Understanding Atopic Dermatitis: Pathophysiology and Management Strategies
Abstract
1. Introduction
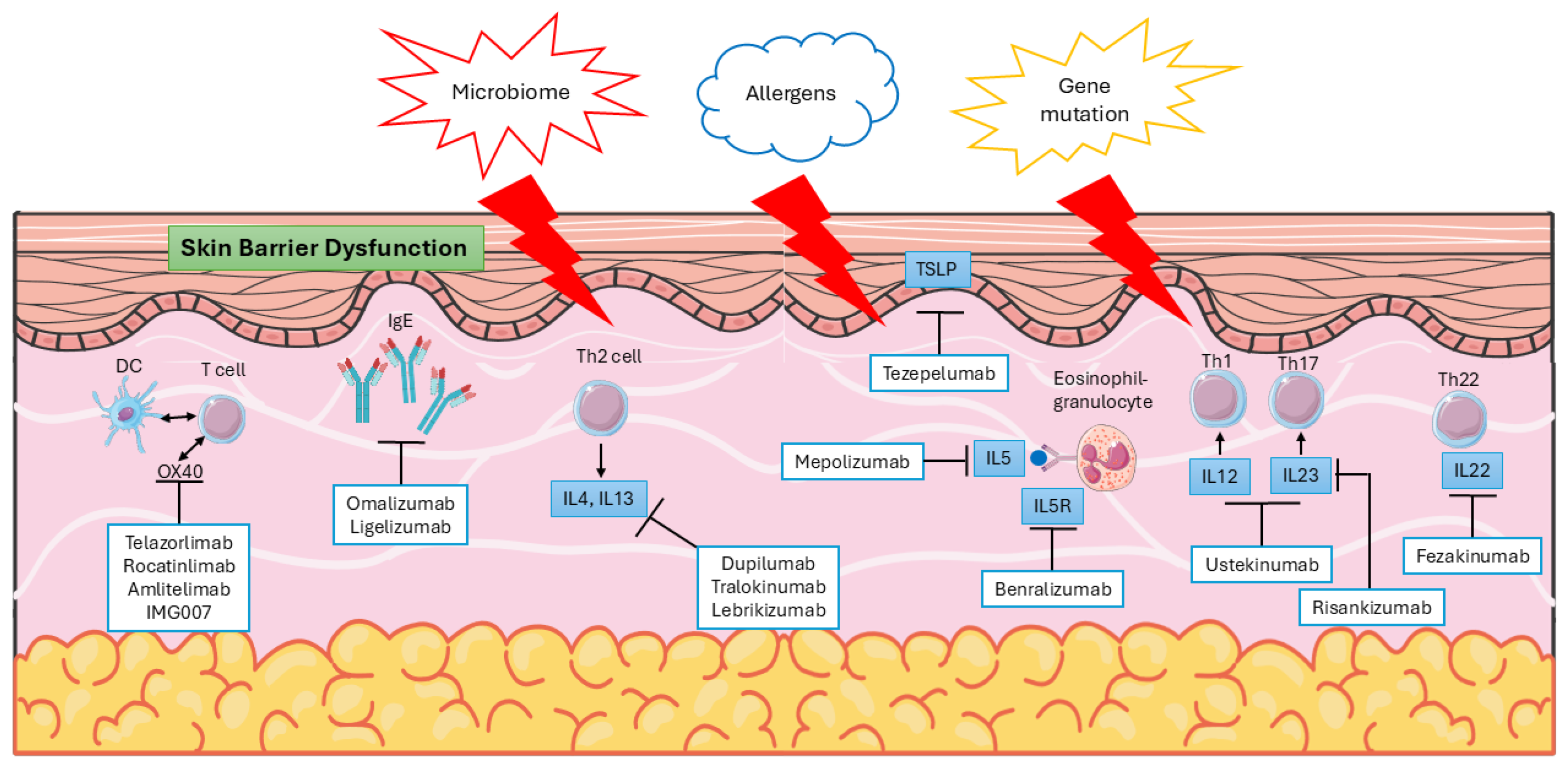
2. Pathophysiology of AD
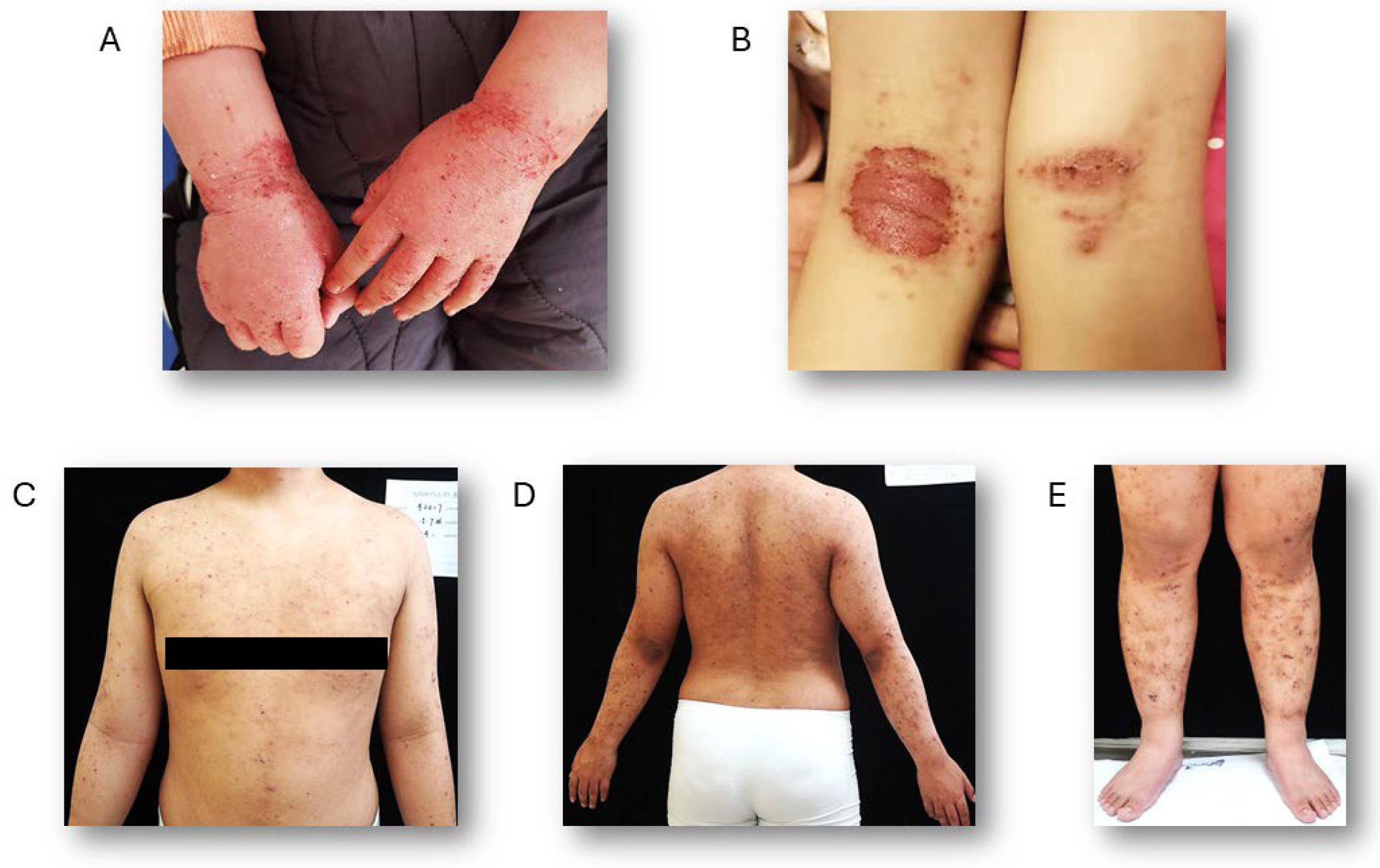
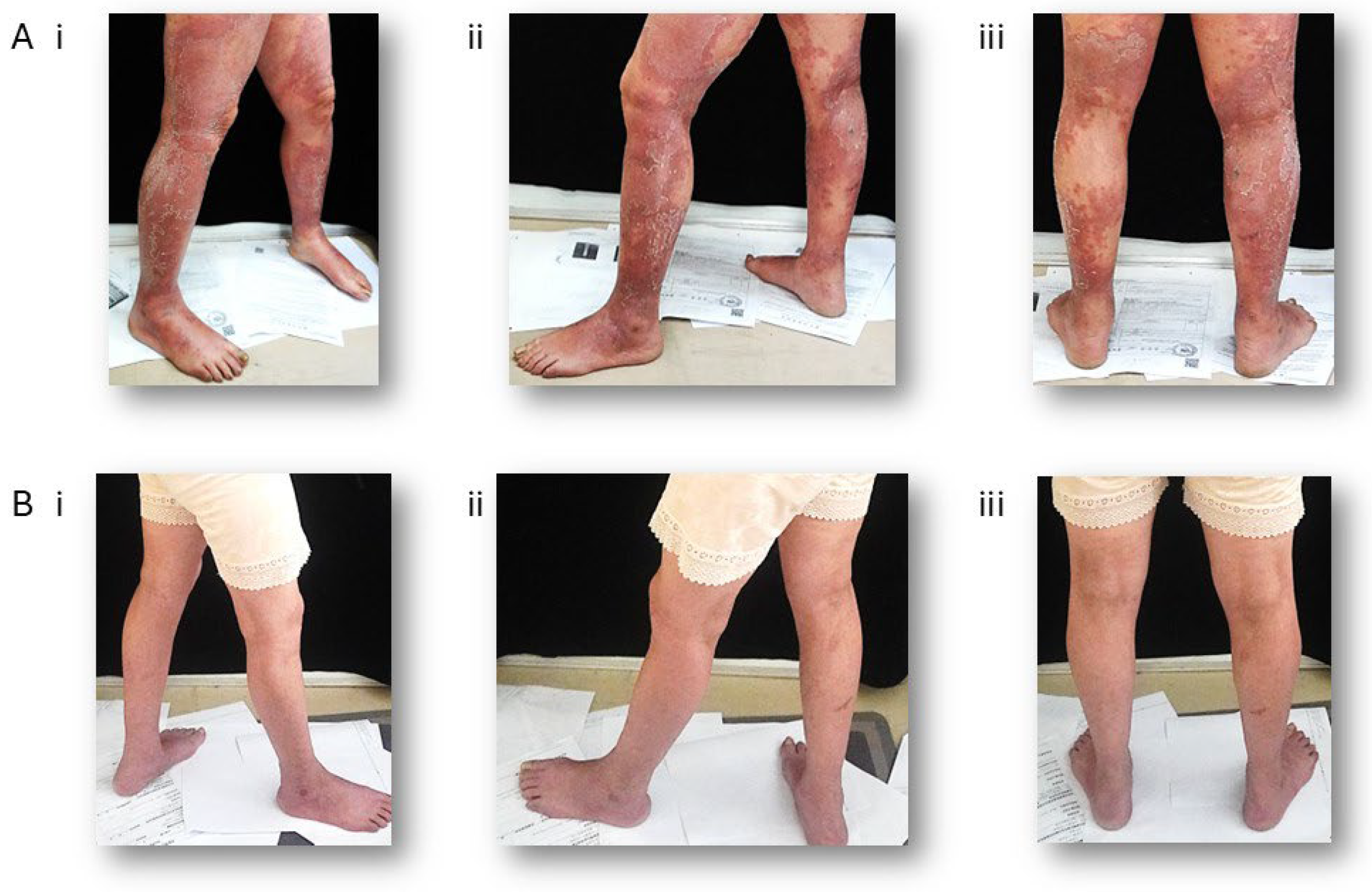
3. Clinical Features
4. Diagnosis
5. Disease Severity and Clinical Outcome Assessments
6. Current Management and Treatment
6.1. Non-Pharmacological Management
6.2. Topical Treatments
6.3. Systemic Therapy
6.3.1. Biologics
6.3.2. Small Molecules
6.3.3. Phototherapy
7. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| AAD | American Academy of Dermatology |
| AhR | aryl hydrocarbon receptor |
| cAMP | cyclic adenosine monophosphate |
| CCL | chemokine C-C motif ligand |
| CXCL | C-X-C motif chemokine ligand 2 |
| DCs | dendritic cells |
| EAD | extrinsic atopic dermatitis |
| EASI | Eczema Area and Severity Index |
| EMA | European Medicines Agency |
| FLG | filaggrin gene |
| IAD | intrinsic atopic dermatitis |
| IFN | interferon |
| IGA | Investigator’s Global Assessment |
| IL | interleukins |
| IMPDH | inosine monophosphate dehydrogenase |
| JAKi | Janus kinase inhibitors |
| MACE | major adverse cardiovascular events |
| MMF | Mycophenolate mofetil |
| NB-UVB | narrowband ultraviolet B |
| OVOL | OVOL ovo like transcriptional repressor |
| OX40L | OX40–OX40 ligand |
| PDE4is | phosphodiesterase-4 inhibitors |
| PM | particulate matter |
| POEM | Patient-Oriented Eczema Measure |
| SASSAD | Six Area, Six Sign Atopic Dermatitis |
| SCORAD | Scoring Atopic Dermatitis |
| SPINK5 | spinous layer protein 5 |
| TCIs | topical calcineurin inhibitors |
| TCS | topical corticosteroids |
| Th | T helper |
| TLA4 | T-lymphocyte-associated protein 4 |
| TNF-α | tumor necrosis factor alpha |
| TSLP | thymic stromal lymphopoietin |
| VTE | venous thromboembolism |
References
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar]
- Tian, J.; Zhang, D.; Yang, Y.; Huang, Y.; Wang, L.; Yao, X.; Lu, Q. Global epidemiology of atopic dermatitis: A comprehensive systematic analysis and modelling study. Br. J. Dermatol. 2023, 190, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Adamson, A.S. The Economics Burden of Atopic Dermatitis. Adv. Exp. Med. Biol. 2017, 1027, 79–92. [Google Scholar] [PubMed]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the Roles of Filaggrin in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]
- Nedoszytko, B.; Reszka, E.; Gutowska-Owsiak, D.; Trzeciak, M.; Lange, M.; Jarczak, J.; Niedoszytko, M.; Jablonska, E.; Romantowski, J.; Strapagiel, D.; et al. Genetic and Epigenetic Aspects of Atopic Dermatitis. Int. J. Mol. Sci. 2020, 21, 6484. [Google Scholar] [CrossRef]
- Manolio, T.A. Genomewide association studies and assessment of the risk of disease. N. Engl. J. Med. 2010, 363, 166–176. [Google Scholar] [CrossRef]
- Budu-Aggrey, A.; Kilanowski, A.; Sobczyk, M.K.; 23andMe Research Team; Shringarpure, S.S.; Mitchell, R.; Reis, K.; Reigo, A.; Estonian Biobank Research Team; Mägi, R.; et al. European and multi-ancestry genome-wide association meta-analysis of atopic dermatitis highlights importance of systemic immune regulation. Nat. Commun. 2023, 14, 6172. [Google Scholar] [CrossRef]
- Li, J.; Su, Y.; Chen, L.; Lin, Y.; Ru, K. Identification of novel mutations in patients with Diamond-Blackfan anemia and literature review of RPS10 and RPS26 mutations. Int. J. Lab. Hematol. 2023, 45, 766–773. [Google Scholar] [CrossRef]
- Song, R.; Xie, L.; Ding, J.; Chen, Y.; Zou, H.; Pang, H.; Peng, Y.; Xia, Y.; Xie, Z.; Li, X.; et al. Association of RPS26 gene polymorphism with different types of diabetes in Chinese individuals. J. Diabetes Investig. 2024, 15, 34–43. [Google Scholar] [PubMed]
