Cuproptosis as a Potential Therapeutic Target for Steatotic Liver Disease
Abstract
1. Introduction
2. Literature Retrieval Strategy
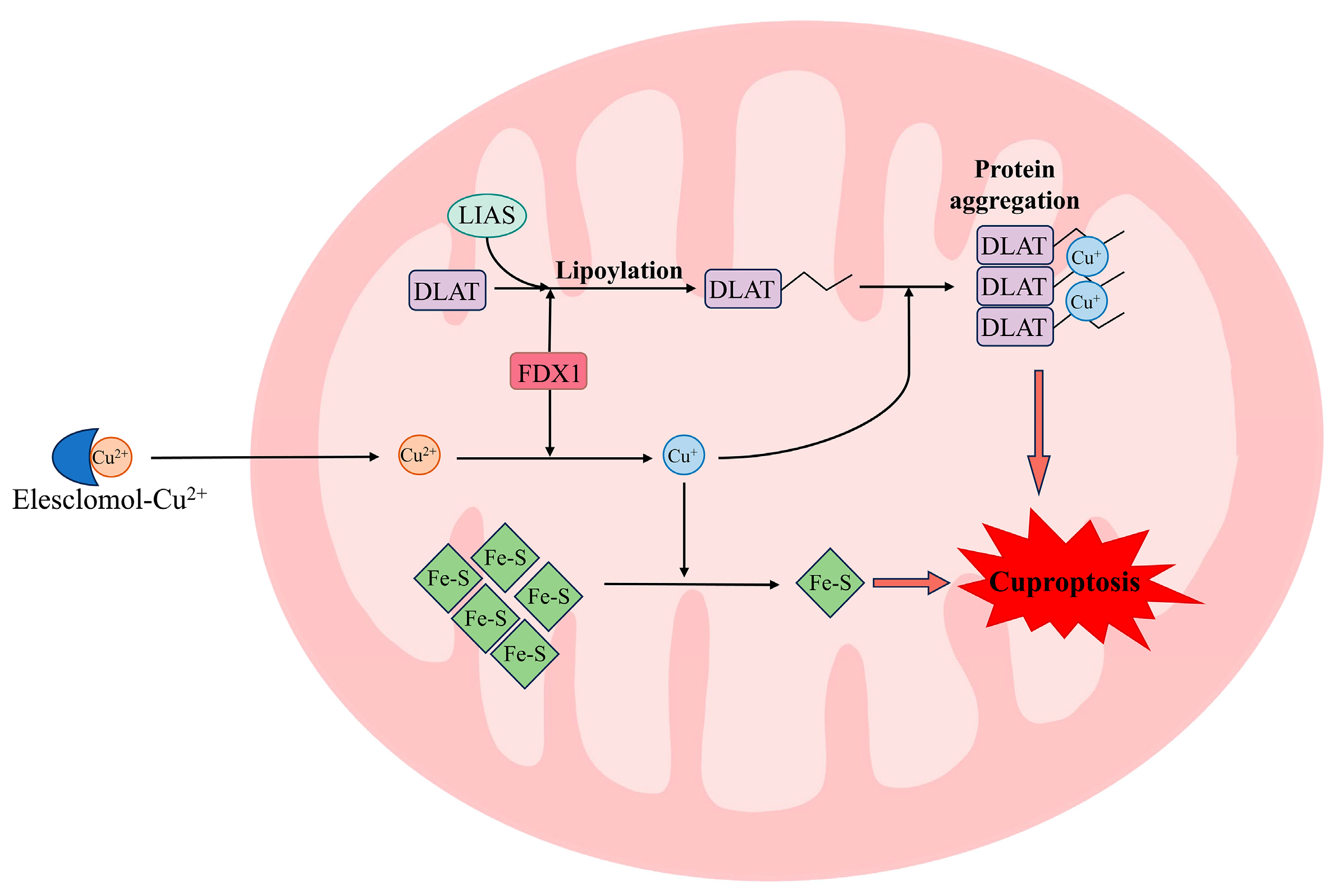
3. Cuproptosis
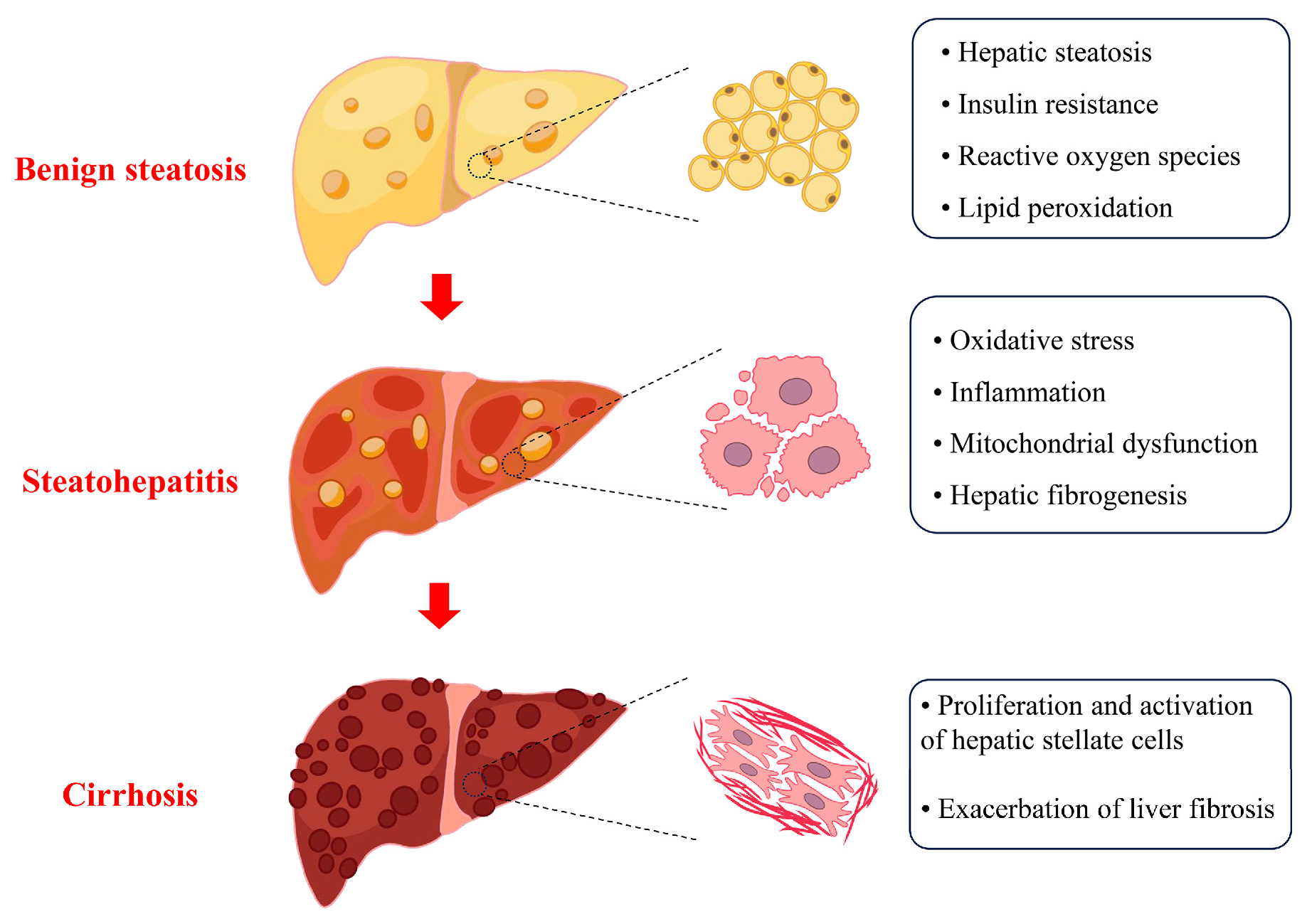
4. Cu Overload in SLD
5. Cuproptosis as a Potential Therapeutic Target for SLD
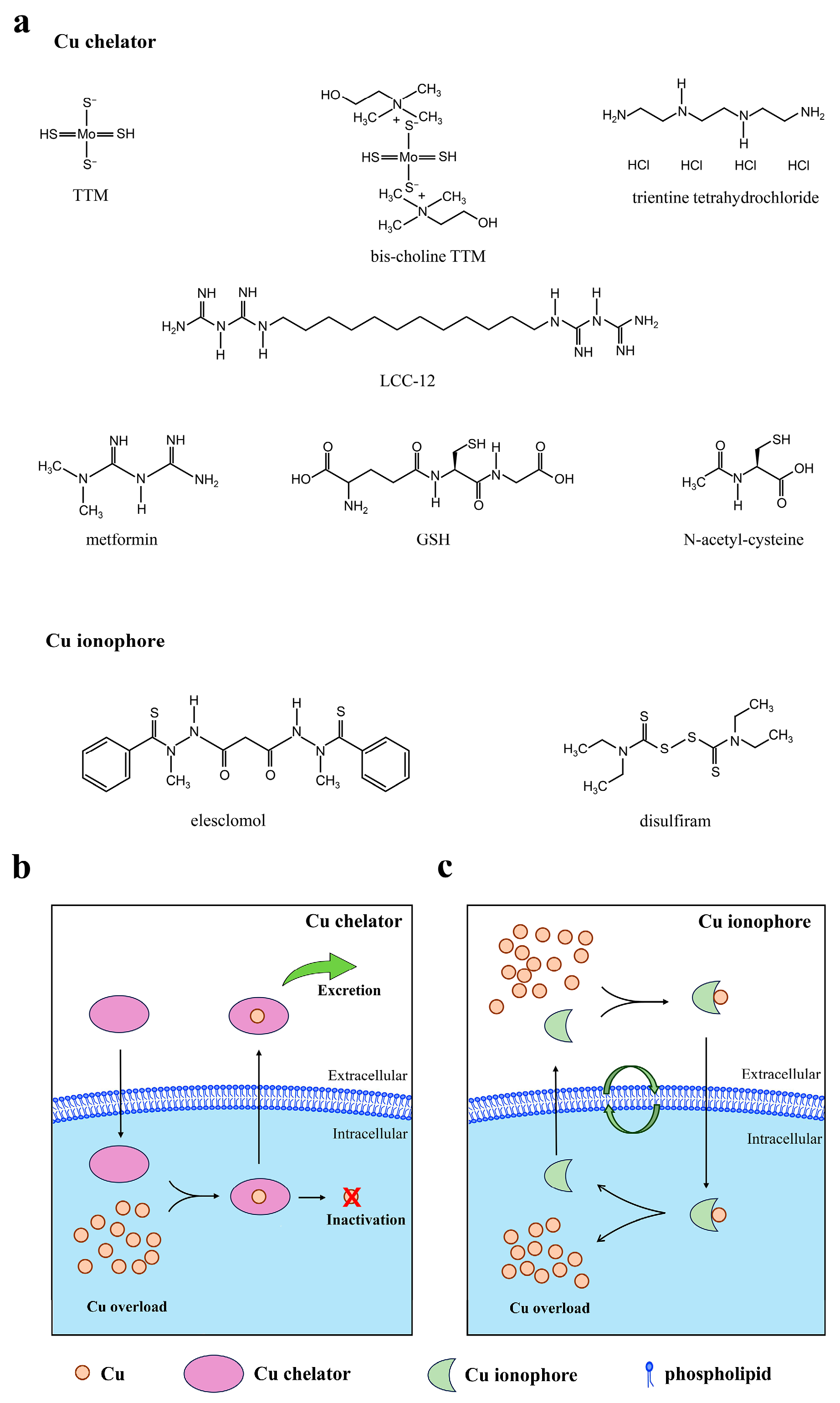
5.1. Cu Chelators
5.2. Cu Ionophores and Cu-Based Nanomedicine
5.3. Targeting Cuproptosis-Dependent Metabolic Pathways
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SLD | Steatotic liver disease |
| Cu | Copper |
| H2O2 | Hydrogen peroxide |
| TCA | Tricarboxylic acid |
| ETC | Electron transport chain |
| FDX1 | Ferredoxin 1 |
| LIAS | Lipoacylation synthase |
| LIPT1 | Lipoyl transferase 1 |
| DLD | Dihydrolipoamide dehydrogenase |
| DLAT | Dihydrolipoamide S-acetyltransferase |
| PDHA1 | Pyruvate dehydrogenase E1 subunit alpha 1 |
| PDHB | Pyruvate dehydrogenase E1 subunit beta |
| ATP7B | Atpase Cu transporters β |
| ROS | Reactive oxygen species |
| TTM | Ammonium tetrathiomolybdate |
| GSH | Glutathione |
| NAC | N-acetyl-cysteine |
| METTL16 | Methyltransferase-like 16 |
| SIRT2 | Sirtuin 2 |
| GPX4 | Glutathione peroxidase 4 |
References
- Israelsen, M.; Francque, S.; Tsochatzis, E.A.; Krag, A. Steatotic liver disease. Lancet 2024, 404, 1761–1778. [Google Scholar] [CrossRef] [PubMed]
- Torp, N.; Israelsen, M.; Thiele, M.; Rinella, M.E.; Krag, A. Phosphatidylethanol in steatotic liver disease. J. Hepatol. 2025, 83, 1189–1203. [Google Scholar] [CrossRef] [PubMed]
- Lekakis, V.; Papatheodoridis, G.V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 3–10. [Google Scholar] [CrossRef]
- Ho, G.J.K.; Tan, F.X.N.; Sasikumar, N.A.; Tham, E.K.J.; Ko, D.; Kim, D.H.; Danpanichkul, P.; Yu, Z.; Xianda, C.; Zhang, Z.X.; et al. High global prevalence of steatotic liver disease and associated subtypes: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2025; in press. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Rehm, J. A public health perspective on mitigating the global burden of chronic liver disease. Hepatology 2024, 79, 451–459. [Google Scholar] [CrossRef]
- Elshaer, A.; Chascsa, D.M.H.; Lizaola-Mayo, B.C. Exploring Varied Treatment Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Life 2024, 14, 884. [Google Scholar] [CrossRef] [PubMed]
