STAMBP Accelerates Progression and Tamoxifen Resistance of Breast Cancer Through Deubiquitinating ERα
Abstract
1. Introduction
2. Materials and Methods
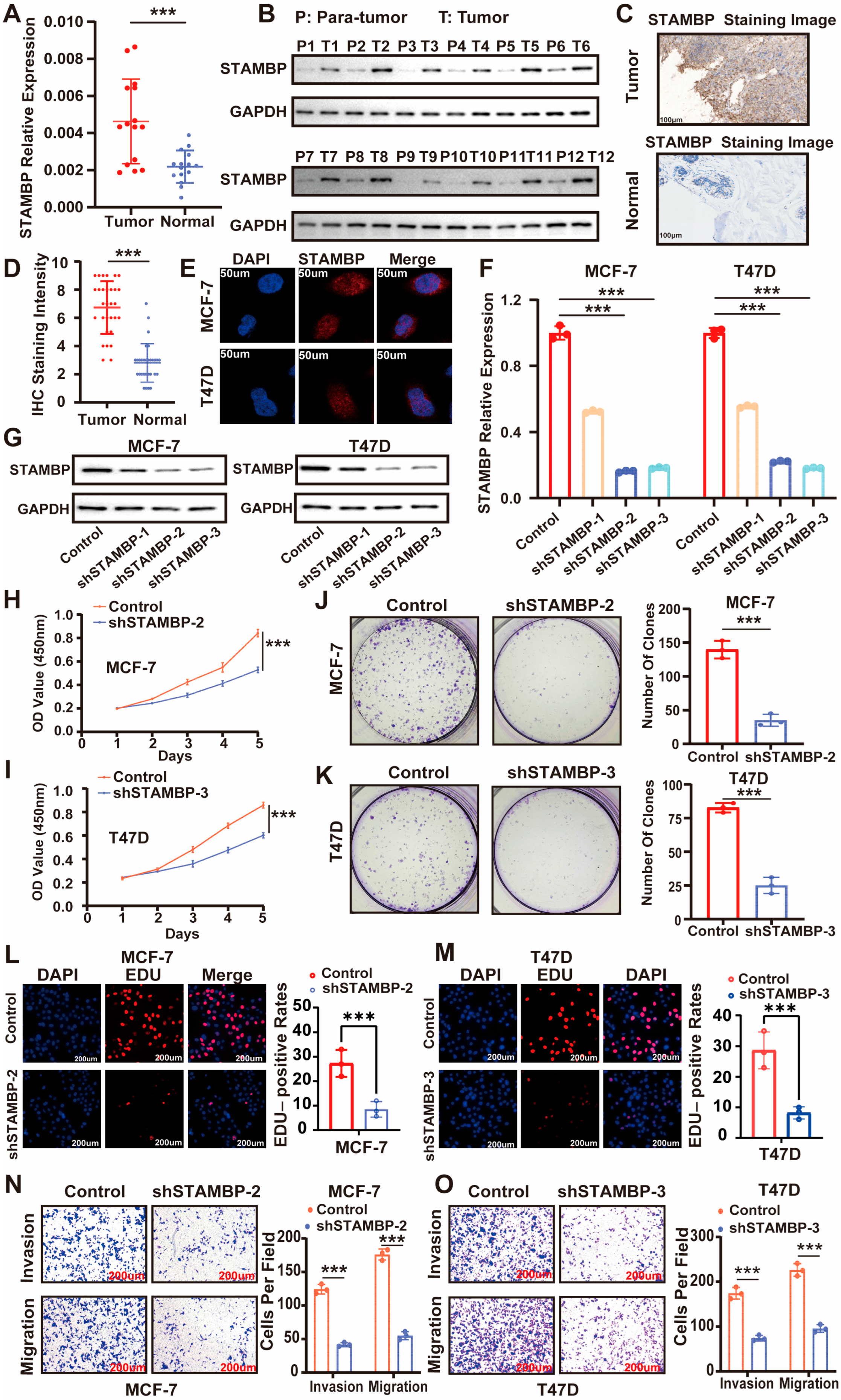
2.1. Clinical Samples Acquisition
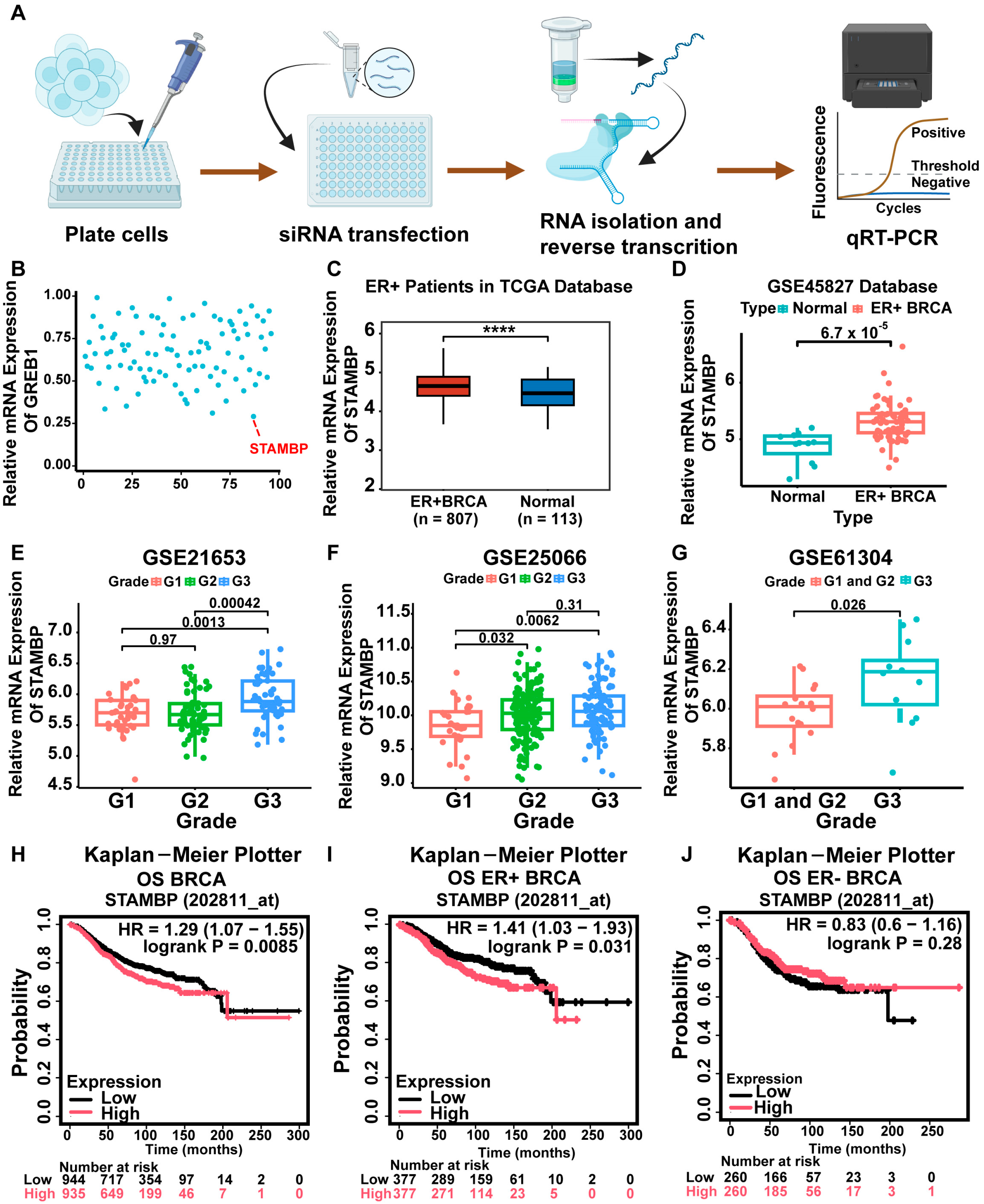
2.2. Acquisition and Analysis of Online Data
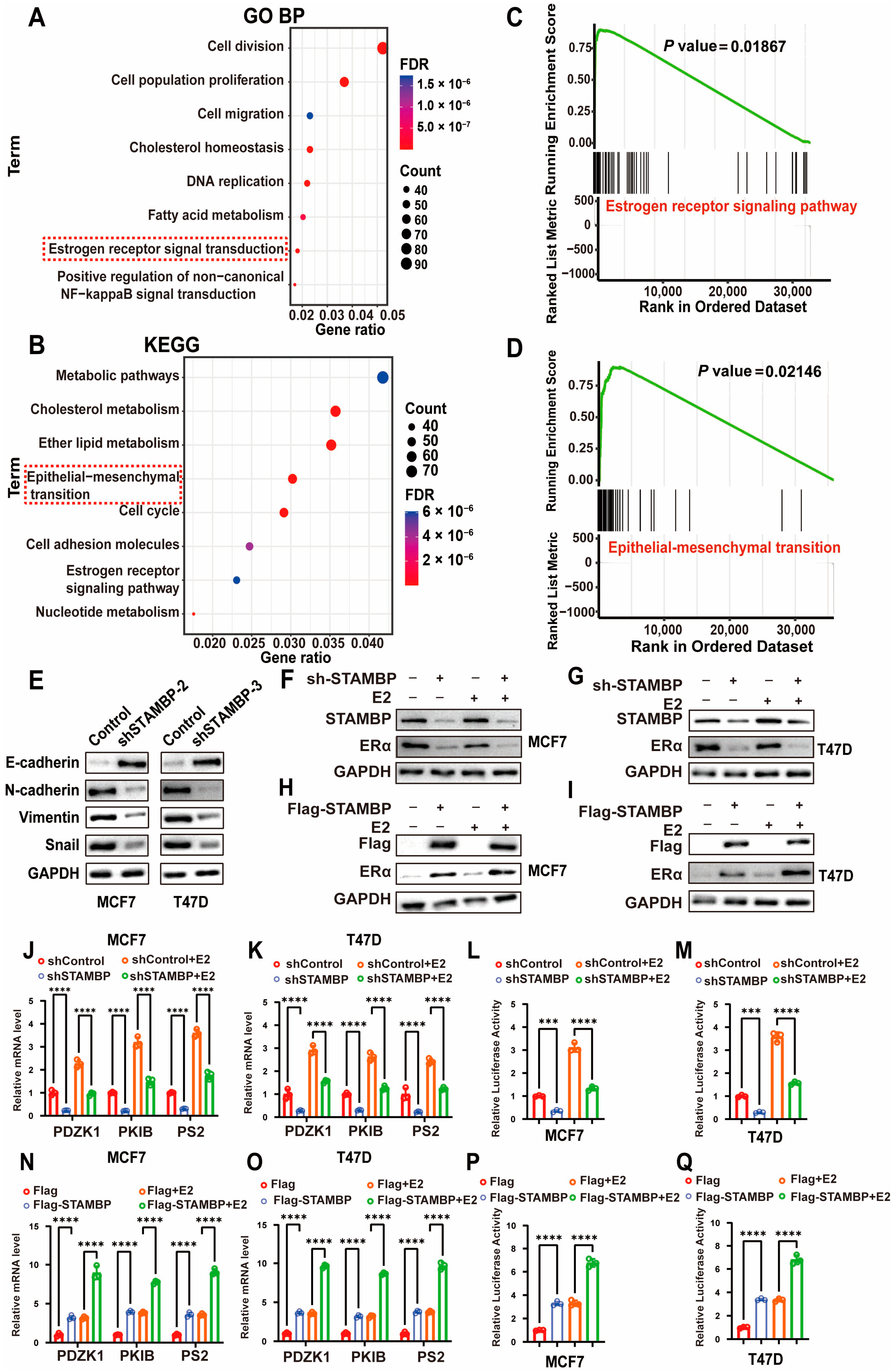
2.3. Functional Enrichment Analyses
2.4. Cell Cultures
2.5. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
2.6. Western Blot (WB)
2.7. Immunohistochemical (IHC) Stain and Immunofluorescence (If)
2.8. Plasmids and Short Hairpin RNA Transfection
2.9. Co-Immunoprecipitation (Co-IP) and Poly-Ubiquitination Assay
2.10. DUB Small Interfering RNA (siRNA) Library
2.11. Protein Stability Assays
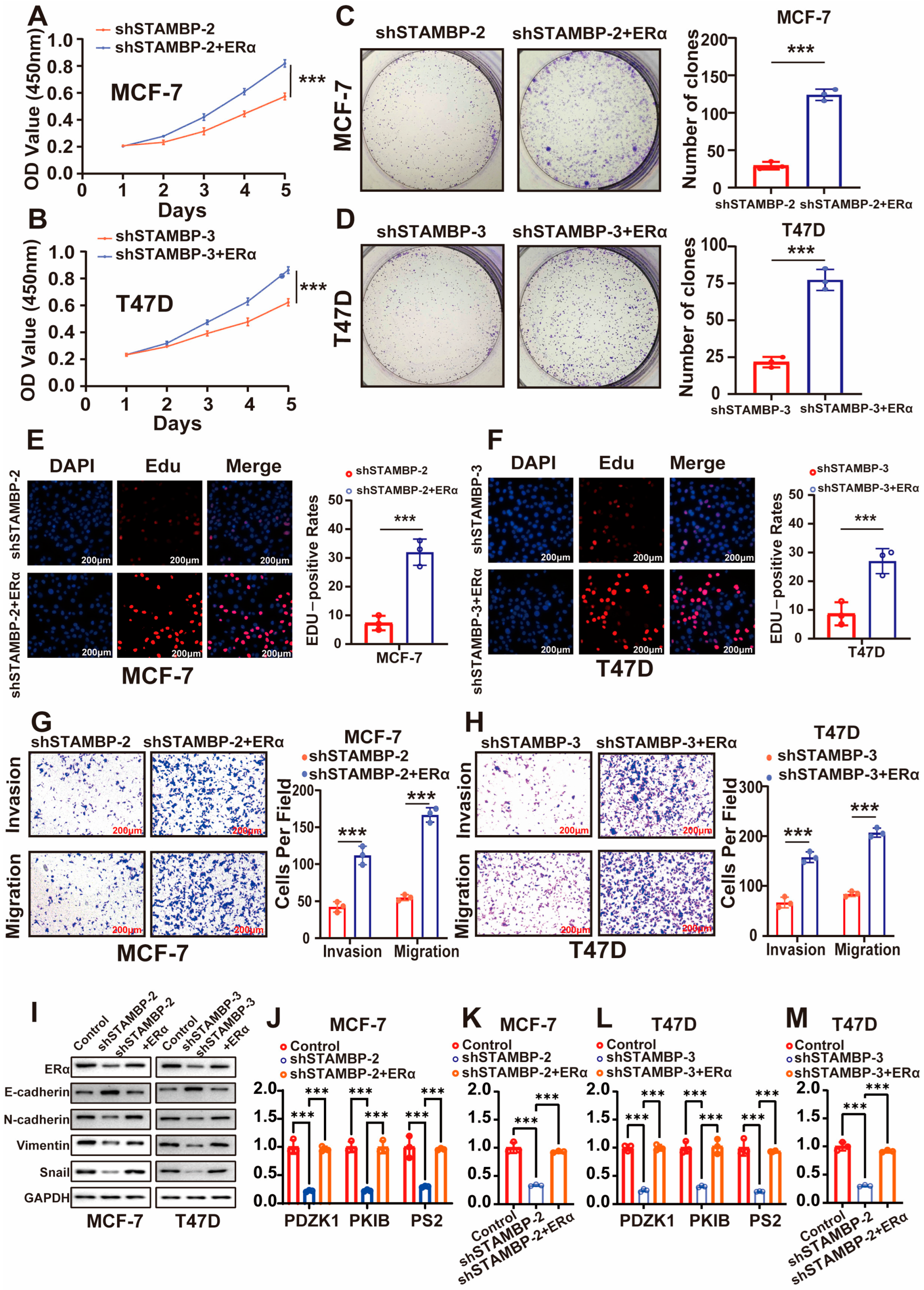
2.12. Cell Proliferative and Migrative Assays
2.13. Luciferase Activity Experiments
2.14. Animal Experiments
2.15. Statistical Analysis
3. Results
3.1. STAMBP Was a Regulator of ER Signaling and Associated with Poor Prognosis in ER-Positive Breast Cancer
3.2. STAMBP Expression Upregulates and Influences Proliferation and Metastasis of ER-Positive BRCA In Vitro
3.3. STAMBP Influences Epithelial–Mesenchymal Transition (EMT) and ER Signaling in ER-Positive BRCA Cells
3.4. STAMBP Accelerates the Progression of BRCA Through ERα
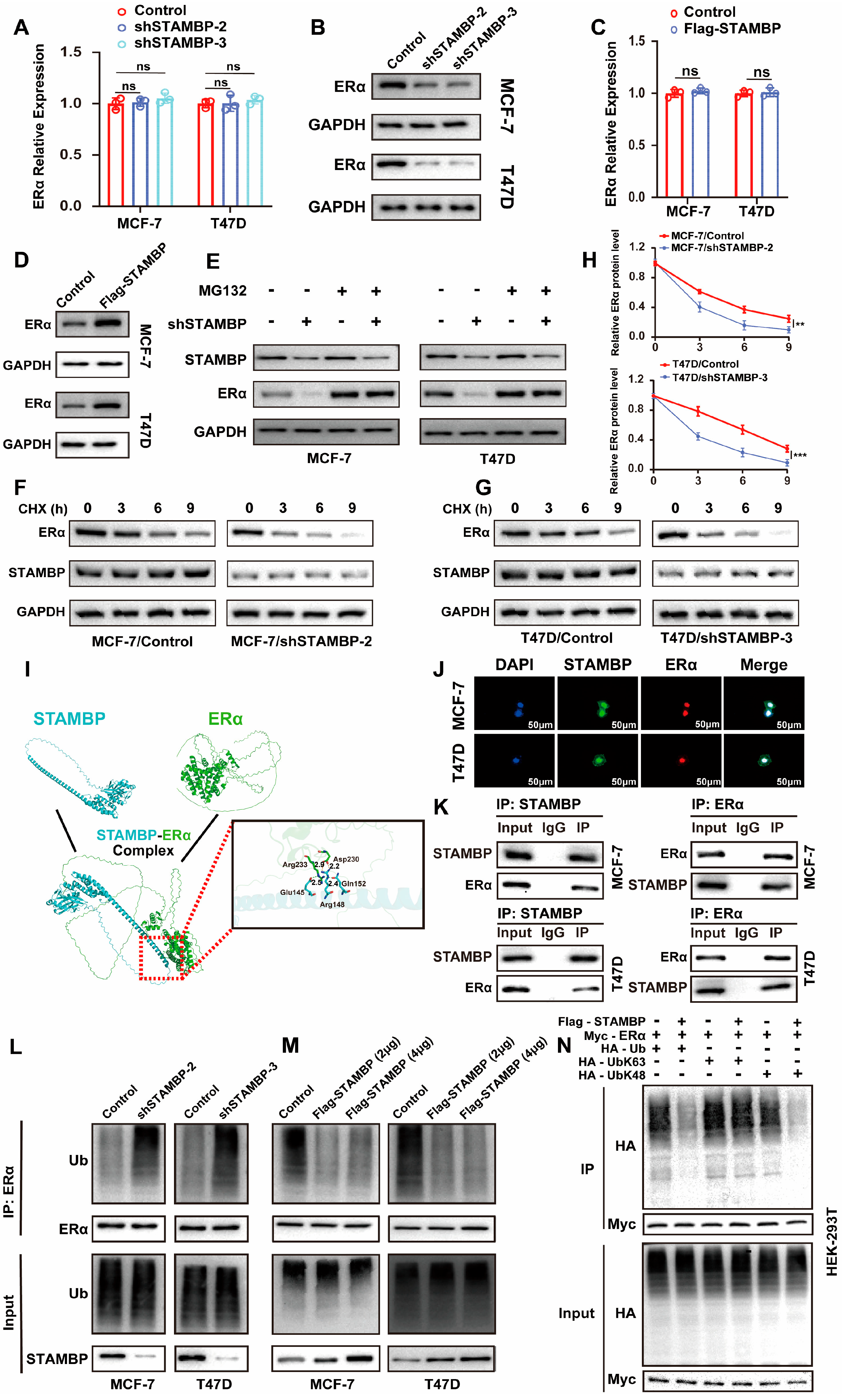
3.5. STAMBP Promotes ERα Protein Stability Through Inhibiting K48-Linking Poly-Ubiquitination
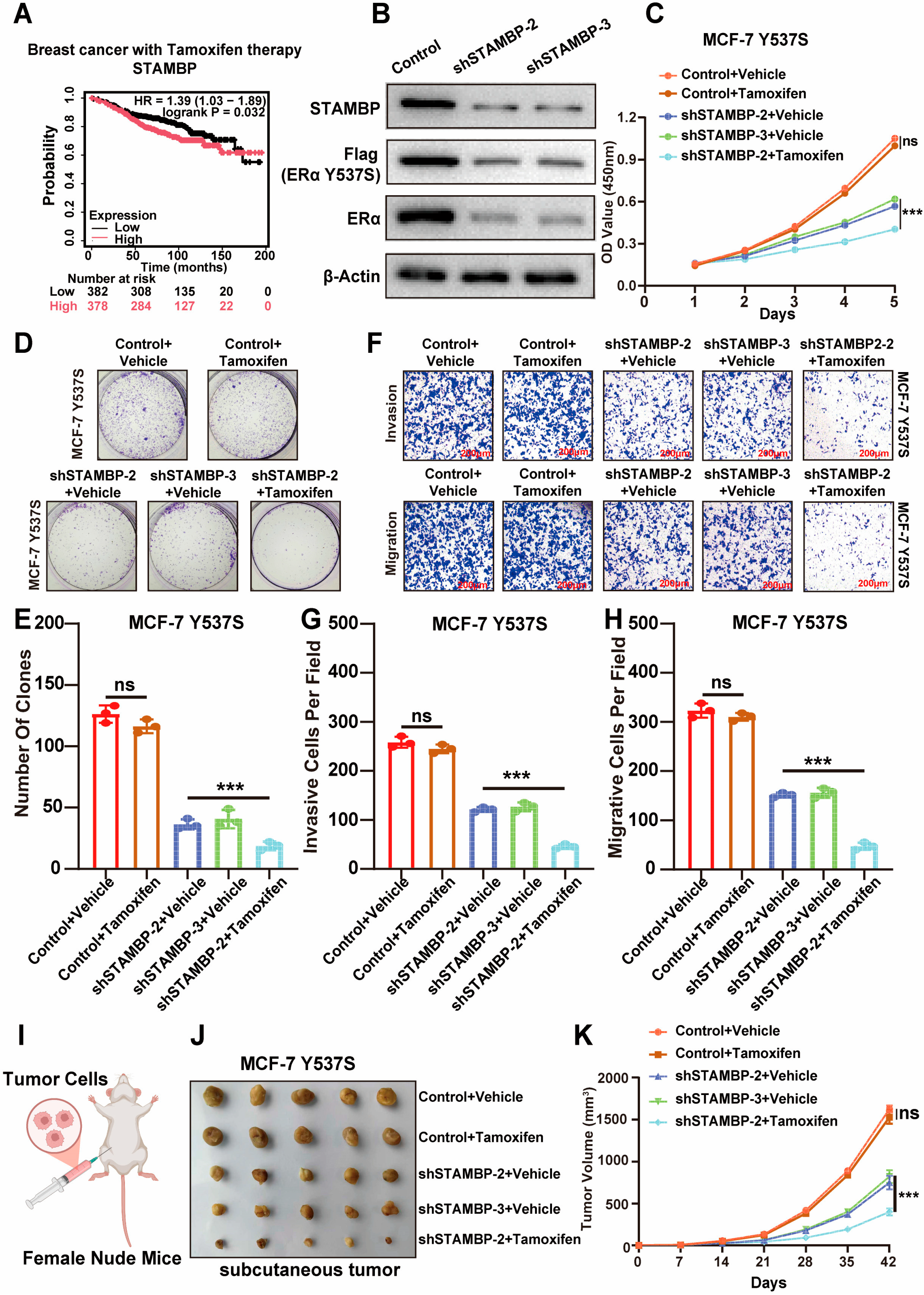
