Cisplatin-Induced Skeletal Muscle Atrophy: Biomolecular Mechanisms and the Protective Role of Exercise-Induced Myokines
Abstract
1. Introduction
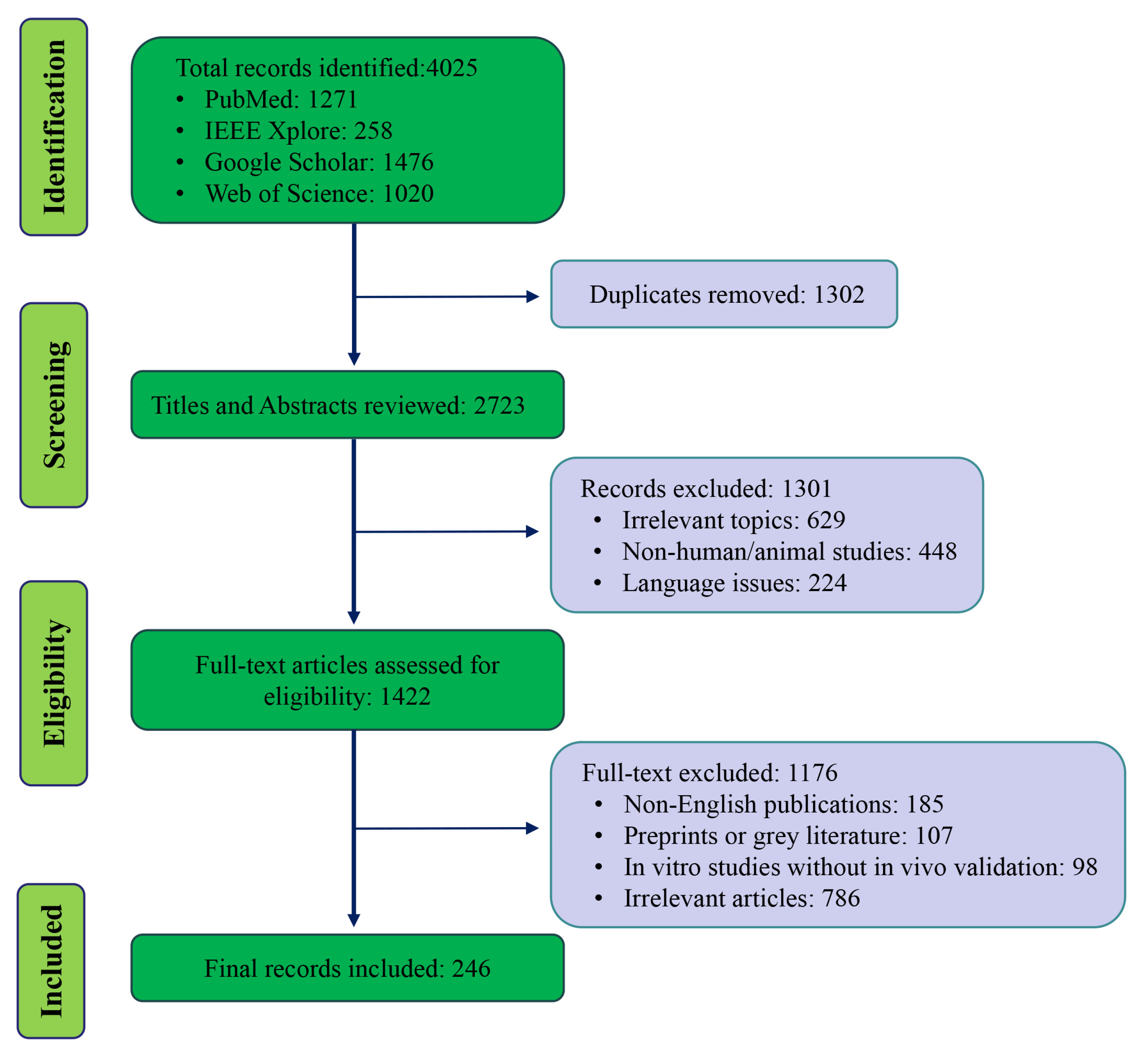
2. Methods
3. Chemotherapy-Induced Skeletal Muscle Atrophy
3.1. Clinical Relevance of Chemotherapy-Associated Muscle Wasting
3.2. Cisplatin and Skeletal Muscle Atrophy: Clinical and Preclinical Evidence
3.3. Chemotherapy-Induced Decreased Motility and Secondary Muscle Disturbance
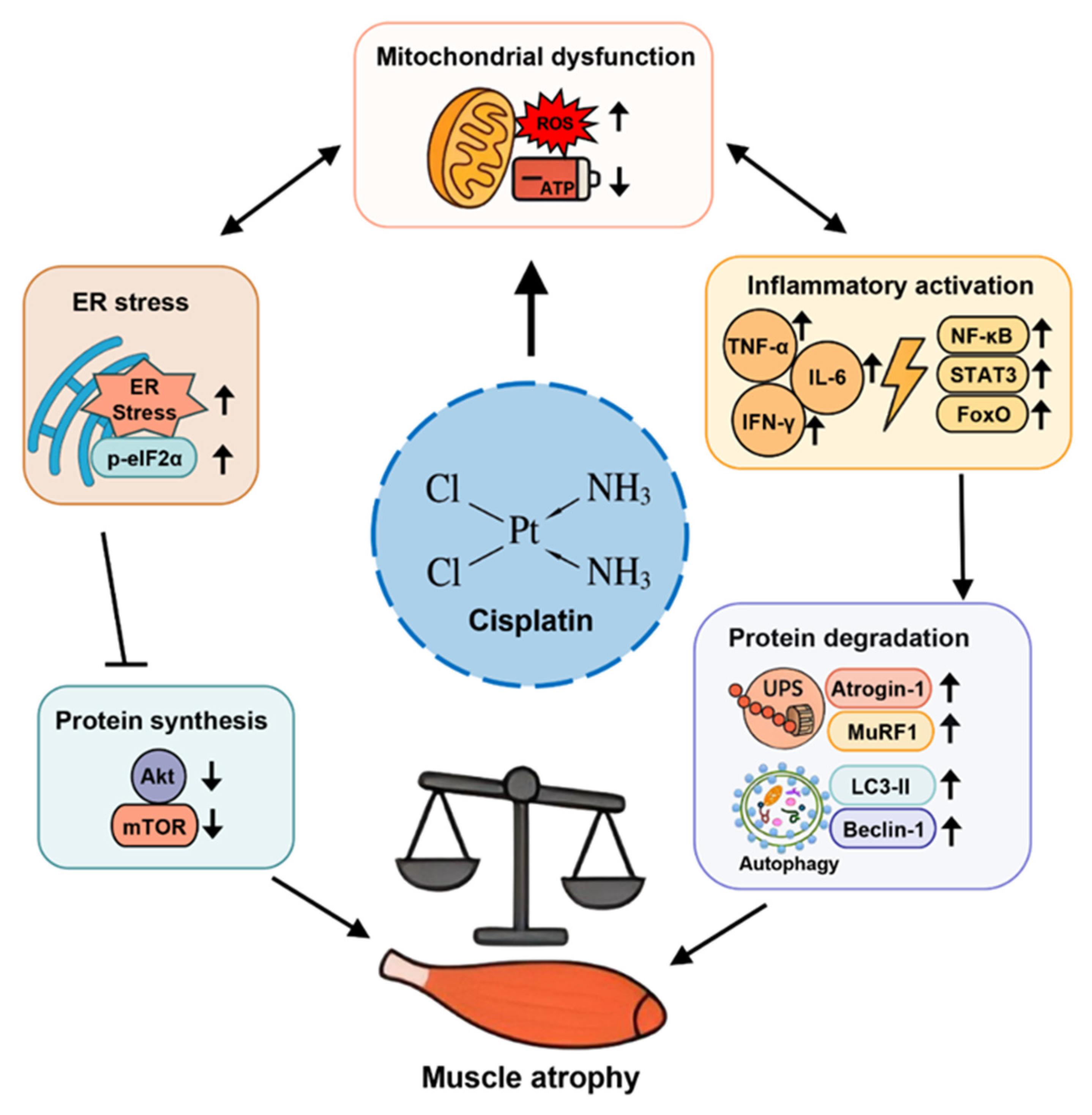
4. Molecular Mechanisms Underlying Muscle Atrophy
4.1. Protein Degradation Pathways
4.2. Suppression of Protein Synthesis
4.3. Mitochondrial Dysfunction and Oxidative Stress
4.4. Inflammatory Signaling
4.5. Crosstalk Between These Pathways
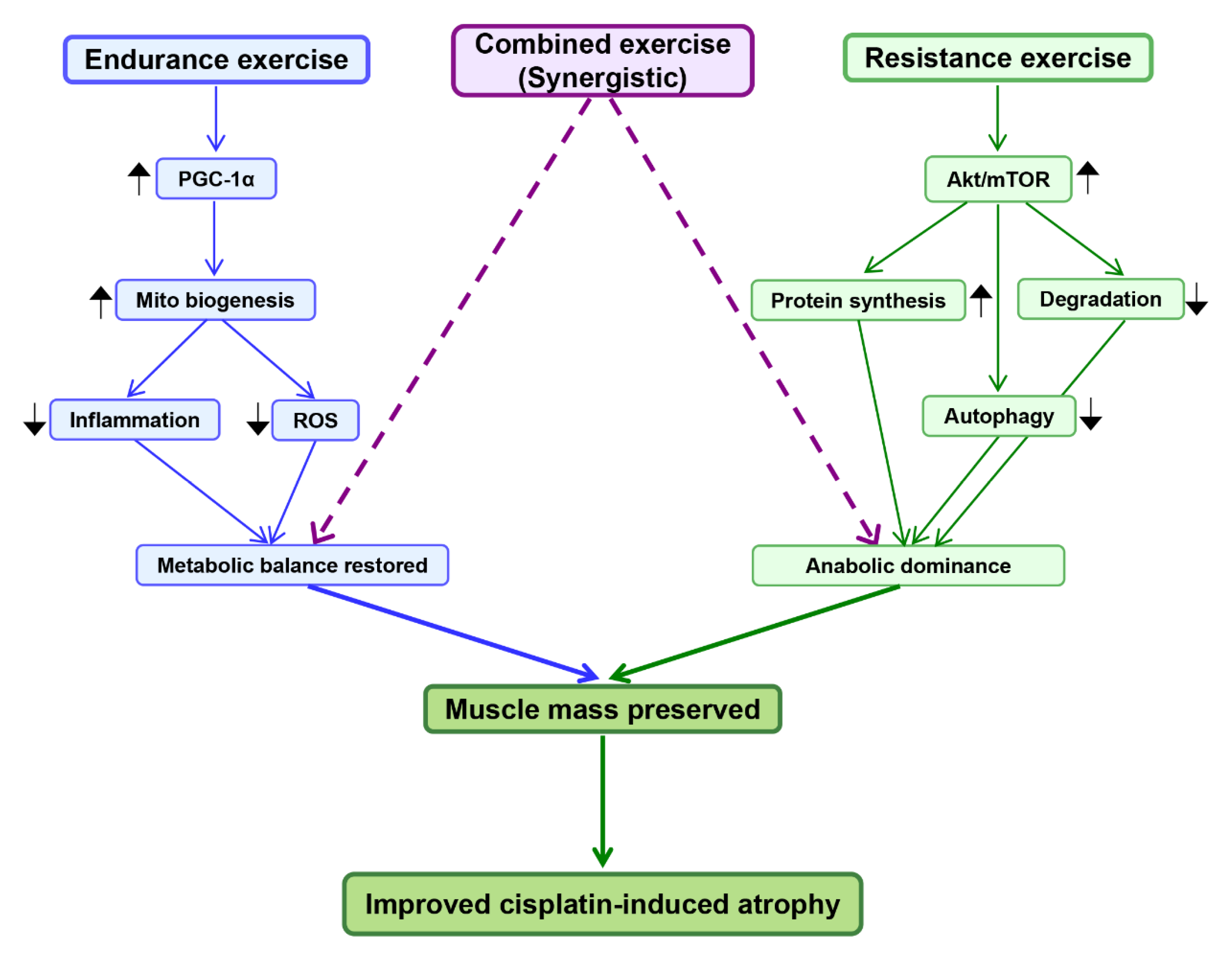
5. Exercise as a Protective Intervention
5.1. Endurance Exercise and Mitochondrial Adaptations
5.2. Resistance Training and Anabolic Signaling
5.3. Combined Exercise and Synergistic Effects
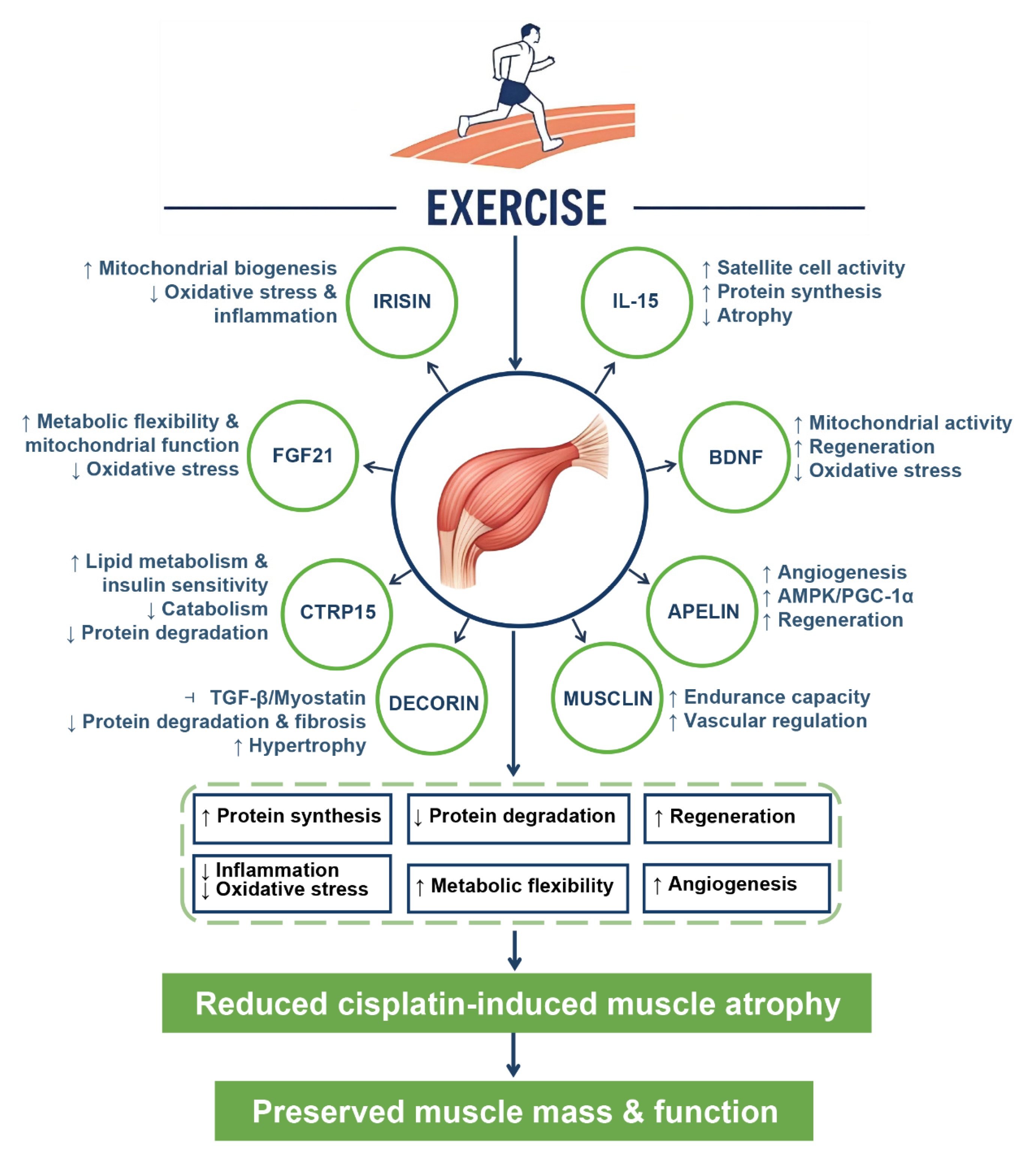
5.4. Myokines as Mediators of Exercise-Induced Protection
5.5. Summary of Protective Mechanisms
6. Future Directions and Challenges
6.1. Standardization of Preclinical Models
6.2. Mechanistic Integration of Exercise Modalities
6.3. Translational Biomarkers and Myokine Profiling
6.4. Clinical Implementation and Personalized Exercise Prescriptions
6.5. Dietary Modulation in Chemotherapy-Induced Skeletal Muscle Atrophy
6.6. Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sartori, R.; Romanello, V.; Sandri, M. Mechanisms of Muscle Atrophy and Hypertrophy: Implications in Health and Disease. Nat. Commun. 2021, 12, 330. [Google Scholar] [CrossRef]
- Wong, J.P.H.; Ng, Y.-K.; Kjærgaard, J.; Blazev, R.; Deshmukh, A.S.; Parker, B.L. Skeletal Muscle Proteomics: Considerations and Opportunities. npj Metab. Health Dis. 2025, 3, 30. [Google Scholar] [CrossRef]
- Bernabe-Ortiz, A.; Carrillo-Larco, R.M.; Gilman, R.H.; Smeeth, L.; Checkley, W.; Miranda, J.J. Skeletal Muscle Mass and All-Cause Mortality: Findings from the CRONICAS Cohort Study. Trop. Med. Int. Health 2023, 28, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Dawson Hughes, B.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional Strategies for Maintaining Muscle Mass and Strength from Middle Age to Later Life: A Narrative Review. Maturitas 2020, 132, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Janssen, T.A.H.; Lowisz, C.V.; Phillips, S. From Molecular to Physical Function: The Aging Trajectory. Curr. Res. Physiol. 2025, 8, 100138. [Google Scholar] [CrossRef]
- Deane, C.S.; Piasecki, M.; Atherton, P.J. Skeletal Muscle Immobilisation-Induced Atrophy: Mechanistic Insights from Human Studies. Clin. Sci. 2024, 138, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, M.; Deng, C.; Qiu, J.; Wang, K.; Chang, M.; Zhou, S.; Gu, Y.; Shen, Y.; Wang, W.; et al. Potential Therapeutic Strategies for Skeletal Muscle Atrophy. Antioxidants 2023, 12, 44. [Google Scholar] [CrossRef]
- Damluji, A.A.; Alfaraidhy, M.; AlHajri, N.; Rohant, N.N.; Kumar, M.; Al Malouf, C.; Bahrainy, S.; Ji Kwak, M.; Batchelor, W.B.; Forman, D.E.; et al. Sarcopenia and Cardiovascular Diseases. Circulation 2023, 147, 1534–1553. [Google Scholar] [CrossRef]
- Lavalle, S.; Valerio, M.R.; Masiello, E.; Gebbia, V.; Scandurra, G. Unveiling the Intricate Dance: How Cancer Orchestrates Muscle Wasting and Sarcopenia. In Vivo 2024, 38, 1520–1529. [Google Scholar] [CrossRef]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer Cachexia: Molecular Mechanisms and Treatment Strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Murphy, E.A.; Carson, J.A. The Impact of Immune Cells on the Skeletal Muscle Microenvironment during Cancer Cachexia. Front. Physiol. 2020, 11, 1037. [Google Scholar] [CrossRef]
- Armstrong, V.S.; Fitzgerald, L.W.; Bathe, O.F. Cancer-Associated Muscle Wasting—Candidate Mechanisms and Molecular Pathways. Int. J. Mol. Sci. 2020, 21, 9268. [Google Scholar] [CrossRef]
- Zhong, P.; Li, X.; Li, J. Mechanisms, Assessment, and Exercise Interventions for Skeletal Muscle Dysfunction Post-Chemotherapy in Breast Cancer: From Inflammation Factors to Clinical Practice. Front. Oncol. 2025, 15, 1551561. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Parry, T.L.; Thomas, G.A.; Khamoui, A.V. Skeletal Muscle Omics Signatures in Cancer Cachexia: Perspectives and Opportunities. J. Natl. Cancer Inst. Monogr. 2023, 2023, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Mantz, L.; Katz-Agranov, N.S.; Ouyang, T.; Hanna, P.E.; Mejia, S.M.; Moreno, D.; Lee, M.; Cao, A.; Dinulos, J.E.; et al. Sarcopenia, Elevated Body Mass Index, and Platinum-Associated Adverse Events in Patients with Lung Cancer. J. Natl. Compr. Cancer Netw. 2025, 23, 393–400. [Google Scholar] [CrossRef]
- Huang, K.; Chiang, Y.; Ali, M.; Hsia, S. Cisplatin-induced Muscle Wasting and Atrophy: Molecular Mechanism and Potential Therapeutic Interventions. J. Cachexia Sarcopenia Muscle 2025, 16, e13817. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Chiang, Y.; Huang, T.; Chen, H.; Lin, P.; Ali, M.; Hsia, S. Capsaicin Alleviates Cisplatin-induced Muscle Loss and Atrophy in Vitro and in Vivo. J. Cachexia Sarcopenia Muscle 2022, 14, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, M.; Yu, Y.; Chen, Y.; Weng, X.; Zhu, L. PD-1 Alleviates Cisplatin-Induced Muscle Atrophy by Regulating Inflammation and Oxidative Stress. Antioxidants 2022, 11, 1839. [Google Scholar] [CrossRef]
- Huot, J.R.; Pin, F.; Chatterjee, R.; Bonetto, A. PGC1α Overexpression Preserves Muscle Mass and Function in Cisplatin-induced Cachexia. J. Cachexia Sarcopenia Muscle 2022, 13, 2480–2491. [Google Scholar] [CrossRef]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; De Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-Induced Skeletal Muscle Dysfunction: Mechanisms and Counteracting Therapeutic Strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, H.; Lee, H.; Hong, M.; Park, S.; Bae, H. Magnolol Attenuates Cisplatin-Induced Muscle Wasting by M2c Macrophage Activation. Front. Immunol. 2020, 11, 77. [Google Scholar] [CrossRef]
- Lønbro, S.; Gam, S.; Hermann, A.P.; Hansen, C.R.; Johansen, J. Accelerated Loss of Lean Body Mass in Head and Neck Cancer Patients during Cisplatin-Based Chemoradiation. Acta Oncol. 2023, 62, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Choi, J.H.; Park, J.Y.; Kim, B.J.; Kim, J.G.; Kim, J.W.; Park, J.-M.; Chi, K.-C.; Hwang, I.G. Loss of Skeletal Muscle Mass during Palliative Chemotherapy Is a Poor Prognostic Factor in Patients with Advanced Gastric Cancer. Sci. Rep. 2020, 10, 17683. [Google Scholar] [CrossRef] [PubMed]
- You, L. Dihydromyricetin Inhibits Ferroptosis to Attenuate Cisplatin-Induced Muscle Atrophy. Physiol. Res. 2024, 73, 405–413. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Chen, P.-Y.; Liao, J.-W.; Huang, R.-L.; Yu, S.-H.; Chen, L.-N.; Lee, M.-H.; Chen, L.-W.; Chen, H.-W.; Yang, Y.-C.; et al. The Protective Effects of Perch Essence against Muscle Atrophy in Cancer Cachexia and Cisplatin Treatment. Curr. Issues Mol. Biol. 2025, 47, 152. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Bao, P.; Wang, Y.J.; Lu, J.; Liu, Y.J. H2S Protects against Immobilization-Induced Muscle Atrophy via Reducing Oxidative Stress and Inflammation. Front. Physiol. 2022, 13, 844539. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Bao, P.; Wang, Y.; Zhu, X.; Liu, Y.; Ni, X.; Lu, J. Skeletal Muscle CSE Deficiency Leads to Insulin Resistance in Mice. Antioxidants 2022, 11, 2216. [Google Scholar] [CrossRef]
- Chen, L.-K. Sarcopenia in the Era of Precision Health: Toward Personalized Interventions for Healthy Longevity. J. Chin. Med. Assoc. 2024, 87, 980. [Google Scholar] [CrossRef]
- Pedrosa, M.B.; Barbosa, S.; Vitorino, R.; Ferreira, R.; Moreira-Gonçalves, D.; Santos, L.L. Chemotherapy-Induced Molecular Changes in Skeletal Muscle. Biomedicines 2023, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, N.; Yamamoto, S.; Hanai, A.; Oiwa, A.; Arao, H. Exercise Intervention for the Management of Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Network Meta-Analysis. Front. Neurol. 2024, 15, 1346099. [Google Scholar] [CrossRef] [PubMed]
- Halle, J.L.; Counts, B.R.; Carson, J.A. Exercise as a Therapy for Cancer-Induced Muscle Wasting. Sports Med. Health Sci. 2020, 2, 186–194. [Google Scholar] [CrossRef]