- Tutak, K.; Broniarek, I.; Zielezinski, A.; Niewiadomska, D.; Skrzypczak, T.; Baud, A.; Sobczak, K. Insufficiency of 40S ribosomal proteins, RPS26 and RPS25, negatively affects biosynthesis of polyglycine-containing proteins in fragile-X associated conditions. eLife 2025, 13, RP98631. [Google Scholar] [PubMed]
- Li, S.C.; Wang, L.J.; Kuo, H.C.; Tsai, C.S.; Chou, W.J.; Li, C.J.; Liu, A.C.; Yeh, H.Y.; Chen, D.W.; Lee, S.Y. Single-cell RNA sequencing study reveals the potential role of the RPS26 gene in attention deficit hyperactivity disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 141, 111444. [Google Scholar]
- Liu, K.; Chen, Z.; Liu, L.; Li, T.; Xing, C.; Han, F.; Mao, H. Causal Effects of Oxidative Stress on Diabetes Mellitus and Microvascular Complications: Insights Integrating Genome-Wide Mendelian Randomization, DNA Methylation, and Proteome. Antioxidants 2024, 13, 903. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Renert-Yuval, Y.; Brunner, P.M. Atopic dermatitis. Lancet 2025, 405, 583–596. [Google Scholar] [CrossRef]
- Sun, Z.; Vattepu, R.; Zhang, S. Chemokines and Innate Lymphoid Cells in Skin Inflammation. Cells 2021, 10, 3074. [Google Scholar] [CrossRef]
- Rojahn, T.B.; Vorstandlechner, V.; Krausgruber, T.; Bauer, W.M.; Alkon, N.; Bangert, C.; Thaler, F.M.; Sadeghyar, F.; Fortelny, N.; Gernedl, V.; et al. Single-cell transcriptomics combined with interstitial fluid proteomics defines cell type-specific immune regulation in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 146, 1056–1069. [Google Scholar] [CrossRef]
- Pareek, A.; Kumari, L.; Pareek, A.; Chaudhary, S.; Ratan, Y.; Janmeda, P.; Chuturgoon, S.; Chuturgoon, A. Unraveling Atopic Dermatitis: Insights into Pathophysiology, Therapeutic Advances, and Future Perspectives. Cells 2024, 13, 425. [Google Scholar] [CrossRef]
- Czarnowicki, T.; Esaki, H.; Gonzalez, J.; Renert-Yuval, Y.; Brunner, P.; Oliva, M.; Estrada, Y.; Xu, H.; Zheng, X.; Talasila, S.; et al. Alterations in B-cell subsets in pediatric patients with early atopic dermatitis. J. Allergy Clin. Immunol. 2017, 140, 134–144.e9. [Google Scholar] [CrossRef]
- Schuler, C.F., 4th; Tsoi, L.C.; Billi, A.C.; Harms, P.W.; Weidinger, S.; Gudjonsson, J.E. Genetic and Immunological Pathogenesis of Atopic Dermatitis. J. Investig. Dermatol. 2024, 144, 954–968. [Google Scholar]
- Mitamura, Y.; Reiger, M.; Kim, J.; Xiao, Y.; Zhakparov, D.; Tan, G.; Rückert, B.; Rinaldi, A.O.; Baerenfaller, K.; Akdis, M.; et al. Spatial transcriptomics combined with single-cell RNA-sequencing unravels the complex inflammatory cell network in atopic dermatitis. Allergy 2023, 78, 2215–2231. [Google Scholar] [CrossRef] [PubMed]
- Grafanaki, K.; Bania, A.; Kaliatsi, E.G.; Vryzaki, E.; Vasilopoulos, Y.; Georgiou, S. The Imprint of Exposome on the Development of Atopic Dermatitis across the Lifespan: A Narrative Review. J. Clin. Med. 2023, 12, 2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, H.; Wang, X.; Dong, E.; Yao, J.; Li, Y.; Yang, Y.; Wang, T. Exploring the role of breastfeeding, antibiotics, and indoor environments in preschool children atopic dermatitis through machine learning and hygiene hypothesis. Sci. Rep. 2025, 15, 9796. [Google Scholar] [CrossRef] [PubMed]
- Suárez, A.L.; Feramisco, J.D.; Koo, J.; Steinhoff, M. Psychoneuroimmunology of psychological stress and atopic dermatitis: Pathophysiologic and therapeutic updates. Acta Derm. Venereol. 2012, 92, 7–15. [Google Scholar]
