Sex Differences in Oxidative Stress Concerning Allergic Diseases
Abstract
1. Introduction
1.1. Sex Differences in Allergy Epidemiology
1.2. Oxidative Stress and Allergy
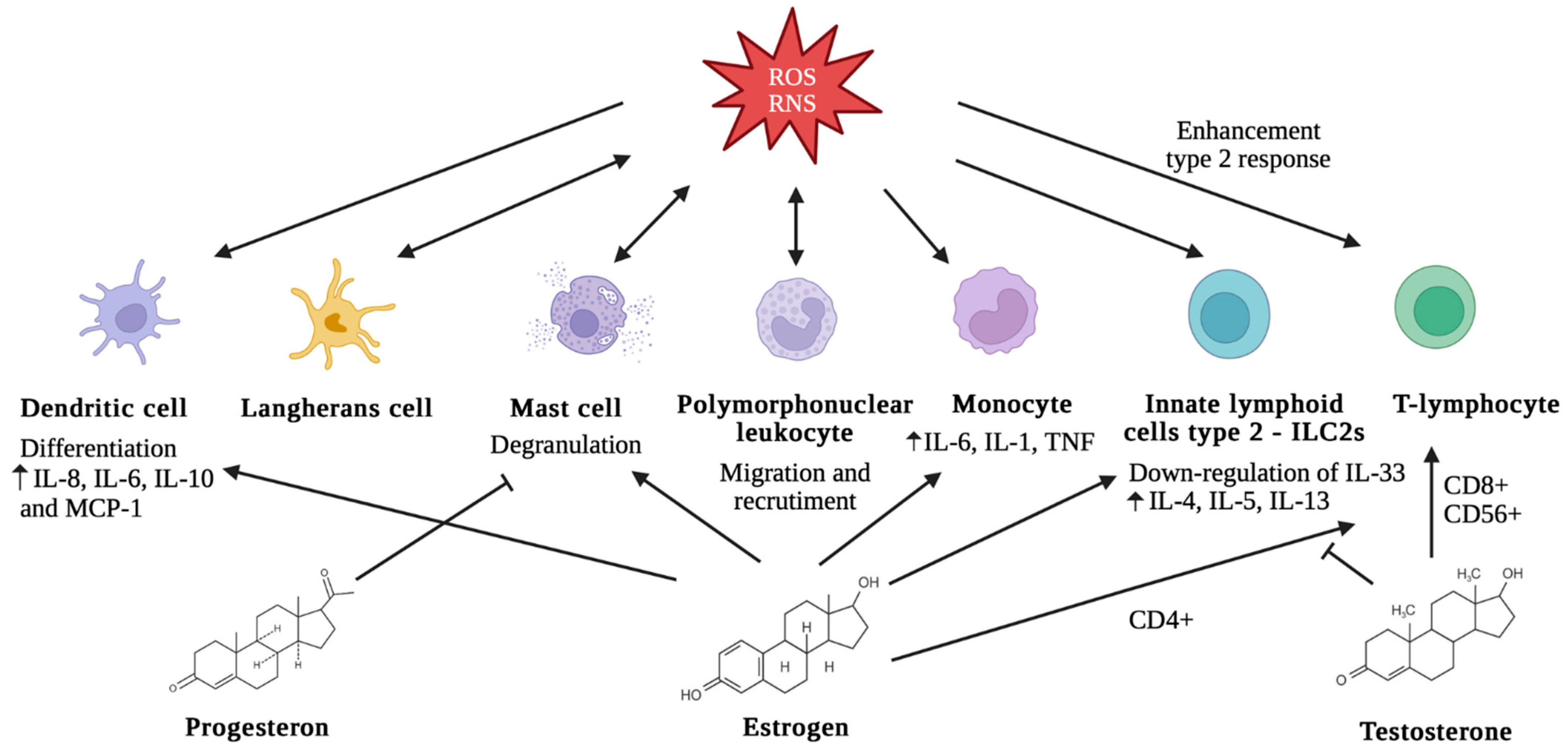
1.3. Sex Differences, Oxidative Stress, and Allergy
2. Sex Differences in Oxidative Stress and Allergic Disease
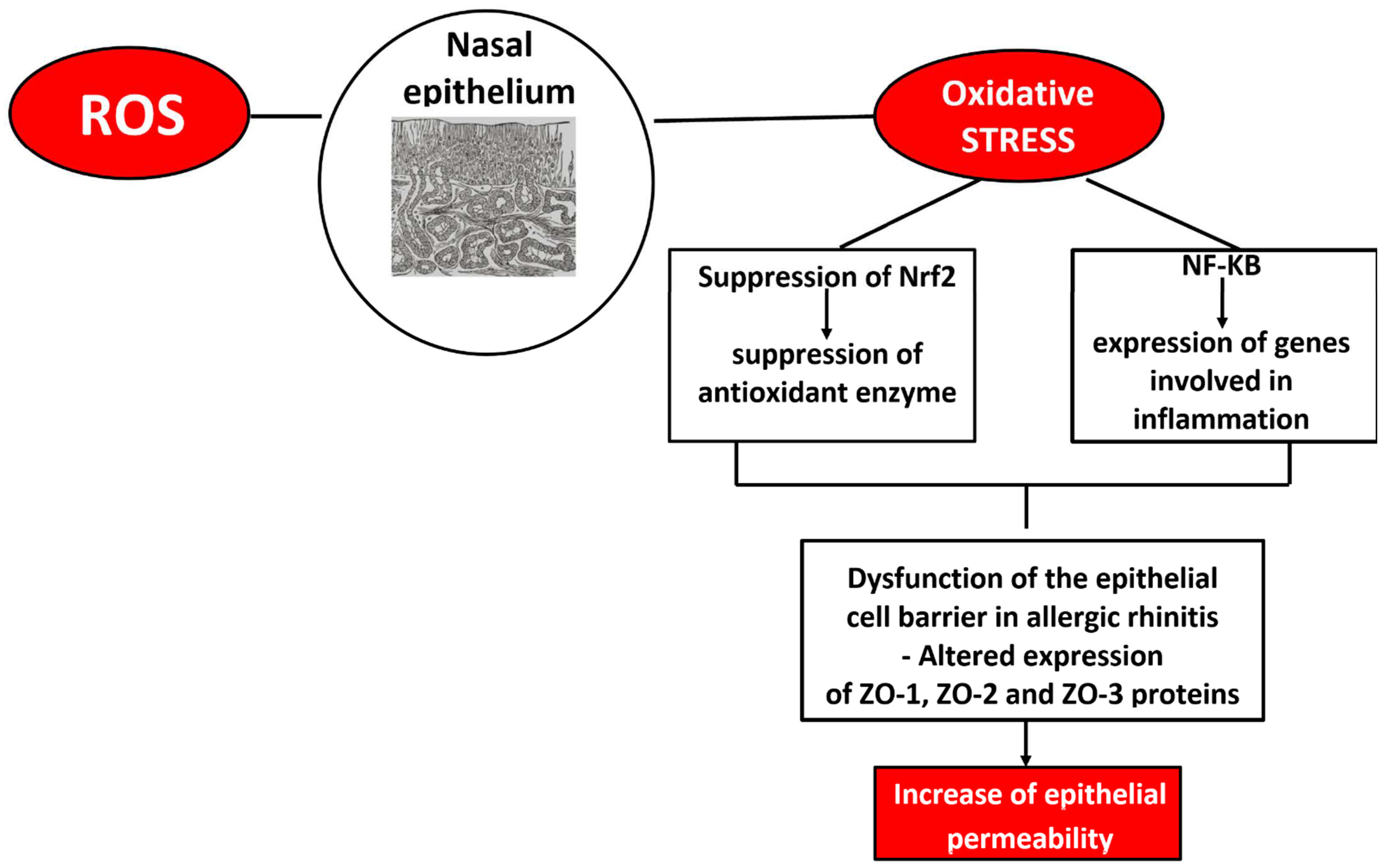
2.1. Allergic Rhinitis, Oxidative Stress, and Sex Differences
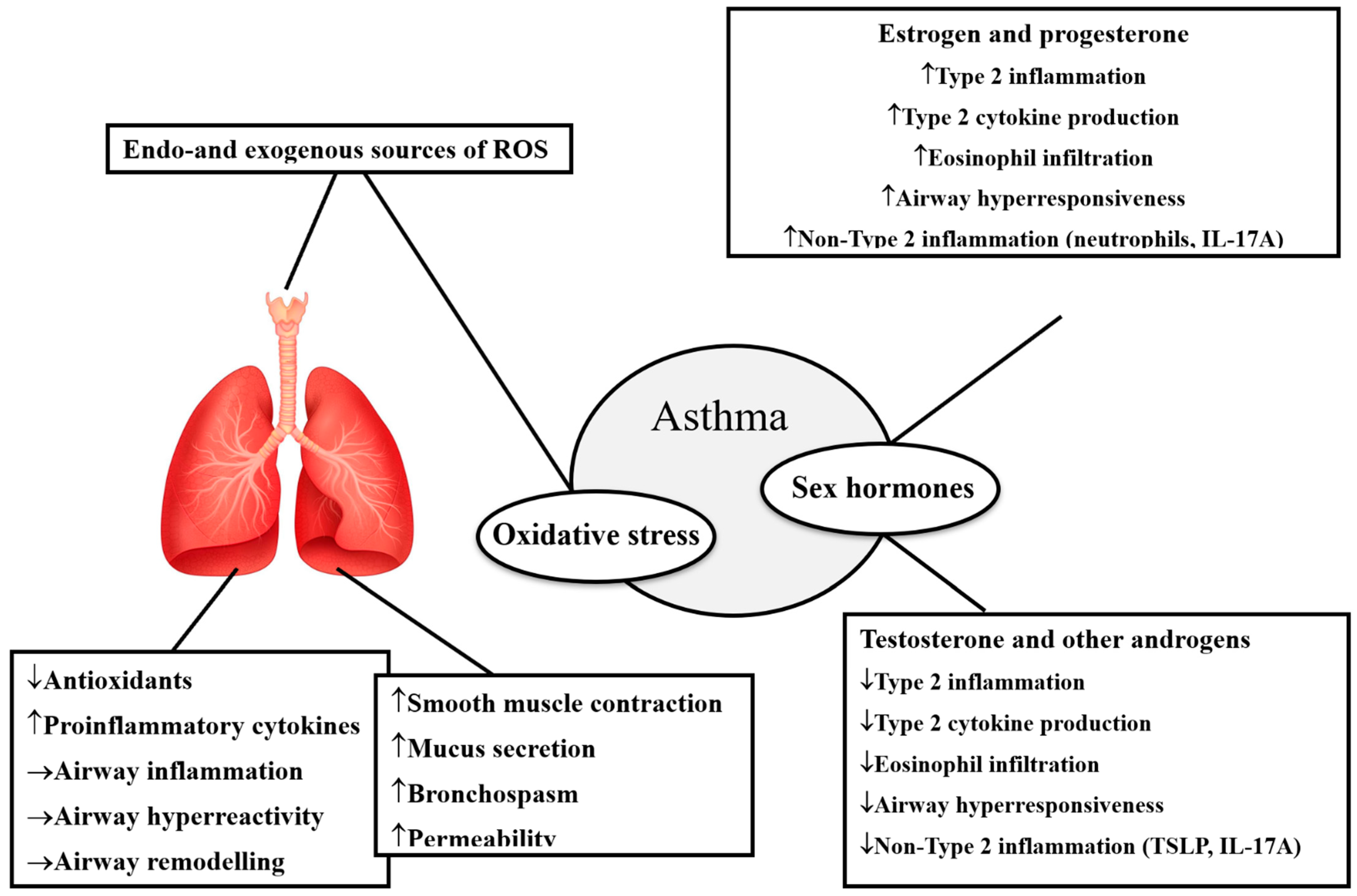
2.2. Asthma, Oxidative Stress, and Sex Differences
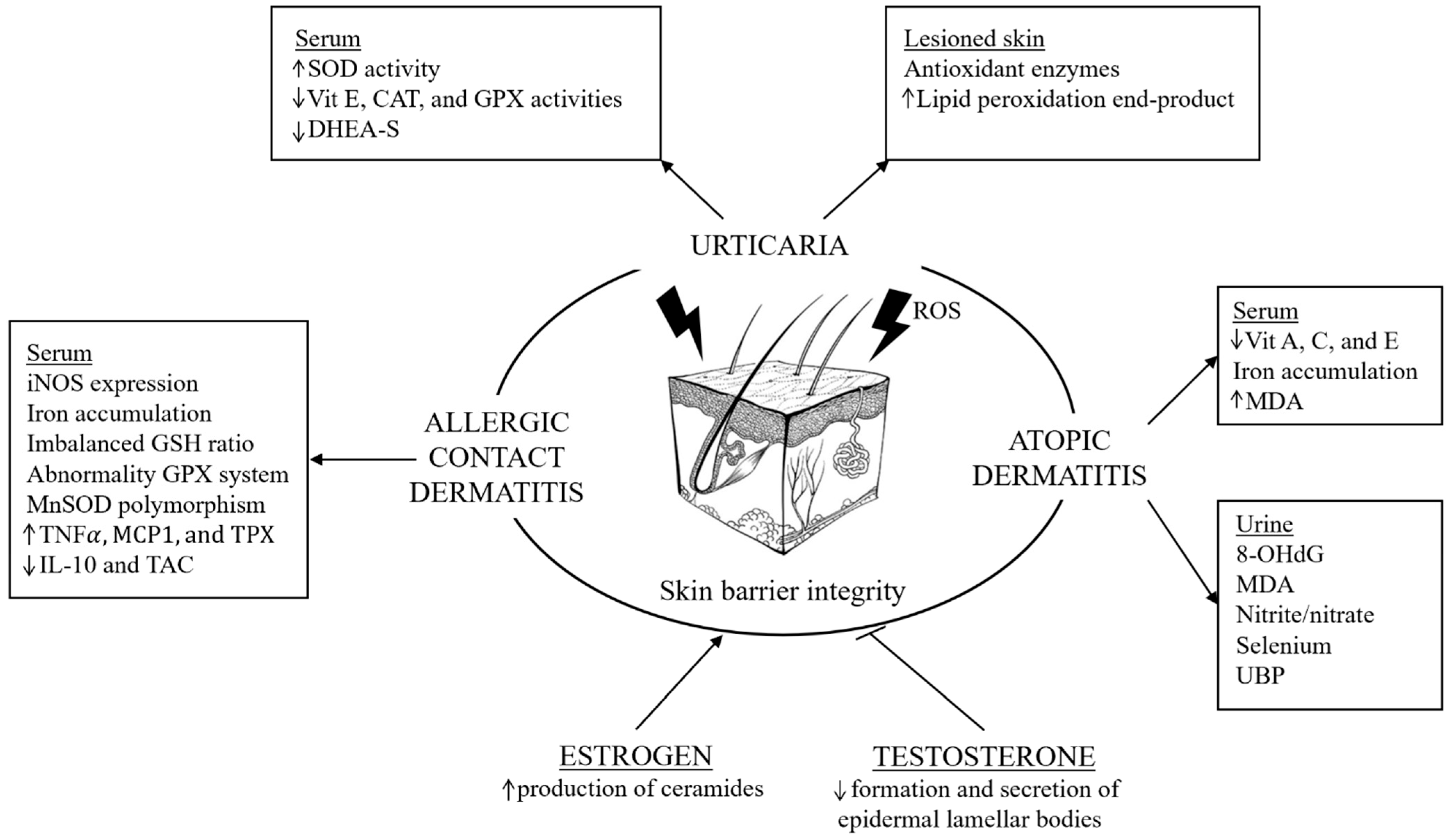
2.3. Urticaria, Oxidative Stress, and Sex Differences
2.4. Atopic Dermatitis, Oxidative Stress, and Sex Differences
2.5. Allergic Contact Dermatitis, Oxidative Stress, and Sex Differences
3. Sex Differences, Oxidative Stress, and Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Y.; Larochelle, L.; Khan, F.A.; Pilote, L. Sex Differences in the Impact of Extreme Heat on Cardiovascular Disease Outcomes: A Systematic Review and Meta-Analysis. Environ. Health 2025, 24, 20. [Google Scholar] [CrossRef]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and Consequences of Sex Differences in Immune Responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef]
- Salvati, L.; Vitiello, G.; Parronchi, P. Gender Differences in Anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 417–424. [Google Scholar] [CrossRef]
- Maurer, M.; Pereira, M.P.; Kolkhir, P. The Definition, Classification, and History of Urticaria. Immunol. Allergy Clin. N. Am. 2024, 44, 407–419. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Ellis, A.; Castells, M. Sex and allergic diseases. Ann. Allergy Asthma Immunol. 2019, 122, 134–135. [Google Scholar] [CrossRef]
- Möhrenschlager, M.; Schäfer, T.; Huss-Marp, J.; Eberlein-König, B.; Weidinger, S.; Ring, J.; Behrendt, H.; Krämer, U. The Course of Eczema in Children Aged 5–7 Years and Its Relation to Atopy: Differences between Boys and Girls. Br. J. Dermatol. 2006, 154, 505–513. [Google Scholar] [CrossRef]
- Lovik, M.; Namork, E.; Faeste, C.; Egaas, E. The Norwegian National Reporting System and Register of Severe Allergic Reactions to Food; Marone, G., Ed.; JGC Publishers: Napoli, Italy, 2003. [Google Scholar]
- Webb, L.M.; Lieberman, P. Anaphylaxis: A Review of 601 Cases. Ann. Allergy Asthma. Immunol. 2006, 97, 39–43. [Google Scholar] [CrossRef]
- Chowdhury, N.U.; Guntur, V.P.; Newcomb, D.C.; Wechsler, M.E. Sex and gender in asthma. Eur. Respir. Rev. 2021, 30, 210067. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Cristani, M.; Speciale, A.; Saija, A.; Gangemi, S.; Minciullo, P.L.; Cimino, F. Circulating Advanced Oxidation Protein Products as Oxidative Stress Biomarkers and Progression Mediators in Pathological Conditions Related to Inflammation and Immune Dysregulation. Curr. Med. Chem. 2016, 23, 3862–3882. [Google Scholar] [CrossRef]
- Allegra, A.; Caserta, S.; Genovese, S.; Pioggia, G.; Gangemi, S. Gender Differences in Oxidative Stress in Relation to Cancer Susceptibility and Survival. Antioxidants 2023, 12, 1255. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and In Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Bede, O.; Nagy, D.; Surányi, A.; Horváth, I.; Szlávik, M.; Gyurkovits, K. Effects of Magnesium Supplementation on the Glutathione Redox System in Atopic Asthmatic Children. Inflamm. Res. 2008, 57, 279–286. [Google Scholar] [CrossRef]
- Kannan, G.; Paul, B.M.; Thangaraj, P. Stimulation, Regulation, and Inflammaging Interventions of Natural Compounds on Nuclear Factor Kappa B (NF-κB) Pathway: A Comprehensive Review. Inflammopharmacology 2025, 33, 145–162. [Google Scholar] [CrossRef]
- Biedrzycki, G.; Wolszczak-Biedrzycka, B.; Dorf, J.; Maciejczyk, M. The Antioxidant Barrier, Oxidative/Nitrosative Stress, and Protein Glycation in Allergy: From Basic Research to Clinical Practice. Front. Immunol. 2024, 15, 1440313. [Google Scholar] [CrossRef]
- Natarajan, K.; Mathialagan, G.D.; Raghavan, S.; Shanmugam, N. The Advanced Lipoxidation End Product Precursor Malon- Dialdehyde Induces IL-17E Expression and Skews Lymphocytes to the Th17 Subset. Cell Mol. Biol. Lett. 2015, 20, 647–662. [Google Scholar] [CrossRef]
- Raghavan, S.; Subramaniyam, G.; Shanmugam, N. Proinflammatory Effects of Malondialdehyde in Lymphocytes. J. Leukoc. Biol. 2012, 92, 1055–1067. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; McKenzie, A.N.J. New Kids on the Block: Group 2 Innate Lymphoid Cells and Type 2 Inflammation in the Lung. Chest 2013, 144, 1681–1686. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Rana, B.M.J.; Walker, J.A.; Kerscher, B.; Knolle, M.D.; Jolin, H.E.; Serrao, E.M.; Haim-Vilmovsky, L.; Teichmann, S.A.; Rodewald, H.-R.; et al. Tissue-Restricted Adaptive Type 2 Immunity Is Orchestrated by Expression of the Costimulatory Molecule OX40L on Group 2 Innate Lymphoid Cells. Immunity 2018, 48, 1195–1207.e6. [Google Scholar] [CrossRef]