- Cortiula, F.; Hendriks, L.E.L.; van de Worp, W.R.P.H.; Schols, A.M.W.J.; Vaes, R.D.W.; Langen, R.C.J.; De Ruysscher, D. Physical Exercise at the Crossroad between Muscle Wasting and the Immune System: Implications for Lung Cancer Cachexia. J. Cachexia Sarcopenia Muscle 2022, 13, 55–67. [Google Scholar] [CrossRef]
- Hardee, J.P.; Carson, J.A. Muscular Contraction’s Therapeutic Potential for Cancer-Induced Wasting. Am. J. Physiol. Cell Physiol. 2022, 323, C378–C384. [Google Scholar] [CrossRef] [PubMed]
- Mavropalias, G.; Sim, M.; Taaffe, D.R.; Galvão, D.A.; Spry, N.; Kraemer, W.J.; Häkkinen, K.; Newton, R.U. Exercise Medicine for Cancer Cachexia: Targeted Exercise to Counteract Mechanisms and Treatment Side Effects. J. Cancer Res. Clin. Oncol. 2022, 148, 1389–1406. [Google Scholar] [CrossRef]
- Aires, I.; Duarte, J.A.; Vitorino, R.; Moreira-Gonçalves, D.; Oliveira, P.; Ferreira, R. Restoring Skeletal Muscle Health through Exercise in Breast Cancer Patients and after Receiving Chemotherapy. Int. J. Mol. Sci. 2024, 25, 7533. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Tan, J.; Zhou, H.-H.; Cao, M.; Zou, Y. Long-Term Exercise Training and Inflammatory Biomarkers in Healthy Subjects: A Meta-Analysis of Randomized Controlled Trials. Front. Psychol. 2023, 14, 1253329. [Google Scholar] [CrossRef]
- Feng, Y.; Rao, Z.; Tian, X.; Hu, Y.; Yue, L.; Meng, Y.; Zhong, Q.; Chen, W.; Xu, W.; Li, H.; et al. Endurance Training Enhances Skeletal Muscle Mitochondrial Respiration by Promoting MOTS-c Secretion. Free Radic. Biol. Med. 2025, 227, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.P.; Raun, S.H.; Havula, E.; Henriquez-Olguín, C.; Rubalcava-Gracia, D.; Frank, E.; Fritzen, A.M.; Jannig, P.R.; Andersen, N.R.; Kruse, R.; et al. The Mitochondrial mRNA-Stabilizing Protein SLIRP Regulates Skeletal Muscle Mitochondrial Structure and Respiration by Exercise-Recoverable Mechanisms. Nat. Commun. 2024, 15, 9826. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Tsai, S.-Y. Mitochondrial Properties in Skeletal Muscle Fiber. Cells 2023, 12, 2183. [Google Scholar] [CrossRef]
- Fu, P.; Zhu, R.; Gao, W.; Gong, L. Effects of Resistance Training on Alleviating Hypoxia-induced Muscle Atrophy: Focus on Acetylation of FoxO1. J. Cell. Mol. Med. 2023, 28, e18096. [Google Scholar] [CrossRef]
- Pang, X.; Zhang, P.; Chen, X.; Liu, W. Ubiquitin-Proteasome Pathway in Skeletal Muscle Atrophy. Front. Physiol. 2023, 14, 1289537. [Google Scholar] [CrossRef]
- Bodine, S.C. The Role of mTORC1 in the Regulation of Skeletal Muscle Mass. Fac. Rev. 2022, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, Z.; Zhang, X.-A.; Ning, K. Myokines May Be the Answer to the Beneficial Immunomodulation of Tailored Exercise—A Narrative Review. Biomolecules 2024, 14, 1205. [Google Scholar] [CrossRef]
- Bettariga, F.; Taaffe, D.R.; Galvão, D.A.; Lopez, P.; Bishop, C.; Markarian, A.M.; Natalucci, V.; Kim, J.-S.; Newton, R.U. Exercise Training Mode Effects on Myokine Expression in Healthy Adults: A Systematic Review with Meta-Analysis. J. Sport Health Sci. 2024, 13, 764–779. [Google Scholar] [CrossRef]
- Trettel, C.d.S.; Pelozin, B.R.d.A.; Barros, M.P.; Bachi, A.L.L.; Braga, P.G.S.; Momesso, C.M.; Furtado, G.E.; Valente, P.A.; Oliveira, E.M.; Hogervorst, E.; et al. Irisin: An Anti-Inflammatory Exerkine in Aging and Redox-Mediated Comorbidities. Front. Endocrinol. 2023, 14, 1106529. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Zhu, S.; Liu, H.; Xu, S. Irisin Protects Musculoskeletal Homeostasis via a Mitochondrial Quality Control Mechanism. Int. J. Mol. Sci. 2024, 25, 10116. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Ding, Z.; Shi, X.; Zhu, Q.; Shen, Q.; Xu, X.; Zhang, J.; Gong, W.; Xiao, W.; Wang, D.; et al. Irisin Inhibits Neutrophil Extracellular Traps Formation and Protects against Acute Pancreatitis in Mice. Redox Biol. 2023, 64, 102787. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, Y.; Qin, M.; Yi, X. Interleukin 15: A New Intermediary in the Effects of Exercise and Training on Skeletal Muscle and Bone Function. J. Cell. Mol. Med. 2024, 28, e70136. [Google Scholar] [CrossRef]
- Khalafi, M.; Maleki, A.H.; Symonds, M.E.; Sakhaei, M.H.; Rosenkranz, S.K.; Ehsanifar, M.; Korivi, M.; Liu, Y. Interleukin-15 Responses to Acute and Chronic Exercise in Adults: A Systematic Review and Meta-Analysis. Front. Immunol. 2024, 14, 1288537. [Google Scholar] [CrossRef] [PubMed]
- Balanyà-Segura, M.; Polishchuk, A.; Just-Borràs, L.; Cilleros-Mañé, V.; Silvera, C.; Ardévol, A.; Tomàs, M.; Lanuza, M.A.; Hurtado, E.; Tomàs, J. Molecular Adaptations of BDNF/NT-4 Neurotrophic and Muscarinic Pathways in Ageing Neuromuscular Synapses. Int. J. Mol. Sci. 2024, 25, 8018. [Google Scholar] [CrossRef]
- Chan, W.S.; Ng, C.F.; Pang, B.P.S.; Hang, M.; Tse, M.C.L.; Iu, E.C.Y.; Ooi, X.C.; Yang, X.; Kim, J.K.; Lee, C.W.; et al. Exercise-Induced BDNF Promotes PPARδ-Dependent Reprogramming of Lipid Metabolism in Skeletal Muscle during Exercise Recovery. Sci. Signal. 2024, 17, eadh2783. [Google Scholar] [CrossRef]
- Thakir, T.M.; Wang, A.R.; Decker-Farrell, A.R.; Ferrer, M.; Guin, R.N.; Kleeman, S.; Levett, L.; Zhao, X.; Janowitz, T. Cancer Therapy and Cachexia. J. Clin. Investig. 2025, 135, e191934. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Ko, A.-T.; Weng, C.-S.; Chang, C.-L.; Jan, Y.-T.; Lin, J.-B.; Chien, H.-J.; Lin, W.-C.; Sun, F.-J.; Wu, K.-P.; et al. Explainable Machine Learning Model for Predicting Skeletal Muscle Loss during Surgery and Adjuvant Chemotherapy in Ovarian Cancer. J. Cachexia Sarcopenia Muscle 2023, 14, 2044–2053. [Google Scholar] [CrossRef]
- Weng, C.; Huang, W.; Chang, C.; Jan, Y.; Chen, T.; Lee, J. Association of Malignant Ascites with Systemic Inflammation and Muscle Loss after Treatment in Advanced-stage Ovarian Cancer. J. Cachexia Sarcopenia Muscle 2023, 14, 2114–2125. [Google Scholar] [CrossRef]
- Amitani, M.; Oba, T.; Kiyosawa, N.; Morikawa, H.; Chino, T.; Soma, A.; Shimizu, T.; Ohno, K.; Ono, M.; Ito, T.; et al. Skeletal Muscle Loss during Neoadjuvant Chemotherapy Predicts Poor Prognosis in Patients with Breast Cancer. BMC Cancer 2022, 22, 327. [Google Scholar] [CrossRef]
- Becker, J.-N.; Hermann, R.; Wichmann, J.; Sonnhoff, M.; Christiansen, H.; Bruns, F. Low Skeletal Muscle Mass Is Predictive of Dose-Limiting Toxicities in Head and Neck Cancer Patients Undergoing Low-Dose Weekly Cisplatin Chemoradiotherapy. PLoS ONE 2023, 18, e0282015. [Google Scholar] [CrossRef]
- Suzuki, S.; Yokota, T.; Notsu, A.; Hamauchi, S.; Onozawa, Y.; Fushiki, K.; Oshima, K.; Kawakami, T.; Tsushima, T.; Yasui, H.; et al. Impact of Relative Cisplatin Dose to Skeletal Muscle Mass on Adverse Events in Patients with Head and Neck Cancer Undergoing Chemoradiotherapy. Oncologist 2024, 29, e1315–e1323. [Google Scholar] [CrossRef]
- Jang, M.K.; Park, S.; Park, C.; Doorenbos, A.Z.; Go, J.; Kim, S. Does Neoadjuvant Chemotherapy Regimen Affect Sarcopenia Status in Patients with Breast Cancer? Breast 2022, 66, 1–7. [Google Scholar] [CrossRef]
- Van der Zanden, V.; van Soolingen, N.J.; Viddeleer, A.R.; Trum, J.W.; Amant, F.; Mourits, M.J.E.; Portielje, J.E.A.; Baalbergen, A.; Souwer, E.T.D.; van Munster, B.C. Loss of Skeletal Muscle Density during Neoadjuvant Chemotherapy in Older Women with Advanced Stage Ovarian Cancer Is Associated with Postoperative Complications. Eur. J. Surg. Oncol. 2022, 48, 896–902. [Google Scholar] [CrossRef]
- Schaeffers, A.W.M.A.; Scholten, H.A.; van Beers, M.A.; Meussen, B.W.; Smid, E.J.; van Gils, C.H.; Devriese, L.A.; de Bree, R. The Effect of Skeletal Muscle Mass on Dose-Limiting Toxicities during (Chemo)Radiotherapy in Patients with Head and Neck Cancer: A Systematic Review and Meta-Analysis. Oral. Oncol. 2024, 157, 106978. [Google Scholar] [CrossRef]
- De Jong, C.; Chargi, N.; Herder, G.J.M.; van Haarlem, S.W.A.; van der Meer, F.; van Lindert, A.S.R.; ten Heuvel, A.; Brouwer, J.; de Jong, P.A.; Devriese, L.A.; et al. The Association between Skeletal Muscle Measures and Chemotherapy-induced Toxicity in Non-small Cell Lung Cancer Patients. J. Cachexia Sarcopenia Muscle 2022, 13, 1554–1564. [Google Scholar] [CrossRef]
- Purcell, S.A.; Kok, D.E.; Ketterl, T.; Garcia, M.B.; Joffe, L.; Brown, J.C.; Dieli-Conwright, C.M.; Williams, G.R. Pharmacokinetics of Cancer Therapeutics and Energy Balance: The Role of Diet Intake, Energy Expenditure, and Body Composition. J. Natl. Cancer Inst. Monogr. 2023, 2023, 3–11. [Google Scholar] [CrossRef]