- Campagna, M.P.; Xavier, A.; Lechner-Scott, J.; Maltby, V.; Scott, R.J.; Butzkueven, H.; Jokubaitis, V.G.; Lea, R.A. Epigenome-wide association studies: Current knowledge, strategies and recommendations. Clin. Epigenet. 2021, 13, 214. [Google Scholar] [CrossRef]
- Yoshida, Y.; Hayakawa, K.; Fujishiro, M.; Ikeda, K.; Tsushima, H.; Hirai, T.; Kawasaki, M.; Tominaga, M.; Suga, Y.; Takamori, K.; et al. Social defeat stress exacerbates atopic dermatitis through downregulation of DNA methyltransferase 1 and upregulation of C-C motif chemokine receptor 7 in skin dendritic cells. Biochem. Biophys. Res. Commun. 2020, 529, 1073–1079. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Sio, Y.Y.; Say, Y.H.; Reginald, K.; Chew, F.T. A Deep Intronic Polymorphism at 9q21.11 Contributes to the Risk of Atopic Dermatitis Through Methylation-Regulated Expression of Tight Junction Protein 2. J. Investig. Allergol. Clin. Immunol. 2025, 35, 179–193. [Google Scholar] [CrossRef]
- Zhang, J.; Dutta, D.; Köttgen, A.; Tin, A.; Schlosser, P.; Grams, M.E.; Harvey, B.; CKDGen Consortium; Yu, B.; Boerwinkle, E.; et al. Plasma proteome analyses in individuals of European and African ancestry identify cis-pQTLs and models for proteome-wide association studies. Nat. Genet. 2022, 54, 593–602. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, Y.; Feng, Q.; Yao, K.; Zheng, L.; Yang, Y.; Zheng, W.; Li, F.; Lv, Y.; Cai, Y. Novel candidate plasma proteins for the pathogenesis and treatment of atopic dermatitis revealed by proteome-wide association study. Sci. Rep. 2024, 14, 30096. [Google Scholar] [CrossRef]
- Nylund, L.; Satokari, R.; Nikkilä, J.; Rajilić-Stojanović, M.; Kalliomäki, M.; Isolauri, E.; Salminen, S.; de Vos, W.M. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol. 2013, 13, 12. [Google Scholar] [CrossRef]
- Pessôa, R.; Clissa, P.B.; Sanabani, S.S. The Interaction between the Host Genome, Epigenome, and the Gut-Skin Axis Microbiome in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 14322. [Google Scholar]
- Khadka, V.D.; Key, F.M.; Romo-González, C.; Martínez-Gayosso, A.; Campos-Cabrera, B.L.; Gerónimo-Gallegos, A.; Lynn, T.C.; Durán-McKinster, C.; Coria-Jiménez, R.; Lieberman, T.D.; et al. The Skin Microbiome of Patients with Atopic Dermatitis Normalizes Gradually During Treatment. Front. Cell. Infect. Microbiol. 2021, 11, 720674. [Google Scholar] [CrossRef]
- Hülpüsch, C.; Tremmel, K.; Hammel, G.; Bhattacharyya, M.; de Tomassi, A.; Nussbaumer, T.; Neumann, A.U.; Reiger, M.; Traidl-Hoffmann, C. Skin pH-dependent Staphylococcus aureus abundance as predictor for increasing atopic dermatitis severity. Allergy 2020, 75, 2888–2898. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.W.; Han, J.; Garcet, S.; Krueger, J.G.; Pavel, A.B.; Guttman-Yassky, E. Improving evaluation of drugs in atopic dermatitis by combining clinical and molecular measures. J. Allergy. Clin. Immunol. Pract. 2020, 8, 3622–3625.e19. [Google Scholar] [CrossRef] [PubMed]
- Bakker, D.; de Bruin-Weller, M.; Drylewicz, J.; van Wijk, F.; Thijs, J. Biomarkers in atopic dermatitis. J. Allergy Clin. Immunol. 2023, 151, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Marín, H.A.; Silverberg, J.I. Differences between pediatric and adult atopic dermatitis. Pediatr. Dermatol. 2022, 39, 345–353. [Google Scholar] [CrossRef]
- Del Duca, E.; Renert-Yuval, Y.; Pavel, A.B.; Mikhaylov, D.; Wu, J.; Lefferdink, R.; Fang, M.; Sheth, A.; Blumstein, A.; Facheris, P.; et al. Proteomic characterization of atopic dermatitis blood from infancy to adulthood. J. Am. Acad. Dermatol. 2023, 88, 1083–1093. [Google Scholar] [CrossRef]
