, P.; Xu, C.-Z.; Zhu, L.; Wei, B.-M. Mesenchymal stem cell derived Extracellular vesicles: Promising Nanomedicine for Cutaneous Wound Treatment. ACS Biomater. Sci. Eng. 2023, 9, 531–541. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, F.; Liu, Z.; Zuo, K.; Wang, B.; Zhang, Y.; Han, X.; Lian, A.; Wang, Y.; Liu, M. MSC-derived exosomes attenuate cell death through suppressing AIF nucleus translocation and enhance cutaneous wound healing. Stem Cell Res. Ther. 2020, 11, 174. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cheung, C.Y.M.; Liu, L.; Cheung, H.P.H.; Tam, K.Y.; Ker, D.F.E.; Cartmell, S.H.; Mao, C.; Zhang, Z.; Wang, D.M. Optimizing biophysical properties of cellular niches to enhance stem cell-derived extracellular vesicle function in musculoskeletal regeneration. BMEMat 2025, e70012. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, E.H.; Yang, Z. Hypoxia-conditioned mesenchymal stem cells in tissue regeneration application. Tissue Eng. Part B Rev. 2022, 28, 966–977. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Yang, D.; Neo, S.; Kadir, N.; Goh, D.; Tan, J.; Denslin, V.; Lee, E.; Yang, Z. Secretive derived from hypoxia preconditioned mesenchymal stem cells promote cartilage regeneration and mitigate joint inflammation via extracellular vesicles. Bioact. Mater. 2023, 27, 98–112. [Google Scholar] [CrossRef]
- Huang, Q.-M.; Zhuo, Y.-Q.; Duan, Z.-X.; Long, Y.-l.; Wang, J.-N.; Zhang, Z.-h.; Fan, S.-Y.; Huang, Y.-M.; Deng, K.-Y.; Xin, H.-B. Long-term hypoxic atmosphere enhances the stemness, immunoregulatory functions, and therapeutic application of human umbilical cord mesenchymal stem cells. Bone Jt. Res. 2024, 13, 764–778. [Google Scholar] [CrossRef]
- Fan, M.-H.; Zhang, X.-Z.; Jiang, Y.-L.; Pi, J.-K.; Zhang, J.-Y.; Zhang, Y.-Q.; Xing, F.; Xie, H.-Q. Exosomes from hypoxic urine-derived stem cells facilitate healing of diabetic wound by targeting SERPINE1 through miR-486-5p. Biomaterials 2025, 314, 122893. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; He, W.; Mu, X.; Wu, X.; Deng, J.; Nie, X. Fibroblasts: Immunomodulatory factors in refractory diabetic wound healing. Front. Immunol. 2022, 13, 918223. [Google Scholar] [CrossRef]
- Wu, H.; Li, F.; Shao, W.; Gao, J.; Ling, D. Promoting angiogenesis in oxidative diabetic wound microenvironment using a nanozyme-reinforced self-protecting hydrogel. ACS Cent. Sci. 2019, 5, 477–485. [Google Scholar] [CrossRef]
- Fan, L.; Jia, X.; Dong, F.; Yang, S.; Li, W.; Zhao, R.; Yin, L.; Zhao, D.; Wang, J. Ginseng-derived nanoparticles accelerate diabetic wound healing by modulating macrophage polarization and restoring endothelial cell function. Mater. Today Bio 2025, 34, 102143. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lv, D.; Qu, S.; Xu, H.; Li, S.; Wang, Z.; Cao, X.; Rong, Y.; Li, X.; Wu, H. A ROS-Responsive Lipid Nanoparticles Release Multifunctional Hydrogel Based on Microenvironment Regulation Promotes Infected Diabetic Wound Healing. Adv. Sci. 2024, 11, 2403219. [Google Scholar] [CrossRef]
- Liu, P.; Xiong, Y.; Chen, L.; Lin, C.; Yang, Y.; Lin, Z.; Yu, Y.; Mi, B.; Liu, G.; Xiao, X. Angiogenesis-based diabetic skin reconstruction through multifunctional hydrogel with sustained releasing of M2 Macrophage-derived exosome. Chem. Eng. J. 2022, 431, 132413. [Google Scholar] [CrossRef]
- Zeng, R.; Lv, B.; Lin, Z.; Chu, X.; Xiong, Y.; Knoedler, S.; Cao, F.; Lin, C.; Chen, L.; Yu, C. Neddylation suppression by a macrophage membrane-coated nanoparticle promotes dual immunomodulatory repair of diabetic wounds. Bioact. Mater. 2024, 34, 366–380. [Google Scholar] [CrossRef]
- Xiao, X.; Zhao, F.; DuBois, D.B.; Liu, Q.; Zhang, Y.L.; Yao, Q.; Zhang, G.-J.; Chen, S. Nanozymes for the therapeutic treatment of diabetic foot ulcers. ACS Biomater. Sci. Eng. 2024, 10, 4195–4226. [Google Scholar] [CrossRef]
- Zhai, M.; Huang, Z.; Xianyu, B.; Bai, X.; Wan, Z.; Fu, Y.; Xu, H.; Lv, L.; Zhou, Y. Selenium-Containing Polyurethane Thermo-Sensitive Hydrogel Accelerates Diabetic Wound Healing by Activating Unfolded Protein Response. Aggregate 2025, 6, e70074. [Google Scholar] [CrossRef]