3.6. Suppressing STAMBP Expression Sensitize Endocrine-Resistance BRCA to Tamoxifen Therapy
3.7. Entrectinib Inhibits STAMBP Expression and Suppresses Tumor Progression in BRCA Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRCA | Breast cancer |
| CCK-8 | Cell Counting Kit-8 |
| CHX | Cycloheximide |
| Co-IP | Co-immunoprecipitation |
| DEG | Differential-expressed gene |
| DFS | Disease-free survival |
| DMFS | Distant metastasis-free survival |
| DUB | Deubiquitinase |
| EDU | 5-ethynyl-2′-deoxyuridine |
| EGA | European Genome-phenome Archive |
| EMT | Epithelial–mesenchymal transition |
| ER | Estrogen receptor |
| ERE | Estrogen response element |
| FDR | False discovery rate |
| GEO | Gene Expression Omnibus |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| JAMM | JAB1/MPN-domain-associated metalloisopeptidase |
| K-M | Kaplan–Meier |
| MCPIP | Monocyte chemotactic protein-induced protein |
| MINDY | Motif-interacting with ubiquitin-containing novel DUB family |
| MJD | Machado–Joseph disease protein domain protease |
| OS | Overall survival |
| OTU | Ovarian tumor-related protease |
| qRT-PCR | Quantitative real-time PCR |
| RFS | Recurrence-free survival |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| STAMBP | STAM binding protein |
| TCGA | The Cancer Genome Atlas Program |
| UCH | Ubiquitin C-terminal hydrolase |
| USP | Ubiquitin-specific protease |