- Schmulenson, E.; Zimmermann, N.; Müller, L.; Kapsa, S.; Sihinevich, I.; Jaehde, U. Influence of the Skeletal Muscle Index on Pharmacokinetics and Toxicity of Fluorouracil. Cancer Med. 2022, 12, 2580–2589. [Google Scholar] [CrossRef]
- Mallard, J.; Hucteau, E.; Schott, R.; Trensz, P.; Pflumio, C.; Kalish-Weindling, M.; Favret, F.; Pivot, X.; Hureau, T.J.; Pagano, A.F. Early Skeletal Muscle Deconditioning and Reduced Exercise Capacity during (Neo)Adjuvant Chemotherapy in Patients with Breast Cancer. Cancer 2023, 129, 215–225. [Google Scholar] [CrossRef]
- Phan, T.T.; Scott, K.S.; Chelette, B.; West, A.P.; Dantzer, R. The Fatigue-Inducing Effects of Cancer and Its Therapy Are Characterized by Decreased Physical Activity in the Absence of Any Motivational Deficit. Brain Behav. Immun. 2024, 117, 205–214. [Google Scholar] [CrossRef]
- Jackson, K.M.; Cole, C.L.; Dunne, R.F. From Bench to Bedside: Updates in Basic Science, Translational and Clinical Research on Muscle Fatigue in Cancer Cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 216–222. [Google Scholar] [CrossRef]
- Anabtawi, N.M.; Pasala, M.S.; Grimshaw, A.A.; Kharel, P.; Bal, S.; Godby, K.; Siwakoti, A.; Buford, T.W.; Bhatia, S.; Costa, L.J.; et al. Low Skeletal Muscle Mass and Treatment Outcomes among Adults with Haematologic Malignancies: A Systematic Review and Meta-analysis. J. Cachexia Sarcopenia Muscle 2024, 15, 1084–1093. [Google Scholar] [CrossRef]
- Luo, K.; Chen, K.; Li, Y.; Ji, Y. Association between Sarcopenia and Outcomes of Surgically Treated Oral Squamous Cell Carcinoma: A Systematic Review and Meta-analysis. Front. Oncol. 2024, 14, 1445956. [Google Scholar] [CrossRef]
- Huiskamp, L.F.J.; Chargi, N.; Devriese, L.A.; de Jong, P.A.; de Bree, R. The Predictive and Prognostic Value of Low Skeletal Muscle Mass for Dose-Limiting Toxicity and Survival in Head and Neck Cancer Patients Receiving Concomitant Cetuximab and Radiotherapy. Eur. Arch. Otorhinolaryngol. 2020, 277, 2847–2858. [Google Scholar] [CrossRef]
- Hatt, J.; Smart, T.F.F.; Hardy, E.J.; Doleman, B.; Lund, J.N.; Philips, B.E. The Impact of Low Muscle Mass on Prognosis Following Neoadjuvant Chemotherapy for Resectable Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis. JCSM Clin. Rep. 2023, 8, 27–35. [Google Scholar] [CrossRef]
- Rinninella, E.; Fagotti, A.; Cintoni, M.; Raoul, P.; Scaletta, G.; Scambia, G.; Gasbarrini, A.; Mele, M.C. Skeletal Muscle Mass as a Prognostic Indicator of Outcomes in Ovarian Cancer: A Systematic Review and Meta-Analysis. Int. J. Gynecol. Cancer 2020, 30, 654–663. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Lee, G.K.-Y.; Au, P.C.-M.; Li, G.H.-Y.; Chan, M.; Li, H.-L.; Cheung, B.M.-Y.; Wong, I.C.-K.; Lee, V.H.-F.; Mok, J.; et al. Systematic Review and Meta-Analysis of Lean Mass and Mortality: Rationale and Study Description. Osteoporos. Sarcopenia 2021, 7, S3–S12. [Google Scholar] [CrossRef]
- Chargi, N.; Wegner, I.; Markazi, N.; Smid, E.; de Jong, P.; Devriese, L.; de Bree, R. Patterns, Predictors, and Prognostic Value of Skeletal Muscle Mass Loss in Patients with Locally Advanced Head and Neck Cancer Undergoing Cisplatin-Based Chemoradiotherapy. J. Clin. Med. 2021, 10, 1762. [Google Scholar] [CrossRef]
- Leão, I.; Garcia, C.; Antunes, P.; Campolargo, A.; Dias, I.; Coimbra, E.; Oliveira, P.; Zenha, H.; Costa, H.; Capela, A.; et al. Acute Impact of Cancer Treatment on Head and Neck Cancer Patients: FIT4TREATMENT. Cancers 2022, 14, 2698. [Google Scholar] [CrossRef]
- Sekine, H.; Matsumoto, C.; Fujitsuka, N.; Mogami, S.; Ohnishi, S.; Takeda, H. Hochuekkito Accelerates Recovery from Cisplatin Induced-Muscle Atrophy Accompanied by Slow-Twitch Fiber-Specific microRNA Upregulation in Mice. Front. Pharmacol. 2025, 16, 1502563. [Google Scholar] [CrossRef]
- Zhang, H.; Chi, M.; Wang, Y.; Chen, L.; Sun, X.; Wan, L.; Yang, Q.; Guo, C. Naringenin Alleviates Cisplatin Induced Muscle Atrophy by Regulating RIPK1/AMPK/NF-κB Pathway. J. Funct. Foods 2021, 86, 104714. [Google Scholar] [CrossRef]
- Seo, D.Y.; Bae, J.H.; Zhang, D.; Song, W.; Kwak, H.-B.; Heo, J.-W.; Jung, S.-J.; Yun, H.R.; Kim, T.N.; Lee, S.H.; et al. Effects of Cisplatin on Mitochondrial Function and Autophagy-Related Proteins in Skeletal Muscle of Rats. BMB Rep. 2021, 54, 575–580. [Google Scholar] [CrossRef]
- Katanić Stanković, J.S.; Selaković, D.; Rosić, G. Oxidative Damage as a Fundament of Systemic Toxicities Induced by Cisplatin—The Crucial Limitation or Potential Therapeutic Target? Int. J. Mol. Sci. 2023, 24, 14574. [Google Scholar] [CrossRef]
- Matsumoto, C.; Sekine, H.; Nahata, M.; Mogami, S.; Ohbuchi, K.; Fujitsuka, N.; Takeda, H. Role of Mitochondrial Dysfunction in the Pathogenesis of Cisplatin-Induced Myotube Atrophy. Biol. Pharm. Bull. 2022, 45, 780–792. [Google Scholar] [CrossRef]
- Hsing, C.-H.; Tsai, C.-C.; Chen, C.-L.; Lin, Y.-H.; Tseng, P.-C.; Satria, R.D.; Lin, C.-F. Pharmacologically Inhibiting Glycogen Synthase Kinase-3β Ameliorates Renal Inflammation and Nephrotoxicity in an Animal Model of Cisplatin-Induced Acute Kidney Injury. Biomedicines 2021, 9, 887. [Google Scholar] [CrossRef]
- Rozhkov, S.V.; Sharlo, K.A.; Shenkman, B.S.; Mirzoev, T.M. The Role of Glycogen Synthase Kinase-3 in the Regulation of Ribosome Biogenesis in Rat Soleus Muscle under Disuse Conditions. Int. J. Mol. Sci. 2022, 23, 2751. [Google Scholar] [CrossRef]
- Qiu, L.; Sun, Y.; Ning, H.; Chen, G.; Zhao, W.; Gao, Y. The Scaffold Protein AXIN1: Gene Ontology, Signal Network, and Physiological Function. Cell Commun. Signaling 2024, 22, 77. [Google Scholar] [CrossRef]
- Sharma, T.; Olea-Flores, M.; Imbalzano, A.N. Regulation of the Wnt Signaling Pathway during Myogenesis by the Mammalian SWI/SNF ATPase BRG1. Front. Cell Dev. Biol. 2023, 11, 1160227. [Google Scholar] [CrossRef]
- Marcella, B.M.; Hockey, B.L.; Braun, J.L.; Whitley, K.C.; Geromella, M.S.; Baranowski, R.W.; Watson, C.J.F.; Silvera, S.; Hamstra, S.I.; Wasilewicz, L.J.; et al. GSK3 Inhibition Improves Skeletal Muscle Function and Whole-Body Metabolism in Male Mouse Models of Duchenne Muscular Dystrophy. Nat. Commun. 2024, 15, 10210. [Google Scholar] [CrossRef]
- Hockey, B.L.; Braun, J.L.; Vandenboom, R.; LeBlanc, P.J.; MacPherson, R.E.K.; Fajardo, V.A. Can the Inhibition of Glycogen Synthase Kinase 3 Mimic the Physiological Effects of Exercise on Muscle and Other Organs? Adv. Exerc. Health Sci. 2025, 2, 108–120. [Google Scholar] [CrossRef]
- Sato, K.; Satoshi, Y.; Miyauchi, Y.; Sato, F.; Kon, R.; Ikarashi, N.; Chiba, Y.; Hosoe, T.; Sakai, H. Downregulation of PGC-1α during Cisplatin-Induced Muscle Atrophy in Murine Skeletal Muscle. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166877. [Google Scholar] [CrossRef]
- Bhatt, B.J.; Ghosh, S.; Mazurak, V.; Brun, A.Q.; Bathe, O.; Baracos, V.E.; Damaraju, S. Molecular Subtypes of Human Skeletal Muscle in Cancer Cachexia. Nature 2025, 646, 973–982. [Google Scholar] [CrossRef]
- Liang, S.-M.; Kuo, C.-L.; Lu, Y.-J.; Chang, T.-C.; Liou, J.-Y. Antrodia Cinnamomea Extract Attenuates Cisplatin-Induced Muscle Atrophy, Apoptosis, and Cell Growth Suppression. J. Food Biochem. 2023, 2023, 5593854. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, B.-O.; Song, N.-Y. The Role of Myokines in Cancer: Crosstalk between Skeletal Muscle and Tumor. BMB Rep. 2023, 56, 365–373. [Google Scholar] [CrossRef]
- De Castro, G.S.; Correia-Lima, J.; Simoes, E.; Orsso, C.E.; Xiao, J.; Gama, L.R.; Gomes, S.P.; Gonçalves, D.C.; Costa, R.G.F.; Radloff, K.; et al. Myokines in Treatment-Naïve Patients with Cancer-Associated Cachexia. Clin. Nutr. 2021, 40, 2443–2455. [Google Scholar] [CrossRef]
- Law, M.L. Cancer Cachexia: Pathophysiology and Association with Cancer-Related Pain. Front. Pain Res. 2022, 3, 971295. [Google Scholar] [CrossRef]
- Ikeno, Y.; Inomata, M.; Tsukimura, Y.; Suzuki, Y.; Takeuchi, H.; Harada, Y.; Kon, R.; Ikarashi, N.; Chiba, Y.; Yamada, T.; et al. Eicosapentaenoic Acid Suppresses Cisplatin-Induced Muscle Atrophy by Attenuating the up-Regulated Gene Expression of Ubiquitin. J. Nutr. Biochem. 2022, 103, 108953. [Google Scholar] [CrossRef]