- Kang, H.J.; Kumar, S.; D’Elia, A.; Dash, B.; Nanda, V.; Hsia, H.C.; Yarmush, M.L.; Berthiaume, F. Self-assembled elastin-like polypeptide fusion protein coacervates as competitive inhibitors of advanced glycation end-products enhance diabetic wound healing. J. Control. Release 2021, 333, 176–187. [Google Scholar] [CrossRef]
- Kang, H.J.; Kumar, S.; Dash, B.C.; Hsia, H.C.; Yarmush, M.L.; Berthiaume, F. Multifunctional elastin-like polypeptide fusion protein coacervates inhibit receptor-mediated proinflammatory signals and promote angiogenesis in mouse diabetic wounds. Adv. Wound Care 2023, 12, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Su, J.; Zhong, H.; Fang, L. Cascade Nanozyme-Loaded Sprayable Hydrogels for Fibroblast Rejuvenation and Diabetic Wound Regeneration. ACS Appl. Mater. Interfaces 2025, 17, 20968–20979. [Google Scholar] [CrossRef] [PubMed]
- Guillon, C.; Ferraro, S.; Clément, S.; Bouschbacher, M.; Sigaudo-Roussel, D.; Bonod, C. Glycation by glyoxal leads to profound changes in the behavior of dermal fibroblasts Short running title: Effect of glyoxal on dermal fibroblasts. BMJ Open Diabetes Res. Care 2021, 9, e002091. [Google Scholar] [CrossRef] [PubMed]
- Samandari, M.; Aghabaglou, F.; Nuutila, K.; Derakhshandeh, H.; Zhang, Y.; Endo, Y.; Harris, S.; Barnum, L.; Kreikemeier-Bower, C.; Arab-Tehrany, E. Miniaturized needle Array-mediated drug delivery accelerates wound healing. Adv. Healthc. Mater. 2021, 10, 2001800. [Google Scholar] [CrossRef]
- Xu, Y.; Jia, Y.; Wu, N.; Wang, J.; He, L.; Yang, D. CD93 ameliorates diabetic wounds by promoting angiogenesis via the p38MAPK/MK2/HSP27 axis. Eur. J. Vasc. Endovasc. Surg. 2023, 66, 707–721. [Google Scholar] [CrossRef]
- Sun, J.; Shen, H.; Shao, L.; Teng, X.; Chen, Y.; Liu, X.; Yang, Z.; Shen, Z. HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res. Ther. 2020, 11, 373. [Google Scholar] [CrossRef]
- Shao, A.; Lang, Y.; Wang, M.; Qin, C.; Kuang, Y.; Mei, Y.; Lin, D.; Zhang, S.; Tang, J. Bclaf1 is a direct target of HIF-1 and critically regulates the stability of HIF-1α under hypoxia. Oncogene 2020, 39, 2807–2818. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Ge, L.; Xun, C.; Li, W.; Jin, S.; Liu, Z.; Zhuo, Y.; Duan, D.; Hu, Z.; Chen, P.; Lu, M. Extracellular vesicles derived from hypoxia-preconditioned olfactory mucosa mesenchymal stem cells enhance angiogenesis via miR-612. J. Nanobiotechnol. 2021, 19, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Potz, B.A.; Malhotra, A.; Sabra, M.; Harris, D.D.; Broadwin, M.; Abid, M.R.; Sellke, F.W. Comparative analysis of normoxia-and hypoxia-modified extracellular vesicle therapy in function, perfusion, and collateralization in chronically ischemic myocardium. Int. J. Mol. Sci. 2023, 24, 2076. [Google Scholar] [CrossRef]
- Zanotti, F.; Zanolla, I.; Trentini, M.; Tiengo, E.; Pusceddu, T.; Licastro, D.; Degasperi, M.; Leo, S.; Tremoli, E.; Ferroni, L. Mitochondrial metabolism and EV cargo of endothelial cells is affected in presence of EVs derived from MSCs on which HIF is activated. Int. J. Mol. Sci. 2023, 24, 6002. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Guan, L.; Guo, W.; Zhang, X.; Wu, S.; Guo, D.; Li, R.; Zvyagin, A.V.; Lin, Q.; Qu, W. Graphene oxide-based injectable conductive hydrogel dressing with immunomodulatory for chronic infected diabetic wounds. Mater. Des. 2022, 224, 111284. [Google Scholar] [CrossRef]
- Liechty, C.; Hu, J.; Zhang, L.; Liechty, K.W.; Xu, J. Role of microRNA-21 and its underlying mechanisms in inflammatory responses in diabetic wounds. Int. J. Mol. Sci. 2020, 21, 3328. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chen, K.; Liu, C.; Qu, X. Harnessing strategies for enhancing diabetic wound healing from the perspective of spatial inflammation patterns. Bioact. Mater. 2023, 28, 243–254. [Google Scholar] [CrossRef]