| WB | western blot |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wei, L.; Gao, H.; Yu, J.; Zhang, H.; Nguyen, T.T.L.; Gu, Y.; Passow, M.R.; Carter, J.M.; Qin, B.; Boughey, J.C.; et al. Pharmacological Targeting of Androgen Receptor Elicits Context-Specific Effects in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2023, 83, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Yang, L.; Jeong, K.W. SETD1A-SOX2 axis is involved in tamoxifen resistance in estrogen receptor α-positive breast cancer cells. Theranostics 2022, 12, 5761–5775. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, M.R.; Jhaveri, K.; Kalinsky, K.; Bardia, A.; Wander, S.A. Precision therapeutics and emerging strategies for HR-positive metastatic breast cancer. Nat. Rev. Clin. Oncol. 2024, 21, 743–761. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, L.; Jiang, S.; Yang, Q. The ubiquitin-proteasome system in breast cancer. Trends Mol. Med. 2023, 29, 599–621. [Google Scholar] [CrossRef]
- Dewson, G.; Eichhorn, P.J.A.; Komander, D. Deubiquitinases in cancer. Nat. Rev. Cancer 2023, 23, 842–862. [Google Scholar] [CrossRef]
- Liu, F.; Chen, J.; Li, K.; Li, H.; Zhu, Y.; Zhai, Y.; Lu, B.; Fan, Y.; Liu, Z.; Chen, X.; et al. Ubiquitination and deubiquitination in cancer: From mechanisms to novel therapeutic approaches. Mol. Cancer 2024, 23, 148. [Google Scholar] [CrossRef]
- Harrigan, J.A.; Jacq, X.; Martin, N.M.; Jackson, S.P. Deubiquitylating enzymes and drug discovery: Emerging opportunities. Nat. Rev. Drug Discov. 2018, 17, 57–78. [Google Scholar] [CrossRef]