- Matchett, K.P.; Paris, J.; Teichmann, S.A.; Henderson, N.C. Spatial genomics: Mapping human steatotic liver disease. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 646–660. [Google Scholar] [CrossRef]
- Ni, K.; Meng, L. Mechanism of PANoptosis in metabolic dysfunction-associated steatotic liver disease. Clin. Res. Hepatol. Gastroenterol. 2024, 48, 102381. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Miao, J. An emerging role of defective copper metabolism in heart disease. Nutrients 2022, 14, 700. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Meyerstein, D. Re-examining Fenton and Fenton-like reactions. Nat. Rev. Chem. 2021, 5, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of copper on mitochondrial function and metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef]
- Gulec, S.; Collins, J.F. Molecular mediators governing iron-copper interactions. Annu. Rev. Nutr. 2014, 34, 95–116. [Google Scholar] [CrossRef]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Dev, S.; Kruse, R.L.; Hamilton, J.P.; Lutsenko, S. Wilson disease: Update on pathophysiology and treatment. Front. Cell Dev. Biol. 2022, 10, 871877. [Google Scholar] [CrossRef]
- Himoto, T.; Masaki, T. Current trends of essential trace elements in patients with chronic liver diseases. Nutrients 2020, 12, 2084. [Google Scholar] [CrossRef]
- Zhong, C.-C.; Zhao, T.; Hogstrand, C.; Chen, F.; Song, C.-C.; Luo, Z. Copper (Cu) induced changes of lipid metabolism through oxidative stress-mediated autophagy and Nrf2/PPARγ pathways. J. Nutr. Biochem. 2022, 100, 108883. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yang, P.; Lip, G.Y.; Ren, J. Copper homeostasis and cuproptosis in cardiovascular disease therapeutics. Trends Pharmacol. Sci. 2023, 44, 573–585. [Google Scholar] [CrossRef]
- Lei, G.; Tang, L.; Yu, Y.; Bian, W.; Yu, L.; Zhou, J.; Li, Y.; Wang, Y.; Du, J. The potential of targeting cuproptosis in the treatment of kidney renal clear cell carcinoma. Biomed. Pharmacother. 2023, 167, 115522. [Google Scholar] [CrossRef]
- Han, J.; Luo, J.; Wang, C.; Kapilevich, L.; Zhang, X.-A. Roles and mechanisms of copper homeostasis and cuproptosis in osteoarticular diseases. Biomed. Pharmacother. 2024, 174, 116570. [Google Scholar] [CrossRef]
- Zhang, X.; Han, X. Targeting cuproptosis for cancer therapy: Focus on the anti-tumor immune system. Cancer Pathog. Ther. 2024, 3, 226–243. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson disease. Nat. Rev. Dis. Primers 2018, 4, 21. [Google Scholar] [CrossRef]