- Zheng, C.; Wu, H.; Lu, Z.; Bi, J.; Wan, X. IL-33-Induced Reactive Oxygen Species Are Required for Optimal Metabolic pro-Gramming in Group 2 Innate Lymphoid Cells. Cell Mol. Immunol. 2020, 17, 1266–1268. [Google Scholar] [CrossRef]
- Patella, V.; Incorvaia, C.; Minciullo, P.L.; Oricchio, C.; Saitta, S.; Florio, G.; Saija, A.; Gangemi, S. Oxidative Stress Markers in Patients with Hymenoptera Venom Allergy. Allergy Asthma Proc. 2015, 36, e9–e13. [Google Scholar] [CrossRef]
- Schauer, U.; Leinhaas, C.; Jäger, R.; Rieger, C.H. Enhanced Superoxide Generation by Eosinophils from Asthmatic Children. Int. Arch. Allergy Appl. Immunol. 1991, 96, 317–321. [Google Scholar] [CrossRef]
- DA SILVA, J.A.P. Sex Hormones and Glucocorticoids: Interactions with the Immune System. Ann. N. Y. Acad. Sci. 1999, 876, 102–118. [Google Scholar] [CrossRef]
- Cutolo, M.; Capellino, S.; Sulli, A.; Serioli, B.; Secchi, M.E.; Villaggio, B.; Straub, R.H. Estrogens and Autoimmune Diseases. Ann. N. Y. Acad. Sci. 2006, 1089, 538–547. [Google Scholar] [CrossRef]
- Ma, L.J.; Guzman, E.A.; DeGuzman, A.; Muller, H.K.; Walker, A.M.; Owen, L.B. Local Cytokine Levels Associated with De-Layed-Type Hypersensitivity Responses: Modulation by Gender, Ovariectomy, and Estrogen Replacement. J. Endocrinol. 2007, 193, 291–297. [Google Scholar] [CrossRef]
- Vasiadi, M.; Kempuraj, D.; Boucher, W.; Kalogeromitros, D.; Theoharides, T.C. Progesterone inhibits mast cell secretion. Int. J. Immunopathol. Pharmacol. 2006, 19, 787–794. [Google Scholar] [CrossRef]
- Vancolen, S.; Sébire, G.; Robaire, B. Influence of Androgens on the Innate Immune System. Andrology 2023, 11, 1237–1244. [Google Scholar] [CrossRef]
- Weinstein, Y.; Berkovich, Z. Testosterone Effect on Bone Marrow, Thymus, and Suppressor T Cells in the (NZB X NZW)F1 Mice: Its Relevance to Autoimmunity. J. Immunol. 1981, 126, 998–1002. [Google Scholar] [CrossRef]
- Amadori, A.; Zamarchi, R.; De Silvestro, G.; Forza, G.; Cavatton, G.; Danieli, G.A.; Clementi, M.; Chieco-Bianchi, L. Genetic Control of the CD4/CD8 T-Cell Ratio in Humans. Nat. Med. 1995, 1, 1279–1283. [Google Scholar] [CrossRef]
- Cunningham, M.; Gilkeson, G. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allerg. Immu. 2011, 40, 66–73. [Google Scholar] [CrossRef]
- Kalogeromitros, D.; Katsarou, A.; Armenaka, M.; Rigopoulos, D.; Zapanti, M.; Stratigos, I. Influence of the Menstrual Cycle on Skin-Prick Test Reactions to Histamine, Morphine and Allergen. Clin. Exp. Allergy 1995, 25, 461–466. [Google Scholar] [CrossRef]
- Nocella, C.; D’Amico, A.; Cammisotto, V.; Bartimoccia, S.; Castellani, V.; Loffredo, L.; Marini, L.; Ferrara, G.; Testa, M.; Motta, G. Structure, Activation, and Regulation of NOX2: At the Crossroad between the Innate Immunity and Oxidative Stress-Mediated Pathologies. Antioxidants 2023, 12, 429. [Google Scholar] [CrossRef]
- Kendall, B.; Eston, R. Exercise-Induced Muscle Damage and the Potential Protective Role of Estrogen. Sport. Med. 2002, 32, 103–123. [Google Scholar] [CrossRef]
- Michos, C.; Kiortsis, D.N.; Evangelou, A.; Karkabounas, S. Antioxidant Protection during the Menstrual Cycle: The Ef-Fects of Estradiol on Ascorbic- Dehydroascorbic Acid Plasma Levels and Total Antioxidant Plasma Status in Eumenorrhoic Women during the Menstrual Cycle. Acta Obstet. Gyne-Cologica Scand. 2006, 85, 960–965. [Google Scholar] [CrossRef]
- Bednarek-Tupikowska, G.; Tupikowski, K.; Bidzinska, B. Serum Lipid Peroxides and Total Antioxidant Status in Postmenopausal Women on Hormone Replacement Therapy. Gynecol. Endocrinol. 2004, 19, 57–63. [Google Scholar] [CrossRef]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef]
- Barp, J.; Araújo, A.S.R.; Fernandes, T.R.G.; Rigatto, K.V.; Llesuy, S.; Belló-Klein, A.; Singal, P. Myocardial Antioxidant and Oxidative Stress Changes Due to Sex Hormones. Braz. J. Med. Biol. Res. 2002, 35, 1075–1081. [Google Scholar] [CrossRef]
- Junker, A.; Wang, J.; Gouspillou, G.; Ehinger, J.K.; Elmér, E.; Sjövall, F.; Fisher-Wellman, K.H.; Neufer, P.D.; Molina, A.J.A.; Ferrucci, L.; et al. Human Studies of Mitochondrial Biology Demonstrate an Overall Lack of Binary Sex Differences: A Multivariate Meta-Analysis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22146. [Google Scholar] [CrossRef]
- Yang, H.; Yang, R.; Liu, H.; Ren, Z.; Kong, F.; Li, D.; Ma, X. Synergism between PGC-1α and Estrogen in the Survival of Endometrial Cancer Cells via the Mitochondrial Pathway. OncoTargets Ther. 2016, 9, 3963–3973. [Google Scholar] [CrossRef]