- Geng, X.; Qi, Y.; Liu, X.; Shi, Y.; Li, H.; Zhao, L. A multifunctional antibacterial and self-healing hydrogel laden with bone marrow mesenchymal stem cell-derived exosomes for accelerating diabetic wound healing. Biomater. Adv. 2022, 133, 112613. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Johnson, T.K.; Wang, Y.; Thomas, M.; Huynh, K.; Yang, Q.; Bond, V.C.; Chen, Y.E.; Liu, D. Macrophage M2 polarization induced by exosomes from adipose-derived stem cells contributes to the exosomal proangiogenic effect on mouse ischemic hindlimb. Stem Cell Res. Ther. 2020, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chen, W.; Xiong, Y.; Li, Q.; Kong, S.; Li, M.; Pang, C.; Qiu, Y.; Xu, Z.; Gong, Q. Small extracellular vesicles derived from hypoxic preconditioned dental pulp stem cells ameliorate inflammatory osteolysis by modulating macrophage polarization and osteoclastogenesis. Bioact. Mater. 2023, 22, 326–342. [Google Scholar] [CrossRef]
- Qian, W.; Huang, L.; Xu, Y.; Lu, W.; Wen, W.; Guo, Z.; Zhu, W.; Li, Y. Hypoxic ASCs-derived exosomes attenuate colitis by regulating macrophage polarization via miR-216a-5p/HMGB1 axis. Inflamm. Bowel Dis. 2023, 29, 602–619. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, S.; Wang, K.; Yang, R.; Liu, D.; Liao, H.; Qi, Y.; Qiu, K.; Hu, Y.; Wen, H. Relieving macrophage dysfunction by inhibiting SREBP2 activity: A hypoxic mesenchymal stem cells-derived exosomes loaded multifunctional hydrogel for accelerated diabetic wound healing. Small 2024, 20, 2309276. [Google Scholar] [CrossRef]
- He, Y.; Liu, K.; Guo, S.; Chang, R.; Zhang, C.; Guan, F.; Yao, M. Multifunctional hydrogel with reactive oxygen species scavenging and photothermal antibacterial activity accelerates infected diabetic wound healing. Acta Biomater. 2023, 155, 199–217. [Google Scholar] [CrossRef]
- Zheng, Q.; Chen, C.; Liu, Y.; Gao, J.; Li, L.; Yin, C.; Yuan, X. Metal nanoparticles: Advanced and promising technology in diabetic wound therapy. Int. J. Nanomed. 2024, 19, 965–992. [Google Scholar] [CrossRef]
- Nouri, Z.; Barfar, A.; Perseh, S.; Motasadizadeh, H.; Maghsoudian, S.; Fatahi, Y.; Nouri, K.; Yektakasmaei, M.P.; Dinarvand, R.; Atyabi, F. Exosomes as therapeutic and drug delivery vehicle for neurodegenerative diseases. J. Nanobiotechnol. 2024, 22, 463. [Google Scholar] [CrossRef]
- Luo, Q.; Xian, P.; Wang, T.; Wu, S.; Sun, T.; Wang, W.; Wang, B.; Yang, H.; Yang, Y.; Wang, H. Antioxidant activity of mesenchymal stem cell-derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics 2021, 11, 5986. [Google Scholar] [CrossRef]
- Li, N.; Zhao, L.; Geng, X.; Liu, J.; Zhang, X.; Hu, Y.; Qi, J.; Chen, H.; Qiu, J.; Zhang, X. Stimulation by exosomes from hypoxia-preconditioned hair follicle mesenchymal stem cells facilitates mitophagy by inhibiting the PI3K/AKT/mTOR signaling pathway to alleviate ulcerative colitis. Theranostics 2024, 14, 4278. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Lu, J.; Liang, F.; Cheng, J. Hypoxia-Induced Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization and Enhance Angiogenesis to Promote Diabetic Wound Healing. Biomolecules 2025, 15, 1504. https://doi.org/10.3390/biom15111504
Su Y, Lu J, Liang F, Cheng J. Hypoxia-Induced Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization and Enhance Angiogenesis to Promote Diabetic Wound Healing. Biomolecules. 2025; 15(11):1504. https://doi.org/10.3390/biom15111504
Chicago/Turabian StyleSu, Yongfeng, Junda Lu, Feiyuan Liang, and Jianwen Cheng. 2025. "Hypoxia-Induced Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization and Enhance Angiogenesis to Promote Diabetic Wound Healing" Biomolecules 15, no. 11: 1504. https://doi.org/10.3390/biom15111504
APA StyleSu, Y., Lu, J., Liang, F., & Cheng, J. (2025). Hypoxia-Induced Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Regulate Macrophage Polarization and Enhance Angiogenesis to Promote Diabetic Wound Healing. Biomolecules, 15(11), 1504. https://doi.org/10.3390/biom15111504