- Chan, W.-Y.; Garnica, A.D.; Rennert, O.M. Cell culture studies of Menkes kinky hair disease. Clin. Chim. Acta 1978, 88, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.M.; Go, Y.Y.; Shin, S.H.; Cho, J.-G.; Woo, J.-S.; Song, J.-J. Anti-cancer effects of disulfiram in head and neck squamous cell carcinoma via autophagic cell death. PLoS ONE 2018, 13, e0203069. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Cho, Y.; Oh, E.; Lee, N.; An, H.; Sung, D.; Cho, T.-M.; Seo, J.H. Disulfiram targets cancer stem-like properties and the HER2/Akt signaling pathway in HER2-positive breast cancer. Cancer Lett. 2016, 379, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, Y.; Zhou, Y.; Hu, W.; Yang, C.; Jing, Q.; Zhou, C.; Wang, X.; Hu, J.; Wang, L. Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol. 2021, 46, 102122. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kroemer, G. Cuproptosis: A copper-triggered modality of mitochondrial cell death. Cell Res. 2022, 32, 417–418. [Google Scholar] [CrossRef]
- Joshi, P.R.; Sadre, S.; Guo, X.A.; McCoy, J.G.; Mootha, V.K. Lipoylation is dependent on the ferredoxin FDX1 and dispensable under hypoxia in human cells. J. Biol. Chem. 2023, 299, 105075. [Google Scholar] [CrossRef]
- Pan, Y.-X.; Zhuo, M.-Q.; Li, D.-D.; Xu, Y.-H.; Wu, K.; Luo, Z. SREBP-1 and LXRα pathways mediated Cu-induced hepatic lipid metabolism in zebrafish Danio rerio. Chemosphere 2019, 215, 370–379. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Q.; Yang, R.; Wu, Z.; Yuan, H.; Zhang, N.; Zhi, M.; Zhang, Y.; Ni, X.; Wang, Z. Copper exposure association with prevalence of non-alcoholic fatty liver disease and insulin resistance among US adults (NHANES 2011–2014). Ecotoxicol. Environ. Saf. 2021, 218, 112295. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Li, Z.; Yan, J.; Li, Y.; Zheng, J.; Zhuang, Z.; Yang, W.; Gong, L.; Shi, J. Activation of HIF-1 signaling ameliorates liver steatosis in zebrafish atp7b deficiency (Wilson’s disease) models. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165842. [Google Scholar] [CrossRef]
- Muchenditsi, A.; Yang, H.; Hamilton, J.P.; Koganti, L.; Housseau, F.; Aronov, L.; Fan, H.; Pierson, H.; Bhattacharjee, A.; Murphy, R. Targeted inactivation of copper transporter Atp7b in hepatocytes causes liver steatosis and obesity in mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 313, G39–G49. [Google Scholar] [CrossRef]