- Zhang, H.; Chi, M.; Chen, L.; Sun, X.; Wan, L.; Yang, Q.; Guo, C. Daidzein Alleviates Cisplatin-Induced Muscle Atrophy by Regulating Glut4/AMPK/FoxO Pathway. Phytother. Res. 2021, 35, 4363–4376. [Google Scholar] [CrossRef]
- Matsumoto, C.; Sekine, H.; Zhang, N.; Mogami, S.; Fujitsuka, N.; Takeda, H. Role of P53 in Cisplatin-Induced Myotube Atrophy. Int. J. Mol. Sci. 2023, 24, 9176. [Google Scholar] [CrossRef]
- Jang, J.; Lee, H.; Song, J.; Bae, T.; Park, M.; Kwon, Y.V.; Lee, D.; Yoon, Y. Paeonia Lactiflora Extract Suppresses Cisplatin-Induced Muscle Wasting via Downregulation of Muscle-Specific Ubiquitin E3 Ligases, NF-κB Signaling, and Cytokine Levels. J. Ethnopharmacol. 2021, 266, 113403. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D.; Kang, C. Muscle Disuse Atrophy Caused by Discord of Intracellular Signaling. Antioxid. Redox Signal. 2020, 33, 727–744. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial Dysfunction: Roles in Skeletal Muscle Atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Mirzoev, T.M. Skeletal Muscle Recovery from Disuse Atrophy: Protein Turnover Signaling and Strategies for Accelerating Muscle Regrowth. Int. J. Mol. Sci. 2020, 21, 7940. [Google Scholar] [CrossRef]
- Misiąg, W.; Piszczyk, A.; Szymańska-Chabowska, A.; Chabowski, M. Physical Activity and Cancer Care—A Review. Cancers 2022, 14, 4154. [Google Scholar] [CrossRef]
- Brook, M.S. Investigating Muscle Protein Synthesis Using Deuterium Oxide: The Impact of Dietary Protein Interventions across the Lifespan. Exp. Physiol. 2025, 110, 949–960. [Google Scholar] [CrossRef]
- Lim, C.; McKendry, J.; Lees, M.; Atherton, P.J.; Burd, N.A.; Holwerda, A.M.; van Loon, L.J.C.; McGlory, C.; Mitchell, C.J.; Smith, K.; et al. Turning over New Ideas in Human Skeletal Muscle Proteostasis: What Do We Know and Where to from Here? Exp. Physiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Gan, M.; Qiu, Y.; Chen, Q.; Wang, X.; Liao, T.; Zhao, M.; Chen, L.; Zhang, S.; Zhao, Y.; et al. The Role of Mitochondrial Dynamics and Mitophagy in Skeletal Muscle Atrophy: From Molecular Mechanisms to Therapeutic Insights. Cell. Mol. Biol. Lett. 2024, 29, 59. [Google Scholar] [CrossRef]
- Sakai, H.; Ikeno, Y.; Tsukimura, Y.; Inomata, M.; Suzuki, Y.; Kon, R.; Ikarashi, N.; Chiba, Y.; Yamada, T.; Kamei, J. Upregulation of Ubiquitinated Proteins and Their Degradation Pathway in Muscle Atrophy Induced by Cisplatin in Mice. Toxicol. Appl. Pharmacol. 2020, 403, 115165. [Google Scholar] [CrossRef]
- Sakai, H.; Zhou, Y.; Miyauchi, Y.; Suzuki, Y.; Ikeno, Y.; Kon, R.; Ikarashi, N.; Chiba, Y.; Hosoe, T.; Kamei, J. Increased 20S Proteasome Expression and the Effect of Bortezomib during Cisplatin-Induced Muscle Atrophy. Biol. Pharm. Bull. 2022, 45, 910–918. [Google Scholar] [CrossRef]
- Bae, J.H.; Seo, D.Y.; Lee, S.H.; Shin, C.; Jamrasi, P.; Han, J.; Song, W. Effects of Exercise on AKT/PGC1-α/FOXO3a Pathway and Muscle Atrophy in Cisplatin-Administered Rat Skeletal Muscle. Korean J. Physiol. Pharmacol. 2021, 25, 585–592. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal Muscle Atrophy: From Mechanisms to Treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Mattingly, M.L.; Anglin, D.A.; Michel, J.M.; Godwin, J.S.; McIntosh, M.C.; Kontos, N.J.; Bergamasco, J.G.A.; Scarpelli, M.C.; Angleri, V.; et al. Skeletal Muscle Myosin Heavy Chain Fragmentation as a Potential Marker of Protein Degradation in Response to Resistance Training and Disuse Atrophy. Exp. Physiol. 2024, 109, 1739–1754. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, G.; Zhang, Z.; Zhu, J.Z.; Li, L.; Zhou, Y.; Rodney, G.G.; Abo-Zahrah, R.S.; Anderson, L.; Garcia, J.M.; et al. UBR2 Targets Myosin Heavy Chain IIb and IIx for Degradation: Molecular Mechanism Essential for Cancer-Induced Muscle Wasting. Proc. Natl. Acad. Sci. USA 2022, 119, e2200215119. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Tian, Y.; Gu, M.; Wang, Y.; Ashrafizadeh, M.; Reza Aref, A.; Cañadas, I.; Klionsky, D.J.; Goel, A.; et al. Autophagy-Driven Regulation of Cisplatin Response in Human Cancers: Exploring Molecular and Cell Death Dynamics. Cancer Lett. 2024, 587, 216659. [Google Scholar] [CrossRef]
- Cao, Z.; Tian, K.; Ran, Y.; Zhou, H.; Zhou, L.; Ding, Y.; Tang, X. Beclin-1: A Therapeutic Target at the Intersection of Autophagy, Immunotherapy, and Cancer Treatment. Front. Immunol. 2024, 15, 1506426. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Huang, X.; Huang, J.; Zheng, Y.; March, M.E.; Li, J.; Wei, Y. The Role of Autophagy in Skeletal Muscle Diseases. Front. Physiol. 2021, 12, 638983. [Google Scholar] [CrossRef]
- Sebastián, D.; Zorzano, A. Self-Eating for Muscle Fitness: Autophagy in the Control of Energy Metabolism. Dev. Cell 2020, 54, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Asami, M.; Naito, H.; Kitora, S.; Suzuki, Y.; Miyauchi, Y.; Tachinooka, R.; Yoshida, S.; Kon, R.; Ikarashi, N.; et al. Exogenous Insulin-like Growth Factor 1 Attenuates Cisplatin-Induced Muscle Atrophy in Mice. J. Cachexia Sarcopenia Muscle 2021, 12, 1570–1581. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhang, H.; Dokudovskaya, S. The Role of mTORC1 Pathway and Autophagy in Resistance to Platinum-Based Chemotherapeutics. Int. J. Mol. Sci. 2023, 24, 10651. [Google Scholar] [CrossRef]
- Cormerais, Y.; Lapp, S.C.; Kalafut, K.C.; Cissé, M.Y.; Shin, J.; Stefadu, B.; Personnaz, J.; Schrötter, S.; Freed, J.; D’Amore, A.; et al. AKT-Mediated Phosphorylation of TSC2 Controls Stimulus- and Tissue-Specific mTORC1 Signaling and Organ Growth. Dev. Cell 2025, 60, 2544–2557. [Google Scholar] [CrossRef]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted Role of mTOR (Mammalian Target of Rapamycin) Signaling Pathway in Human Health and Disease. Sig. Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Maharati, A.; Rajabloo, Y.; Moghbeli, M. Molecular Mechanisms of mTOR-Mediated Cisplatin Response in Tumor Cells. Heliyon 2024, 11, e41483. [Google Scholar] [CrossRef]
- Lu, W.; Ni, K.; Li, Z.; Xiao, L.; Li, Y.; Jiang, Y.; Zhang, J.; Shi, H. Salubrinal Protects against Cisplatin-Induced Cochlear Hair Cell Endoplasmic Reticulum Stress by Regulating Eukaryotic Translation Initiation Factor 2α Signalling. Front. Mol. Neurosci. 2022, 15, 916458. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Rui, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Chen, R.; Chen, Y.; Wang, Y.; Li, S.; et al. GSDMD Enhances Cisplatin-Induced Apoptosis by Promoting the Phosphorylation of eIF2α and Activating the ER-Stress Response. Cell Death Discov. 2022, 8, 114. [Google Scholar] [CrossRef]
- Shu, S.; Wang, H.; Zhu, J.; Fu, Y.; Cai, J.; Chen, A.; Tang, C.; Dong, Z. Endoplasmic Reticulum Stress Contributes to Cisplatin-Induced Chronic Kidney Disease via the PERK–PKCδ Pathway. Cell. Mol. Life Sci. 2022, 79, 452. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Zhi, Y.; Zhou, J.; Wang, C.; Chai, K.; Fan, Z.; Lv, G. Endoplasmic Reticulum Stress and Quality Control in Relation to Cisplatin Resistance in Tumor Cells. Front. Pharmacol. 2024, 15, 1419468. [Google Scholar] [CrossRef]
- Kim, M.-C.; Hwang, S.-H.; Yang, Y.; Kim, N.-Y.; Kim, Y. Reduction in Mitochondrial Oxidative Stress Mediates Hypoxia-Induced Resistance to Cisplatin in Human Transitional Cell Carcinoma Cells. Neoplasia 2021, 23, 653–662. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, B.; Huang, Y.; Zhang, Y.; Jiang, Y.; Ma, L.; Shen, Y.-Q. Mitochondrial DNA-Targeted Therapy: A Novel Approach to Combat Cancer. Cell Insight 2023, 2, 100113. [Google Scholar] [CrossRef]
- Elmorsy, E.A.; Saber, S.; Hamad, R.S.; Abdel-Reheim, M.A.; El-Kott, A.F.; AlShehri, M.A.; Morsy, K.; Salama, S.A.; Youssef, M.E. Advances in Understanding Cisplatin-Induced Toxicity: Molecular Mechanisms and Protective Strategies. Eur. J. Pharm. Sci. 2024, 203, 106939. [Google Scholar] [CrossRef]
- Li, F.; Zhu, F.; Wang, S.; Hu, H.; Zhang, D.; He, Z.; Chen, J.; Li, X.; Cheng, L.; Zhong, F. Icariin Alleviates Cisplatin-Induced Premature Ovarian Failure by Inhibiting Ferroptosis through Activation of the Nrf2/ARE Pathway. Sci. Rep. 2024, 14, 17318. [Google Scholar] [CrossRef]