- Lu, J.P.; Monardo, L.; Bryskin, I.; Hou, Z.F.; Trachtenberg, J.; Wilson, B.C.; Pinthus, J.H. Androgens Induce Oxidative Stress and Radiation Resistance in Prostate Cancer Cells Though NADPH Oxidase. Prostate Cancer Prostatic Dis. 2010, 13, 39–46. [Google Scholar] [CrossRef]
- Huang, X.; Pan, C.-H.; Yin, F.; Peng, J.; Yang, L. The Role of Estrogen in Mitochondrial Disease. Cell. Mol. Neurobiol. 2025, 45, 68. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Bellanti, F.; Matteo, M.; Rollo, T.; Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex Hormones Modulate Circulating Antioxidant Enzymes: Impact of Estrogen Therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, L.L.; Liu, T.Y.; Wang, Z.T. Evaluation of Gender-Related Differences in Various Oxidative Stress Enzymes in Mice. Chin. J. Physiol. 2011, 54, 385–390. [Google Scholar]
- Alkazemi, D.; Rahman, A.; Habra, B. Alterations in Glutathione Redox Homeostasis among Adolescents with Obesity and Anemia. Sci. Rep. 2021, 11, 3034. [Google Scholar] [CrossRef]
- Chełchowska, M.; Jurczewska, J.; Gajewska, J.; Mazur, J.; Szostak-Węgierek, D.; Rudnicka, E.; Ambroszkiewicz, J. Antioxidant Defense Expressed as Glutathione Status and Keap1-Nrf2 System Action in Relation to Anthropometric Parameters and Body Composition in Young Women with Polycystic Ovary Syndrome. Antioxidants 2023, 12, 730. [Google Scholar] [CrossRef]
- Escalante Gómez, C.; Quesada Mora, S. HRT Decreases DNA and Lipid Oxidation in Postmenopausal Women. Climacteric 2012, 16, 104–110. [Google Scholar] [CrossRef]
- Bousquet, J.; Khaltaev, N.; Cruz, A.A.; Denburg, J.; Fokkens, W.J.; Togias, A.; Zuberbier, T.; Baena-Cagnani, C.E.; Canonica, G.W.; Weel, C. Allergic Rhinitis and Its Impact on Asthma (Aria) 2008 Update (in Collaboration with the World Health Organization, Ga(2)Len and Allergen. Allergy 2008, 63, 8–160. [Google Scholar] [CrossRef]
- Portelli, M.A.; Hodge, E.; Sayers, I. Genetic Risk Factors for the Development of Allergic Disease Identified by Genome-wide Association. Clin. Exp. Allergy 2014, 45, 21–31. [Google Scholar] [CrossRef]
- Heindl, B.; Braunsteiner, T.; Klug, L.; Wantke, F.; Hemmer, W.; Wöhrl, S. Frequency of Positive Allergy Tests in Children, Adults and Seniors. Allergo J. Int. 2022, 31, 81–87. [Google Scholar] [CrossRef]
- Fröhlich, M.; Pinart, M.; Keller, T.; Reich, A.; Cabieses, B.; Hohmann, C.; Keller, T.; Hohmann, C.; Standl, M.; Wijga, A.H.; et al. Differences in the Prevalence of Adults with Allergic Rhinitis by Gender. Clin. Transl. Allergy Internet 2017, 7, 1–9. [Google Scholar]
- Leffler, J.; Stumbles, P.A.; Strickland, D.H. Immunological Processes Driving IgE Sensitisation and Disease Development in Males and Females. Int. J. Mol. Sci. 2018, 19, 1554. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Cebula, M.; Schmidt, E.E.; Arnér, E.S.J. TrxR1 as a Potent Regulator of the Nrf2-Keap1 Response System. Antioxid. Redox Signal. 2015, 23, 823–853. [Google Scholar] [CrossRef]
- Zhang, W.; Tang, R.; Ba, G.; Li, M.; Lin, H. Anti-Allergic and Anti-Inflammatory Effects of Resveratrol via Inhibiting Txnip-Oxidative Stress Pathway in a Mouse Model of Allergic Rhinitis. World Allergy Organ. J. 2020, 13, 100473. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.J.; Nguyen, T.V.; Song, C.H.; Chai, O.H. Mangiferin Alleviates Ovalbumin-Induced Allergic Rhinitis via Nrf2/HO-1/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2020, 21, 3415. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Shin, H.S.; Kim, H.T.; Song, C.H.; Chai, O.H. PM2.5 Exacerbates Oxidative Stress and Inflammatory Response through the Nrf2/NF-κB Signaling Pathway in OVA-Induced Allergic Rhinitis Mouse Model. Int. J. Mol. Sci. 2021, 22, 8173. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Fan, Y.; Piao, C.H.; Nguyen, T.V.; Shin, D.U.; Jung, S.Y.; Hyeon, E.; Song, C.H.; Lee, S.Y.; Shin, H.S. Piper Nigrum Extract Improves Ova-Induced Nasal Epithelial Barrier Dysfunction via Activating Nrf2/Ho-1 Signaling. Cell Immunol. 2020, 351, 104035. [Google Scholar] [CrossRef]
- Ober, C.; Yao, T.-C. The Genetics of Asthma and Allergic Disease: A 21st Century Perspective. Immunol. Rev. 2011, 242, 10–30. [Google Scholar] [CrossRef]
- Mak, J.C.; Chan-Yeung, M.M. Reactive Oxidant Species in Asthma. Curr. Opin. Pulm. Med. 2006, 12, 7–11. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Ivanov, V.P.; Bogomazov, A.D.; Freidin, M.B.; Illig, T.; Solodilova, M.A. Antioxidant Defense Enzyme Genes and Asthma Susceptibility: Gender-Specific Effects and Heterogeneity in Gene-Gene Interactions between Pathogenetic Variants of the Disease. BioMed Res. Int. 2014, 2014, 708903. [Google Scholar] [CrossRef]