- Zhong, C.-C.; Zhao, T.; Hogstrand, C.; Song, C.-C.; Zito, E.; Tan, X.-Y.; Xu, Y.-C.; Song, Y.-F.; Wei, X.-L.; Luo, Z. Copper induces liver lipotoxicity disease by up-regulating Nrf2 expression via the activation of MTF-1 and inhibition of SP1/Fyn pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166752. [Google Scholar] [CrossRef]
- Li, H.; Gong, W.; Wang, G.; Yu, E.; Tian, J.; Xia, Y.; Li, Z.; Zhang, K.; Xie, J. Role of nuclear pregnane X receptor in Cu-induced lipid metabolism and xenobiotic responses in largemouth bass (Micropterus salmoides). Front. Endocrinol. 2022, 13, 950985. [Google Scholar] [CrossRef]
- Tan, P.Y.; Roy, M.S. Dietary copper and selenium are associated with insulin resistance in overweight and obese Malaysian adults. Nutr. Res. 2021, 93, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Kaneto, H.; Miyatsuka, T.; Yamamoto, K.; Yoshiuchi, K.; Yamasaki, Y.; Shimomura, I.; Matsuoka, T.-A.; Matsuhisa, M. Role of copper ion in the pathogenesis of type 2 diabetes. Endocr. J. 2009, 56, 699–706. [Google Scholar] [CrossRef]
- Hilário-Souza, E.; Cuillel, M.; Mintz, E.; Charbonnier, P.; Vieyra, A.; Cassio, D.; Lowe, J. Modulation of hepatic copper-ATPase activity by insulin and glucagon involves protein kinase A (PKA) signaling pathway. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 2086–2097. [Google Scholar] [CrossRef]
- Manne, V.; Handa, P.; Kowdley, K.V. Pathophysiology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin. Liver Dis. 2018, 22, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef]
- Adams, L.A.; Ratziu, V. Non-alcoholic fatty liver–perhaps not so benign. J. Hepatol. 2015, 62, 1002–1004. [Google Scholar] [CrossRef]
- Pafili, K.; Roden, M. Nonalcoholic fatty liver disease (NAFLD) from pathogenesis to treatment concepts in humans. Mol. Metab. 2021, 50, 101122. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Bora, H.K.; Misra, U.K. Relationship of antioxidant and oxidative stress markers in different organs following copper toxicity in a rat model. Toxicol. Appl. Pharmacol. 2016, 293, 37–43. [Google Scholar] [CrossRef]
- Baker, Z.N.; Cobine, P.A.; Leary, S.C. The mitochondrion: A central architect of copper homeostasis. Metallomics 2017, 9, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.C.; Winge, D.R.; Cobine, P.A. “Pulling the plug” on cellular copper: The role of mitochondria in copper export. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zischka, H.; Einer, C. Mitochondrial copper homeostasis and its derailment in Wilson disease. Int. J. Biochem. Cell Biol. 2018, 102, 71–75. [Google Scholar] [CrossRef]