- Ou, Y.; Zhang, K.; Shuai, Q.; Wang, C.; Hu, H.; Cao, L.; Qi, C.; Guo, M.; Li, Z.; Shi, J.; et al. USP51/GRP78/ABCB1 axis confers chemoresistance through decreasing doxorubicin accumulation in triple-negative breast cancer cells. Acta Pharm. Sin. B 2025, 15, 2593–2611. [Google Scholar] [CrossRef]
- Yang, L.; An, X.; Yang, S.; Lin, X.; Chen, Z.; Xue, Q.; Chen, X.; Wang, Y.; Yan, D.; Chen, S.; et al. The deubiquitinase USP24 suppresses ferroptosis in triple-negative breast cancer by stabilizing DHODH protein. Cell Death Dis. 2025, 16, 564. [Google Scholar] [CrossRef]
- He, X.; Xia, X.; Lei, Z.; Tang, M.; Zhang, J.; Liao, Y.; Huang, H. The deubiquitinase USP7 stabilizes HER2 expression and promotes breast cancer progression. Neoplasia 2025, 66, 101192. [Google Scholar] [CrossRef]
- He, L.; He, J.; Jiang, T.; Gong, R.; Wan, X.; Duan, M.; Chen, Z.; Cheng, Y. Inhibition of UCH-L1 enhances immunotherapy efficacy in triple-negative breast cancer by stabilizing PD-L1. Eur. J. Pharmacol. 2025, 1000, 177743. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Dong, K.; An, J.; Liu, Z.; Zhao, Q.; Lv, Y. OTUD3 inhibits breast cancer cell metastasis by regulating TGF-β pathway through deubiquitinating SMAD7. Cancer Cell Int. 2025, 25, 181. [Google Scholar] [CrossRef]
- Bednash, J.S.; Johns, F.; Patel, N.; Smail, T.R.; Londino, J.D.; Mallampalli, R.K. The deubiquitinase STAMBP modulates cytokine secretion through the NLRP3 inflammasome. Cell. Signal. 2021, 79, 109859. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Xuan, X.; Wu, D.; Zhang, J.; Xu, X.; Zhao, Y.; Ma, C.; Li, D. STAMBP promotes lung adenocarcinoma metastasis by regulating the EGFR/MAPK signaling pathway. Neoplasia 2021, 23, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xu, Z.; Du, Y.; Liu, T.; Xiong, Z.; Hu, J.; Chen, L.; Peng, X.; Zhou, F. Identification of STAM-binding protein as a target for the treatment of gemcitabine resistance pancreatic cancer in a nutrient-poor microenvironment. Cell Death Dis. 2024, 15, 657. [Google Scholar] [CrossRef]
- Yang, Q.; Yan, D.; Zou, C.; Xue, Q.; Lin, S.; Huang, Q.; Li, X.; Tang, D.; Chen, X.; Liu, J. The deubiquitinating enzyme STAMBP is a newly discovered driver of triple-negative breast cancer progression that maintains RAI14 protein stability. Exp. Mol. Med. 2022, 54, 2047–2059. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Shen, Y.; Zhu, C.; Qin, X.; Gao, Y. Liquidambaric acid inhibits cholangiocarcinoma progression by disrupting the STAMBPL1/NRF2 positive feedback loop. Phytomedicine 2025, 136, 156303. [Google Scholar] [CrossRef]
- Fanning, S.W.; Mayne, C.G.; Dharmarajan, V.; Carlson, K.E.; Martin, T.A.; Novick, S.J.; Toy, W.; Green, B.; Panchamukhi, S.; Katzenellenbogen, B.S.; et al. Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. eLife 2016, 5, e12792. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Martin, A.R.; Kurnia, P.T.; Gómez Tejeda Zañudo, J.; Abravanel, D.L.; Hughes, M.E.; Parker, T.; Tarantino, P.; Curigliano, G.; King, T.A.; et al. Clinicopathological features and genomics of ER-positive/HER2-negative breast cancer relapsing on adjuvant abemaciclib. ESMO Open 2025, 10, 105126. [Google Scholar] [CrossRef]