- Sahiner, U.M.; Birben, E.; Erzurum, S.; Sackesen, C.; Kalayci, Ö. Oxidative Stress in Asthma: Part of the Puzzle. Pediatr. Allergy Immunol. 2018, 29, 789–800. [Google Scholar] [CrossRef]
- Skalska, M.; Kleniewska, P.; Pawliczak, R. Participation of Reactive Oxygen Species in the Pathogenesis of Atopic Dermatitis, Allergic Rhinitis and Asthma. Alergol. Pol.-Pol. J. Allergol. 2023, 10, 26–31. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Li, N.; Yang, X.; Sun, X.; Tian, H.; Zhang, Y. Mechanism of Action and Therapeutic Implications of Nrf2/HO-1 in Inflammatory Bowel Disease. Antioxidants 2024, 13, 1012. [Google Scholar] [CrossRef]
- Hasanvand, D.; Amiri, I.; Soleimani Asl, S.; Saidijam, M.; Shabab, N.; Artimani, T. Effects of CeO2 Nanoparticles on the HO-1, NQO1, and GCLC Expression in the Testes of Diabetic Rats. Can. J. Physiol. Pharmacol. 2018, 96, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, F. Linoleic Acid Induces Human Ovarian Granulosa Cell Inflammation and Apoptosis through the ER-FOXO1-ROS-NFκB Pathway. Sci. Rep. 2024, 14, 6392. [Google Scholar] [CrossRef] [PubMed]
- Konwar, C.; Asiimwe, R.; Inkster, A.M.; Merrill, S.M.; Negri, G.L.; Aristizabal, M.J.; Rider, C.F.; MacIsaac, J.L.; Carlsten, C.; Kobor, M.S. Risk-Focused Differences in Molecular Processes Implicated in SARS-CoV-2 Infection: Corollaries in DNA Methylation and Gene Expression. Epigenetics Chromatin 2021, 14, 54. [Google Scholar] [CrossRef]
- Farha, S.; Asosingh, K.; Laskowski, D. Effects of the Menstrual Cycle on Lung Function Variables in Women with Asthma. Am. J. Respir. Crit. Care Med. 2009, 180, 304–310. [Google Scholar] [CrossRef]
- Shames, R.S.; Heilbron, D.C.; Janson, S.L.; Kishiyama, J.L.; Au, D.S.; Adelman, D.C. Clinical Differences among Women with and without Self-Reported Perimenstrual Asthma. Ann Allergy Asthma Immunol 1998, 81, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dweik, R.A.; Boggs, P.B.; Erzurum, S.C.; Irvin, C.G.; Leigh, M.W.; Lundberg, J.O.; Olin, A.-C.; Plummer, A.L.; Taylor, D.R. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am. J. Respir. Crit. Care Med. 2011, 184, 602–615. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Sheikh, A. Hormonal Contraceptives and Asthma in Women of Reproductive Age: Analysis of Data from Serial National Scottish Health Surveys. J. R. Soc. Med. 2015, 108, 358–371. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Pillinger, R.; Tibble, H.; Nwaru, B.I.; Tibble, H.; Shah, S.A. Hormonal Contraceptives and Onset of Asthma in Reproductive-Age Women: Population-Based Cohort Study. J. Allergy Clin. Immunol. 2020, 146, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Yung, J.A.; Fuseini, H.; Newcomb, D.C.; Ambhore, N.S.; Kalidhindi, R.S.R.; Loganathan, J. Hormones, Sex, and Asthma. Ann. Allergy Asthma Immunol. 2018, 120, 488–494. [Google Scholar] [CrossRef]
- Fuseini, H.; Newcomb, D.C. Mechanisms Driving Gender Differences in Asthma. Curr. Allergy Asthma Rep. 2017, 17, 19. [Google Scholar] [CrossRef]
- Gandhi, V.D.; Cephus, J.-Y.; Norlander, A.E.; Chowdhury, N.U.; Zhang, J.; Ceneviva, Z.J.; Tannous, E.; Polosukhin, V.V.; Putz, N.D.; Wickersham, N.; et al. Androgen Receptor Signaling Promotes Treg Suppressive Function during Allergic Airway Inflammation. J. Clin. Invest. 2022, 132, e153397. [Google Scholar] [CrossRef]
- Fuseini, H.; Yung, J.A.; Cephus, J.Y.; Zhang, J.; Goleniewska, K.; Polosukhin, V.V.; Peebles, R.S.; Newcomb, D.C. Testosterone Decreases House Dust Mite–Induced Type 2 and IL-17A–Mediated Airway Inflammation. J. Immunol. 2018, 201, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.E.; Robinson, C.B.; Leonard, J.M.; Panettieri, R.A. Nebulized Dehydroepiandrosterone-3-Sulfate Improves Asthma Control in the Moderate-to-Severe Asthma Results of a 6-Week, Randomized, Double-Blind, Placebo-Controlled Study. Allergy Asthma Proc. 2010, 31, 461–471. [Google Scholar] [CrossRef]
- Canguven, O.; Albayrak, S. Do Low Testosterone Levels Contribute to the Pathogenesis of Asthma? Med. Hypotheses 2011, 76, 585–588. [Google Scholar] [CrossRef]
- Maurer, M.; Zuberbier, T.; Metz, M. The Classification, Pathogenesis, Diagnostic Workup, and Management of Urticaria: An Update. In Allergic Diseases–From Basic Mechanisms to Comprehensive Management and Prevention; Springer: Berlin/Heidelberg, Germany, 2021; pp. 117–133. [Google Scholar]
- Tong, L.J.; Balakrishnan, G.; Kochan, J.P.; Kinét, J.-P.; Kaplan, A.P. Assessment of Autoimmunity in Patients with Chronic Urticaria. J. Allergy Clin. Immunol. 1997, 99, 461–465. [Google Scholar] [CrossRef]