- Bar, J.; Del Duca, E.; David, E.; Bose, S.; Chefitz, G.; Brunner, P.M.; Bissonnette, R.; Guttman-Yassky, E. Skin Tape Stripping Reveals Distinct Biomarker Profiles in Chronic Hand Eczema of Patients with and Without Comorbid Atopic Dermatitis. Allergy 2025, 80, 2271–2285. [Google Scholar] [CrossRef]
- Correa da Rosa, J.; Malajian, D.; Shemer, A.; Rozenblit, M.; Dhingra, N.; Czarnowicki, T.; Khattri, S.; Ungar, B.; Finney, R.; Xu, H.; et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J. Allergy Clin. Immunol. 2015, 135, 712–720. [Google Scholar] [CrossRef]
- Garcovich, S.; Maurelli, M.; Gisondi, P.; Peris, K.; Yosipovitch, G.; Girolomoni, G. Pruritus as a Distinctive Feature of Type 2 Inflammation. Vaccines 2021, 9, 303. [Google Scholar] [CrossRef]
- Robinson, L.; Strowd, L.C. American Academy of Dermatology Guidelines for Managing Atopic Dermatitis. Adv. Exp. Med. Biol. 2024, 1447, 217–225. [Google Scholar] [PubMed]
- Lobefaro, F.; Gualdi, G.; Di Nuzzo, S.; Amerio, P. Atopic Dermatitis: Clinical Aspects and Unmet Needs. Biomedicines 2022, 10, 2927. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y.; Hayano, S. Subtypes of atopic dermatitis: From phenotype to endotype. Allergol. Int. 2022, 71, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, Z.; Deng, Y. Clinical features and biomarker differences of severe intrinsic and extrinsic atopic dermatitis. Cutan. Ocul. Toxicol. 2024, 43, 97–103. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Silverberg, J.I.; Wilson, E.J.; Ong, P.Y. Update on Atopic Dermatitis: Diagnosis, Severity Assessment, and Treatment Selection. J. Allergy Clin. Immunol. Pract. 2020, 8, 91–101. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef]
- Williams, H.C.; Burney, P.G.; Pembroke, A.C.; Hay, R.J. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br. J. Dermatol. 1994, 131, 406–416. [Google Scholar] [CrossRef]
- Fernando, D.D.; Mounsey, K.E.; Bernigaud, C.; Surve, N.; Estrada Chávez, G.E.; Hay, R.J.; Currie, B.J.; Chosidow, O.; Fischer, K. Scabies. Nat. Rev. Dis. Primers 2024, 10, 74. [Google Scholar] [CrossRef]
- Tucker, D.; Masood, S. Seborrheic Dermatitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Scheinman, P.L.; Vocanson, M.; Thyssen, J.P.; Johansen, J.D.; Nixon, R.L.; Dear, K.; Botto, N.C.; Morot, J.; Goldminz, A.M. Contact dermatitis. Nat. Rev. Dis. Primers 2021, 7, 38. [Google Scholar] [CrossRef]
- Gutiérrez-Cerrajero, C.; Sprecher, E.; Paller, A.S.; Akiyama, M.; Mazereeuw-Hautier, J.; Hernández-Martín, A.; González-Sarmiento, R. Ichthyosis. Nat. Rev. Dis. Primers 2023, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J.; Kim, Y.H.; Stonesifer, C.; Tensen, C.P.; Geskin, L.J.; Quaglino, P.; Ramelyte, E. Cutaneous T cell lymphoma. Nat. Rev. Dis. Primers 2021, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, S.; Worswick, S. Photosensitizing drug reactions. Clin. Dermatol. 2022, 40, 57–63. [Google Scholar] [CrossRef]
- Lehman, H.; Gordon, C. The Skin as a Window into Primary Immune Deficiency Diseases: Atopic Dermatitis and Chronic Mucocutaneous Candidiasis. J. Allergy Clin. Immunol. Pract. 2019, 7, 788–798. [Google Scholar]
- Tso, S.; Satchwell, F.; Moiz, H.; Hari, T.; Dhariwal, S.; Barlow, R.; Forbat, E.; Randeva, H.; Tan, Y.T.; Ilchyshyn, A.; et al. Erythroderma (exfoliative dermatitis). Part 1: Underlying causes, clinical presentation and pathogenesis. Clin. Exp. Dermatol. 2021, 46, 1001–1010. [Google Scholar] [CrossRef]