- Chi, M.; Zhang, H.; Wang, Y.; Sun, X.; Yang, Q.; Guo, C. Silibinin Alleviates Muscle Atrophy Caused by Oxidative Stress Induced by Cisplatin through ERK/FoxO and JNK/FoxO Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 5694223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chi, M.; Chen, L.; Sun, X.; Wan, L.; Yang, Q.; Guo, C. Linalool Prevents Cisplatin Induced Muscle Atrophy by Regulating IGF-1/Akt/FoxO Pathway. Front. Pharmacol. 2020, 11, 598166. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.A.; Graham, M.Q.; Adam, A.M.; Juracic, E.S.; Tupling, A.R.; Quadrilatero, J. Mitophagy Is Required to Protect against Excessive Skeletal Muscle Atrophy Following Hindlimb Immobilization. J. Biomed. Sci. 2025, 32, 29. [Google Scholar] [CrossRef]
- Domingo, I.K.; Latif, A.; Bhavsar, A.P. Pro-Inflammatory Signalling PRRopels Cisplatin-Induced Toxicity. Int. J. Mol. Sci. 2022, 23, 7227. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, M.; Chang, M.; Liu, R.; Qiu, J.; Wang, K.; Deng, C.; Shen, Y.; Zhu, J.; Wang, W.; et al. Inflammation: Roles in Skeletal Muscle Atrophy. Antioxidants 2022, 11, 1686. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, L.; Zhu, J.; Xu, H.; Ma, W.; Zhang, L.; Shen, Y.; Law, B.Y.-K.; Ding, F.; Gu, X.; et al. Inhibition of IL-6/JAK/STAT3 Pathway Rescues Denervation-Induced Skeletal Muscle Atrophy. Ann. Transl. Med. 2020, 8, 1681. [Google Scholar] [CrossRef]
- Xie, T.; Lv, T.; Zhang, T.; Feng, D.; Zhu, F.; Xu, Y.; Zhang, L.; Gu, L.; Guo, Z.; Ding, C.; et al. Interleukin-6 Promotes Skeletal Muscle Catabolism by Activating Tryptophan–Indoleamine 2,3-Dioxygenase 1–Kynurenine Pathway during Intra-Abdominal Sepsis. J. Cachexia Sarcopenia Muscle 2023, 14, 1046–1059. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ding, S. Unraveling the Role of STAT3 in Cancer Cachexia: Pathogenic Mechanisms and Therapeutic Opportunities. Front. Endocrinol. 2025, 16, 1608612. [Google Scholar] [CrossRef]
- Chen, Z.; Lan, H.; Liao, Z.; Huang, J.; Jian, X.; Hu, J.; Liao, H. Regulatory T Cells-Centered Regulatory Networks of Skeletal Muscle Inflammation and Regeneration. Cell Biosci. 2022, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Giovarelli, M.; Mocciaro, E.; Carnovale, C.; Cervia, D.; Perrotta, C.; Clementi, E. Immunosenescence in Skeletal Muscle: The Role-Play in Cancer Cachexia Chessboard. Semin. Cancer Biol. 2025, 111, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Piñeiro, L.J.; Wu, Y.; Popsuj, S.; José-Edwards, D.S.; Stolfi, A.; Di Gregorio, A. Cis-Regulatory Interfaces Reveal the Molecular Mechanisms Underlying the Notochord Gene Regulatory Network of Ciona. Nat. Commun. 2024, 15, 3025. [Google Scholar] [CrossRef]
- Ali, S.I.M.; Alrashid, S.Z. A Review of Methods for Gene Regulatory Networks Reconstruction and Analysis. Artif. Intell. Rev. 2025, 58, 256. [Google Scholar] [CrossRef]
- Lian, D.; Chen, M.-M.; Wu, H.; Deng, S.; Hu, X. The Role of Oxidative Stress in Skeletal Muscle Myogenesis and Muscle Disease. Antioxidants 2022, 11, 755. [Google Scholar] [CrossRef]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in Oxidative Stress, Inflammation and Aging: From Mechanisms to Therapeutic Advances. Sig. Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of Oxidative Stress and Inflammation-Related Signaling Pathways in Doxorubicin-Induced Cardiomyopathy. Cell Commun. Signal. 2023, 21, 61. [Google Scholar] [CrossRef]
- Chen, P.; Jia, F.; Wang, M.; Yang, S. Analysis of the Mechanism of Skeletal Muscle Atrophy from the Pathway of Decreased Protein Synthesis. Front. Physiol. 2025, 16, 1533394. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic Reticulum Stress: Molecular Mechanism and Therapeutic Targets. Sig. Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef]
- Vietri, M.; Miranda, M.R.; Amodio, G.; Ciaglia, T.; Bertamino, A.; Campiglia, P.; Remondelli, P.; Vestuto, V.; Moltedo, O. The Link between Endoplasmic Reticulum Stress and Lysosomal Dysfunction under Oxidative Stress in Cancer Cells. Biomolecules 2025, 15, 930. [Google Scholar] [CrossRef] [PubMed]
- Pigg, Q.W.; Harris, D.R.; Inoue, D.S.; Janini Gomes, M. Mitigating Doxorubicin-Induced Skeletal Muscle Toxicity: A Review of Oxidative Stress Mechanisms and the Therapeutic Role of Exercise. Antioxidants 2025, 14, 870. [Google Scholar] [CrossRef]
- Sanft, T.; Harrigan, M.; McGowan, C.; Cartmel, B.; Zupa, M.; Li, F.-Y.; Ferrucci, L.M.; Puklin, L.; Cao, A.; Nguyen, T.H.; et al. Randomized Trial of Exercise and Nutrition on Chemotherapy Completion and Pathologic Complete Response in Women with Breast Cancer: The Lifestyle, Exercise, and Nutrition Early after Diagnosis Study. J. Clin. Oncol. 2023, 41, 5285–5295. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Szlachcic, A.; Ptak-Belowska, A.; Brzozowski, T. Physical Activity, Exerkines, and Their Role in Cancer Cachexia. Int. J. Mol. Sci. 2025, 26, 8011. [Google Scholar] [CrossRef]
- Pradhan, R.; Dieterich, W.; Natarajan, A.; Schwappacher, R.; Reljic, D.; Herrmann, H.J.; Neurath, M.F.; Zopf, Y. Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia. Cancers 2024, 16, 1921. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Lee, H.S.; Kim, J.-H. Resistance Exercise and Skeletal Muscle: Protein Synthesis, Degradation, and Controversies. Eur. J. Appl. Physiol. 2025, 125, 2353–2382. [Google Scholar] [CrossRef]
- Thomas, A.C.Q.; Stead, C.A.; Burniston, J.G.; Phillips, S.M. Exercise-Specific Adaptations in Human Skeletal Muscle: Molecular Mechanisms of Making Muscles Fit and Mighty. Free Radic. Biol. Med. 2024, 223, 341–356. [Google Scholar] [CrossRef]
- Stec, M.J.; Graham, Z.A.; Su, Q.; Adler, C.; Ni, M.; Rouzic, V.L.; Golann, D.R.; Ferrara, P.J.; Halasz, G.; Sleeman, M.W.; et al. Combined Endurance and Resistance Exercise Training Alters the Spatial Transcriptome of Skeletal Muscle in Young Adults. iScience 2025, 28, 113301. [Google Scholar] [CrossRef]
- Furrer, R.; Hawley, J.A.; Handschin, C. The Molecular Athlete: Exercise Physiology from Mechanisms to Medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef]
- Wang, J.; Jia, D.; Zhang, Z.; Wang, D. Exerkines and Sarcopenia: Unveiling the Mechanism behind Exercise-Induced Mitochondrial Homeostasis. Metabolites 2025, 15, 59. [Google Scholar] [CrossRef]
- Letukienė, A.; Hendrixson, V.; Ginevičienė, V. Current Knowledge and Scientific Trends in Myokines and Exercise Research in the Context of Obesity. Front. Med. 2024, 11, 1421962. [Google Scholar] [CrossRef]
- Khemraj, P.; Kuznyetsova, A.; Hood, D.A. Adaptations in Mitochondrial Quality Control and Interactions with Innate Immune Signaling within Skeletal Muscle: A Narrative Review. J. Sport Health Sci. 2025, 101049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Zhang, J.; Jia, D. Exercise Alleviates Cardiovascular Diseases by Improving Mitochondrial Homeostasis. J. Am. Heart Assoc. 2024, 13, e036555. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, K.; Galganski, L.; Budzinska, A.; Woyda-Ploszczyca, A.; Zoladz, J.A.; Jarmuszkiewicz, W. Effects of Endurance Training on the Coenzyme Q Redox State in Rat Heart, Liver, and Brain at the Tissue and Mitochondrial Levels: Implications for Reactive Oxygen Species Formation and Respiratory Chain Remodeling. Int. J. Mol. Sci. 2022, 23, 896. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Li, C.; Zhang, X.; Shan, Y.; Zhang, Z.; Bo, H.; Zhang, Y. Endurance Exercise-Induced Histone Methylation Modification Involved in Skeletal Muscle Fiber Type Transition and Mitochondrial Biogenesis. Sci. Rep. 2024, 14, 21154. [Google Scholar] [CrossRef]
- Gao, T.; Hu, Y.; Zhang, H.; Shi, R.; Song, Y.; Ding, M.; Gao, F. Aerobic Capacity beyond Cardiorespiratory Fitness Linking Mitochondrial Function, Disease Resilience and Healthy Aging. FASEB J. 2025, 39, e70655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yuan, Y.; Liu, F.; Li, L.; Liu, J.; Chen, Y.; Cheng, J.; Lu, Y. PGC-1α Alleviates Mitochondrial Dysfunction via TFEB-Mediated Autophagy in Cisplatin-Induced Acute Kidney Injury. Aging 2021, 13, 8421–8439. [Google Scholar] [CrossRef]
- Craige, S.M.; Mammel, R.K.; Amiri, N.; Willoughby, O.S.; Drake, J.C. Interplay of ROS, Mitochondrial Quality, and Exercise in Aging: Potential Role of Spatially Discrete Signaling. Redox Biol. 2024, 77, 103371. [Google Scholar] [CrossRef]
- Memme, J.M.; Slavin, M.; Moradi, N.; Hood, D.A. Mitochondrial Bioenergetics and Turnover during Chronic Muscle Disuse. Int. J. Mol. Sci. 2021, 22, 5179. [Google Scholar] [CrossRef]
- Tayebi, S.M.; Poorhabibi, H.; Heidary, D.; Amini, M.A.; Sadeghi, A. Impact of Aerobic Exercise on Chronic Inflammation in Older Adults: A Systematic Review and Meta-Analysis. BMC Sports Sci. Med. Rehabil. 2025, 17, 229. [Google Scholar] [CrossRef] [PubMed]