- Yonemori, K.; Shimoi, T.; Yamamoto, K.; Kogawa, T.; Kitano, S.; Kobayashi, S.; Yanai, K.; Yamaguchi, K.; Otake, Y.; Hojo, S.; et al. Phase I study of H3B-6545 in patients with estrogen receptor-positive breast cancer. ESMO Open 2025, 10, 105290. [Google Scholar] [CrossRef]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Graff, S.L.; Tolaney, S.M.; Hart, L.L.; Razavi, P.; Janni, W.; Schwartzberg, L.S.; Danyliv, A.; Akdere, M.; Ferrusi, I.; Adhikary, R.R.; et al. Correlation analysis of invasive disease-free survival and overall survival in a real-world population of patients with HR+/HER2- early breast cancer. Cancer 2025, 131, e35817. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, X.; Sun, W.; Xu, F.; Kou, H.; Hu, W.; Zhang, Y.; Jiang, Q.; Tang, J.; Xu, Y. RelB-activated GPX4 inhibits ferroptosis and confers tamoxifen resistance in breast cancer. Redox Biol. 2023, 68, 102952. [Google Scholar] [CrossRef]
- Kim, H.; Whitman, A.A.; Wisniewska, K.; Kakati, R.T.; Garcia-Recio, S.; Calhoun, B.C.; Franco, H.L.; Perou, C.M.; Spanheimer, P.M. Tamoxifen Response at Single-Cell Resolution in Estrogen Receptor-Positive Primary Human Breast Tumors. Clin. Cancer Res. 2023, 29, 4894–4907. [Google Scholar] [CrossRef]
- Nelin, T.D.; Lorch, S.; Jensen, E.A.; Alexiou, S.; Gibbs, K.; Napolitano, N.; Monk, H.M.; Furth, S.; Shults, J.; Bamat, N.A. The association between diuretic class exposures and enteral electrolyte use in infants developing grade 2 or 3 bronchopulmonary dysplasia in United States children’s hospitals. J. Perinatol. 2021, 41, 779–785. [Google Scholar] [CrossRef]