- Chopra, R.; Vakharia, P.P.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y.; Silverberg, J.I. Severity strata for Eczema Area and Severity Index (EASI), modified EASI, Scoring Atopic Dermatitis (SCORAD), objective SCORAD, Atopic Dermatitis Severity Index and body surface area in adolescents and adults with atopic dermatitis. Br. J. Dermatol. 2017, 177, 1316–1321. [Google Scholar] [CrossRef]
- Ridd, M.J.; Gaunt, D.M.; Guy, R.H.; Redmond, N.M.; Garfield, K.; Hollinghurst, S.; Ball, N.; Shaw, L.; Purdy, S.; Metcalfe, C. Comparison of patient (POEM), observer (EASI, SASSAD, TIS) and corneometry measures of emollient effectiveness in children with eczema: Findings from the COMET feasibility trial. Br. J. Dermatol. 2018, 179, 362–370. [Google Scholar] [CrossRef]
- Staumont-Sallé, D.; Taieb, C.; Merhand, S.; Shourick, J. The Atopic Dermatitis Control Tool: A High-Performance Tool for Optimal Support. Acta Derm. Venereol. 2021, 101, adv00618. [Google Scholar]
- Kwatra, S.G.; Rodriguez, D.; Dias-Barbosa, C.; Budhiarso, I.; Fofana, F.; Vernon, M.; Gabriel, S.; Piketty, C.; Puelles, J. Validation of the Peak Pruritus Numerical Rating Scale as a Patient-Reported Outcome Measure in Prurigo Nodularis. Dermatol. Ther. 2023, 13, 2403–2416. [Google Scholar] [CrossRef]
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef]
- AAAAI/ACAAI JTF Atopic Dermatitis Guideline Panel; Chu, D.K.; Schneider, L.; Asiniwasis, R.N.; Boguniewicz, M.; De Benedetto, A.; Ellison, K.; Frazier, W.T.; Greenhawt, M.; Huynh, J.; et al. Atopic dermatitis (eczema) guidelines: 2023 American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force on Practice Parameters GRADE- and Institute of Medicine-based recommendations. Ann. Allergy Asthma Immunol. 2024, 132, 274–312. [Google Scholar] [CrossRef] [PubMed]
- Oykhman, P.; Dookie, J.; Al-Rammahy, H.; de Benedetto, A.; Asiniwasis, R.N.; LeBovidge, J.; Wang, J.; Ong, P.Y.; Lio, P.; Gutierrez, A.; et al. Dietary Elimination for the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2022, 10, 2657–2666.e8. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Alikhan, A.; Bercovitch, L.; Cohen, D.E.; Darr, J.M.; Drucker, A.M.; Eichenfield, L.F.; Frazer-Green, L.; Paller, A.S.; Schwarzenberger, K.; et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J. Am. Acad. Dermatol. 2023, 89, e1–e20. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, T.D.; Aleman, S.J.; Bao-Loc-Trung, M.; Forte, M.V.; Brandt, W.; Armstrong, C.; Howard, J.; Mosieri, C.N.; Ahmadzadeh, S.; Varrassi, G.; et al. Advancing Treatment in Atopic Dermatitis: A Comprehensive Review of Clinical Efficacy, Safety, and Comparative Insights Into Corticosteroids, Calcineurin Inhibitors, and Phosphodiesterase-4 Inhibitors as Topical Therapies. Cureus 2024, 16, e55393. [Google Scholar] [CrossRef]
- Woo, T.E.; Kuzel, P. Crisaborole 2% Ointment (Eucrisa) for Atopic Dermatitis. Ski. Ther. Lett. 2019, 24, 4–6. [Google Scholar]
- Kirima, K.; Hoshino, Y.; Arichika, N.; Wadatsu, T.; Kakumoto, Y.; Shibamori, M.; Hiyama, H. Difamilast topically ameliorates pruritus, dermatitis, and barrier dysfunction in atopic dermatitis model mice by inhibiting phosphodiesterase 4, especially the 4B subtype. J. Pharmacol. Exp. Ther. 2025, 392, 103582. [Google Scholar] [CrossRef]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Eichenfield, L.F.; Hebert, A.A.; Simpson, E.L.; Stein Gold, L.; Bissonnette, R.; Papp, K.A.; Browning, J.; Kwong, P.; Korman, N.J.; et al. Tapinarof cream 1% once daily: Significant efficacy in the treatment of moderate to severe atopic dermatitis in adults and children down to 2 years of age in the pivotal phase 3 ADORING trials. J. Am. Acad. Dermatol. 2024, 91, 457–465. [Google Scholar] [CrossRef]