- Magni, O.; Arnaoutis, G.; Panagiotakos, D. The Impact of Exercise on Chronic Systemic Inflammation: A Systematic Review and Meta–Meta-Analysis. Sport. Sci. Health 2025, 21, 1405–1417. [Google Scholar] [CrossRef]
- Wang, J.; He, Y.; Wang, Z.; Wang, Z.; Miao, Y.; Choi, J.-Y. Effects of Different Exercise Prescription Parameters on Metabolic and Inflammatory Biomarkers in Cancer Patients: A Systematic Review, Meta-Analysis, and Meta-Regression. Front. Immunol. 2025, 16, 1663560. [Google Scholar] [CrossRef] [PubMed]
- YAO, B.; LI, L.; GUAN, X.; ZHU, J.; LIU, Q.; QU, B.; DING, H. Endurance Training Inhibits the JAK2/STAT3 Pathway to Alleviate Sarcopenia. Physiol. Res. 2024, 73, 295–304. [Google Scholar] [CrossRef]
- Ducharme, J.B.; Specht, J.W.; Bailly, A.R.; Deyhle, M.R. Serum Cytokines and Their Soluble Receptors Are Differently Regulated between Trained and Untrained Men after Vigorous Endurance Exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2025, 328, R581–R587. [Google Scholar] [CrossRef]
- Currier, B.S.; Mcleod, J.C.; Banfield, L.; Beyene, J.; Welton, N.J.; D’Souza, A.C.; Keogh, J.A.J.; Lin, L.; Coletta, G.; Yang, A.; et al. Resistance Training Prescription for Muscle Strength and Hypertrophy in Healthy Adults: A Systematic Review and Bayesian Network Meta-Analysis. Br. J. Sports Med. 2023, 57, 1211–1220. [Google Scholar] [CrossRef]
- Ugurlu, D.; Gülü, M.; Yapici, H.; Yagin, F.H.; Comertpay, E.; Eroglu, O.; Afonso, J.; Aldhahi, M.I. Dose-Response Effects of 8-Week Resistance Training on Body Composition and Muscular Performance in Untrained Young Women: A Quasi-Experimental Design. Medicine 2024, 103, e40322. [Google Scholar] [CrossRef]
- Mcleod, J.C.; Currier, B.S.; Lowisz, C.V.; Phillips, S.M. The Influence of Resistance Exercise Training Prescription Variables on Skeletal Muscle Mass, Strength, and Physical Function in Healthy Adults: An Umbrella Review. J. Sport. Health Sci. 2024, 13, 47–60. [Google Scholar] [CrossRef]
- Abou Sawan, S.; Nunes, E.A.; Lim, C.; McKendry, J.; Phillips, S.M. The Health Benefits of Resistance Exercise: Beyond Hypertrophy and Big Weights. Exerc. Sport Mov. 2023, 1, e00001. [Google Scholar] [CrossRef]
- D’Hulst, G.; Masschelein, E.; De Bock, K. Resistance Exercise Enhances Long-Term mTORC1 Sensitivity to Leucine. Mol. Metab. 2022, 66, 101615. [Google Scholar] [CrossRef]
- Pietras, P.; Aulas, A.; Fay, M.M.; Leśniczak-Staszak, M.; Sowiński, M.; Lyons, S.M.; Szaflarski, W.; Ivanov, P. Translation Inhibition and Suppression of Stress Granules Formation by Cisplatin. Biomed. Pharmacother. 2022, 145, 112382. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, Y.; Yu, X.-Y.; Pan, X.; Zhang, Y.; Tu, J.; Song, Y.-H.; Li, Y. Ribosome Biogenesis in Disease: New Players and Therapeutic Targets. Sig. Transduct. Target. Ther. 2023, 8, 15. [Google Scholar] [CrossRef]
- Scarpelli, M.C.; Bergamasco, J.G.A.; Godwin, J.S.; Mesquita, P.H.C.; Chaves, T.S.; Silva, D.G.; Bittencourt, D.; Dias, N.F.; Medalha Junior, R.A.; Carello Filho, P.C.; et al. Resistance Training-Induced Changes in Muscle Proteolysis and Extracellular Matrix Remodeling Biomarkers in the Untrained and Trained States. Eur. J. Appl. Physiol. 2024, 124, 2749–2762. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.M.; Godwin, J.S.; Plotkin, D.L.; Mesquita, P.H.C.; McIntosh, M.C.; Ruple, B.A.; Libardi, C.A.; Mobley, C.B.; Kavazis, A.N.; Roberts, M.D. Proteolytic Markers Associated with a Gain and Loss of Leg Muscle Mass with Resistance Training Followed by High-Intensity Interval Training. Exp. Physiol. 2023, 108, 1268–1281. [Google Scholar] [CrossRef]
- Moradi, Y.; Zehsaz, F.; Nourazar, M.A. Concurrent Exercise Training and Murf-l and Atrogin-1 Gene Expression in the Vastus Lateralis Muscle of Male Wistar Rats. Apunt. Med. Esport. 2020, 55, 21–27. [Google Scholar] [CrossRef]
- Cardiel-Gutiérrez, S.; Villicaña-Gómez, E.A.; Márquez-Gamiño, S.; Vera-Delgado, K.S.; Sotelo-Barroso, F.; Caudillo-Cisneros, C.; Sánchez-Duarte, E. Moderate-Intensity Training Reduces the Expression of Atrogin-1 and Murf-1 in Gastrocnemius Muscle of Diabetic Rats: 1428. Med. Sci. Sports Exerc. 2021, 53, 467. [Google Scholar] [CrossRef]
- Chu, Y.; Yuan, X.; Tao, Y.; Yang, B.; Luo, J. Autophagy in Muscle Regeneration: Mechanisms, Targets, and Therapeutic Perspective. Int. J. Mol. Sci. 2024, 25, 11901. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhou, J.; Quan, H.; Li, W.; Li, T.; Wang, L. Resistance Training Alleviates Muscle Atrophy and Muscle Dysfunction by Reducing Inflammation and Regulating Compromised Autophagy in Aged Skeletal Muscle. Front. Immunol. 2025, 16, 1597222. [Google Scholar] [CrossRef]
- Schwendinger, F.; Pocecco, E. Counteracting Physical Inactivity during the COVID-19 Pandemic: Evidence-Based Recommendations for Home-Based Exercise. Int. J. Environ. Res. Public Health 2020, 17, 3909. [Google Scholar] [CrossRef]
- Correale, L.; Buzzachera, C.F.; Liberali, G.; Codrons, E.; Mallucci, G.; Vandoni, M.; Montomoli, C.; Bergamaschi, R. Effects of Combined Endurance and Resistance Training in Women with Multiple Sclerosis: A Randomized Controlled Study. Front. Neurol. 2021, 12, 698460. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qi, Y.; Chen, X.; Li, J.; Zhang, J.; Li, P.; Zhou, Z. Synergistic Effects of Concurrent Aerobic and Strength Training on Fitness in Children and Adolescents: A Multivariate and Network Meta-Analysis. Scand. J. Med. Sci. Sports 2024, 34, e14764. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; Batrakoulis, A.; Norhayati, M.N.; Mohamed, M.; Drenowatz, C.; Irekeola, A.A.; Afolabi, H.A.; Gülü, M.; Alkhamees, N.H.; Wan Ghazali, W.S. Combined Aerobic and Resistance Training Improves Body Composition, Alters Cardiometabolic Risk, and Ameliorates Cancer-Related Indicators in Breast Cancer Patients and Survivors with Overweight/Obesity: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Sports Sci. Med. 2024, 23, 366–395. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, H.; He, A.; Li, Y.; He, C.; Xia, Y. Exercise in Cancer Prevention and Anticancer Therapy: Efficacy, Molecular Mechanisms and Clinical Information. Cancer Lett. 2022, 544, 215814. [Google Scholar] [CrossRef]
- Wen, H.; Deng, H.; Li, B.; Chen, J.; Zhu, J.; Zhang, X.; Yoshida, S.; Zhou, Y. Mitochondrial Diseases: From Molecular Mechanisms to Therapeutic Advances. Sig. Transduct. Target. Ther. 2025, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Burtscher, J.; Romani, M.; Bernardo, G.; Popa, T.; Ziviani, E.; Hummel, F.C.; Sorrentino, V.; Millet, G.P. Boosting Mitochondrial Health to Counteract Neurodegeneration. Prog. Neurobiol. 2022, 215, 102289. [Google Scholar] [CrossRef]
- Liu, R.; Gao, X.; Wang, L. Network Meta-Analysis of the Intervention Effects of Different Exercise Measures on Sarcopenia in Cancer Patients. BMC Public Health 2024, 24, 1281. [Google Scholar] [CrossRef]
- Yen, C.-J.; Hung, C.-H.; Tsai, W.-M.; Cheng, H.-C.; Yang, H.-L.; Lu, Y.-J.; Tsai, K.-L. Effect of Exercise Training on Exercise Tolerance and Level of Oxidative Stress for Head and Neck Cancer Patients Following Chemotherapy. Front. Oncol. 2020, 10, 1536. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Cheng, H.-C.; Yen, C.-J.; Hung, C.-H.; Huang, Y.-T.; Yang, H.-L.; Cheng, W.-T.; Tsai, K.-L. Effects of Exercise in Patients Undergoing Chemotherapy for Head and Neck Cancer: A Pilot Randomized Controlled Trial. Int. J. Environ. Res. Public. Health 2021, 18, 1291. [Google Scholar] [CrossRef]
- Iglesias, P. Muscle in Endocrinology: From Skeletal Muscle Hormone Regulation to Myokine Secretion and Its Implications in Endocrine–Metabolic Diseases. J. Clin. Med. 2025, 14, 4490. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, W.; Chen, P.; Wang, H.; Wang, H.; Zhu, L.; Liu, X. Exerkines and Myokines in Aging Sarcopenia. Front. Endocrinol. 2025, 16, 1592491. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in Health, Resilience and Disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Wang, Z.; Li, L.; Yang, M.; Li, B.; Hu, S. From Skeletal Muscle to Myocardium: Molecular Mechanisms of Exercise-Induced Irisin Regulation of Cardiac Fibrosis. Int. J. Mol. Sci. 2025, 26, 3550. [Google Scholar] [CrossRef]