- Xu, S.-Q.; Zhu, H.-Y.; Lin, J.-G.; Su, T.-F.; Liu, Y.; Luo, X.-P. Copper ions stimulate the proliferation of hepatic stellate cells via oxygen stress in vitro. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 2013, 33, 75–80. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Zhang, F.; Gao, Y.; Li, J.; Ji, Y. Copper homeostasis dysregulation promoting cell damage and the association with liver diseases. Chin. Med. J. 2023, 136, 1653–1662. [Google Scholar] [CrossRef]
- Li, L.; Yi, Y.; Shu, X.; Li, J.; Kang, H.; Chang, Y. The Correlation Between Serum Copper and Non-alcoholic Fatty Liver Disease in American Adults: An Analysis Based on NHANES 2011 to 2016. Biol. Trace Elem. Res. 2024, 202, 4398–4409. [Google Scholar] [CrossRef] [PubMed]
- Amedeo, L.; Ralf, W. Copper and liver fibrosis in MASLD: The two-edged sword of copper deficiency and toxicity. Metab. Target Organ Damage 2024, 4, 33. [Google Scholar] [CrossRef]
- Pan, T.-T.; Huang, J.-Y.; Wang, X.-D.; Chen, D.-Z.; Chen, Y.-P. Copper’s dual role: Reviewing its impact on liver health and disease. Int. Immunopharmacol. 2025, 152, 114391. [Google Scholar] [CrossRef]
- Yu, L.; Yousuf, S.; Yousuf, S.; Yeh, J.; Biggins, S.W.; Morishima, C.; Shyu, I.; O’Shea-Stone, G.; Eilers, B.; Waldum, A.; et al. Copper deficiency is an independent risk factor for mortality in patients with advanced liver disease. Hepatol. Commun. 2023, 7, e0076. [Google Scholar] [CrossRef] [PubMed]
- Liggi, M.; Murgia, D.; Civolani, A.; Demelia, E.; Sorbello, O.; Demelia, L. The relationship between copper and steatosis in Wilson’s disease. Clin. Res. Hepatol. Gastroenterol. 2013, 37, 36–40. [Google Scholar] [CrossRef]
- Ouyang, G.; Wu, Z.; Liu, Z.; Pan, G.; Wang, Y.; Liu, J.; Guo, J.; Liu, T.; Huang, G.; Zeng, Y. Identification and validation of potential diagnostic signature and immune cell infiltration for NAFLD based on cuproptosis-related genes by bioinformatics analysis and machine learning. Front. Immunol. 2023, 14, 1251750. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Zhong, L.; Zhou, Y.; Long, L.; Yi, T.; Chen, S.; Li, Y.; Chen, Y.; Shen, L. Identification of cuproptosis-related genes in nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2023, 2023, 9245667. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ma, X.; Liu, R.; Xu, C.; He, Q.; Dong, M. Identification and validation of cuproptosis-related genes for diagnosis and therapy in nonalcoholic fatty liver disease. Mol. Cell. Biochem. 2025, 480, 473–489. [Google Scholar] [CrossRef]