- Shirley, M. Dupilumab: First Global Approval. Drugs 2017, 77, 1115–1121. [Google Scholar] [CrossRef]
- Seegräber, M.; Srour, J.; Walter, A.; Knop, M.; Wollenberg, A. Dupilumab for treatment of atopic dermatitis. Expert Rev. Clin. Pharmacol. 2018, 11, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Simpson, E.L.; Siegfried, E.C.; Cork, M.J.; Wollenberg, A.; Arkwright, P.D.; Soong, W.; Gonzalez, M.E.; Schneider, L.C.; Sidbury, R.; et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2022, 400, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Regeneron. Dupixent® (Dupilumab) Prescribing Information; Regeneron: Tarrytown, NY, USA, 2017. [Google Scholar]
- Blair, H.A. Tralokinumab in Atopic Dermatitis: A Profile of Its Use. Clin. Drug Investig. 2022, 42, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Flohr, C.; Cork, M.; Bewley, A.; Blauvelt, A.; Hong, H.C.; Imafuku, S.; Schuttelaar, M.L.A.; Simpson, E.L.; Soong, W.; et al. Efficacy and Safety of Tralokinumab in Adolescents with Moderate to Severe Atopic Dermatitis: The Phase 3 ECZTRA 6 Randomized Clinical Trial. JAMA Dermatol. 2023, 159, 596–605. [Google Scholar] [CrossRef]
- Chovatiya, R.; Ribero, S.; Wollenberg, A.; Park, C.O.; Silvestre, J.F.; Hong, H.C.; Seneschal, J.; Saeki, H.; Thyssen, J.P.; Øland, C.B.; et al. Long-Term Disease Control and Minimal Disease Activity of Head and Neck Atopic Dermatitis in Patients Treated with Tralokinumab up to 4 Years. Am. J. Clin. Dermatol. 2025, 26, 587–601. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Toth, D.; Bieber, T.; Alexis, A.F.; Elewski, B.E.; Pink, A.E.; Hijnen, D.; Jensen, T.N.; Bang, B.; Olsen, C.K.; et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: Results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br. J. Dermatol. 2021, 184, 450–463. [Google Scholar]
- Wollenberg, A.; Beck, L.A.; de Bruin Weller, M.; Simpson, E.L.; Imafuku, S.; Boguniewicz, M.; Zachariae, R.; Olsen, C.K.; Thyssen, J.P. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: Results from five tralokinumab clinical trials. Br. J. Dermatol. 2022, 186, 453–465. [Google Scholar] [CrossRef]
- Stingeni, L.; Ferrucci, S.; Amerio, P.; Foti, C.; Patruno, C.; Girolomoni, G. Lebrikizumab: A new anti-IL-13 agent for treating moderate-to-severe atopic dermatitis. Expert Opin. Biol. Ther. 2025, 25, 15–20. [Google Scholar]
- Guttman-Yassky, E.; Simpson, E.L.; Reich, K.; Kabashima, K.; Igawa, K.; Suzuki, T.; Mano, H.; Matsui, T.; Esfandiari, E.; Furue, M. An anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: A multicentre, double-blind, placebo-controlled phase 2b study. Lancet 2023, 401, 204–214. [Google Scholar]
- Hoy, S.M. Baricitinib: A Review in Moderate to Severe Atopic Dermatitis. Am. J. Clin. Dermatol. 2022, 23, 409–420. [Google Scholar] [CrossRef]
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020, 9, 2020-8-5. [Google Scholar] [CrossRef]
- Edwards, S.J.; Karner, C.; Jhita, T.; Barton, S.; Marceniuk, G.; Yiu, Z.Z.N.; Wittmann, M. Abrocitinib, tralokinumab and upadacitinib for treating moderate-to-severe atopic dermatitis. Health Technol. Assess 2024, 28, 1–113. [Google Scholar] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Haag, C.; Alexis, A.; Aoki, V.; Bissonnette, R.; Blauvelt, A.; Chovatiya, R.; Cork, M.J.; Danby, S.G.; Eichenfield, L.F.; Eyerich, K.; et al. A practical guide to using oral Janus kinase inhibitors for atopic dermatitis from the International Eczema Council. Br. J. Dermatol. 2024, 192, 135–143. [Google Scholar] [CrossRef]
- Mikhaylov, D.; Ungar, B.; Renert-Yuval, Y.; Guttman-Yassky, E. Oral Janus kinase inhibitors for atopic dermatitis. Ann. Allergy Asthma Immunol. 2023, 130, 577–592. [Google Scholar] [CrossRef]