- Brown, A.D.; Marko, A.D.; Marko, D.M.; Baranowski, B.J.; Silvera, S.; Finch, M.S.; Yang, A.J.; Dhaliwal, R.; Ryan, C.R.; Roy, B.D.; et al. Brain-Derived Neurotrophic Factor Drives Muscle Adaptation Similar to Aerobic Training in Mice. FASEB J. 2025, 39, e70321. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Hekmatikar, A.; Nelson, A.; Petersen, A. Highlighting the Idea of Exerkines in the Management of Cancer Patients with Cachexia: Novel Insights and a Critical Review. BMC Cancer 2023, 23, 889. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, J.Y.; Lee, M.H.; Ju, S.-H.; Yi, H.-S.; Shong, M. Mitochondrial Stress and Mitokines: Therapeutic Perspectives for the Treatment of Metabolic Diseases. Diabetes Metab. J. 2024, 48, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arias-Calderón, M.; Casas, M.; Balanta-Melo, J.; Morales-Jiménez, C.; Hernández, N.; Llanos, P.; Jaimovich, E.; Buvinic, S. Fibroblast Growth Factor 21 Is Expressed and Secreted from Skeletal Muscle Following Electrical Stimulation via Extracellular ATP Activation of the PI3K/Akt/mTOR Signaling Pathway. Front. Endocrinol. 2023, 14, 1059020. [Google Scholar] [CrossRef]
- Kilpiö, T.; Skarp, S.; Perjés, Á.; Swan, J.; Kaikkonen, L.; Saarimäki, S.; Szokodi, I.; Penninger, J.M.; Szabó, Z.; Magga, J.; et al. Apelin Regulates Skeletal Muscle Adaptation to Exercise in a High-Intensity Interval Training Model. Am. J. Physiol. Cell Physiol. 2024, 326, C1437–C1450. [Google Scholar] [CrossRef]
- Le Moal, E.; Liu, Y.; Collerette-Tremblay, J.; Dumontier, S.; Fabre, P.; Molina, T.; Dort, J.; Orfi, Z.; Denault, N.; Boutin, J.; et al. Apelin Stimulation of the Vascular Skeletal Muscle Stem Cell Niche Enhances Endogenous Repair in Dystrophic Mice. Sci. Transl. Med. 2024, 16, eabn8529. [Google Scholar] [CrossRef]
- Walzik, D.; Wences Chirino, T.Y.; Zimmer, P.; Joisten, N. Molecular Insights of Exercise Therapy in Disease Prevention and Treatment. Sig. Transduct. Target. Ther. 2024, 9, 138. [Google Scholar] [CrossRef]
- Broniec, M.N.; Norland, K.; Thomas, J.; Wang, X.; Harris, R.A. The Decorin and Myostatin Response to Acute Whole Body Vibration: Impact of Adiposity, Sex, and Race. Int. J. Obes. 2024, 48, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Baghy, K.; Szakadáti, H.; Kovalszky, I. Decorin the Antifibrotic Proteoglycan and Its Progression in Therapy. Am. J. Physiol.-Cell Physiol. 2025, 328, C1853–C1865. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Y.; Shi, Y.; Shi, Y.; Su, X.; Chen, P.; Wu, D.; Shi, H. Exercise and Exerkines: Mechanisms and Roles in Anti-Aging and Disease Prevention. Exp. Gerontol. 2025, 200, 112685. [Google Scholar] [CrossRef]
- Petro, J.L.; Gallo-Villegas, J.; Calderón, J.C. Myonectin and Metabolic Health: A Systematic Review. Front. Endocrinol. 2025, 16, 1557142. [Google Scholar] [CrossRef]
- Saw, E.L.; Werner, L.D.; Cooper, H.L.; Pimental, D.R.; Zamani, P.; Chirinos, J.A.; Valero-Muñoz, M.; Sam, F. Musclin Counteracts Skeletal Muscle Dysfunction and Exercise Intolerance in Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2025, 18, e012350. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Song, X.; Wang, H.; Yan, Y.; Liu, B. The Role of Exercise-Induced Myokines in Promoting Angiogenesis. Front. Physiol. 2022, 13, 981577. [Google Scholar] [CrossRef] [PubMed]
- Bucarey, J.L.; Trujillo-González, I.; Paules, E.M.; Espinosa, A. Myokines and Their Potential Protective Role against Oxidative Stress in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Antioxidants 2024, 13, 1363. [Google Scholar] [CrossRef]
- Rathor, R.; Suryakumar, G. Myokines: A Central Point in Managing Redox Homeostasis and Quality of Life. BioFactors 2024, 50, 885–909. [Google Scholar] [CrossRef]
- Uurasmaa, T.-M.; Ricardo, C.; Autio, A.; Heinonen, I.H.A.; Rundqvist, H.; Anttila, K. Voluntary Wheel Running Reduces Tumor Growth and Increases Capillarity in the Heart during Doxorubicin Chemotherapy in a Murine Model of Breast Cancer. Front. Physiol. 2024, 15, 1347347. [Google Scholar] [CrossRef]
- Halle, J.L.; Counts, B.R.; Zhang, Q.; James, K.M.; Puppa, M.J.; Alway, S.E.; Carson, J.A. Mouse Skeletal Muscle Adaptations to Different Durations of Treadmill Exercise after the Cessation of FOLFOX Chemotherapy. Front. Physiol. 2023, 14, 1283674. [Google Scholar] [CrossRef]
- Nguyen, B.L.; Baumfalk, D.R.; Lapierre-Nguyen, S.S.; Zhong, R.; Doerr, V.; Montalvo, R.N.; Wei-LaPierre, L.; Smuder, A.J. Effects of Exercise and Doxorubicin on Acute Diaphragm Neuromuscular Transmission Failure. Exp. Neurol. 2024, 378, 114818. [Google Scholar] [CrossRef]
- Tamayo-Torres, E.; Garrido, A.; de Cabo, R.; Carretero, J.; Gómez-Cabrera, M.C. Molecular Mechanisms of Cancer Cachexia. Role of Exercise Training. Mol. Asp. Med. 2024, 99, 101293. [Google Scholar] [CrossRef] [PubMed]
- Frühwein, H.; Paul, N.W. “Lost in Translation?” Animal Research in the Era of Precision Medicine. J. Transl. Med. 2025, 23, 152. [Google Scholar] [CrossRef] [PubMed]
- Ineichen, B.V.; Furrer, E.; Grüninger, S.L.; Zürrer, W.E.; Macleod, M.R. Analysis of Animal-to-Human Translation Shows That Only 5% of Animal-Tested Therapeutic Interventions Obtain Regulatory Approval for Human Applications. PLoS Biol. 2024, 22, e3002667. [Google Scholar] [CrossRef]
- Berg, I.; Härvelid, P.; Zürrer, W.E.; Rosso, M.; Reich, D.S.; Ineichen, B.V. Which Experimental Factors Govern Successful Animal-to-Human Translation in Multiple Sclerosis Drug Development? A Systematic Review and Meta-Analysis. eBioMedicine 2024, 110, 105434. [Google Scholar] [CrossRef]
- Bishop, D.J.; Lee, M.J.-C.; Picard, M. Exercise as Mitochondrial Medicine: How Does the Exercise Prescription Affect Mitochondrial Adaptations to Training? Annu. Rev. Physiol. 2025, 87, 107–129. [Google Scholar] [CrossRef]
- Fairman, C.M.; Lønbro, S.; Cardaci, T.D.; VanderVeen, B.N.; Nilsen, T.S.; Murphy, A.E. Muscle Wasting in Cancer: Opportunities and Challenges for Exercise in Clinical Cancer Trials. JCSM Rapid Commun. 2022, 5, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Tsitkanou, S.; Murach, K.A.; Washington, T.A.; Greene, N.P. Exercise Counteracts the Deleterious Effects of Cancer Cachexia. Cancers 2022, 14, 2512. [Google Scholar] [CrossRef]
- Kearney, N.; Connolly, D.; Begic, S.; Mockler, D.; Guinan, E. Feasibility Metrics of Exercise Interventions during Chemotherapy: A Systematic Review. Crit. Rev. Oncol. Hematol. 2024, 195, 104272. [Google Scholar] [CrossRef]
- Perše, M. Cisplatin Mouse Models: Treatment, Toxicity and Translatability. Biomedicines 2021, 9, 1406. [Google Scholar] [CrossRef]
- Amarelo, A.; Mota, M.; Amarelo, B.; Ferreira, M.C.; Fernandes, C.S. Technological Resources for Physical Rehabilitation in Cancer Patients Undergoing Chemotherapy: A Scoping Review. Cancers 2024, 16, 3949. [Google Scholar] [CrossRef]
- Underwood, W.P.; Michalski, M.G.; Lee, C.P.; Fickera, G.A.; Chun, S.S.; Eng, S.E.; Liu, L.Y.; Tsai, B.L.; Moskowitz, C.S.; Lavery, J.A.; et al. A Digital, Decentralized Trial of Exercise Therapy in Patients with Cancer. npj Digit. Med. 2024, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, K.M.; Turner, K.L.; Siwik, C.; Gonzalez, B.D.; Upasani, R.; Glazer, J.V.; Ferguson, R.J.; Joshua, C.; Low, C.A. Digital Health and Telehealth in Cancer Care: A Scoping Review of Reviews. Lancet Digit. Health 2023, 5, e316–e327. [Google Scholar] [CrossRef] [PubMed]
- De Vleeschauwer, S.I.; van de Ven, M.; Oudin, A.; Debusschere, K.; Connor, K.; Byrne, A.T.; Ram, D.; Rhebergen, A.M.; Raeves, Y.D.; Dahlhoff, M.; et al. OBSERVE: Guidelines for the Refinement of Rodent Cancer Models. Nat. Protoc. 2024, 19, 2571–2596. [Google Scholar] [CrossRef]
- Delphan, M.; Delfan, N.; West, D.; Delfan, M. Exercise Protocols: The Gap between Preclinical and Clinical Exercise Oncology Studies. Metab. Open 2022, 13, 100165. [Google Scholar] [CrossRef]
- Avancini, A.; Borsati, A.; Toniolo, L.; Ciurnelli, C.; Belluomini, L.; Budolfsen, T.; Lillelund, C.; Milella, M.; Quist, M.; Pilotto, S. Physical Activity Guidelines in Oncology: A Systematic Review of the Current Recommendations. Crit. Rev. Oncol./Hematol. 2025, 210, 104718. [Google Scholar] [CrossRef]
- Saleh, A.Y.; Shareef, A.; Bishoyi, A.K.; Jyothi, S.R.; Panigrahi, R.; Pargaien, A.; Garg, G.; Hafizova, M.; Sameer, H.N.; Yaseen, A.; et al. Personalized Exercise Programs in Oncology. Oncol. Rev. 2025, 19, 1645505. [Google Scholar] [CrossRef] [PubMed]