- Yoshimaru, T.; Suzuki, Y.; Matsui, T.; Yamashita, K.; Ochiai, T.; Yamaki, M.; Shimizu, K. Blockade of Superoxide Generation Prevents High-affinity Immunoglobulin E Receptor-mediated Release of Allergic Mediators by Rat Mast Cell Line and Human Basophils. Clin. Exp. Allergy 2002, 32, 612–618. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Polaniak, R.; Rogala, B.; Birkner, E. Markers of Antioxidant Defence System and Lipid Peroxidation in Peripheral Blood of Female Patients with Chronic Idiopathic Urticaria. Arch. Dermatol. Res. 2006, 298, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Cristaudo, A.; D’Argento, V.; Cassano, N.; Turbino, L.; Guarrera, M.; Vena, G.; Picardo, M. Oxidative Stress in Physical Urticarias. Clin. Exp. Dermatol. 2001, 26, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Eberlein-König, B.; Placzek, M.; Przybilla, B. Protective Effect against Sunburn of Combined Systemic Ascorbic Acid (Vitamin C) and d-Alpha-Tocopherol (Vitamin, E). J. Am. Acad. Dermatol. 1998, 38, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, D.S.; Wood, S.; Watson, R.R. Modulation of Immune Function and Cytokine Production by Various Levels of Vitamin e Supplementation during Murine AIDS. Immunopharmacology 1995, 29, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bonetta, R. Potential Therapeutic Applications of MnSODs and SOD-Mimetics. Chem.-Eur. J. 2017, 24, 5032–5041. [Google Scholar] [CrossRef]
- Gregoriou, S.; Rigopoulos, D.; Katsambas, A.; Katsarou, A.; Papaioannou, D.; Gkouvi, A.; Kontochristopoulos, G.; Danopoulou, I.; Stavrianeas, N.; Kalogeromitros, D. Etiologic Aspects and Prognostic Factors of Patients with Chronic Urticaria: Nonrandomized, Prospective, Descriptive Study. J. Cutan. Med. Surg. 2009, 13, 198–203. [Google Scholar] [CrossRef]
- Fricke, J.; Ávila, G.; Keller, T.; Weller, K.; Lau, S.; Maurer, M.; Zuberbier, T.; Keil, T. Prevalence of Chronic Urticaria in Children and Adults across the Globe: Systematic Review with Meta-Analysis. Allergy 2020, 75, 423–432. [Google Scholar] [CrossRef]
- Cocchiara, R.; Albeggiani, G.; Di Trapani, G.; Azzolina, A.; Lampiasi, N.; Rizzo, F.; Geraci, D. Modulation of Rat Peritoneal Mast Cell and Human Basophil Histamine Release by Estrogens. Int. Arch. Allergy Immunol. 1990, 93, 192–197. [Google Scholar] [CrossRef]
- Zaitsu, M.; Narita, S.-I.; Lambert, K.C.; Grady, J.J.; Estes, D.M.; Curran, E.M.; Brooks, E.G.; Watson, C.S.; Goldblum, R.M.; Midoro-Horiuti, T. Estradiol Activates Mast Cells via a Non-Genomic Estrogen Receptor-α and Calcium Influx. Mol. Immunol. 2007, 44, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Nguyen Ba, G.; Tew, K.D. Estrogens and Environmental Estrogens. Biomed. Pharmacother. 2002, 56, 36–44. [Google Scholar] [CrossRef]
- Gibbs, C.J.; Coutts, I.I.; Lock, R.; Finnegan, O.C.; White, R.J. Premenstrual Exacerbation of Asthma. Thorax 1984, 39, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Schoepke, N.; Asero, R.; Ellrich, A. Biomarkers and Clinical Characteristics of Autoimmune Chronic Spontaneous Urticaria: Results of the PURIST Study. Allergy 2019, 74, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Ornek, S.A.; Suroji Alkilinc, A.; Kızıltac, U.; Kızıltac, K.; Kocaturk, E. Effect of Puberty, Menstruation, Pregnancy, Lactation, and Menopause on Chronic Urticaria Activity. J. Cutan. Med. Surg. 2023, 27, 466–471. [Google Scholar] [CrossRef]
- Kocatürk, E.; Al-Ahmad, M.; Krause, K.; Gimenez-Arnau, A.M.; Thomsen, S.F.; Conlon, N.; Marsland, A.; Savk, E.; Criado, R.F.; Danilycheva, I.; et al. Effects of Pregnancy on Chronic Urticaria: Results of the PREG-CU UCARE Study. Allergy 2021, 76, 3133–3144. [Google Scholar] [CrossRef]
- Kocatürk, E.; Podder, I.; Zenclussen, A.C.; Kasperska Zajac, A.; Elieh-Ali-Komi, D.; Church, M.K.; Maurer, M. Urticaria in Pregnancy and Lactation. Front. Allergy 2022, 3, 892673. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Rogala, B. Sex Hormones and Urticaria. J. Dermatol. Sci. 2008, 52, 79–86. [Google Scholar] [CrossRef]
- Kasperska-Zajac, A.; Brzoza, Z.; Badura-Brzoza, K.; Matysia-kiewicz, J.; Hese, R.T.; Rogala, B. Does the Dehydroepiandros- Terone Sulphate Decline in Chronic Urticaria Result from Psychological Distress? Allergy 2007, 62, 1640. [Google Scholar]
- Labrie, F.; Luu-The, V.; Bélanger, A.; Lin, S.-X.; Simard, J.; Pelletier, G.; Labrie, C. Is Dehydroepiandrosterone a Hormone? J. Endocrinol. 2005, 187, 169–196. [Google Scholar] [CrossRef]
- Otsuka, A.; Nomura, T.; Rerknimitr, P.; Seidel, J.A.; Honda, T.; Kabashima, K. The Interplay between Genetic and Environmental Factors in the Pathogenesis of Atopic Dermatitis. Immunol. Rev. 2017, 278, 246–262. [Google Scholar] [CrossRef]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative Stress and Atopic Dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Sivaranjani, N.; Venkata Rao, S.; Rajeev, G. Role of Reactive Oxygen Species and Antioxidants in Atopic Dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Ji, H.; Li, X.-K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell. Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Chung, J.; Oh, S.Y.; Shin, Y.K. Association of Glutathione-S-Transferase Polymorphisms with Atopic Dermatitis Risk in Preschool Age Children. Clin. Chem. Lab. Med. 2009, 47, 1475–1481. [Google Scholar] [CrossRef]