- Bracho-Borro, M.; Franco-Ruiz, P.A.; Magaña, M. The use of azathioprine in atopic dermatitis: A review. Dermatol. Ther. 2022, 35, e15665. [Google Scholar] [CrossRef]
- Khattri, S.; Shemer, A.; Rozenblit, M.; Dhingra, N.; Czarnowicki, T.; Finney, R.; Gilleaudeau, P.; Sullivan-Whalen, M.; Zheng, X.; Xu, H.; et al. Cyclosporine in patients with atopic dermatitis modulates activated inflammatory pathways and reverses epidermal pathology. J. Allergy Clin. Immunol. 2014, 133, 1626–1634. [Google Scholar] [CrossRef]
- Maksimovic, V.; Pavlovic-Popovic, Z.; Vukmirovic, S.; Cvejic, J.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Molecular mechanism of action and pharmacokinetic properties of methotrexate. Mol. Biol. Rep. 2020, 47, 4699–4708. [Google Scholar] [CrossRef]
- Prussick, L.; Plotnikova, N.; Gottlieb, A. Mycophenolate Mofetil in Severe Atopic Dermatitis: A Review. J. Drugs Dermatol. 2016, 15, 715–718. [Google Scholar]
- Molla, A. A Comprehensive Review of Phototherapy in Atopic Dermatitis: Mechanisms, Modalities, and Clinical Efficacy. Cureus 2024, 16, e56890. [Google Scholar] [CrossRef]
- Davis, D.M.R.; Drucker, A.M.; Alikhan, A.; Bercovitch, L.; Cohen, D.E.; Darr, J.M.; Eichenfield, L.F.; Frazer-Green, L.; Paller, A.S.; Schwarzenberger, K.; et al. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. J. Am. Acad. Dermatol. 2024, 90, e43–e56. [Google Scholar] [CrossRef]
| Major criteria (at least 3 must be met) |
| 1. Pruritus |
| 2. Typical morphology and distribution |
| Adults: Flexural lichenification |
| Infancy: Facial and extensor involvement |
| 3. Chronic or chronically relapsing dermatitis |
| 4. Personal or family history of atopic disease (asthma, allergic rhinitis, AD) |
| Minor criteria (at least 3 must be met) |
| 1. Xerosis |
| 2. Ichthyosis/hyperlinear palms/keratosis pilaris |
| 3. Immediate skin test reactivity |
| 4. Elevated serum IgE |
| 5. Early age of onset |
| 6. Tendency for cutaneous infections |
| 7. Tendency to nonspecific hand/foot dermatitis |
| 8. Nipple eczema |
| 9. Cheilitis |
| 10. Recurrent conjunctivitis |
| 11. Dennie-Morgan infraorbital folds |
| 12. Keratoconus |
| 13. Anterior subscapsular cataracts |
| 14. Orbital darkening |
| 15. Facial pallor/facial erythema |
| 16. Pityriasis alba |
| 17. Anterior neck folds |
| 18. Pruritus when sweating |
| 19. Intolerance to wool and lipid solvents |
| 20. Perifollicular accentuation |
| 21. Food hypersensitivity |
| 22. Course influenced by environmental and/or emotional factors |
| 23. White dermatographism or delayed blanch to cholinergic agent |
| Essential features (must be present) |
| Pruritus |
| Eczema (acute, subacute, chronic) |
| Typical morphology and age-specific patterns * |
| Chronic or relapsing history |
| Important features (seen in most cases, adding support to the diagnosis) |
| Early age of onset |
| Atopy |
| Personal and/or family history |
| IgE reactivity |
| Xerosis |
| Associated features (suggest the diagnosis, but not for defining or detecting AD) |
| Atypical vascular responses (e.g., facial pallor, while dermographism, delayed blanch response) |
| Keratosis pilaris/pityriasis alba/hyperlinear palms/ichthyosis |
| Ocular/periorbital changes |
| Other regional findings (e.g., perioral changes/periauricular lesions) |
| Perifollicular accentuation/lichenification/prurigo lesions |
| Exclusionary conditions |
| Scabies |
| Seborrheic dermatitis |
| Contact dermatitis |
| Ichthyoses |
| Cutaneous T-cell lymphoma |
| Psoriasis |
| Photosensitivity dermatoses |