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Tomiguchi, M.; Sueta, A.; Murakami, K.; Omoto, Y.; Iwase, H. Comparison of ESR1 Mutations in Tumor Tissue and Matched Plasma Samples from Metastatic Breast Cancer Patients. Transl. Oncol. 2017, 10, 766–771. [Google Scholar] [CrossRef]
- Zhuang, T.; Zhang, S.; Liu, D.; Li, Z.; Li, X.; Li, J.; Yang, P.; Zhang, C.; Cui, J.; Fu, M.; et al. USP36 promotes tumorigenesis and tamoxifen resistance in breast cancer by deubiquitinating and stabilizing ERα. J. Exp. Clin. Cancer Res. CR 2024, 43, 249. [Google Scholar] [CrossRef]
- Yang, P.; Yang, X.; Wang, D.; Yang, H.; Li, Z.; Zhang, C.; Zhang, S.; Zhu, J.; Li, X.; Su, P.; et al. PSMD14 stabilizes estrogen signaling and facilitates breast cancer progression via deubiquitinating ERα. Oncogene 2024, 43, 248–264. [Google Scholar] [CrossRef]
- Bednash, J.S.; Weathington, N.; Londino, J.; Rojas, M.; Gulick, D.L.; Fort, R.; Han, S.; McKelvey, A.C.; Chen, B.B.; Mallampalli, R.K. Targeting the deubiquitinase STAMBP inhibits NALP7 inflammasome activity. Nat. Commun. 2017, 8, 15203. [Google Scholar] [CrossRef]
- McDonell, L.M.; Mirzaa, G.M.; Alcantara, D.; Schwartzentruber, J.; Carter, M.T.; Lee, L.J.; Clericuzio, C.L.; Graham, J.M., Jr.; Morris-Rosendahl, D.J.; Polster, T.; et al. Mutations in STAMBP, encoding a deubiquitinating enzyme, cause microcephaly-capillary malformation syndrome. Nat. Genet. 