- de Oliveira da Silva, B.; Ramos, L.F.; Moraes, K.C. Molecular interplays in hepatic stellate cells: Apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol. Int. 2017, 41, 946–959. [Google Scholar] [CrossRef]
- Siegmund, S.V.; Uchinami, H.; Osawa, Y.; Brenner, D.A.; Schwabe, R.F. Anandamide induces necrosis in primary hepatic stellate cells. Hepatology 2005, 41, 1085–1095. [Google Scholar] [CrossRef]
- Wijayasiri, P.; Astbury, S.; Kaye, P.; Oakley, F.; Alexander, G.J.; Kendall, T.J.; Aravinthan, A.D. Role of hepatocyte senescence in the activation of hepatic stellate cells and liver fibrosis progression. Cells 2022, 11, 2221. [Google Scholar] [CrossRef]
- Stremmel, W.; Weiskirchen, R. Therapeutic strategies in Wilson disease: Pathophysiology and mode of action. Ann. Transl. Med. 2020, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Kamlin, C.O.F.; Jenkins, T.M.; L Heise, J.; Amin, N.S. Trientine Tetrahydrochloride, From Bench to Bedside: A Narrative Review. Drugs 2024, 84, 1509–1518. [Google Scholar] [CrossRef]
- Kirk, F.T.; Munk, D.E.; Swenson, E.S.; Quicquaro, A.M.; Vendelbo, M.H.; Larsen, A.; Schilsky, M.L.; Ott, P.; Sandahl, T.D. Effects of tetrathiomolybdate on copper metabolism in healthy volunteers and in patients with Wilson disease. J. Hepatol. 2024, 80, 586–595. [Google Scholar] [CrossRef]
- Borchard, S.; Raschke, S.; Zak, K.M.; Eberhagen, C.; Einer, C.; Weber, E.; Müller, S.M.; Michalke, B.; Lichtmannegger, J.; Wieser, A.; et al. Bis-choline tetrathiomolybdate prevents copper-induced blood–brain barrier damage. Life Sci. Alliance 2022, 5, e202101164. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.H.; Askari, F.K.; Czlonkowska, A.; Ferenci, P.; Bronstein, J.M.; Bega, D.; Ala, A.; Nicholl, D.; Flint, S.; Olsson, L.; et al. Bis-choline tetrathiomolybdate in patients with Wilson’s disease: An open-label, multicentre, phase 2 study. Lancet Gastroenterol. Hepatol. 2017, 2, 869–876. [Google Scholar] [CrossRef]