| Immune deficiency diseases |
| Erythroderma of other causes |
| Diagnosis | Main Features | Ref. |
| Scabies | Increased nocturnal pruritus, a small, short (3–7 mm) and linear-to-serpiginous burrow visible in the skin surface caused by scabies mites | [49] |
| Seborrheic dermatitis | Tend to coexist with AD in infants, salmon-colored papules and greasy scale crust in scalp and face | [50] |
| Contact dermatitis | Shown in the exposure area to allergens or irritants. Acute: oedema, erythema and vesicles; chronic: xerosis (dry skin), scales, hyperkeratosis and fissures | [51] |
| Ichthyoses | Impaired keratinocyte differentiation and abnormal formation of the epidermal barrier result in itching, frequent infections, reduced sweating (hypohidrosis) accompanied by heat intolerance, as well as various complications related to vision, hearing, and nutrition | [52] |
| Cutaneous T-cell lymphoma | Erythematous, dry patches, skin biopsy, PCR and other laboratory tests are needed for diagnosis | [53] |
| Psoriasis | Immune-mediated erythematous patches with silvery scale, nail can be affected | [54] |
| Photosensitivity dermatoses | Cutaneous eruptions when exposed to ultraviolet or visible radiation can be induced by drugs | [55] |
| Immune deficiency diseases | AD can be skin manifestation; genetic testing is needed | [56] |
| Erythroderma of other causes | Skin inflammatory state, with associated skin barrier and metabolic dysfunctions, clinical history, biopsies and other tests are needed | [57] |
| Scoring System | Parameters | Severity Rating | Ref. |
| SCORAD | six signs—erythema, excoriation, swelling, oozing/crusting, lichenification and dryness | Clear (0–9.9) | [58] |
| Mild (10.0–28.9) | |||
| on eight body sites, and pruritus and sleeplessness | Moderate (29.0–48.9) | ||
| Severe (49.0–103) | |||
| EASI | four signs—erythema, excoriation, swelling and lichenification | Clear (0) | |
| Almost clear (0.1–1.0) | |||
| Mild (1.1–7) | |||
| on four body sites | Moderate (7.1–21) | ||
| Severe (21.1–50) | |||
| Very severe (50.1–72) | |||
| IGA | FDA categorization of AD severity based on the investigator’s subjective assessment of a representative lesion | 0 = clear | |
| 1 = almost clear | |||
| 2 = mild | |||
| 3 = moderate | |||
| 4 = severe | |||
| SASSAD | six signs (erythema, exudation, excoriation, dryness, cracking and lichenification | 0 = absent | [59] |
| 1 = mild | |||
| on six areas (head and neck, trunk, hands, arms, legs and feet | 2 = moderate | ||
| 3 = severe | |||
| POEM | seven symptoms scored over past week (itch, sleep, bleeding, weeping/oozing, cracking, flaking, and dryness/roughness) | Clear/almost clear (0–2) | |
| Mild (3–7) | |||
| Moderate (8–16) | |||
| Severe (17–24) | |||
| Very severe (25–28) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, H.; Siu, W.S.; Ma, H.; Li, Y. Understanding Atopic Dermatitis: Pathophysiology and Management Strategies. Biomolecules 2025, 15, 1500. https://doi.org/10.3390/biom15111500
Chai H, Siu WS, Ma H, Li Y. Understanding Atopic Dermatitis: Pathophysiology and Management Strategies. Biomolecules. 2025; 15(11):1500. https://doi.org/10.3390/biom15111500
Chicago/Turabian StyleChai, Heng, Wing Sum Siu, Hui Ma, and Yuzhen Li. 2025. "Understanding Atopic Dermatitis: Pathophysiology and Management Strategies" Biomolecules 15, no. 11: 1500. https://doi.org/10.3390/biom15111500
APA StyleChai, H., Siu, W. S., Ma, H., & Li, Y. (2025). Understanding Atopic Dermatitis: Pathophysiology and Management Strategies. Biomolecules, 15(11), 1500. https://doi.org/10.3390/biom15111500