- Novinger, L.J.; Weinzierl, N.M.; Bonetto, A. Diversity in Chemotherapy-Induced Cachexia. Am. J. Physiol. Cell Physiol. 2025, 328, C139–C147. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, J.R.; Han, J. Exercise Alleviates Cisplatin-Induced Toxicity in the Hippocampus of Mice by Inhibiting Neuroinflammation and Improving Synaptic Plasticity. Korean J. Physiol. Pharmacol. 2024, 28, 145–152. [Google Scholar] [CrossRef]
- Collao, N.; Sanders, O.; Caminiti, T.; Messeiller, L.; De Lisio, M. Resistance and Endurance Exercise Training Improves Muscle Mass and the Inflammatory/Fibrotic Transcriptome in a Rhabdomyosarcoma Model. J. Cachexia Sarcopenia Muscle 2023, 14, 781–793. [Google Scholar] [CrossRef]
- Tuğral, A.; Arıbaş, Z.; Kaya Uçar, G.; Arslan, F.D.; Bakar, Y.; Karakoyun, I.; Akyol, M. The Effect of Supervised Aerobic Exercise on Adipokine and Myokine Biomarkers in Patients with Cancer during Systemic Chemotherapy: A Single-Blinded Prospective Controlled Trial. Support. Care Cancer 2025, 33, 741. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Crossland, H. Metabolomic and Proteomic Applications to Exercise Biomedicine. Transl. Exerc. Biomed. 2024, 1, 9–22. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Li, N.; Du, J.; Qin, X.; Sun, X.; Wang, Y.; Li, C. Integrated Proteomic and Metabolomic Analysis of Muscle Atrophy Induced by Hindlimb Unloading. Biomolecules 2024, 15, 14. [Google Scholar] [CrossRef]
- Murach, K.A.; Peterson, C.A. A Muscle Exercise Research Revolution Powered by -Omics at Single Cell and Nucleus Resolution. BMC Biol. 2023, 21, 298. [Google Scholar] [CrossRef]
- Yin, L.; Wu, S.; Bai, P.; Wang, X. Combination of Transcriptomics and Proteomics for Analyzing Potential Biomarker and Molecular Mechanism Underlying Skeletal Muscle Atrophy. J. Proteom. 2024, 309, 105283. [Google Scholar] [CrossRef]
- Li, X.; Fang, L.; Zhou, R.; Yao, L.; Clayton, S.W.; Muscat, S.; Kamm, D.R.; Wang, C.; Liu, C.-J.; Qin, L.; et al. Current Cutting-Edge Omics Techniques on Musculoskeletal Tissues and Diseases. Bone Res. 2025, 13, 59. [Google Scholar] [CrossRef]
- Moreno-Justicia, R.; Van der Stede, T.; Stocks, B.; Laitila, J.; Seaborne, R.A.; Van de Loock, A.; Lievens, E.; Samodova, D.; Marín-Arraiza, L.; Dmytriyeva, O.; et al. Human Skeletal Muscle Fiber Heterogeneity beyond Myosin Heavy Chains. Nat. Commun. 2025, 16, 1764. [Google Scholar] [CrossRef]
- Aquila, G.; Re Cecconi, A.D.; Brault, J.J.; Corli, O.; Piccirillo, R. Nutraceuticals and Exercise against Muscle Wasting during Cancer Cachexia. Cells 2020, 9, 2536. [Google Scholar] [CrossRef] [PubMed]
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Woldeselassie, M.; Tamene, A. Therapeutic Controversies over Use of Antioxidant Supplements during Cancer Treatment: A Scoping Review. Front. Nutr. 2024, 11, 1480780. [Google Scholar] [CrossRef]
- Pfister, C.; Schoenemann, J. Selenium in Cancer Rehabilitation—A Retrospective Study from a Specialized Clinic. Nutrients 2023, 15, 3827. [Google Scholar] [CrossRef]
- Sugimoto, R.; Lee, L.; Tanaka, Y.; Morita, Y.; Hijioka, M.; Hisano, T.; Furukawa, M. Zinc Deficiency as a General Feature of Cancer: A Review of the Literature. Biol. Trace Elem. Res. 2024, 202, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Nie, R.; Yin, Y.; Cai, X.; Xie, D.; Cai, M. Protective Effect of Natural Antioxidants on Reducing Cisplatin-Induced Nephrotoxicity. Dis. Markers 2022, 2022, 1612348. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Strasser, F.; Gonella, S.; Solheim, T.S.; Madeddu, C.; Ravasco, P.; Buonaccorso, L.; de van der Schueren, M.A.E.; Baldwin, C.; Chasen, M.; et al. Cancer Cachexia in Adult Patients: ESMO Clinical Practice Guidelines. Esmo Open 2021, 6, 100092. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, X.; Wan, R.; Luo, Z.; Qu, L.; Wang, Q. Impact of Exercise on Cancer: Mechanistic Perspectives and New Insights. Front. Immunol. 2024, 15, 1474770. [Google Scholar] [CrossRef]
| Feature/Mechanism | Cisplatin-Induced Atrophy | Disuse/Denervation Atrophy | Cancer Cachexia/Systemic Atrophy | References |
|---|---|---|---|---|
| Primary cause | Chemotherapy toxicity and systemic stress | Mechanical unloading or loss of neural input | Chronic inflammation and tumor–host interaction | [16,20] |
| Mitochondrial effect | Direct mtDNA damage and oxidative stress via cisplatin–DNA adducts | Reduced mitochondrial activity due to inactivity | Impaired mitochondrial biogenesis secondary to inflammation | [1,77,86] |
| Inflammatory cytokines | Marked systemic IL-6 and TNF-α elevation following chemotherapy | Mild local inflammation | Severe systemic inflammation (IL-1β, IL-6, TNF-α) | [16,18] |
| Myokine regulation | Decreased secretion of IL-15 and irisin; imbalance in myostatin signaling | Slightly reduced myokine production due to inactivity | Altered myokine profile driven by tumor–host crosstalk | [89,90] |
| PGC-1α and mitochondrial biogenesis | Markedly suppressed, leading to energy deficit and impaired regeneration | Reduced but recoverable with reloading | Suppressed by chronic inflammatory signaling | [1,86] |
| Dominant mechanism | Dual mechanism: direct cytotoxic injury and systemic metabolic dysregulation | Local disuse-induced proteolysis | Systemic inflammation–driven catabolism | [1,20] |
| Reversibility | Partially reversible with exercise or nutritional interventions | Fully reversible with reloading | Poorly reversible without treating the underlying disease | [75,88,91] |
| Study Type | Subjects | Key Findings | References |
|---|---|---|---|
| Clinical | Head and Neck Cancer Patients | Cisplatin chemotherapy results in significant muscle mass loss and muscle atrophy symptoms. | [1] |
| Clinical | Lung Cancer Patients | Cisplatin treatment leads to substantial muscle mass and strength decline, impacting quality of life. | [6] |
| Clinical | Ovarian Cancer Patients | Cisplatin treatment decreases muscle index, resulting in impaired physical function. | [53] |
| Preclinical | Mouse Model | Cisplatin induces muscle mass reduction, with a significant decrease in muscle fiber cross-sectional area. | [7] |
| Preclinical | Mouse Model | Cisplatin causes a decrease in muscle protein synthesis, accompanied by activation of the protein degradation system. | [16] |
| Preclinical | Mouse Model | Cisplatin induces muscle atrophy with increased oxidative stress and inflammation. | [18] |
| Challenges | Future Directions | References |
|---|---|---|
| Patient heterogeneity | Develop personalized exercise prescriptions tailored to cancer type, treatment regimen, baseline physical status, and genetic background. | [220,221,222] |
| Treatment side effects | Focus on understanding how different exercise modalities can reduce treatment-related muscle wasting and improve overall health. | [9,10,34] |
| Adherence to exercise programs | Integrate digital health tools, such as wearables and tele-rehabilitation, to enhance feasibility and patient compliance. | [220,223,224] |
| Lack of standardized exercise protocols | Standardize exercise protocols and establish reproducible interventions in preclinical and clinical research. | [220,225,226] |
| Limited clinical trials on exercise interventions | Conduct large-scale clinical trials to validate the efficacy of exercise interventions during cisplatin-based chemotherapy. | [189,219,220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, X. Cisplatin-Induced Skeletal Muscle Atrophy: Biomolecular Mechanisms and the Protective Role of Exercise-Induced Myokines. Biomolecules 2025, 15, 1495. https://doi.org/10.3390/biom15111495
Xu M, Liu X. Cisplatin-Induced Skeletal Muscle Atrophy: Biomolecular Mechanisms and the Protective Role of Exercise-Induced Myokines. Biomolecules. 2025; 15(11):1495. https://doi.org/10.3390/biom15111495
Chicago/Turabian StyleXu, Miaomiao, and Xiaoguang Liu. 2025. "Cisplatin-Induced Skeletal Muscle Atrophy: Biomolecular Mechanisms and the Protective Role of Exercise-Induced Myokines" Biomolecules 15, no. 11: 1495. https://doi.org/10.3390/biom15111495
APA StyleXu, M., & Liu, X. (2025). Cisplatin-Induced Skeletal Muscle Atrophy: Biomolecular Mechanisms and the Protective Role of Exercise-Induced Myokines. Biomolecules, 15(11), 1495. https://doi.org/10.3390/biom15111495