- Babu Balagopal, P.; Kohli, R.; Uppal, V.; Averill, L.; Shah, C.; McGoogan, K.; Di Guglielmo, M.; Goran, M.; Hossain, M.J. Effect of N-acetyl cysteine in children with metabolic dysfunction-associated steatotic liver disease—A pilot study. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Moreno, M.; Monroy-Ramirez, H.C.; Caloca-Camarena, F.; Arceo-Orozco, S.; Muriel, P.; Sandoval-Rodriguez, A.; García-Bañuelos, J.; García-González, A.; Navarro-Partida, J.; Armendariz-Borunda, J. A new opportunity for N-acetylcysteine. An outline of its classic antioxidant effects and its pharmacological potential as an epigenetic modulator in liver diseases treatment. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 398, 2365–2386. [Google Scholar] [CrossRef]
- Petrie, J.R. Metformin beyond type 2 diabetes: Emerging and potential new indications. Diabetes Obes. Metab. 2024, 26, 31–41. [Google Scholar] [CrossRef]
- Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D’Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F.; et al. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14, 186. [Google Scholar] [CrossRef]
- Logie, L.; Harthill, J.; Patel, K.; Bacon, S.; Hamilton, D.L.; Macrae, K.; McDougall, G.; Wang, H.-H.; Xue, L.; Jiang, H. Cellular responses to the metal-binding properties of metformin. Diabetes 2012, 61, 1423–1433. [Google Scholar] [CrossRef]
- Solier, S.; Müller, S.; Cañeque, T.; Versini, A.; Mansart, A.; Sindikubwabo, F.; Baron, L.; Emam, L.; Gestraud, P.; Pantoș, G.D. A druggable copper-signalling pathway that drives inflammation. Nature 2023, 617, 386–394. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Brenner, D.A. Hepatic stellate cells: Balancing homeostasis, hepatoprotection and fibrogenesis in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 481–499. [Google Scholar] [CrossRef]
- Sekar, V.; Vp, V.; Vijay, V.; Br, A.; Vijayan, N.; Perumal, M.K. Inhibition of hepatic stellate cell activation by nutraceuticals: An emphasis on mechanisms of action. J. Food Sci. Technol. 2024, 61, 2046–2056. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G.; Kang, R. Targeting cuproplasia and cuproptosis in cancer. Nat. Rev. Clin. Oncol. 2024, 21, 370–388. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, T.; Li, L. Targeting cuproptosis for cancer therapy: Mechanistic insights and clinical perspectives. J. Hematol. Oncol. 2024, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Lee, Y.; Park, Y.-K.; Shin, D.-G.; Joshi, P.; Hong, S.-H.; Alder, N.; Koo, S.I.; Lee, J.-Y. Astaxanthin attenuates the increase in mitochondrial respiration during the activation of hepatic stellate cells. J. Nutr. Biochem. 