- Raimondo, A.; Serio, B.; Lembo, S. Oxidative Stress in Atopic Dermatitis and Possible Biomarkers: Present and Future. Indian. J. Dermatol. 2023, 68, 657–660. [Google Scholar] [CrossRef]
- Peppers, J.; Paller, A.S.; Maeda-Chubachi, T.; Wu, S.; Robbins, K.; Gallagher, K. A Phase 2, Randomized Dose-Finding Study of Tapinarof (GSK2894512 Cream) for the Treatment of Atopic Dermatitis. J. Am. Acad. Dermatol. 2019, 80, 89–98. [Google Scholar] [CrossRef]
- Shibama, S.; Ugajin, T.; Yamaguchi, T.; Yokozeki, H. Bilirubin Oxidation Derived from Oxidative Stress Is Associated with Disease Severity of Atopic Dermatitis in Adults. Clin. Exp. Dermatol. 2019, 44, 153–160. [Google Scholar] [CrossRef]
- Niwa, Y.; Sumi, H.; Kawahira, K.; Terashima, T.; Nakamura, T.; Akamatsu, H. Protein Oxidative Damage in the Stratum Corneum: Evidence for a Link between Environmental Oxidants and the Changing Prevalence and Nature of Atopic Dermatitis in Japan. Br. J. Dermatol. 2003, 149, 248–254. [Google Scholar] [CrossRef]
- Silvestre Salvador, J.; Romero-Pérez, D.; Encabo-Durán, B. Atopic Dermatitis in Adults: A Diagnostic Challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. The Roles of Sex Hormones in the Course of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 4660. [Google Scholar] [CrossRef]
- Roved, J.; Westerdahl, H.; Hasselquist, D. Sex Differences in Immune Responses: Hormonal Effects, Antagonistic Se-Lection, and Evolutionary Consequences. Horm. Behav. 2017, 88, 95–105. [Google Scholar] [CrossRef]
- Namazi, M.R. The Th1-Promoting Effects of Dehydroepiandrosterone Can Provide an Explanation for the Stronger Th1-Immune Response of Women. Iran. J. Allergy Asthma Immunol. 2009, 8, 65–69. [Google Scholar]
- Chen, Y.; Yokozeki, H.; Katagiri, K. Physiological and Functional Changes in the Stratum Corneum Restored by Oestrogen in an Ovariectomized Mice Model of Climacterium. Exp. Dermatol. 2017, 26, 394–401. [Google Scholar] [CrossRef]
- Ormerod, A.; Dwyer, C.; Reid, A.; Copeland, P.; Thompson, W.D. Inducible Nitric Oxide Synthase Demonstrated in Allergic and Irritant Contact Dermatitis. Acta Derm. Venereol. 1997, 77, 436–440. [Google Scholar] [CrossRef]
- Okayama, Y. Oxidative Stress in Allergic and Inflammatory Skin Diseases. Curr. Drug Target.-Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Albanesi, C. Keratinocytes in Allergic Skin Diseases. Curr. Opin. Allergy Clin. Immunol. 2010, 10, 452–456. [Google Scholar] [CrossRef]
- Corsini, E.; Galbiati, V.; Nikitovic, D.; Tsatsakis, A.M. Role of Oxidative Stress in Chemical Allergens Induced Skin Cells Activation. Food Chem. Toxicol. 2013, 61, 74–81. [Google Scholar] [CrossRef]
- Kerstan, A.; Bröcker, E.-B.; Trautmann, A. Decisive Role of Tumor Necrosis Factor-α for Spongiosis Formation in Acute Eczematous Dermatitis. Arch. Dermatol. Res. 2011, 303, 651–658. [Google Scholar] [CrossRef]
- Kerstan, A.; Leverkus, M.; Trautmann, A. Effector Pathways during Eczematous Dermatitis: Where Inflammation Meets Cell Death. Exp. Dermatol. 2009, 18, 893–899. [Google Scholar] [CrossRef]
- Martın, A.P.; Gagliardi, J.; Baena-Cagnani, C.E. Expres- Sion of CS-1 Fibronectin Precedes Monocyte Chemoat-Tractant Protein-1 Production during Elicitation of Allergic Contact Der-Matitis. Clin. Exp. Allergy 2003, 33, 1118–1124. [Google Scholar] [CrossRef]
- Sander, C.S.; Thiele, J.J. Oxidative Stress. In Irritant Dermatitis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 375–382. [Google Scholar] [CrossRef]
- Gangemi, S.; Ricciardi, L.; Minciullo, P.L.; Cristani, M.; Saitta, S.; Chirafisi, J.; Spatari, G.; Santoro, G.; Saija, A. Serum Levels of Protein Oxidation Products in Patients with Nickel Allergy. Allergy Asthma Proc. 2009, 30, 552–557. [Google Scholar] [CrossRef]
- Brans, R.; Dickel, H.; Bruckner, T.; Coenraads, P.J.; Heesen, M.; Merk, H.F.; Blömeke, B. MnSOD Polymorphisms in Sensitized Patients with Delayed-Type Hypersensitivity Reactions to the Chemical Allergen Para-Phenylene Diamine: A Case-Control Study. Toxicology 2005, 212, 148–154. [Google Scholar] [CrossRef]
- Kaur, S.; Zilmer, K.; Leping, V.; Zilmer, M. Allergic Contact Dermatitis Is Associated with Significant Oxidative Stress. Dermatol. Res. Pract. 2014, 2014, 415638. [Google Scholar] [CrossRef]