2013, 45, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.A.; Chica-Parrado, M.R.; Unni, N.; Jaeger, E.; Fang, Y.V.; Guo, L.; Napolitano, F.; Luna, P.; Harris, M.; Chao, C.; et al. ESR1 Y537S and D538G Mutations Drive Resistance to CDK4/6 Inhibitors in Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2025, 31, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Huggins, R.J.; Greene, G.L. ERα/PR crosstalk is altered in the context of the ERα Y537S mutation and contributes to endocrine therapy-resistant tumor proliferation. NPJ Breast Cancer 2023, 9, 96. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Gu, L.; Yang, M.; Zhou, Y.; Qin, X.; Xiong, C. STAMBP Accelerates Progression and Tamoxifen Resistance of Breast Cancer Through Deubiquitinating ERα. Biomolecules 2025, 15, 1502. https://doi.org/10.3390/biom15111502
Wang Z, Gu L, Yang M, Zhou Y, Qin X, Xiong C. STAMBP Accelerates Progression and Tamoxifen Resistance of Breast Cancer Through Deubiquitinating ERα. Biomolecules. 2025; 15(11):1502. https://doi.org/10.3390/biom15111502
Chicago/Turabian StyleWang, Zhihuai, Likai Gu, Mei Yang, Yi Zhou, Xihu Qin, and Chen Xiong. 2025. "STAMBP Accelerates Progression and Tamoxifen Resistance of Breast Cancer Through Deubiquitinating ERα" Biomolecules 15, no. 11: 1502. https://doi.org/10.3390/biom15111502
APA StyleWang, Z., Gu, L., Yang, M., Zhou, Y., Qin, X., & Xiong, C. (2025). STAMBP Accelerates Progression and Tamoxifen Resistance of Breast Cancer Through Deubiquitinating ERα. Biomolecules, 15(11), 1502. https://doi.org/10.3390/biom15111502