2019, 71, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Fondevila, M.F.; Fernandez, U.; Heras, V.; Parracho, T.; Gonzalez-Rellan, M.J.; Novoa, E.; Porteiro, B.; Alonso, C.; Mayo, R.; da Silva Lima, N. Inhibition of carnitine palmitoyltransferase 1A in hepatic stellate cells protects against fibrosis. J. Hepatol. 2022, 77, 15–28. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Yang, B.; Sun, S.; Zhang, P.; Luo, Z.; Feng, T.; Cui, Z.; Zhu, T.; Li, Y. Lactylation of METTL16 promotes cuproptosis via m6A-modification on FDX1 mRNA in gastric cancer. Nat. Commun. 2023, 14, 6523. [Google Scholar] [CrossRef]
- Wang, X.; Xue, Y.; Chang, L.; Zhu, X.; Liu, W.; Liang, T. The Regulation of Trace Metal Elements in Cancer Ferroptosis. Adv. Biol. 2025, 9, 2400821. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; ChenLiu, Z.; Wang, D.; Tang, D. Cross-talks of GSH, mitochondria, RNA m6A modification, NRF2, and p53 between ferroptosis and cuproptosis in HCC: A review. Int. J. Biol. Macromol. 2025, 302, 140523. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, K.; Jiang, X.; Wei, Q.; Zhu, L.; Wang, X.; Jin, H.; Feng, L. Ferroptosis inducers enhanced cuproptosis induced by copper ionophores in primary liver cancer. J. Exp. Clin. Cancer Res. 2023, 42, 142. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wei, C.; Liu, G.; Zhang, L.; Li, J.; Li, L.; Cai, S.; Fang, L. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1α/SLC7A11 pathway. Cell Prolif. 2022, 55, e13158. [Google Scholar] [CrossRef]
| Therapeutic Strategies | Mechanism | Targeting of SLD Stage |
|---|---|---|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Luo, C.; Guo, Q.; Duan, Q.; Wu, Z.; Li, Y. Cuproptosis as a Potential Therapeutic Target for Steatotic Liver Disease. Biomolecules 2025, 15, 1490. https://doi.org/10.3390/biom15111490
Pan Y, Luo C, Guo Q, Duan Q, Wu Z, Li Y. Cuproptosis as a Potential Therapeutic Target for Steatotic Liver Disease. Biomolecules. 2025; 15(11):1490. https://doi.org/10.3390/biom15111490
Chicago/Turabian StylePan, Yujie, Cheng Luo, Qitao Guo, Qifei Duan, Ziyan Wu, and Yan Li. 2025. "Cuproptosis as a Potential Therapeutic Target for Steatotic Liver Disease" Biomolecules 15, no. 11: 1490. https://doi.org/10.3390/biom15111490
APA StylePan, Y., Luo, C., Guo, Q., Duan, Q., Wu, Z., & Li, Y. (2025). Cuproptosis as a Potential Therapeutic Target for Steatotic Liver Disease. Biomolecules, 15(11), 1490. https://doi.org/10.3390/biom15111490