- Cavani, A.; Pità, O.; Girolomoni, G. New Aspects of the Molecular Basis of Contact Allergy. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 404–408. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in Inflammation and Metabolic Disease. Nat. Rev. Immunology 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Tilg, H. Adipocytokines in Nonalcoholic Fatty Liver Disease: Key Players Regulating Steatosis, Inflammation and Fibro-Sis. Curr. Pharm. Des. 2010, 16, 1893–1894. [Google Scholar] [CrossRef]
- Martin, S.S.; Qasim, A.N.; Rader, D.J.; Reilly, M.P. C- Reactive Protein Modifies the Association of Plasma Leptin with Coronary Calcium in Asymptomatic Overweight Individuals. Obesity 2012, 20, 856–861. [Google Scholar] [CrossRef]
- Boonchai, W.; Likittanasombat, S.; Viriyaskultorn, N.; Kanokrungsee, S. Gender Differences in Allergic Contact Dermatitis to Common Allergens. Contact Dermat. 2024, 90, 458–465. [Google Scholar] [CrossRef]
- Koskelo, M.; Sinikumpu, S.P.; Jokelainen, J.; Huilaja, L. Risk Factors of Hand Eczema: A Population-Based Study among 900 Subjects. Contact-Matitis 2022, 87, 485–491. [Google Scholar] [CrossRef]
- Mauro, M.; Bovenzi, M.; Larese, F.F. Occupational Contact Dermatitis in a Gender Perspective: North East Italian Data 1996–2016. Med. Lav. 2021, 112, 34–43. [Google Scholar] [CrossRef]
- Scherrer, M.A.R.; Abreu, É.P.; Rocha, V.B. Neomycin: Sources of Contact and Sensitization Evaluation in 1162 Patients Treated at a Tertiary Service. An. Bras. Dermatol. 2023, 98, 487–492. [Google Scholar] [CrossRef]
- Brasch, J.; Schnuch, A.; Uter, W. The Profile of Patch Test Reactions to Common Contact Allergens Is Related to Sex. Contact Dermat. 2008, 58, 37–41. [Google Scholar] [CrossRef]
- Ahlström, M.G.; Thyssen, J.P.; Wennervaldt, M.; Menné, T.; Johansen, J.D. Nickel Allergy and Allergic Contact Dermatitis: A Clinical Review of Immunology, Epidemiology, Exposure, and Treatment. Contact Dermat. 2019, 81, 227–241. [Google Scholar] [CrossRef]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; Di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef]
- Aronson, J.K. Defining ‘Nutraceuticals’: Neither Nutritious nor Pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8–19. [Google Scholar] [CrossRef]
- Alessandrello, C.; Sanfilippo, S.; Minciullo, P.L.; Gangemi, S. An Overview on Atopic Dermatitis, Oxidative Stress, and Psychological Stress: Possible Role of Nutraceuticals as an Additional Therapeutic Strategy. Int. J. Mol. Sci. 2024, 25, 5020. [Google Scholar] [CrossRef]
- London, N.R., Jr.; Tharakan, A.; Lane, A.P.; Biswal, S.; Ramanathan, M., Jr. Nuclear Erythroid 2-Related Factor 2 Acti-Vation Inhibits House Dust Mite-Induced Sinonasal Epithelial Cell Barrier Dysfunction. Int. Forum Allergy Rhinol. 2017, 7, 536–541. [Google Scholar] [CrossRef]
- Yusin, J.; Wang, V.; Henning, S.M.; Yang, J.; Tseng, C.H.; Thames, G.; Arnold, I.; Heber, D.; Lee, R.P.; Sanavio, L. The Effect of Broccoli Sprout Extract on Seasonal Grass Pollen-Induced Allergic Rhinitis. Nutrients 2021, 13, 1337. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Shen, L. Preliminary Clinical Effect Evaluation of Resveratrol in Adults with Allergic Rhinitis. Int. Arch. Allergy Immunol. 2018, 175, 231–236. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Y.; Li, F.; Wu, W.; Xie, D.; Feng, Y. In Vitro and in Vivo Antiallergic Effects of Taurine on Allergic Rhinitis. Int. Arch. Allergy Immunol. 2020, 181, 404–416. [Google Scholar] [CrossRef]
- Norton, R.L.; Hoffmann, P.R. Selenium and Asthma. Mol. Asp. Med. 2012, 33, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mabalirajan, U.; Aich, J.; Leishangthem, G.D.; Sharma, S.K.; Dinda, A.K.; Ghosh, B. Effects of Vitamin E on Mitochondrial Dysfunction and Asthma Features in an Experimental Allergic Murine Model. J. Appl. Physiol. 1985, 107, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.; Kwon, O.-K.; Oh, S.-R.; Lee, H.-K.; Ahn, K. Anti-Inflammatory and Anti-Asthmatic Effects of Resveratrol, a Polyphenolic Stilbene, in a Mouse Model of Allergic Asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar] [CrossRef]
- Baïz, N.; Chastang, J.; Ibanez, G.; Annesi-Maesano, I. Prenatal Exposure to Selenium May Protect against Wheezing in Children by the Age of 3. Immun. Inflamm. Dis. 2016, 5, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Y.; Feng, J.; Jiang, X.-F.; Xiao, W.-F.; Chen, X.-X. Antioxidant and Anti-Inflammatory Effects of Schisandra and Paeonia Extracts in the Treatment of Asthma. Exp. Ther. Med. 2014, 8, 1479–1483. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Luo, J. The Protective Role of Zingerone in a Murine Asthma Model via Activation of the AMPK/ Nrf2/HO-1 Pathway. Food Funct. 2021, 12, 3120–3131. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, J.; Ma, Z.; Ma, S. Anti-Asthmatic Effects of Matrine in a Mouse Model of Allergic Asthma. Fitoterapia 2014, 94, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Landi, C.; Cameli, P.; Vantaggiato, L.; Bergantini, L.; d’Alessandro, M.; Perruzza, M.; Carleo, A.; Shaba, E.; Di Giuseppe, F.; Angelucci, S.; et al. Ceruloplasmin and Oxidative Stress in Severe Eosinophilic Asthma Patients Treated with Mepolizumab and Benralizumab. Biochim. Biophys. Acta BBA-Proteins Proteom. 2021, 1869, 140563. [Google Scholar] [CrossRef]
- Bousquet, J.; Cabrera, P.; Berkman, N.; Viswanathan, R.K.; Moss, M.H.; Mathur, S.K. The Effect of Treatment with Omalizumab, an Anti-IgE Antibody, on Asthma Exacerbations and Emergency Medical Visits in Patients with Severe Persistent Asthma. Allergy 2005, 60, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pace, S.; Pergola, C.; Dehm, F. Androgen-mediated sex bias impairs efficiency of leukotriene biosynthesis inhibitors in males. J. Clin. Investig. 2017, 127, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Cassano, N.; Raho, G.; Filieri, M.; D’Argento, V.; Amoruso, A.; Filotico, R.; Vena, G.A. Influence of Desloratadine on Oxidative Stress Markers in Patients with Chronic Idiopathic Urticaria. Int. J. Dermatol. 2006, 45, 394–396. [Google Scholar] [CrossRef]
- Yang, H.; Chen, J.S.; Luo, X.Y.; Wang, H. Efficacy and Safety Profile of Antioxidants in the Treatment of Atopic Dermatitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Dermatol. Ther. 2022, 35, e15549. [Google Scholar] [CrossRef]
- Gehlsen, K.R. Methods and Compositions for Topical Treatment of Damaged Tissue Using Reactive Oxygen Metabolite Production or Release Inhibitors. US6270781B1, 7 August 2001. [Google Scholar]
- Fujii, K.; Kawabe, T.; Hosoe, K.; Hidaka, T. Dermal Compositions Containing Coenzyme Q as the Active Ingredient. US20050070610A1, 2011. [Google Scholar]
- Murphy, M.P.; Smith, R.A.J.; Taylor, K.M. Compositions and Methods for Skin Care. US20090258841A1, 2009. [Google Scholar]
- Travica, N.; Teasdale, S.; Marx, W. Nutraceuticals in Mood Disorders: Current Knowledge and Future Directions. Curr. Opin. Psychiatry 2022, 36, 54–59. [Google Scholar] [CrossRef]
- Bar-Sela, G.; Cohen, M.; Ben-Arye, E.; Epelbaum, R. The Medical Use of Wheatgrass: Review of the Gap Between Basic and Clinical Applications. Mini-Rev. Med. Chem. 2015, 15, 1002–1010. [Google Scholar] [CrossRef]
- Jurendić, T.; Ščetar, M. Aronia Melanocarpa Products and By-Products for Health and Nutrition: A Review. Antioxidants 2021, 10, 1052. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Park, N.-J.; Jegal, H.; Paik, J.-H.; Choi, S.; Kim, S.-N.; Yang, M.H. Nymphoides Peltata Root Extracts Improve Atopic Dermatitis by Regulating Skin Inflammatory and Anti-Oxidative Enzymes in 2,4-Dinitrochlorobenzene (DNCB)-Induced SKH-1 Hairless Mice. Antioxidants 2023, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.X.; Lyu, J.L.; Wu, P.Y.; Wen, K.C.; Chang, C.C.; Chiang, H.M. Coffea Arabica Extract Attenuates Atopic Dermatitis-like Skin Lesions by Regulating NLRP3 Inflammasome Expression and Skin Barrier Functions. Int. J. Mol. Sci. 2023, 24, 12367. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Kang, M.C.; Jin, M.; Lee, T.H.; Lim, B.O.; Kim, S.Y. Fermented Blueberry and Black Rice Containing Lactobacillus Plantarum MG4221: A Novel Functional Food for Particulate Matter (PM2.5)/Dinitrochlorobenzene (DNCB)-Induced Atopic Dermatitis. Food Funct. 2021, 12, 3611–3623. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Hashimoto-Hachiya, A.; Tsuji, G.; Murai, M.; Yan, X.; Furue, M. Upregulation of FLG, LOR, and IVL Expression by Rhodiola Crenulata Root Extract via Aryl Hydrocarbon Receptor: Differential Involvement of OVOL1. Int. J. Mol. Sci. 2018, 19, 1654. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristallo, M.; Furci, F.; Casciaro, M.; Gangemi, S.; Nettis, E. Sex Differences in Oxidative Stress Concerning Allergic Diseases. Biomolecules 2025, 15, 1461. https://doi.org/10.3390/biom15101461
Cristallo M, Furci F, Casciaro M, Gangemi S, Nettis E. Sex Differences in Oxidative Stress Concerning Allergic Diseases. Biomolecules. 2025; 15(10):1461. https://doi.org/10.3390/biom15101461
Chicago/Turabian StyleCristallo, Mattia, Fabiana Furci, Marco Casciaro, Sebastiano Gangemi, and Eustachio Nettis. 2025. "Sex Differences in Oxidative Stress Concerning Allergic Diseases" Biomolecules 15, no. 10: 1461. https://doi.org/10.3390/biom15101461
APA StyleCristallo, M., Furci, F., Casciaro, M., Gangemi, S., & Nettis, E. (2025). Sex Differences in Oxidative Stress Concerning Allergic Diseases. Biomolecules, 15(10), 1461. https://doi.org/10.3390/biom15101461





